Abstract
Nicotine exposure during development can alter behavior in adulthood in mice. One route of nicotine administration that can mimic some of the dynamics of human smoking is administration of the drug to pregnant and nursing mice through the drinking water. It is critical to determine if nicotine administration has an impact on maternal behavior as such changes could lead to persistent behavioral alterations in the offspring, independent of the neuropharmacological effects of the drug. While a number of studies have detected nicotine exposure-induced changes, the effects of nicotine administration through the drinking water on maternal behavior in mice have not been examined comprehensively. In the current study we have compared maternal behaviors of C57BL6/J mice exposed to nicotine in the drinking water to behaviors of animals exposed to saccharin (vehicle) in the drinking water for the first 7 days after birth of their litters and find no significant between-group differences in any behaviors measured except passive nursing. We have also assessed the effects of nicotine administration through the drinking water on postnatal weight gain of the pups and find no significant differences between groups. Open-field locomotor activity differences between exposed and unexposed offspring in adolescence were also assessed, with transient hyperactivity detected in nicotine-exposed mice. These data suggest that behavioral differences identified between animals exposed to nicotine through maternal drinking water administration are primarily due to the neuropharmacological effects of the drug and not due to effects of exposure on maternal behavior.
Keywords: Nicotine, drinking water administration, maternal behavior
Introduction
Tobacco smoke exposure during pregnancy results in a number of deleterious health effects for both mother and offspring (1). Prominent among these is an increased risk for attention deficit hyperactivity disorder (ADHD) and other non-clinical cognitive issues in children of smoking mothers, suggesting that a component in tobacco can adversely affect neurodevelopment (1). Of the approximately 4000 substances in tobacco (2), nicotine is a known neuromodulator, with psychoactive properties (3), likely due to the widespread expression of nicotinic acetylcholine receptors (nAChRs) throughout the developing central nervous system (4).
A number of methods of administration during pregnancy and the early postnatal period have been employed in rodents to assess the potential of nicotine to alter neurodevelopment (1). Among these is administration by drinking water, which results in significant blood cotinine levels in the dam and the offspring prenatally via the placenta (5). Nicotine exposure also continues postnatally via maternal milk consumption by the pups (6,7). Administration through the drinking water minimizes nicotine-induced hypoxia, maternal stress and injury risk and is suggested to be a good model for human exposure patterns as mice consume water, and therefore nicotine, primarily during their active phase (5,8). Studies of offspring exposed to nicotine using this method have identified differences in several behavioral and physiological parameters including locomotor activity, preference for cocaine and nicotine, trace fear conditioning, learned helplessness, genital development and hypothermic response to acute nicotine exposure (5,7–9).
One potential confound of studies using maternal drinking water exposure to evaluate the neurodevelopmental effects of nicotine in offspring is that drug exposure could alter maternal behavior. To date, no comprehensive evaluation of the maternal behavior of mice exposed to nicotine via drinking water has been conducted. Maternal behavior, particularly licking and grooming of the pups during the first postnatal week, can have profound effects on the subsequent behavior and molecular-level characteristics of the offspring, with accumulating evidence indicating that persistent alterations in epigenetic regulatory mechanisms, and therefore gene expression, can be induced by altered maternal behavior (10–13). Therefore, if drinking water nicotine consumption has persistent effects on maternal behavior during this critical period, it is conceivable that this could induce the alterations observed in exposed offspring, independent of any direct neuropharmacological effect mediated by nicotine itself.
To assess this possibility, we have analyzed the maternal behaviors of nursing C57BL6/J mice administered nicotine in the drinking water, or a saccharin (vehicle) control solution. Treated drinking water was provided throughout pregnancy and continued until the offspring were weaned at postnatal day 21. The maternal behavior analysis was conducted during the first 7 postnatal days, the period during which differences in these behaviors have the most prominent effects on behavior of the offspring (10). The postnatal weight gain of both exposed and unexposed offspring was also monitored and adolescent open-field locomotor activity was subsequently assessed as a measure of developmental nicotine-mediated behavioral change in the offspring.
Material and Methods
Animals and oral nicotine administration paradigm
All procedures involving animals in this study were approved by the Yale University Institutional Animal Care and Use Committee in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. C57BL6/J mice (Jackson Laboratories, Bar Harbor, ME) were initially housed in a colony room with ad libitum access to standard laboratory chow and untreated drinking water on a 12 hour light-dark cycle (lights on: 7AM). Following acclimation after delivery (at least 7 days), mice used for analysis of maternal behavior were transferred to a satellite housing facility with an altered 12 hour light-dark cycle (lights on: 1PM) to facilitate behavioral observations during both the light and dark cycles. Following acclimation to the light-dark cycle (at least 14 days), females were mated in harems and singly-housed upon identification of a vaginal sperm plug. Plugged females were immediately given ad libitum access to drinking water containing 200 μg/mL nicotine (hydrogen tartrate salt calculated with respect to the free base) and 2% saccharin (w/v) or pH-matched 2% saccharin (w/v) and 0.2% (v/v) tartaric acid (Sigma Aldrich, St. Louis, MO) as their sole water supply. Drinking solutions were changed twice weekly.
The 200 μg/mL nicotine dose was selected as it has been shown previously to have both behavioral and biochemical efficacy in the adult mouse (14–17) and can induce alterations in neurodevelopment (5). This dose also has minimal effects on pregnancy dynamics in the C57BL/6J strain, inducing a modest reduction in maternal fluid volume intake, but with no effect on maternal food intake or weight gain during pregnancy or on offspring in utero weight (5), therefore eliminating these as potential confounding factors in our maternal behavior analysis. While lower doses have been administered to mice via this route and have been found to alter offspring development (7,8), we hypothesized that the probability of detecting changes in maternal care induced by nicotine consumption would be maximized at this higher dose. All animals had ad libitum access to standard laboratory chow at all times.
Measurement of maternal behavior
Births were monitored daily and the day of birth was designated as postnatal day (PND) 0. Aside from routine husbandry, animals were left undisturbed from PND 0 to PND 7. Maternal behavior was monitored twice daily on PND 0-7 at 10AM (with lights on) and 4PM (lights off with the room under red light illumination) for 60 min/session. During each session, maternal behavior was assessed by a scorer blind to treatment group based on previously published methods (13). Briefly, every 4 mins the scorer noted the behavior(s) in which the dam was engaged, for a total of 15 observations of each dam for each session. Behaviors monitored were: licking and grooming of pups, snout contact with pups, nest building/arrangement, passive nursing, arched back nursing and no contact with pups. Any nursing behavior which could not be scored definitively as arched back due to maternal posture was classified as ‘questionable arched back nursing’ (see Table 1 for further details of the monitored behaviors).
Table 1. Descriptions of observed behaviors scored for this study.
Summary of characteristics necessary for each defined behavior to be counted.
|
Maternal behaviors | |
| Licking/grooming | Dam licks pups |
| Snout contact | Dam touches pups with snout with no licking apparent |
| Nesting | Any nest rearrangement/modification behavior |
| Passive nursing | Pups nursing while dam lying prone/on top of/beside pups with no muscle tone |
| Questionable arched back nursing | Nursing posture intermediate between passive and arched back |
| Arched back nursing | Active nursing posture with dam exhibiting significant muscle tone with pups collected beneath arch |
| Total nursing | Time spent in any nursing activity listed above |
|
Non-maternal behaviors | |
| Self-grooming | Dam cleaning own fur |
| No contact | Dam engaged in activity with no pup contact (feeding/drinking etc.) |
In an independent cohort of nicotine-exposed and control litters, pup weights were recorded on PND 0, 7, 14 and 21 (weaning). At weaning, offspring were housed in same sex groups (maximum of 5 animals per group) and received ad libitum access to standard laboratory chow and untreated drinking water. Mice were again weighed at PND 31-32 and open-field locomotor activity was assessed.
Open-field locomotor activity
On PND 31-32, mice were weighed and open-field locomotor activity was assessed. Animals were placed individually in clear rat cages with no bedding material and locomotor activity was measured in 5 min bins for a 30 min session using an Opto-Max v2.27B infra-red beam locomotor activity meter system (Columbus Instruments Inc., Columbus, OH, USA). After mice were removed, fecal boli and urine patches were counted as a measure of open-field anxiety (18–23).
Statistical Analysis
Maternal behaviors are presented as the percentage of time spent engaged in each scored behavior, (i.e., the number of recorded instances for each session divided by the total number of observation windows per session). Maternal behaviors, offspring weight and open-field locomotor activity were analyzed by repeated measures analysis of variance (rmANOVA) followed by post-hoc one-way ANOVA. Within-subject effects are reported using Greenhouse-Geisser corrected terms when data were found to be non-spherical according to Mauchly’s test of sphericity (i.e., when Mauchly’s p<0.05). All data are presented as means ± standard error of mean (SEM) and p-values of less than 0.05 were considered significant.
Results
Drinking water nicotine administration has a minimal effect on maternal behaviors
Analysis of licking and grooming of pups revealed no significant difference between dams administered nicotine in the drinking water (n = 9) and dams administered saccharin alone (n = 6), indicating that this critical non-nursing maternal behavior was unaltered by nicotine exposure (Fig. 1). Similarly, snout contact with pups and time spent engaged in nest building/arrangement behaviors were found to be unaffected by nicotine consumption (Table 2). Within-subject analyses of each of these behaviors indicated a significant effect of day, with the percentage of the observation sessions spent performing these three behaviors significantly reduced in both treatment groups by PND 7 (Table 2).
Figure 1. Maternal licking and grooming of pups is not altered by nicotine administration in the drinking water.
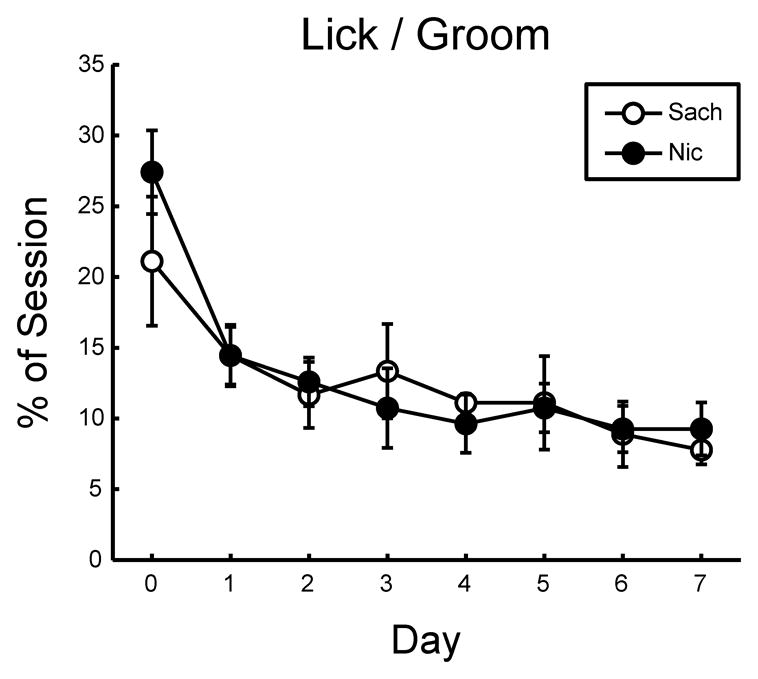
Percent time spent licking and grooming pups during observation sessions was not significantly different between nicotine-drinking (Nic) and saccharin-drinking (Sach) dams (F(1,25) = 0.073; p > 0.05). No main effect of observation time (AM or PM) was observed, so the two sessions are shown collapsed for each day within each treatment group. Within-subject analysis indicated a significant reduction in pup licking and grooming over days (Mauchly’s p = 0.85; F(7,175) = 9.135; p < 0.05).
Table 2. Summary of other behaviors scored during maternal care analysis.
Means and SEM are shown for each treatment group in each daily scoring session for the first (PND 0) and last (PND 7) days of observation. Results of the rmANOVA across all scoring sessions and all observation days (PND 0–7) are shown, with day as the repeated measure.
| Behavior | Time | Day 0 |
Day 7 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sach | Sach | Nic | Nic | Sach | Sach | Nic | Nic | rmANOVA (Days 0–7) | ||
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | Mauchly, F-stat, p-value | ||
| Snout Contact | AM | 3.33 | 2.28 | 8.15 | 1.48 | 0.00 | 0.00 | 1.48 | 0.98 | Ttmt: -, 1.11, 0.30 |
| PM | 4.45 | 1.41 | 2.96 | 1.61 | 3.33 | 2.28 | 0.74 | 0.74 | Day: 0.014, 3.54, 0.006 * | |
| Time: -, 0.001, 0.98 | ||||||||||
| Nesting | AM | 6.67 | 3.44 | 9.63 | 5.10 | 3.33 | 2.28 | 0.74 | 0.74 | Ttmt: -, 0.64, 0.43 |
| PM | 5.56 | 2.05 | 3.70 | 2.25 | 1.11 | 1.11 | 1.48 | 0.98 | Day: 0.000, 3.91, 0.005 * | |
| Time: -, 0.54, 0.47 | ||||||||||
| Self-Grooming | AM | 11.11 | 2.22 | 11.11 | 1.57 | 10.00 | 5.09 | 7.41 | 2.34 | Ttmt: -, 2.64, 0.12 |
| PM | 14.45 | 6.76 | 8.89 | 1.57 | 12.22 | 5.28 | 5.93 | 2.34 | Day: 0.32, 1.50, 0.17 | |
| Time: -, 0.071, 0.79 | ||||||||||
| No Contact | AM | 3.33 | 2.28 | 3.70 | 2.96 | 18.89 | 9.18 | 3.70 | 3.70 | Ttmt: -, 1.44, 0.24 |
| PM | 8.89 | 5.07 | 6.67 | 2.48 | 35.56 | 3.72 | 29.63 | 11.39 | Day: 0.000, 2.16, 0.075 | |
| Time: -, 21.1, 0.000* | ||||||||||
p<0.05
With regard to nursing behaviors, only 7–12% of nursing time was spent in passive nursing, but a significant effect of treatment was detected on this measure with nicotine-exposed dams performing this behavior for a significantly greater percentage of time during the observation sessions than controls (Fig. 2A). A significant effect of day was detected in both ‘questionable’ arched back nursing and arched back nursing behaviors (Fig. 2B–C), with an effect of observation time also observed in arched back nursing. No effect of nicotine treatment group was observed on these measures.
Figure 2. Passive nursing is increased in dams drinking nicotine.
A. A main effect of treatment on the percentage of time spent passive nursing was detected (F(1, 25) = 8.193; p<0.05). Inset: Post-hoc one-way ANOVA on passive nursing data collapsed across days confirmed a significant main effect of treatment on this behavior (F(1, 14) = 4.905; p<0.05). B. No effect of treatment group on questionable arched back nursing was detected (F(1,25) = 0.20; p>0.05). As no effect of time of observation (AM or PM) was detected, data from both AM and PM sessions have been combined. Within-subject analysis indicated a significant increase in questionable arched back nursing across days (Mauchly’s p = 0.12; F(7, 175) = 7.356; p<0.05). C. A main effect of time of observation (AM or PM) was detected on arched back nursing (F(1, 25) = 4.691; p<0.05). No main effect of treatment or interaction was detected (F(1,25) = 0.14; p>0.05). Within-subject analysis indicated a significant effect of day (Mauchly’s p = 0.16; F(7, 175) = 4.709; p<0.05), with significantly less time spent engaged in arched back nursing as the pups grew older. D. Analysis of all nursing behavior subtypes combined indicated no significant differences between treatment groups (F(1,25) = 1.95; p>0.05) but did identify a main effect of time of observation, with significantly more nursing behavior observed in the AM sessions (F(1,25) = 8.365; p<0.05).
To determine if the amount of time engaged in any nursing behavior overall was different between treatment groups, the three behavioral subtypes were combined into a ‘total nursing’ parameter. No effect of treatment was detected in this analysis, though a main effect of observation time was observed, with significantly less time spent engaged in nursing behavior during the 4PM observation sessions by both treatment groups (Fig. 2D).
Regarding non-maternal behaviors, no effects of nicotine consumption were detected on self-grooming behavior or on time spent by dams not in contact with pups (Table 2).
Maternal drinking water nicotine exposure does not affect litter size or offspring sex balance
In an independent cohort of animals (Nicotine = 6 litters; Saccharin = 7 litters), maternal drinking water nicotine consumption had no significant effect on the number of pups born (Sach = 7.14 ± 0.80 pups vs. Nic = 8.33 ± 0.84; t(10.8) = −1.02; p = 0.33), or on the number of female (Sach = 3.43 ± 0.72 vs. Nic = 4.00 ± 0.58; t(10.8) = −0.62; p = 0.55) or male (Sach = 3.71 ± 0.36 vs. Nic = 4.33 ± 0.76; t(7.19) = −0.74; p = 0.49) pups born per litter. Among these litters, only 1 pup, from a nicotine-treated dam, was found dead immediately after birth.
Assessment of postnatal weight gain, mortality and locomotor activity
Between birth and PND 31-32 (the time of locomotor activity testing) for the independent cohort described above, an average of 0.43 ± 0.30 saccharin-treated and 0.67 ± 0.49 nicotine-treated pups died per litter, with no significant effect of treatment on postnatal mortality (t(8.36) = −0.41; p = 0.69).
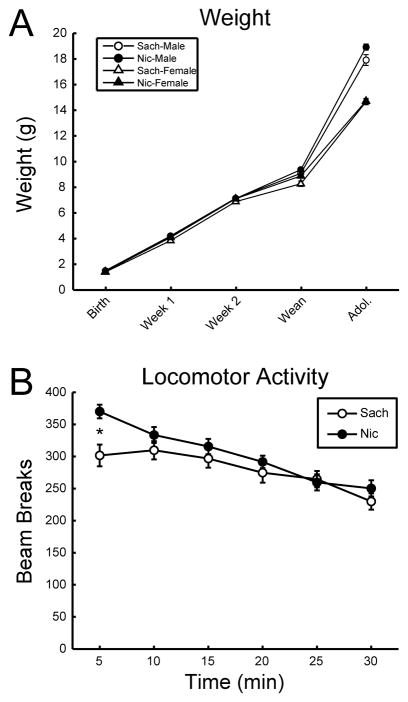
As an index of overall postnatal development, the weight gain of these offspring was monitored from birth every 7 days until weaning at PND 21 and also in early adolescence (PND 31-32). As expected, a significant main effect of sex was detected, with male offspring significantly heavier than female offspring; however, no significant difference in weight gain was detected between the treatment groups (Fig. 3A).
Figure 3. Exposure to nicotine through maternal drinking water has no significant effect on pup growth but induces an increased response to novelty during adolescence.
A. Postnatal weight of nicotine-exposed and saccharin-exposed offspring was recorded at PND 0, 7, 14, 21 (weaning) and 31-32 (adolescence). A significant main effect of sex was detected (F(1, 89) = 55.464; p<0.05). No main effect of treatment was detected (F(1,89) = 2.48; p>0.05). B. Open-field locomotor activity of adolescent (PND 31-32) mice exposed to nicotine (Nic) or saccharin (Sach) from conception through weaning shows no differences between the treatment groups (F(1,89) = 2.56; p>0.05). A significant interaction between recording bin and treatment was detected (Mauchly’s p<0.05; F(3.737, 332.607) = 4.372; p<0.05) and subsequent post-hoc analysis of each bin indicated significant hyperactivity in the nicotine group in the first 5 min bin (F(1, 92) = 11.576; p<0.05).
At the time of locomotor activity testing (PND 31-32), there was no effect of treatment on the number of pups per litter (6.71 ± 0.78 pups per saccharin-treated litter vs. 7.67 ± 0.92 pups per nicotine litter, (t(10.3) = −0.79; p = 0.45) or in the number of female (Sach = 3.29 ± 0.75 vs. Nic = 3.67 ± 0.76; t(10.9) = −0.36; p = 0.73) or male (Sach = 3.43 ± 0.20 vs. Nic = 4.00 ± 0.77; t(5.68) = −0.71; p = 0.50) pups per litter. All surviving offspring (Sach = 24 males and 23 females; Nic = 24 males and 22 females) were tested for locomotor activity.
Analysis of adolescent open-field locomotor activity in these mice indicated no overall differences between nicotine-exposed and control animals. However, within-subject analysis detected a significant interaction between treatment group and recording bin (Fig. 3B). Subsequent one-way ANOVA indicated nicotine-exposed animals were significantly more active, relative to controls, in the first 5 min recording bin, suggesting a heightened response to novelty in these mice (Fig. 3B). Male mice also produced a significantly greater number of fecal boli in the open-field apparatus (6.67 ± 0.30 boli), relative to females (5.33 ± 0.31 boli), potentially suggesting increased generalized anxiety (F(1,89) = 10.1; p = 0.002), though no effect of treatment was observed on this parameter (Sach = 6.28 ± 0.27 vs. Nic = 5.76 ± 0.36; F(1,89) = 1.68; p = 0.20) or in the number of urine patches observed (Sach = 1.55 ± 0.14 vs. Nic = 1.87 ± 0.13; F(1,89) = 2.56; p = 0.11).
Discussion
The experiments presented here show that nicotine administration through drinking water in mice has a very limited impact on maternal behavior. Specifically, no changes were seen in pup licking and grooming during the first postnatal week, the time period in which maternal contact is known to result in the most profound changes in later behavior of pups. Changes in overall tactile stimulation of rodent pups have persistent effects on stress-related behaviors in the offspring (10–13,24). Thus it is particularly critical that no changes in maternal licking and grooming of the offspring, maternal snouting or nest arrangement/maintenance behaviors (in which the dam also adjusts the positions of members of her litter) were observed, suggesting that the overall amount of maternal tactile stimulation of the pups was also consistent, regardless of treatment group. The finding that both licking/grooming and nest maintenance/arrangement behaviors decreased significantly across the first postnatal week is also consistent with a maternal care analysis of untreated C57BL/6J dams at baseline (25) and therefore suggests that oral nicotine and/or saccharin administration has little effect on these critical maternal care behaviors in this strain. The consistency between maternal behaviors assessed by a physically present investigator in this and other studies (13) and by remote camera monitoring in another (25) also indicates minimal maternal behavior disruption is caused by the presence of an investigator.
The only significant difference detected between the treatment groups was an increase in the time spent by nicotine-exposed dams in a passive nursing posture, while no significant differences in the time spent in arched back nursing or nursing behavior in general was observed. This is of interest since both tobacco smoke and nicotine can affect maternal prolactin levels and therefore milk production (26–28). Therefore, an increase in passive nursing, although only a small percentage of total nursing time, may represent a behavioral modification to compensate for any nicotine-induced changes in milk production.
Regarding the postnatal analysis of the pups, the lack of a weight gain difference between pups from nicotine and control litters and the increased weight of male compared to female offspring is consistent with other reports using this nicotine delivery method at a much lower dose (50 vs. 200 μg/mL) (7). This suggests that, while nicotine exposure may induce growth retardation during fetal development through a number of mechanisms, including placental damage, hypoxia and anorexic effects in the mother (5,29), these effects are minimal when this dose range is administered to mice in the drinking water. This is consistent with the lack of an effect of drinking water nicotine exposure on maternal food consumption and weight gain during pregnancy and offspring in utero weight reported previously (5).
The elevated horizontal locomotor activity observed in the nicotine-exposed offspring after 5 minutes in a novel environment potentially suggests a heightened response to novelty in animals exposed to nicotine during perinatal development. This is consistent with studies showing increased activity and reduced habituation to a novel environment in rats exposed to nicotine during gestation via maternal osmotic mini-pump (30,31). The elevation in response to novelty in adolescence may therefore be an important marker of the neurodevelopmental consequences of nicotine and indicates that administration of nicotine through maternal drinking water resulted in significant exposure of the pups, as it induced persistent behavioral effects.
Taken together, this study shows that nicotine administration through the maternal drinking water results in significant exposure to the pups with little change in maternal care. Critically, these data indicate that changes in maternal behaviors are not likely to contribute significantly to persistent behavioral changes in the offspring following drinking water administration of nicotine. Furthermore, this study demonstrates that maternal nicotine consumption via this route does not induce growth retardation in the exposed pups. By excluding altered maternal care and mechanisms such as fetal hypoxia which could underlie offspring growth retardation, any physiological, biochemical or behavioral differences detected in mice exposed to nicotine during pre- and early postnatal development through maternal drinking water, such as the heightened novelty response reported here, are likely due to the direct neuropharmacological effects of nicotine. This provides a useful method for identifying the specific consequences of nicotine on neuronal development, as opposed to the other constituents of tobacco smoke.
Acknowledgments
This work was supported by NIH grants DA10455 and DA00436 to MRP and the State of Connecticut Department of Mental Health and Addiction Services. NKH was supported by a post-doctoral fellowship from the Yale University Interdisciplinary Research Consortium on Stress, Self-Control and Addiction (Research Education (RL5, 12 of 14) NIH IRL5 DA024858). The authors would like to thank Dr. Arie Kaffman (Department of Psychiatry, Yale University School of Medicine) for information and advice on the maternal care behavioral analysis protocol.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heath C, Picciotto M. Nicotine-induced plasticity during development: modulation of the cholinergic system and long-term consequences for circuits involved in attention and sensory processing. Neuropharmacology. 2009;56 (Suppl 1):254–262. doi: 10.1016/j.neuropharm.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith C, Perfetti T, Garg R, Hansch C. IARC carcinogens reported in cigarette mainstream smoke and their calculated log P values. Food Chem Toxicol. 2003;41(6):807–817. doi: 10.1016/s0278-6915(03)00021-8. [DOI] [PubMed] [Google Scholar]
- 3.Stolerman I, Jarvis M. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117(1):2–10. doi: 10.1007/BF02245088. discussion 14–20. [DOI] [PubMed] [Google Scholar]
- 4.Zoli M, Le Novère N, Hill JJ, Changeux J. Developmental regulation of nicotinic ACh receptor subunit mRNAs in the rat central and peripheral nervous systems. J Neurosci. 1995;15(3 Pt 1):1912–1939. doi: 10.1523/JNEUROSCI.15-03-01912.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pauly J, Sparks J, Hauser K, Pauly T. In utero nicotine exposure causes persistent, gender-dependant changes in locomotor activity and sensitivity to nicotine in C57Bl/6 mice. Int J Dev Neurosci. 2004;22(5–6):329–337. doi: 10.1016/j.ijdevneu.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Narayanan U, Birru S, Vaglenova J, Breese C. Nicotinic receptor expression following nicotine exposure via maternal milk. Neuroreport. 2002;13(7):961–963. doi: 10.1097/00001756-200205240-00012. [DOI] [PubMed] [Google Scholar]
- 7.Gyekis J, Anthony K, Foreman J, Klein L, Vandenbergh D. Perinatal nicotine exposure delays genital development in mice. Reprod Toxicol. 2010;29(3):378–380. doi: 10.1016/j.reprotox.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Klein L, Stine M, Pfaff D, Vandenbergh D. Laternal nicotine exposure increases nicotine preference in periadolescent male but not female C57B1/6J mice. Nicotine Tob Res. 2003;5(1):117–124. doi: 10.1080/14622200307257. [DOI] [PubMed] [Google Scholar]
- 9.Paz R, Barsness B, Martenson T, Tanner D, Allan A. Behavioral teratogenicity induced by nonforced maternal nicotine consumption. Neuropsychopharmacology. 2007;32(3):693–699. doi: 10.1038/sj.npp.1301066. [DOI] [PubMed] [Google Scholar]
- 10.Kaffman A, Meaney M. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J Child Psychol Psychiatry. 2007;48(3–4):224–244. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- 11.Meaney M. Epigenetics and the biological definition of gene x environment interactions. Child Dev. 2010;81(1):41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang T, Meaney M. Epigenetics and the environmental regulation of the genome and its function. Annu Rev Psychol. 2010;61:439–466. C431–433. doi: 10.1146/annurev.psych.60.110707.163625. [DOI] [PubMed] [Google Scholar]
- 13.Wei L, David A, Duman R, Anisman H, Kaffman A. Early life stress increases anxiety-like behavior in Balbc mice despite a compensatory increase in levels of postnatal maternal care. Horm Behav. 2010;57(4–5):396–404. doi: 10.1016/j.yhbeh.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunzell D, Russell D, Picciotto M. In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6J mice. J Neurochem. 2003;84(6):1431–1441. doi: 10.1046/j.1471-4159.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- 15.Brunzell D, Chang J, Schneider B, Olausson P, Taylor J, Picciotto M. beta2-Subunit-containing nicotinic acetylcholine receptors are involved in nicotine-induced increases in conditioned reinforcement but not progressive ratio responding for food in C57BL/6 mice. Psychopharmacology (Berl) 2006;184(3–4):328–338. doi: 10.1007/s00213-005-0099-z. [DOI] [PubMed] [Google Scholar]
- 16.Caldarone B, King S, Picciotto M. Sex differences in anxiety-like behavior and locomotor activity following chronic nicotine exposure in mice. Neurosci Lett. 2008;439(2):187–191. doi: 10.1016/j.neulet.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King S, Caldarone B, Picciotto M. Beta2-subunit-containing nicotinic acetylcholine receptors are critical for dopamine-dependent locomotor activation following repeated nicotine administration. Neuropharmacology. 2004;47 (Suppl 1):132–139. doi: 10.1016/j.neuropharm.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 18.Hall CS. Emotional behavior in the rat: I. Defecation and urination as measures of individual differences in emotionality. Journal of comparative psychology. 1934;18(3):385–403. [Google Scholar]
- 19.Choleris E, Thomas A, Kavaliers M, Prato F. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25(3):235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- 20.Misslin R, Cigrang M. Does neophobia necessarily imply fear or anxiety? Behavioural Processes. 1986;12(1):45–50. doi: 10.1016/0376-6357(86)90069-0. [DOI] [PubMed] [Google Scholar]
- 21.DeFries J, Wilson J, McClearn G. Open-field behavior in mice: selection response and situational generality. Behav Genet. 1970;1(3):195–211. doi: 10.1007/BF01074652. [DOI] [PubMed] [Google Scholar]
- 22.Guy J, Hendrich B, Holmes M, Martin J, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27(3):322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 23.Miyakawa T, Yagi T, Kagiyama A, Niki H. Radial maze performance, open-field and elevated plus-maze behaviors in Fyn-kinase deficient mice: further evidence for increased fearfulness. Brain Res Mol Brain Res. 1996;37(1–2):145–150. doi: 10.1016/0169-328x(95)00300-h. [DOI] [PubMed] [Google Scholar]
- 24.Burton C, Chatterjee D, Chatterjee-Chakraborty M, Lovic V, Grella S, Steiner M, Fleming A. Prenatal restraint stress and motherless rearing disrupts expression of plasticity markers and stress-induced corticosterone release in adult female Sprague-Dawley rats. Brain Res. 2007;1158:28–38. doi: 10.1016/j.brainres.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 25.van der Veen R, Abrous D, de Kloet E, Piazza P, Koehl M. Impact of intra- and interstrain cross-fostering on mouse maternal care. Genes Brain Behav. 2008;7(2):184–192. doi: 10.1111/j.1601-183X.2007.00337.x. [DOI] [PubMed] [Google Scholar]
- 26.Oliveira E, Pinheiro C, Santos-Silva A, Trevenzoli I, Abreu-Villaça Y, Nogueira Neto J, Reis A, Passos M, Moura E, Lisboa P. Nicotine exposure affects mother’s and pup’s nutritional, biochemical, and hormonal profiles during lactation in rats. J Endocrinol. 2010;205(2):159–170. doi: 10.1677/JOE-09-0430. [DOI] [PubMed] [Google Scholar]
- 27.Blake C, Sawyer C. Nicotine blocks the suckling-induced rise in circulating prolactin in lactating rats. Science. 1972;177(49):619–621. doi: 10.1126/science.177.4049.619. [DOI] [PubMed] [Google Scholar]
- 28.Ferry J, McLean B, Nikitovitch-Winer M, Rees E. Tobacco-smoke inhalation delays suckling-induced prolactin release in the rat. Proc Soc Exp Biol Med. 1974;147(1):110–113. doi: 10.3181/00379727-147-38291. [DOI] [PubMed] [Google Scholar]
- 29.Huang L, Hsiao S, Trzeciakowski J, Frye G, Winzer-Serhan U. Chronic nicotine induces growth retardation in neonatal rat pups. Life Sci. 2006;78(13):1483–1493. doi: 10.1016/j.lfs.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 30.Vaglenova J, Birru S, Pandiella N, Breese C. An assessment of the long-term developmental and behavioral teratogenicity of prenatal nicotine exposure. Behav Brain Res. 2004;150(1–2):159–170. doi: 10.1016/j.bbr.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Vaglenova J, Parameshwaran K, Suppiramaniam V, Breese C, Pandiella N, Birru S. Long-lasting teratogenic effects of nicotine on cognition: gender specificity and role of AMPA receptor function. Neurobiol Learn Mem. 2008;90(3):527–536. doi: 10.1016/j.nlm.2008.06.009. [DOI] [PubMed] [Google Scholar]