Abstract
BTBR T+ tf/J (BTBR) is an inbred mouse strain that shows behavioral traits with analogies to the three diagnostic symptoms of autism spectrum disorder (ASD); deficits in social interaction, impaired communication, and repetitive behaviors with restricted interests. Previous findings reveal that when compared to C57BL/6J (B6) and other inbred strains, BTBR exhibit normal to low anxiety-like traits in paradigms designed to assess anxiety-related behaviors. The current study assessed the generality of these anxiety findings. In experiment 1, B6 and BTBR mice were tested in the elevated plus maze (EPM), mouse defense test battery (MDTB) and elevated zero-maze. BTBR mice exhibited an anxiogenic profile in the EPM, with a reduction in open arm time and an increase in risk assessment behaviors, as compared to B6. In the MDTB, BTBR showed enhanced vocalization to the predator, and significantly less locomotor activity than B6 in the pre-threat situation, but significantly more locomotion than B6 following exposure to a predator threat, suggesting enhanced defensiveness to the predator. In the zero-maze, BTBR mice showed a significantly higher number of entries and time spent in the open segments of the apparatus, when compared to B6. In experiment 2, a three-chambered social preference test was used to evaluate effects of the systemic administration of an anxiolytic compound, diazepam, on B6 and BTBR social approach. Diazepam consistently increased time in the compartment containing the social stimulus, for both B6 and BTBR mice. However, in the vehicle treated groups, B6 mice spent significantly more time while BTBR mice spent significantly less time in the social stimulus compartment; after diazepam administration both B6 and BTBR strains significantly preferred the social stimulus chamber. These results suggest that while the anxiety responses of BTBR mice to novel situations (EPM and zero-maze) are inconsistent, BTBR mice appear to be more defensive to animate threat stimuli (predator or another mouse). Reduction of anxiety by diazepam normalized the social preference of BTBR for a mouse stimulus in the three-chambered test.
Keywords: anxiety, autism, social behavior, mouse models, BTBR
1. Introduction
Autism spectrum disorders (ASD) are a group of neurodevelopmental conditions that affect approximately 1 in 150 children across the US [16,34,60]. ASD are characterized by social interaction and communication deficits, and ritualistic-repetitive behaviors that are typically detectable in early childhood and continue throughout the lifespan [2,20,35,58]. While the etiology of ASD is not yet established, a strong genetic component is evident from the extremely high (up to 90%) concordance rate for monozygotic twins, along with a 4:1 male/female ratio [4,19,21,28,45]. Nevertheless, genetic studies to determine specific heritable factors underlying susceptibility for ASD have demonstrated that the great majority of cases involve multiple gene interactions with possible environmental factors [1,47,56,57].
ASD are diagnosed on the basis of an aberrant behavioral phenotype, rather than by a biochemical or physiological biomarker or a specific neuropathology [2,27]. Therefore, suitable animal models for ASD require a relationship to the types of behavioral deficits that are considered the core symptoms of such disorder, including impairments in social interaction and deficiencies in other functional domains [14,40,55]. Given the many genetic resources available for mice, and the high level of homology between the mouse and human genomes, mice are especially useful for analysis of genetic disorders [11,18,29,53].
There are two basic ways to harness the power of mouse genetics for ASD mouse model research. Reverse genetic approaches involve the analysis of the phenotypes of specific mouse strains with mutations in candidate genes for ASD [see for instance 23,52,61,66]. Forward genetic approaches identify inbred strains of mice with phenotypes relevant to ASD, and explore mechanisms responsible for these phenotypes [see for instance 10,41-44]. The use of inbred strains of mice offers useful translational tools for the testing of genetic hypotheses and evaluation of potential therapeutic strategies without directly manipulating specific genes. This is especially useful for the study of polygenic based phenotypes such as anxiety or social behavior. Results obtained using the latter approaches have indicated that the BTBR T+ tf/J (BTBR) inbred strain displays behavioral traits with face validity for all three diagnostic symptoms of ASD. Compared to the commonly used C57BL/6J (B6) strain, BTBR mice display lower reciprocal social interactions, reduced social approach and impaired juvenile play [9,39,65], unusual patterns or reduced vocalization [50,51,62], and higher levels of repetitive self-grooming throughout developmental stages [39,64,65].
In addition, BTBR mice display a variable profile of anxiety-like behaviors in comparison to B6 and other inbred strains. In the elevated plus maze (EPM) Moy et al. [44] found no differences between B6 and BTBR on the proportion of open arm entries and time. Yang et al. [63] also reported no differences in the proportion of open arm time but noted a significant increase in the total number of open arm entries by male BTBR mice compared to B6. A significant strain difference was seen in the elevated zero-maze [39], with BTBR spending a higher percentage in the open segments of the apparatus than B6.
The present study was aimed at extending these anxiety findings, and on evaluating if defensiveness to social threat might be involved in the well-established reductions in social preference for BTBR mice [39,44]. In the first experiment, B6 and BTBR mice were tested in the EPM, mouse defense test battery (MDTB) and zero-maze. These paradigms vary in terms of threatening stimuli: the plus and zero mazes provide a novel, elevated and exposed space as the threat, whereas the MDTB threat is a predatory stimulus, a hand-held anesthetized rat that first approaches, then chases, and finally contacts the mouse [for review see 7]. In the second experiment, we used an extensively investigated three-chambered test [41] to verify the effect of systemic administration of an anxiolytic compound, diazepam, on B6 and BTBR social preferences.
2. Materials and methods
2.1. Animals
Male B6 mice between the ages of 13 and 17 weeks and male BTBR mice between the ages of 14 and 17 weeks were used as subjects. B6 and BTBR mice for this study were offspring of breeding pairs obtained from the Jackson Laboratory (Bar Harbor, ME) and subjects were bred in the animal facilities of the University of Hawaii Laboratory Animal Service. Out-bred CD-1 stimulus mice were purchased from Charles River Labs (Company Location). Breeding pairs from both B6 and BTBR inbred strains were maintained by sibling mating. Subject and stimulus mice were reared in standard polypropylene cages, 26.5 cm × 17 cm × 11.5 cm (H), in a group of three to five male littermates after weaning at the 25 days of age, in a temperature-controlled room (22 ± 1°C). All subjects were maintained on a 12-h light/dark cycle (lights on at 06:00 am), with free access to food and water in their home cages. All procedures were conducted in accordance with protocols approved by the University of Hawaii Institutional Animal Care and Use Committee.
2.2. Drugs
Diazepam (RBI, USA) was prepared as a suspension in physiological sterile saline containing 2% of Tween-80. It was administered through intraperitoneal (i.p.) injection (20 ml/kg) in a dose of 2 mg/kg 30 min before the start of the three-chambered test. The dose used and the time interval between drug injection and testing were chosen based on previously published studies [25,26,32].
2.3. Apparatus
2.3.1. Elevated plus maze
The test apparatus is based on that described by Lister [33] and comprised of two open arms (30 cm × 7 cm × 2 cm) and two closed arms (30 cm × 7 cm × 20 cm) that extend from a common central platform (5 × 5 cm). The apparatus was constructed from wood and Plexiglas and was raised to a height of 40 cm above floor level. To prevent mice falling off, a rim of Plexiglas (0.25 cm high) surrounded the perimeter of the open arms. One ceiling-mounted video camera was used to record mouse behavior during the test and the experimental room was illuminated with standard fluorescent lamps. The mean intensity of luminosity on the open arms of the EPM was 55 lx.
2.3.2. Mouse defense test battery
The MDTB test was conducted in an oval runway, 0.40 m wide, 0.30 m high and 4.8 m in total length, consisting of two 2-m straight segments joined by two 0.4 m curved segments and separated by a median wall (2.0 m long × 0.30 m high). The apparatus is elevated 0.8 m from the floor to enable the experimenter to hold the rat and move with ease, while minimizing the subjects’ ability to view the experimenter. All parts of the apparatus were made of black Plexiglas. The floor of the apparatus was marked every 20 cm with white lines to facilitate measurement of locomotion distances. Two ceiling-mounted video cameras were used to record behaviors during the test and the room was illuminated with a red light. The mean intensity of luminosity at the level of the MDTB apparatus was 20 lx.
2.3.3. Elevated zero-maze
The test apparatus consisted of an acrylic circular platform (6 cm in width) composed of open and closed segments, with the later being surrounded by 20 cm high acrylic walls. The platform was raised to a height of 50 cm above floor level and the diameter of the maze is 65 cm. To prevent mice falling off, a rim of Plexiglas (0.25 cm high) surrounded the perimeter of the open segments. One ceiling-mounted video camera was used to record the behavior during the test and the experimental room was illuminated with standard fluorescent lamps. The mean intensity of luminosity on the open segments of the zero-maze was 70 lx.
2.3.4. Three-chambered apparatus
A 41 cm L × 70 cm W × 28 cm H three-chambered arena was used to assess sociability. Since subject mice were black, white Plexiglas panels were installed on the back walls and the entire arena was placed on a white Plexiglas floor to provide a contrasting background. The two outside chambers contained an inverted empty black wire cup (Galaxy Pencil/Utility Cup Spectrum Diversified Designs, Inc., Streetsboro, OH) which could contain stimulus mice. The experimental room was illuminated with standard fluorescent lamps. The mean intensity of luminosity on the outside chambers was 30 lx.
2.4. Behavioral test schedule
In the first experiment, B6 and BTBR mice were tested in the EPM, MDTB and zero-maze. In the second experiment, mice from both strains received an i.p. injection of vehicle or diazepam 30 min before the three-chambered test. All tests were conducted during the light phase of the light/dark cycle from 9:00 am to 4:00 pm. Scorers were blind to treatment and strain conditions.
2.5. Behavioral tests
2.5.1. Elevated plus-maze
B6 and BTBR mice were transported to the experimental room and left undisturbed for 60 min prior to testing. Testing was initiated by placing the subject on the central platform of the maze, facing one of the enclosed arms, following which the experimenter immediately withdrew to an adjacent room. Test sessions lasted 5 min and, between subjects, the maze was thoroughly cleaned with 10% ethanol and dry paper towels. The results were expressed as mean percentage of entries into open arms over total entries into both open and closed arms, mean percentage of time spent in open arms over total time spent in both open and closed arms, mean frequency of entries and time in the central platform and total number of open and closed arms entries. Ethological measures comprised frequency scores for stretched attend postures (mouse stretches forward and retracts to original position), head-dips (mouse protrudes its head over the edge of an open arm and down toward the floor), head-outs (mouse orients its head toward the ceiling) and stretched head-outs (mouse orients its head toward the ceiling with body stretched).
2.5.2. Mouse defense test battery
One week after the EPM, mice from both strains were transported to the experimental room and left undisturbed for 60 min prior to testing. The apparatus was cleaned with 10% ethanol and dried with paper towels in between trials. Ten minutes before the test session began, the Sprague-Dawley rat (average weight of 450 g) used as the predator stimulus was deeply anesthetized with sodium pentobarbital (80 mg/kg, i.p.) in order to minimize its discomfort.
The MDTB consists of the following subtests:
Pre-test: subjects were placed into the MDTB apparatus for a 3-min familiarization period during which total line crossings and wall rears were recorded.
Predator avoidance test: avoidance and escape distances were measured when a predator stimulus (a hand-held rat) was brought up to the subject at a speed of approximately 0.5 m/s. Approach was terminated when contact with the subject was made or if the subject ran away from the approaching rat. This was repeated five times.
Chase/flight test: the hand-held rat was brought up to the subject at a speed of approximately 2.0 m/s. Chase was initiated only when the subject was standstill with its head oriented toward the rat, and completed when the subject had traveled a distance of 14.4 m (three laps in the runway). The time spent by the mouse to travel this distance was recorded. With this latency, mean flight speed (m/s) was calculated. In addition, the number of stops (pauses in locomotion), orientations (subject orients its head toward the oncoming rat) and reversals (subject turned and ran in the rat direction) were also recorded.
Straight alley test: the runway is then converted into a straight alley, 80 cm long, by the closing of a door at one end and the placement of a removable barrier at the other. The rat is placed at one end while the mouse begins the test at the other. During a 30-s period, the number of approach-withdrawals (subject moves toward the rat more than 20 cm and then returns), as well as voluntary contacts with the rat stimulus were recorded. Another measures scored included immobility time (freezing) and the frequency of defensive uprights.
Forced contact test: in this situation, the straight alley length was reduced to 40 cm. The rat was brought up by the experimenter in five sudden contacts directed toward the subject. For each such contact, the number of vocalizations, defensive uprights, jump attacks, jump escapes and bites were recorded. This procedure was repeated three times.
Post-test: upon completion of the forced contact test, the alley doors were opened allowing the subject locomotion around the oval runway. The subject exploratory activity was recorded for another 3-min period during which the experimenter and the rat stimulus were out of sight. Line crossings and wall rears were recorded.
2.5.3. Elevated zero-maze
Three weeks after the MDTB, B6 and BTBR mice were transported to the experimental room and left for 60 min prior to testing in the zero-maze. Testing was initiated by placing the subject facing one of the closed segments, following which the experimenter immediately withdrew to an adjacent room. Test sessions lasted 5 min and, between subjects, the maze was thoroughly cleaned with 10% ethanol and dry paper towels. The results were expressed as mean percentage of entries into open segments over total entries into both open and closed segments, mean percentage of time spent in open segments over total time spent in both open and closed segments and total number of open and closed segments entries.
2.5.4. Three-chambered social approach test
Independent groups of B6 and BTBR mice were assessed for sociability in a three-chambered apparatus [41]. B6 mice with an average weight of 29 g and BTBR mice with an average weight of 35 g received the i.p. injection of vehicle or diazepam 30 min before the three-chambered test. Briefly, during a 10 min habituation period, a subject mouse was placed in the middle chamber, the sliding doors were opened and the mouse given free access to the entire arena during which the duration of time in each of the two outside stimulus compartments was hand scored with stopwatches. Following the habituation phase, mice were placed back into the center, the doors were closed and a single unfamiliar male CD-1 mouse was placed in one of the two cups. The duration of time spent in each chamber was measured in a 10 min session and required all four paws to be in the compartment to be counted. Placement of the stimulus mouse was successively alternated between trials. The duration of time in which all four paws of the subject mouse was in each of the two compartments was compared.
2.6. Statistical Analysis
The behavioral data obtained in the EPM, MDTB and zero-maze were analyzed by one-way analysis of variance (ANOVA), with strain (B6 or BTBR) as the independent variable, followed by unpaired t-tests. Data from the three-chambered test were analyzed using within-group repeated measures ANOVA for comparison of time spent in the empty cage side with time spent in the CD-1 mouse side. Time spent in the center chamber is shown in the graph for illustrative purposes. Entries into both side compartments of the three-chambered apparatus were summed as total entries and analyzed by one-way ANOVA across groups, as a measure of general exploratory activity. For all statistical analyses, a probability level of p<0.05 was considered significant but probabilities meeting more stringent criteria (e.g. p<0.01) are marked as such.
3. Results
3.1 Experiment 1
3.1.1 Elevated plus maze
Table 1 shows the results (means ± SEM and F value) obtained when B6 and BTBR mice were tested in the EPM. No significant strain effects were found for the number of open arm entries and percentage of open arm over total arm entries. However, there was a significant strain effect on the total number of entries into arms of the plus maze, with BTBR mice showing a decrease in this variable. One-way ANOVA revealed a significant strain effect on time spent in the open arms of the plus maze: BTBR showed a significant decrease in this measure. There was a borderline effect (p=0.056) for the percentage of time in the open arms over total time in open and closed arms: this ratio was reduced in the BTBR strain. No significant strain effect was verified for time spent in the center of the plus maze. However, there was a significant strain effect on the number of center entries, with BTBR showing a reduction in this measure.
Table 1.
Behavioral data (means ± SEM and F value) obtained when B6 and BTBR mice were tested in the EPM
| B6 | BTBR | F (1,22) | |
|---|---|---|---|
| Open arm entries | 12.42 ± 1.22 | 9.92 ± 1.78 | 1.34 |
|
Open arm entries /
total entries (%) |
34.66 ± 2.15 | 33.38 ± 4.19 | 0.07 |
| Open arm time (s) | 50.88 ± 5.45 | 31.88 ± 5.66* | 5.84 |
|
Open arm time /
total time (%) |
23.34 ± 2.54 | 15.29 ± 2.71 f | 4.07 |
| Center entries | 34.33 ± 1.74 | 26.83 ± 2.64* | 5.63 |
| Center time (s) | 80.21 ± 4.53 | 82.42 ± 11.21 | 0.03 |
| Total arm entries | 34.83 ± 1.75 | 27.25 ± 2.69* | 5.57 |
n=12 for each group
p<0.05
0.05<p<0.1 compared to B6 by unpaired t-test.
Data on the ethological variables measured in the EPM is displayed in Fig. 1. One-way ANOVA revealed a significant strain effect on the frequencies of stretched attend postures and stretched head-outs: BTBR mice displayed a significant increase in the occurrence of these behaviors, when compared to B6. No other significant effects were obtained.
Fig. 1.
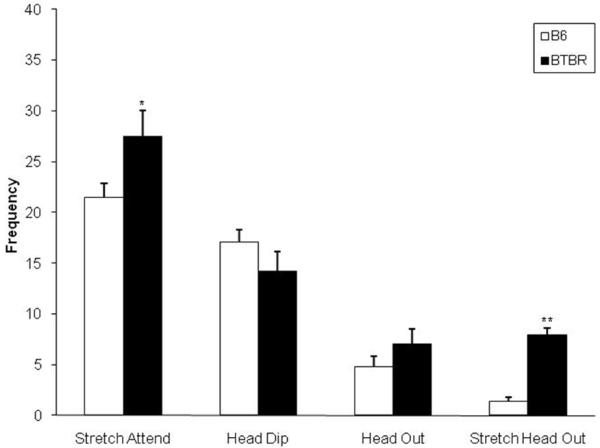
Frequency (mean ± SEM) of stretched attend posture, head-dip, head-out and stretched head-out behaviors of B6 and BTBR mice tested in the EPM; **p<0.01, *p<0.05 compared to B6 by unpaired t-test; n=12 for each group.
3.1.2 MDTB
All behavioral data (means ± SEM and F value) for B6 and BTBR in the MDTB are displayed in Table 2. One-way ANOVA revealed significant strain effects on the frequency of line crossings and wall rears assessed in the pre-test: BTBR mice were lower on both measures. A significant strain effect was also obtained in the predator avoidance test, with reductions in escape distances traveled by BTBR mice. In the forced contact test, BTBR mice made significantly more vocalizations and fewer defensive uprights. Finally, in the post-test situation BTBR mice exhibited a significant increase in the number of line crossings and a decrease in the frequency of wall rears.
Table 2.
Frequency (mean ± SEM) of behaviors obtained when B6 and BTBR mice were confronted with a rat in the MDTB
| B6 | BTBR | F (1,22) | |
|---|---|---|---|
| Pre-test activity | |||
| Line crossings | 152.17 ± 8.46 | 109.83 ± 15.00* | 6.04 |
| Rears | 8.83 ± 1.15 | 1.83 ± 0.65* | 27.96 |
| Predator avoidance test | |||
| Avoidance distance (cm) | 54.65 ± 13.23 | 61.39 ± 8.75 | 0.18 |
| Avoidance frequency | 2.00 ± 0.44 | 2.66 ± 0.26 | 1.69 |
| Escape distance (cm) | 103.86 ± 12.35 | 62.30 ± 8.82* | 7.50 |
| Escape frequency | 4.33 ± 0.28 | 3.83 ± 0.32 | 1.35 |
| Chase/flight test | |||
| Flight speed (m/s) | 0.39 ± 0.03 | 0.30 ± 0.04 | 3.52 |
| Stops | 8.83 ± 1.06 | 10.16 ± 0.76 | 1.05 |
| Orientations | 3.58 ± 0.56 | 3.16 ± 0.55 | 0.28 |
| Reversals | 1.00 ± 0.28 | 1.91 ± 0.60 | 1.94 |
| Straight alley test | |||
| Approaches/withdrawals | 1.25 ± 0.22 | 1.75 ± 0.37 | 1.34 |
| Contacts | 0.58 ± 0.23 | 0.16 ± 0.11 | 2.67 |
| Freezing | 1.92 ± 0.60 | 2.83 ± 0.68 | 1.02 |
| Uprights | 4.17 ± 0.71 | 3.33 ± 0.40 | 1.06 |
| Forced contact test | |||
| Vocalizations | 1.50 ± 0.44 | 5.00 ± 1.31* | 6.39 |
| Uprights | 7.17 ± 0.82 | 3.42 ± 1.05* | 7.84 |
| Jump attacks | 0 ± 0 | 1.00 ± 0.75 | 1.78 |
| Jump escapes | 2.92 ± 0.56 | 3.00 ± 1.27 | 0.00 |
| Bites | 0.08 ± 0.08 | 0.42 ± 0.29 | 1.23 |
| Post-test | |||
| Line crossings | 123.75 ± 4.45 | 176.08 ± 12.77* | 14.97 |
| Rears | 20.25 ± 1.41 | 12.50 ± 1.79* | 11.57 |
n=12 for each group
p<0.05 compared to B6 by unpaired t-test.
3.1.3 Elevated zero-maze
Table 3 shows results (means ± SEM and F value) for B6 and BTBR mice in the elevated zero-maze. One-way ANOVA revealed a significant strain effect on the number of entries and duration of time spent in the open segments of the zero-maze apparatus: both measures were significantly increased in BTBR mice. A significant strain effect was also found for the percentage of open segments time relative to total time: this percentage was enhanced in the BTBR strain. Finally, one-way ANOVA revealed a significant increase in the frequency of entries into both open and closed segments of the apparatus for BTBR mice.
Table 3.
Behavioral data (means ± SEM and F value) obtained when B6 and BTBR mice were tested in the elevated zero-maze
| B6 | BTBR | F (1,14) | |
|---|---|---|---|
| Open segments entries | 6.88 ± 1.91 | 13.5 ± 1.89* | 6.07 |
|
Open segments entries /
total entries (%) |
44.89 ± 1.88 | 47.90 ± 0.38 | 2.41 |
| Open segments time (s) | 37.25 ± 8.57 | 68.88 ± 8.94* | 6.52 |
|
Open segments time /
total time (%) |
12.42 ± 2.86 | 22.96 ± 2.98* | 6.53 |
| Total entries | 14.63 ± 3.85 | 28.00 ± 3.78* | 6.13 |
n=8 for each group
p<0.05 compared to B6 by unpaired t-test.
3.2 Experiment 2: effect of systemic administration of diazepam on B6 and BTBR sociability measured in the three-chambered test
Fig. 2 shows the effect of systemic administration of diazepam on the duration of time that B6 and BTBR mice spent in both side compartments of the three-chambered apparatus. B6 mice treated with vehicle spent more time in the chamber containing the conspecific stimulus than in the chamber containing the empty cup (F1,10 = 118.41; p<0.05). In contrast, BTBR mice treated with vehicle spent more time in the chamber containing the empty cup than in the chamber with the CD-1 mouse (F1,10 = 8.19; p<0.05). With systemic administration of diazepam, both B6 (F1,10 = 80.11; p<0.05) and BTBR (F1,10 = 14.86; p<0.05) strains showed an increased time in the compartment containing the CD-1 mouse used as social stimulus. Time in the social stimulus compartment was not different for B6 and BTBR mice following diazepam administration (p>0.05). No significant group effect was found for total entries into the side compartments of the three-chambered apparatus (Table 4).
Fig. 2.
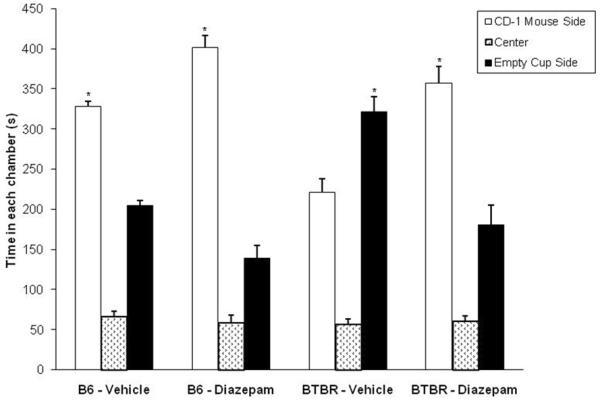
Effect (mean ± S.E.M.) of systemic administration of diazepam on B6 and BTBR sociability measured in the three-chambered apparatus; *p<0.05, preference for social stimulus chamber over the other stimulus chamber for each group; BTBR-Vehicle mice spent more time in the chamber with the empty cup than in the chamber containing the CD-1 mouse; n=11 for each group.
Table 4.
Number (mean ± SEM) of entries into both side compartments of the three-chambered apparatus in B6 and BTBR mice treated with vehicle and diazepam
| Groups | Entries |
|---|---|
| B6 -Vehicle | 22.18 ± 1.43 |
| B6 - Diazepam | 25.55 ± 4.05 |
| BTBR - Vehicle | 19.28 ± 1.42 |
| BTBR - Diazepam | 25.91 ± 3.24 |
n=11 for each group.
4. Discussion
In this study, three experimental models (EPM, MDTB and zero-maze) were used to assess levels of anxiety in B6 and BTBR mice. These two mouse strains were previously compared on the EPM, in the context of an evaluation of 10 mouse strains on tasks relevant to autism [44] with findings that B6 and BTBR show very similar anxiety-like profiles on this test, each of them being ranked as second or third highest of the 10 mouse strains on the percentage of time on the open arms, number of open arm entries and total arm entries. Yang et al. [63] reported enhanced open arm entries for BTBR compared to B6 mice, suggesting an anxiolytic-like BTBR profile. However, this was accompanied by a nonsignificant enhancement of total arm entries, and by a finding of no differences in time on the open arms for the two strains, both of which moderate an interpretation that BTBR show less anxiety than B6 on the EPM. Benno et al. [5] reported that a tail suspension test prior to the EPM disproportionately increased anxiety like behaviors of BTBR as opposed to B6 mice. They suggested that BTBR may be unusually stress-reactive.
In the context of these reports, the present findings that BTBR spent less time in the open arms of the plus maze and showed an increase in the frequency of risk assessment behaviors (stretched attend postures and stretched head-outs) may suggest the presence of higher levels of stress factors in that test than were present in the Yang et al. [63] comparisons. One possibility is the light level in the test room, potentially a major determinant of the aversiveness of the unshielded open arms. Yang et al. [63] used a very low level light (20 lx), whereas the present study involved a considerably brighter light (55 lx). This difference supports an interpretation that the EPM differences of BTBR and B6 mice may be very sensitive to the aversiveness of the stimuli to which the animals are reacting, with BTBR being the more responsive of the two strains.
In the MDTB, increases in the frequency of line crossings in the test situation after the predator stimulus is removed, compared to those before it was presented, are interpreted as representing escape attempts, an indication of enhanced defensiveness or anxiety [7]. This pattern was clear for the present BTBR mice and absent in B6 mice. BTBR showed a reduced escape distance compared to B6 mice, a measure that, however, appears to be less responsive to anxiety-impacting drugs than to panicolytic and panicogenic agents [6]. In the forced contact test of the MDTB, BTBR mice displayed an increase in the number of vocalizations but a decrease in the occurrence of defensive uprights, when compared to B6. Enhanced vocalizations are again compatible with a view that BTBR are more responsive to threat stimuli, and are part of a defensive attack pattern that is specifically responsive to anxiolytic drugs [6]. Although defensive uprights also appear to be responsive to anxiolytics, upright behaviors are reduced in the BTBR strain in a number of other situations as well (in preparation), potentially reflecting motoric or other changes in these mice. In summary of this pattern of findings, BTBR mice show enhancement of several measures selectively relating to anxiety, albeit in the context of some potentially contradictory results, in response to a predator (rat) stimulus.
In contrast to the present EPM findings, BTBR mice showed a consistent increase in the frequency of entries and time spent in the open segments of the zero-maze, as well as an increase in the total number of open and closed segment entries in the apparatus. These results suggest an anxiolytic profile for BTBR. The zero-maze was modeled on the EPM and developed specifically to avoid the computational complications arising from the center area of the latter [54]. Results from the EPM and the zero-maze are typically very similar [e.g. 8,30,31]. Here the BTBR-B6 differences in the zero-maze are very different from those in the EPM. Lighting levels were not different between the two tests (55 lx vs. 70 lx, respectively) and the same animals were run in both. It is notable that the zero-maze was the last of three tests, distributed over 4 weeks of testing, for BTBR and B6 mice. Thus, additional handling and testing may have been a factor in the differences between the present EPM and zero-maze results, possibly by affecting the responsivity of B6 mice, more than those of the BTBR strain: MacPherson et al. [36] found that compared to B6, BTBR mice display severely impaired contextual fear memory: they froze significantly less than B6 when re-exposed to a chamber where they received an unconditioned stimulus (electric foot-shocks). However, these zero-maze results are in agreement with prior reports of anxiolytic-like behaviors for BTBR compared to B6 mice [39] in this paradigm, and mice in that study had not been run previously in the EPM. Thus, although it is possible that prior EPM exposure may influence subsequent zero maze performance, this effect is not necessary for BTBR-B6 differences on this test.
In summary of the anxiety test data, these reveal a somewhat inconsistent set of anxiety findings for BTBR vs. B6 mice, with results suggesting enhanced anxiety in the EPM and MDTB, but reduced anxiety in the zero-maze. These results may be understandable in the context of findings (44) indicating that the anxiety-like behaviors of BTBR and B6 mice, under low stress conditions, are very similar, such that minor variations in test conditions may impact their relative positions. The differences between the present and previous [63] EPM findings are in agreement with a view that BTBR mice may react more strongly to stress or anxiety-eliciting stimuli than do B6 mice. Their enhanced stress-induced levels of corticosterone, compared to B6 [5,22] are compatible with that view. However, it is notable that the differences reported here for all three anxiety tests were relatively mild or moderate, suggesting that in these tests the anxiety-eliciting stimulus was not one that elicited a strong difference in responsivity between BTBR and B6 mice. The situation may have been different for the social preference test, in which mice were responding to conspecific stimuli.
Experiment 2 found that systemic administration of diazepam increased time in the compartment containing the social stimulus (CD-1 mouse), in agreement with previous studies demonstrating that systemic administration of diazepam robustly enhances sociability in several rodent species [see for instance 12,17]. In particular, Riedel et al. [48] reported that B6 female mice treated with diazepam consistently spent more time with a stranger mouse in the three-chambered test, as compared to a vehicle injected group. Such results are typically interpreted as indicating that diazepam reduces social anxiety, consistent with the clinical effects of diazepam [38], and its attenuation of anxiety-like measures in mice, on an array of tests such as the EPM [49].
However, the focal finding of Experiment 2 was that diazepam changed the significant preference of BTBR mice for the empty cup side of the three-chambered apparatus to a significant preference for the mouse side, in effect flipping that preference from the nonsocial to the social stimulus chamber. Moreover, time spent in the stimulus mouse chamber was not significantly different for BTBR and B6 groups under diazepam, indicating that, on this measure, the drug had normalized the social preference of BTBR mice.
These findings are consistent with an interpretation that under control conditions BTBR mice may be inhibited in their approach to conspecifics because of defensiveness or anxiety, and that by reducing this anxiety, diazepam sharply increases preference for the social stimulus chamber. In the context of the BTBR mouse as a model for autism-like behaviors, it is notable that anxiety is also common in children with autism [59], as is a reduced gaze fixation for human faces that may potentially reflect avoidance of these social stimuli [15]. Although there appear to be no systematic and controlled studies of the effects of diazepam on behaviors of autistic individuals, one small scale study indicated that “unsocialized” or “explosive” aggression increased in seven autistic children receiving the drug [37]. This is particularly interesting as findings of enhanced aggression following administration of diazepam or other benzodiazepines are not uncommon in both clinical settings and animal models [e.g. 3,24,46], a phenomenon that, like many other measures of anxiolytic action, may reflect a reduction in behavioral inhibition [13].
In conjunction with the somewhat inconsistent and moderate findings of anxiety differences for B6 and BTBR mice in three anxiety tests using nonsocial stimuli, the results of experiment 2, in the three-chambered social preference test, suggest that BTBR mice may show a particularly enhanced defensiveness or anxiety-like response to conspecific stimuli. Such response is compatible with findings of reduced gaze times in autistic individuals, and with a general view that enhanced anxiety to conspecific stimuli may be one of the factors involved in social deficiencies of individuals with autism.
Research Highlights.
-
➢
BTBR showed enhanced defensiveness to animate threat stimuli (predator or another mouse).
-
➢
Reduction of anxiety by diazepam normalized the social preference of BTBR.
Acknowledgements
The present study was supported by the National Institute of Mental Health (NIMH) grant MH081845-01A2 to RJB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].APA . Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- [3].Arnone M, Dantzer R. Effects of diazepam on extinction induced aggression in pigs. Pharmacol Biochem Behav. 1980;13:27–30. doi: 10.1016/0091-3057(80)90115-x. [DOI] [PubMed] [Google Scholar]
- [4].Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- [5].Benno R, Smirnova Y, Vera S, Ligget A, Schanz N. Exaggerated responses to stress in the BTBR T+tf/J mouse: an unusual behavioral phenotype. Behav Brain Res. 2009;197:462–465. doi: 10.1016/j.bbr.2008.09.041. [DOI] [PubMed] [Google Scholar]
- [6].Blanchard DC, Griebel G, Blanchard RJ. Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci Biobehav Rev. 2001;25:205–218. doi: 10.1016/s0149-7634(01)00009-4. [DOI] [PubMed] [Google Scholar]
- [7].Blanchard DC, Griebel G, Blanchard RJ. The mouse defense test battery: pharmacological and behavioral assays for anxiety and panic. Eur J Pharmacol. 2003;463:97–116. doi: 10.1016/s0014-2999(03)01276-7. [DOI] [PubMed] [Google Scholar]
- [8].Bleickardt CJ, Mullins DE, Macsweeney CP, Werner BJ, Pond AJ, Guzzi MF, Martin FD, Varty GB, Hodgson RA. Characterization of the V1a antagonist, JNJ-17308616, in rodent models of anxiety-like behavior. Psychopharmacology (Berl) 2009;202:711–718. doi: 10.1007/s00213-008-1354-x. [DOI] [PubMed] [Google Scholar]
- [9].Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brodkin ES. BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behav Brain Res. 2007:53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- [11].Bucan M, Abel T. The mouse: genetics meets behaviour. Nat Rev Genet. 2002;3(2):114–123. doi: 10.1038/nrg728. [DOI] [PubMed] [Google Scholar]
- [12].Costall B, Domeney AM, Farre AJ, Kelly ME, Martinez L, Naylor RJ. Profile of action of a novel 5-hydroxytryptamine1A receptor ligand E-4424 to inhibit aversive behavior in the mouse, rat and marmoset. J Pharmacol Exp Ther. 1992;262:90–98. [PubMed] [Google Scholar]
- [13].Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- [14].Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, et al. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897–6906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].File SE, Cheeta S, Akanezi C. Diazepam and nicotine increase social interaction in gerbils: a test for anxiolytic action. Brain Res. 2001;888:311–313. doi: 10.1016/s0006-8993(00)03102-4. [DOI] [PubMed] [Google Scholar]
- [18].Flint J, Mott R. Applying mouse complex-trait resources to behavioural genetics. Nature. 2008;456:724–727. doi: 10.1038/nature07630. [DOI] [PubMed] [Google Scholar]
- [19].Folstein SE, Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet. 2001;2:943–955. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- [20].Fombonne E, Heavey L, Smeeth L, Rodrigues LC, Cook C, Smith PG, et al. Validation of the diagnosis of autism in general practitioner records. BMC Public Health. 2004;3:4–5. doi: 10.1186/1471-2458-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Freitag CM. The genetics of autistic disorders and its clinical relevance: a review of the literature. Mol Psychiatry. 2007;12:2–22. doi: 10.1038/sj.mp.4001896. [DOI] [PubMed] [Google Scholar]
- [22].Frye CA, Llaneza DC. Corticosteroid and neurosteroid dysregulation in an animal model of autism, BTBR mice. Physiol Behav. 2010;100:264–267. doi: 10.1016/j.physbeh.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Glessner JT, Wang K, Cai G, Korvatska O, Kim CE, Wood S, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gordon EB. Letter: Tranquillizers causing aggression. Br Med J. 1975;2:36–37. doi: 10.1136/bmj.2.5961.36-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- [26].Griebel G, Perrault G, Sanger DJ. Characterization of the behavioral profile of the non-peptide CRF receptor antagonist CP-154,526 in anxiety models in rodents: comparison with diazepam and buspirone. Psychopharmacology (Berl) 1998;138:55–66. doi: 10.1007/s002130050645. [DOI] [PubMed] [Google Scholar]
- [27].Halladay AK, Amaral D, Aschner M, Bolivar VJ, Bowman A, DiCicco-Bloom E, et al. Animal models of autism spectrum disorders: information for neurotoxicologists. NeuroToxicology. 2009;30:811–821. doi: 10.1016/j.neuro.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hoekstra RA, Bartels M, Verweij CJ, Boomsma DI. Heritability of autistic traits in the general population. Arch Pediatr Adolesc Med. 2007;161:372–377. doi: 10.1001/archpedi.161.4.372. [DOI] [PubMed] [Google Scholar]
- [29].Insel TR. Mouse models for autism: report from a meeting. Mamm Genome. 2001;12:755–757. doi: 10.1007/s00335-001-4006. [DOI] [PubMed] [Google Scholar]
- [30].Kumar V, Jaiswal AK, Singh PN, Bhattacharya SK. Anxiolytic activity of Indian Hypericum perforatum Linn: an experimental study. Indian J Exp Biol. 2000;38:36–41. [PubMed] [Google Scholar]
- [31].Kumar V, Singh RK, Jaiswal AK, Bhattacharya SK, Acharya SB. Anxiolytic activity of Indian Abies pindrow Royle leaves in rodents: an experimental study. Indian J Exp Biol. 2000;38:343–346. [PubMed] [Google Scholar]
- [32].Lepicard EM, Joubert C, Hagneau I, Perez-Diaz F, Chapouthier G. Differences in anxiety-related behavior and response to diazepam in BALB/cByJ and C57BL/6J strains of mice. Pharmacol Biochem Behav. 2000;67:739–748. doi: 10.1016/s0091-3057(00)00419-6. [DOI] [PubMed] [Google Scholar]
- [33].Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- [34].LLaneza DC, DeLuke SV, Batista M, Crawley JN, Christodulu KV, Frye CA. Communications, interventions and scientific advances in autism: a commentary. Physiol Behav. 2010;100:268–276. doi: 10.1016/j.physbeh.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Arch Gen Psychiatry. 2006;63:694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- [36].MacPherson P, McGaffigan R, Wahlsten D, Nguyen PV. Impaired fear memory, altered object memory and modified hippocampal synaptic plasticity in split-brain mice. Brain Res. 2008;1210:179–188. doi: 10.1016/j.brainres.2008.03.008. [DOI] [PubMed] [Google Scholar]
- [37].Marrosu F, Marrosu G, Rachel MG, Biggio G. Paradoxical reactions elicited by diazepam in children with classical autism. Funct Neurol. 1987;2:355–361. [PubMed] [Google Scholar]
- [38].Martin JL, Sainz-Pardo M, Furukawa TA, Martin-Sanchez E, Seoane T, Galan C. Benzodiazepines in generalized anxiety disorder: heterogeneity of outcomes based on a systematic review and meta-analysis of clinical trials. J Psychopharmacol. 2007;21:774–782. doi: 10.1177/0269881107077355. [DOI] [PubMed] [Google Scholar]
- [39].McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+ tf/J mice. Genes Brain Behav. 2008;7:152–263. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- [40].Moy SS, Nadler JJ. Advances in behavioral genetics: mouse models of autism. Mol Psychiatry. 2008;13:4–26. doi: 10.1038/sj.mp.4002082. [DOI] [PubMed] [Google Scholar]
- [41].Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- [42].Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, et al. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav. 2009;8:129–142. doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, et al. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008;191:118–129. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113:472–486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- [46].Olivier B, Mos J, Rasmussen D. Behavioural pharmacology of the serenic, eltoprazine. Drug Metabol Drug Interact. 1990;8:31–83. doi: 10.1515/dmdi.1990.8.1-2.31. [DOI] [PubMed] [Google Scholar]
- [47].Persico AM, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends Neurosci. 2006;29(7):349–358. doi: 10.1016/j.tins.2006.05.010. [DOI] [PubMed] [Google Scholar]
- [48].Riedel G, Kang SH, Choi DY, Platt B. Scopolamine-induced deficits in social memory in mice: reversal by donepezil. Behav Brain Res. 2009;204:217–225. doi: 10.1016/j.bbr.2009.06.012. [DOI] [PubMed] [Google Scholar]
- [49].Rodgers RJ, Cao BJ, Dalvi A, Holmes A. Animal models of anxiety: an ethological perspective. Braz J Med Biol Res. 1997;30:289–304. doi: 10.1590/s0100-879x1997000300002. [DOI] [PubMed] [Google Scholar]
- [50].Roullet FI, Wohr M, Crawley JN. Female urine-induced male mice ultrasonic vocalizations, but not scent-marking, is modulated by social experience. Behav Brain Res. 2010 doi: 10.1016/j.bbr.2010.06.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008;3(8):e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Scearce-Levie K, Roberson ED, Gerstein H, Cholfin JA, Mandiyan VS, Shah NM, et al. Abnormal social behaviors in mice lacking Fgf17. Genes Brain Behav. 2008;7:344–354. doi: 10.1111/j.1601-183X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- [53].Seong E, Seasholtz AF, Burmeister M. Mouse models for psychiatric disorders. Trends Genet. 2002;18:643–650. doi: 10.1016/s0168-9525(02)02807-x. [DOI] [PubMed] [Google Scholar]
- [54].Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterization of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology (Berl) 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- [55].Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sung YJ, Dawson G, Munson J, Estes A, Schellenberg GD, Wijsman EM. Genetic investigation of quantitative traits related to autism: use of multivariate polygenic models with ascertainment adjustment. Am J Hum Genet. 2005;76:68–81. doi: 10.1086/426951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Szatmari P, Maziade M, Zwaigenbaum L, Merette C, Roy MA, Joober R, et al. Informative phenotypes for genetic studies of psychiatric disorders. Am J Med Genet B Neuropsychiatr Genet. 2007;144:581–588. doi: 10.1002/ajmg.b.30426. [DOI] [PubMed] [Google Scholar]
- [58].Volkmar FR, Lord C, Bailey A, Schultz RT, Klin A. Autism and pervasive developmental disorders. J Child Psychol Psychiatry. 2004;45:135–170. doi: 10.1046/j.0021-9630.2003.00317.x. [DOI] [PubMed] [Google Scholar]
- [59].White SW, Oswald D, Ollendick T, Scahill L. Anxiety in children and adolescents with autism spectrum disorders. Clin Psychol Rev. 2009;29:216–229. doi: 10.1016/j.cpr.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wing L, Potter D. The epidemiology of autistic spectrum disorders: is the prevalence rising? Ment Retard Dev Disabil Res Rev. 2002;8:151–161. doi: 10.1002/mrdd.10029. [DOI] [PubMed] [Google Scholar]
- [61].Winslow JT, Insel TR. The social deficits of the oxytocin knockout mouse. Neuropeptides. 2002;36:221–220. doi: 10.1054/npep.2002.0909. [DOI] [PubMed] [Google Scholar]
- [62].Wohr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2010 doi: 10.1111/j.1601-183X.2010.00582.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009:1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, et al. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front Behav Neurosci. 2007b;1:1–9. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+ tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Devl Neuroscience. 2007a;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Young LJ. Oxytocin and vasopressin as candidate genes for psychiatric disorders: lessons from animal models. Am J Med Genet. 2001;105:53–54. [PubMed] [Google Scholar]


