Abstract
To address limitations of conventional influenza vaccine manufacturing and delivery, this study investigated administration of virus-like particle (VLP) influenza vaccine using a microneedle patch. The goal was to determine if skin immunization with influenza VLP vaccine using microneedles enables dose sparing. We found that low-dose influenza (A/PR/8/34 H1N1) VLP vaccination using microneedles was more immunogenic than low-dose intramuscular (IM) vaccination and similarly immunogenic as high-dose IM vaccination in a mouse model. With 1 µg dose of vaccine, both routes showed similar immune responses and protective efficacy, with microneedle vaccination being more effective in inducing recall antibody responses in lungs and antibody secreting cells in bone marrow. With a low dose of vaccine (0.3 µg), microneedle vaccination induced significantly superior protective immunity, which included binding and functional antibodies as well as complete protection against a high dose of lethal infection with A/PR/8/34 virus, whereas IM immunization provided only partial (40%) protection. Therefore, this study demonstrates that microneedle vaccination in the skin confers more effective protective immunity at a lower dose, thus providing vaccine dose sparing effects.
Keywords: influenza, microneedle, virus-like particle vaccine, dose-sparing, skin
1. Introduction
Influenza is a major threat to public health that is responsible for approximately 500,000 deaths worldwide each year [1]. Especially due to the emergence of influenza strains resistant to antiviral agents, vaccination is an indispensible method to prevent the spread of influenza [2, 3]. Currently, the egg-based trivalent inactivated virus vaccine is broadly used for seasonal influenza vaccination campaigns, but it has several limitations including problems in mass-production, egg allergy, and handling live influenza viruses [4].
To overcome these disadvantages, novel cell-based vaccines have been suggested. Virus-like particles (VLPs) without viral replication characteristics have been produced in mammalian and insect cell systems and large-scale bioprocesses for VLPs production have been studied [5]. VLPs lack the RNA genome of the virus, which improves the safety of the vaccine [6]. Influenza VLP vaccines of various strains have conferred good protection from lethal influenza virus challenge [7–14].
The limitations of vaccine manufacturing could be further addressed by reducing the required dose and thereby reducing the amount of vaccine manufactured. In this study, we hypothesized that low-dose influenza VLP vaccination via the skin would be more immunogenic than low-dose IM vaccination and similarly immunogenic as high-dose IM vaccination. We tested this hypothesis using a low-dose vaccination that is three-fold lower than the high-dose vaccination. We propose this hypothesis because skin has two bone marrow-derived antigen-presenting cell types, i.e., Langerhans cells and dermal dendritic cells, which play a critical role in the immune system [15].
Increased immunogenicity has been demonstrated for a number of vaccines when given by intradermal (ID) injection compared to intramuscular (IM) injection. WHO recommends ID injection of rabies vaccine as a dose-sparing and, thereby, cost-saving approach [16]. Other vaccines, such as smallpox and tuberculosis (BCG), are also commonly administered ID, although not for dose-sparing purposes [17, 18]. Recently, ID influenza vaccination was approved in Europe and shown to increase immunogenicity in the elderly at the same dose relative to IM injection [19, 20].
A number of studies have assessed the dose-sparing potential of ID influenza vaccination and have reached different conclusions. A number of studies have compared regular-dose IM vaccination to low-dose ID vaccination and found similar immunogenicity, which suggested dose-sparing effects [21–26]. However, these studies have been criticized for lacking a low-dose IM vaccination comparison group, which would more clearly show the role of the ID route of administration. Other studies have included the low-dose IM comparator and did not show dose sparing associated with the ID route [27]. Differences in the doses at which the comparisons were made may help explain these varied results.
Most previous studies assessing the dose-sparing potential of ID vaccination have used hypodermic needles, which are difficult and unreliable to use for ID injection [28]. To enable simple vaccination in the skin, we have developed patches containing antigen-coated microneedles that can be simply and painlessly inserted into the skin [29]. The vaccine then dissolves off the microneedles into the skin within minutes. Coated microneedles used in this way have been shown to enable induction of strong immune responses against influenza vaccines [30–35]. Dose-sparing of coated microneedles was demonstrated using ovalbumin as a model antigen [36, 37]. Other types of microneedle systems have also been used for vaccination [20, 23, 38–41].
In this study, we determined the immunogenicity and protective efficacy of different doses of influenza VLP vaccine delivered to the skin using coated microneedles in comparison with IM vaccination. We found that microneedle vaccination in the skin with a low dose of influenza VLP vaccine induced comparable protection to IM immunization with a three-fold higher dose of influenza VLPs and much stronger protection compared to IM immunization at the same low dose. These findings indicate significant dose sparing effects of microneedle vaccination in the skin.
2. Materials and methods
2.1. Preparation of microneedle vaccines and coating with VLP
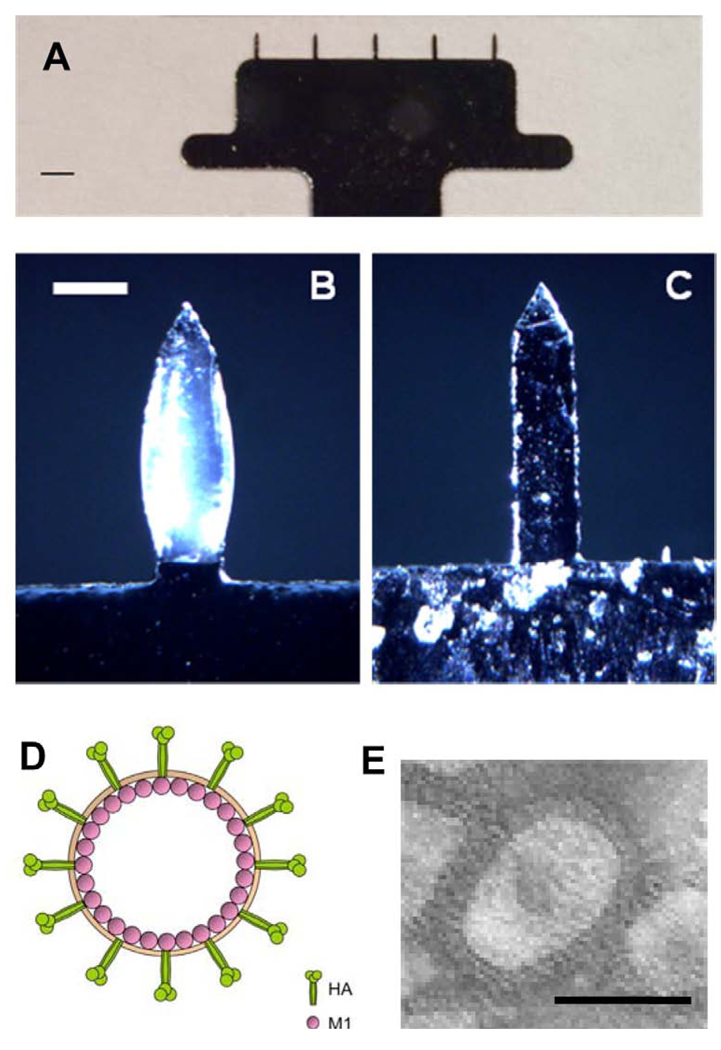
Microneedle preparations and coatings were performed as previously described [30]. Rows of stainless-steel (SS304, 75 µm thickness, McMaster-Carr, Atlanta, GA) microneedles were produced by laser-cutting (Resonetics Maestro, Nashua, NH). (Fig. 1A) These microneedles were cleaned and electropolished in a bath containing a 6:3:1 mixture by volume of glycerin, phosphoric acid, and water to remove debris [42]. The dimensions of the microneedles were 700 µm in length, 160 µm in width at the base, and 50 µm in thickness. For a vaccine coating on microneedles, five-microneedle arrays were dipped six times using coating device containing coating solution at room temperature and dried in ambient air [30]. The coating solution was composed of 1% (w/v) carboxymethylcellulose (CMC) sodium salt (Carbo-Mer, San Diego, CA), 0.5% (w/v) Lutrol F-68 NF (BASF, Mt.Olive, NJ), 15% (w/v) D-(+)-trehalose dihydrate (Sigma Aldrich, St. Louis, MO) and 0.75 – 2.5 mg/ml influenza VLPs in phosphate buffered saline (PBS). In order to determine the dose of VLPs coated on microneedles, vaccine-coated microneedles were incubated in PBS solution for 12 h at 4°C and the amount of released protein was measured by a BCA protein assay kit (Pierce Biotechnology, Rockford, IL). Microneedle arrays were imaged by bright-field microscopy (Olympus SZX12 stereo microscope, Tokyo, Japan) with a CCD camera (Leica DC 300, Leica Microsystems, Wetzlar, Germany). To image microneedle arrays after delivery, microneedles coated with influenza VLPs were inserted into mouse cadaver skin for 5 min and then were imaged.
Fig. 1.
Microneedles and virus-like particles (VLP) for vaccination. (A) Image of five-microneedle array shown by brightfield microscopy (scale bar = 1 mm). (B) Microneedle coated with influenza virus-like particle vaccine and (C) microneedle after insertion into mouse skin for 10 min shown by bright field microscopy (scale bar = 200 µm). (D) Schematic diagram of influenza VLPs containing hemmaglutinin (HA) and matrix (M1) proteins. (E) Transmission electron micrographs of negatively stained influenza VLPs in water (scale bar = 100 nm).
2.2. Preparation of influenza virus and VLPs
A/PR/8/1934 (H1N1; A/PR8) influenza virus was cultivated in 10-day old embryonated hen’s eggs and purified from allantoic fluid. The purified virus was inactivated by mixing the virus with formalin at a final concentration of 1:4000 (v/v) as described previously [14]. Spodoptera frugiperda Sf9 cells were maintained in suspension in serum-free SF900II medium (GIBCO-BRL, Carlsbad, CA ). MDCK cells were grown and maintained in Dulbecco’s modified Eagle’s medium (DMEM). Influenza VLPs containing HA and M1 proteins derived from A/PR8 were prepared as described previously [14]. Briefly, the Sf9 insect cells were co-infected with recombinant baculoviruses expressing HA and M1 proteins at an infection multiplication of 2 and 1, respectively. Influenza VLPs in the culture supernatants were purified by using discontinuous sucrose gradients (15%, 30% and 60%) layers, and characterized by western blot and hemagglutination activity analysis [43]. The HA content was approximately 10% of total proteins of influenza VLPs determined as previously described [44]. For negative staining of VLPs for electron microscopy, sucrose gradient-purified VLPs were applied to a carbon-coated Formvar grid for 30 s as described previously [14]. The grid was immediately stained with 1% uranyl acetate and the samples were examined using a transmission electron microscope (H-7500, Hitachi, Pleasanton, CA).
2.3. Immunization and challenge infection
Female inbred BALB/c mice (Charles River, Wilmington, MA) aged 6 to 8 weeks were used. Groups of mice (12 mice per group) were immunized with a microneedle array coated with VLP vaccine at a dose of either 1 µg or 0.3 µg total VLP proteins for delivery to the skin or immunized by IM injection with intact vaccine (1 µg and 0.3 µg/100 µl) in the upper quadriceps muscles of mice (both legs, each with 50 µl).
The experimental groups included mice immunized at a high dose (1 µg) using microneedles (MN(H)) or IM injection (IM(H)) or at a low dose (0.3 µg) using microneedles (MN(L)) or IM injection (IM(L)). During microneedle delivery, mice were anesthetized with ketamine (110 mg/kg, Abbott Laboratories, Abbott Park, IL) mixed with xylaxine (11 mg/kg, Phoenix Scientific, St. Joseph, MO). Hair on the dorsal surface of mice was removed by depilatory cream (Nair, Princeton, NJ) with a moist cotton stick. After cleaning with an ethanol-soaked cotton ball and drying with a hair dryer, an array of vaccine-coated microneedle was inserted into the skin and held for 10 min to release the vaccine antigen from the coated microneedles.
A preliminary challenge dose test was performed with all vaccinated groups (n=3) in advance to find the optimal challenge dose (data not shown). For formal challenge studies, mice (n=9) lightly anesthetized with isoflurane were intranasally infected with a lethal dose of mouse-adapted A/PR8 virus (100 × LD50) in 50 µl of PBS at 5 weeks after immunization with a single VLP vaccine dose. Some of the challenged mice (n=4 out of 9) were sacrificed 4 days after challenge for post-challenge assays and recall immune responses. Mice (n=5 out of 9) were observed daily to monitor changes in body weight and to record mortality for 14 days. We followed an approved Emory IACUC protocol with 25% loss in body weight as the end point. All animal studies were approved by the Emory University Institutional Animal Care and Use Committee (IACUC).
2.4. Antibody response and hemagglutination inhibition titer
Influenza virus-specific antibody (IgG) in serum and lung samples were determined by enzyme-linked immunosorbent assay (ELISA) plates coated with A/PR8 viral antigen and by using anti-mouse IgG-specific secondary antibodies, as described previously [14]. Antibody levels are presented as the averages of individual mouse serum samples in a group (Serum samples were collected from 6 mice out of 12 in each group). To determine hemagglutination-inhibition (HAI) titers, serum samples were first treated with a receptor-destroying enzyme (Denka Seiken, Tokyo, Japan) by incubation overnight at 37°C and then for 30 min at 56°C. Sera were serially diluted, mixed with 4 HA units (HAU) of influenza A/PR8 virus, and incubated for 30 min at room temperature prior to adding 0.5% chicken red blood cells. The reciprocal of highest serum dilution preventing hemagglutination was scored as the HAI titer.
2.5. Neutralization, lung viral titer and lung inflammatory cytokine assay
Virus neutralization assay was performed using MDCK cells (American Type Culture Collection, VA, USA) following a previously described procedure [14]. Lung viral titers at day 4 post challenge were determined by counting plaques formed on the MDCK cells, as described previously [44]. Inflammatory cytokines (IL-6) in lungs collected at day 4 post challenge were analyzed by Ready-Set-Go cytokine kits (eBioscience, San Diego, CA) following the manufacturer’s procedure, as previously described [14].
2.6. Antibody secreting cell response (ASC)
ASC responses were determined from mouse bone marrow cells at day 4 post-challenge. Mouse bone marrow cells were collected and cultured in vitro for 2 days on plates coated with inactivated A/PR/8/34 virus. PR8-specific IgG antibodies bound to the ELISA plates were determined.
2.7. Statistical analysis
All parameters were recorded for individual mice within all groups. When comparing three or more conditions, a one-way analysis of variance (ANOVA) was performed using PC-SAS software (SAS Institute Inc, Cary, NC). A p-value less than 0.05 was considered to be significant. The mean and standard deviation of the mean were calculated.
3. Results
3.1. Microneedles coated with influenza VLPs
After coating with a formulation containing influenza VLPs as antigen, microneedles showed uniform coating with a slightly bulky shape (Fig. 1B). After insertion into mouse skin, microneedles showed almost complete dissolution of the coated antigen (Fig. 1C). These findings are in agreement with our previous study of microneedle delivery of inactivated influenza virus vaccine, which showed efficient vaccine delivery into the skin , as well as well-distributed antigen through epidermal and dermal layers along the microneedle tract [30].
A schematic diagram of the influenza VLP vaccine is shown in Fig. 1D, exhibiting HA and M1 proteins on its surface. An electron micrograph of the actual VLP vaccine is shown in Fig. 1E. The morphology of VLPs resembles that of wild-type influenza virus particles, also displaying HA spikes on their surfaces, but with M1 proteins inside the virus particle. Taken together, these results show that microneedles can be coated with influenza VLPs, a particulate vaccine structurally similar to the influenza virus
3.2. Dosage effects on virus-specific total IgG and isotype responses
To assess possible dose-sparing effects of ID delivery using microneedles compared to IM delivery using a hypodermic needle, we administered influenza VLPs at doses of 0.3 µg and 1 µg of total proteins by these two methods.
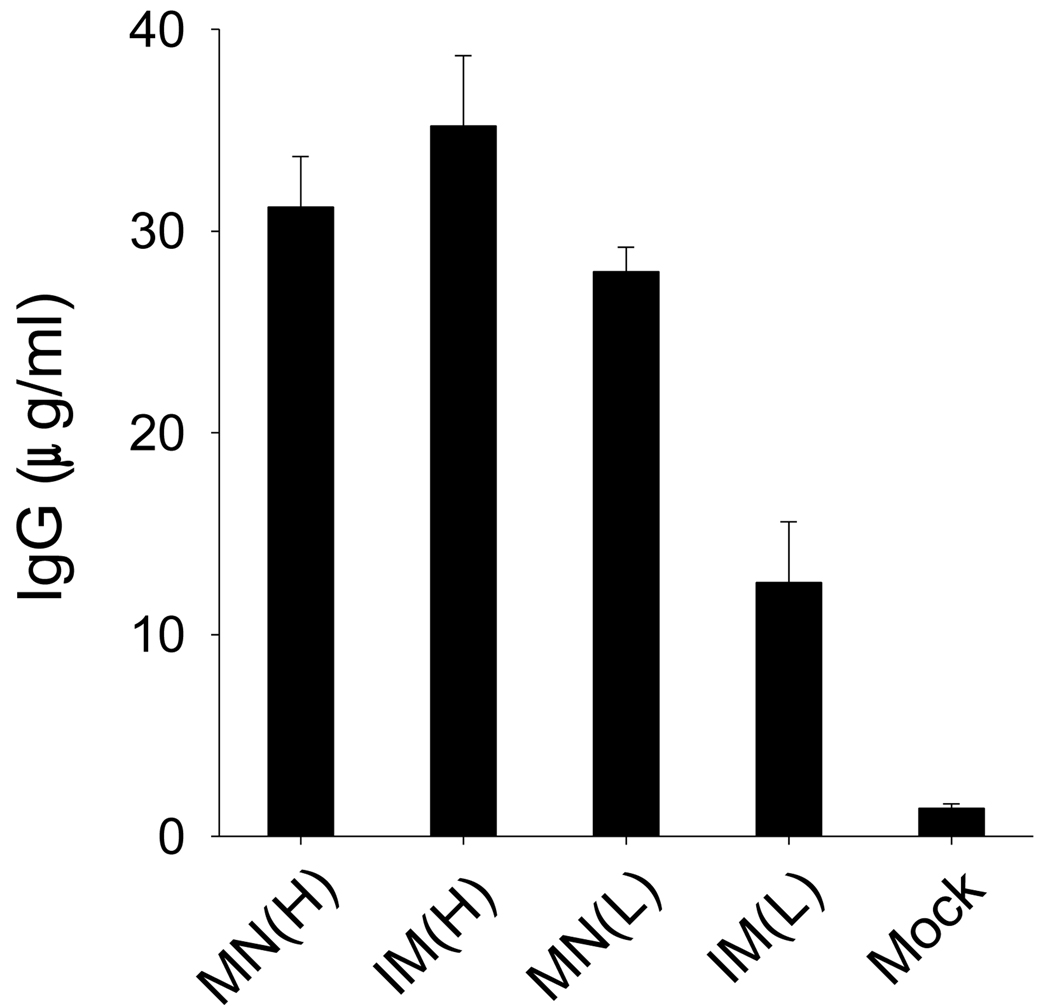
After a single dose of influenza VLPs by microneedles in the skin or by IM injection, virus-specific total IgG antibodies were evaluated in serum samples collected at week 4 post immunization. As shown in Fig. 2, total IgG was similarly enhanced in both the microneedle (MN(H)) and IM (IM(H)) immunization groups at the high VLP dose (1 µg). Remarkably, total IgG for the lower VLP dose (0.3 µg) administered using microneedles (MN(L)) was not significantly different from those of the high-dose vaccinations. In contrast, low-dose vaccination by the IM route (IM(L)) induced significantly lower IgG antibody response compared to the other three groups. These results show that low-dose microneedle vaccination in the skin (MN(L)) induced responses that were stronger than low-dose IM immunization (IM(L)) and similar to high-dose immunization by both routes (IM(H), MN(H)). These data demonstrate the dose-sparing effect of influenza VLP vaccination using microneedles in the skin.
Fig. 2.
IgG antibody responses specific to influenza A/PR8 virus. Groups of mice (n=12) were immunized with a high (1 µg) or low (0.3 µg) dose of VLPs using microneedles or intramuscular injection. Blood samples (n=6) were collected at week 4 after immunization and diluted sera (100-fold) were used to determined PR8-specific total IgG by ELISA. MN(H): high-dose microneedle, IM(H): high-dose intramuscular injection, MN(L): low dose microneedle, IM(L): low-dose intramuscular injection. Data presented as an average standard deviation. ANOVA with multiple comparisons showed significant differences among groups (p<0.001). Duncan or Turkey methods of ANOVA showed no significant differences among groups MN(H), IM(H), and MN(L); however, a significant difference was found between groups MN(L) and IM(L).
3.3. HAI titers
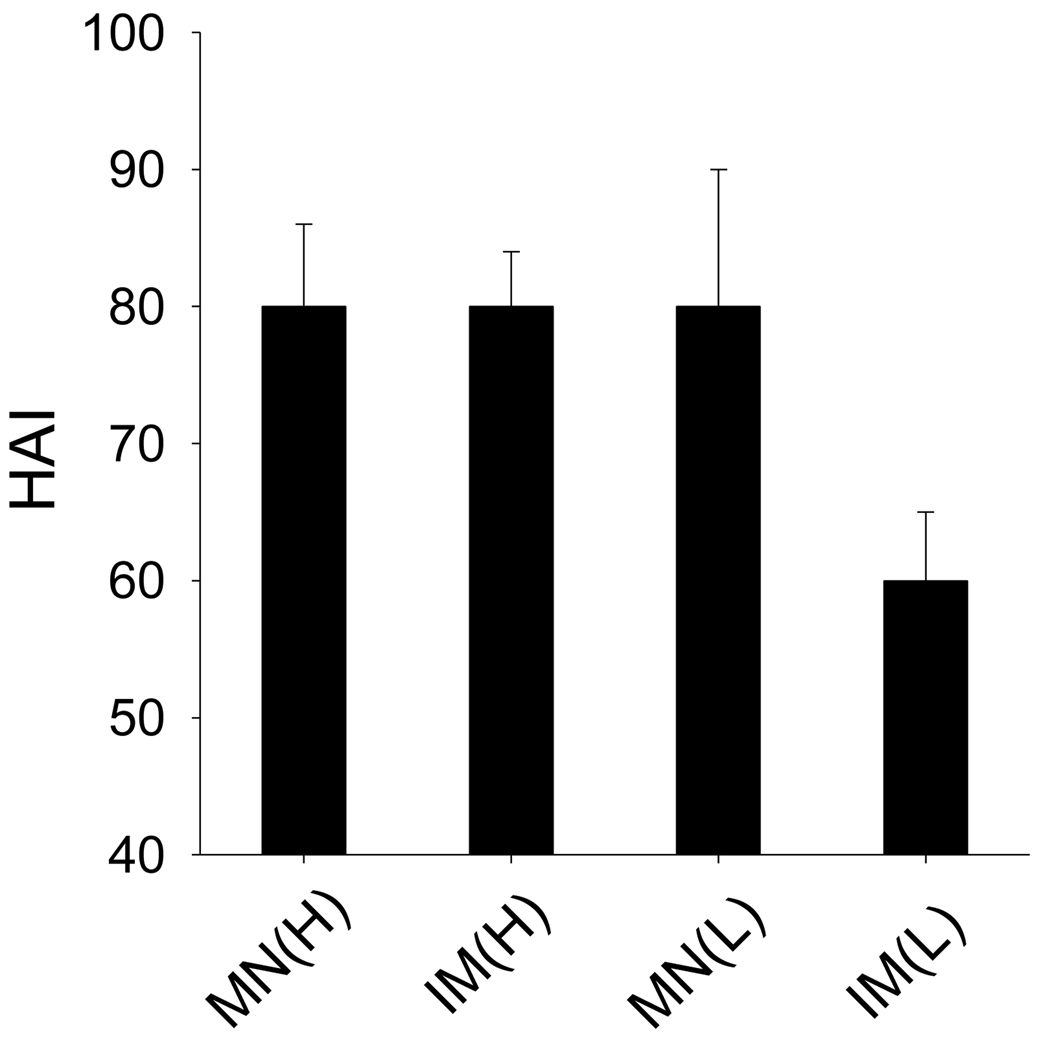
To better understand the dosage effects on microneedle vaccination, HAI titers were determined in serum at week 4 after immunization (Fig. 3). Similar to the findings for total IgG antibody responses, low-dose microneedle vaccination (MN(L)) produced HAI titers just as strong as high-dose vaccination by either route (IM(H) and MN(H)). In contrast, HAI responses by low-dose IM vaccination (IM(L)) were significantly lower. These data further demonstrate the dose sparing effect on inducing HAI titers by influenza VLP vaccination in the skin using microneedles.
Fig. 3.
Hemaglutination inhibition (HAI) titers against PR8-specific virus. HAI titers against A/PR8/34 virus at week 4 after vaccination were determined. Blood samples (n=6) were collected at week 4 after immunization. Groups of mice were the same as described in Fig 2. ANOVA showed no significant differences among groups MN(H), IM(H) and MN(L). A significant difference was found between groups MN(L) and IM(L) (p<0.05).
3.4. Protective vaccine efficacy
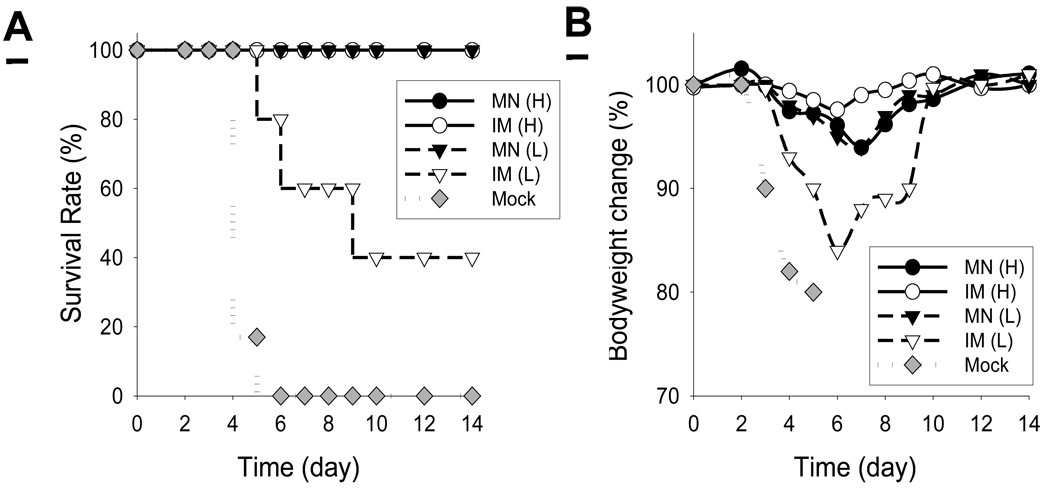
To evaluate protective efficacy, groups of mice immunized with influenza VLPs IM or using microneedles in the skin were challenged with a high lethal dose of influenza A/PR/8/34 virus (100 × LD50) at 7 weeks post vaccination. With a high dose of influenza VLPs, both groups of microneedle (MN(H)) and IM immunization (IM(H)) were 100% protected (Fig. 4A). However, with a low dose (0.3 µg) of influenza VLPs administered IM (IM(L)), only 40% of mice were protected (Fig. 4A). In addition these mice experienced more than 15% body weight loss (Fig. 4B), indicating that the surviving mice suffered severe illness. In contrast, the low-dose microneedle group (MN(L)) showed 100% protection against a high lethal challenge and approximately 5% body weight loss, which demonstrated similar vaccine efficacy to the high-dose vaccinations (Fig. 4A, B). All mice in the mock control died or had to be euthanized by day 5. This further demonstrates a significant dose-sparing effect of influenza VLP vaccine delivery to the skin using microneedles.
Fig. 4.
(A) Survival rates and (B) mouse body weight change after lethal virus challenge. At week 7 after a single immunization, mock and immunized mice were infected with a high lethal dose of mouse-adapted A/PR8 virus (100 × LD50). Mice were monitored daily to determine body weight changes as an indicator of morbidity and the percentage mortality rates (n=5). Groups of mice were the same as described in Fig 2.
3.5. Recall neutralizing activities
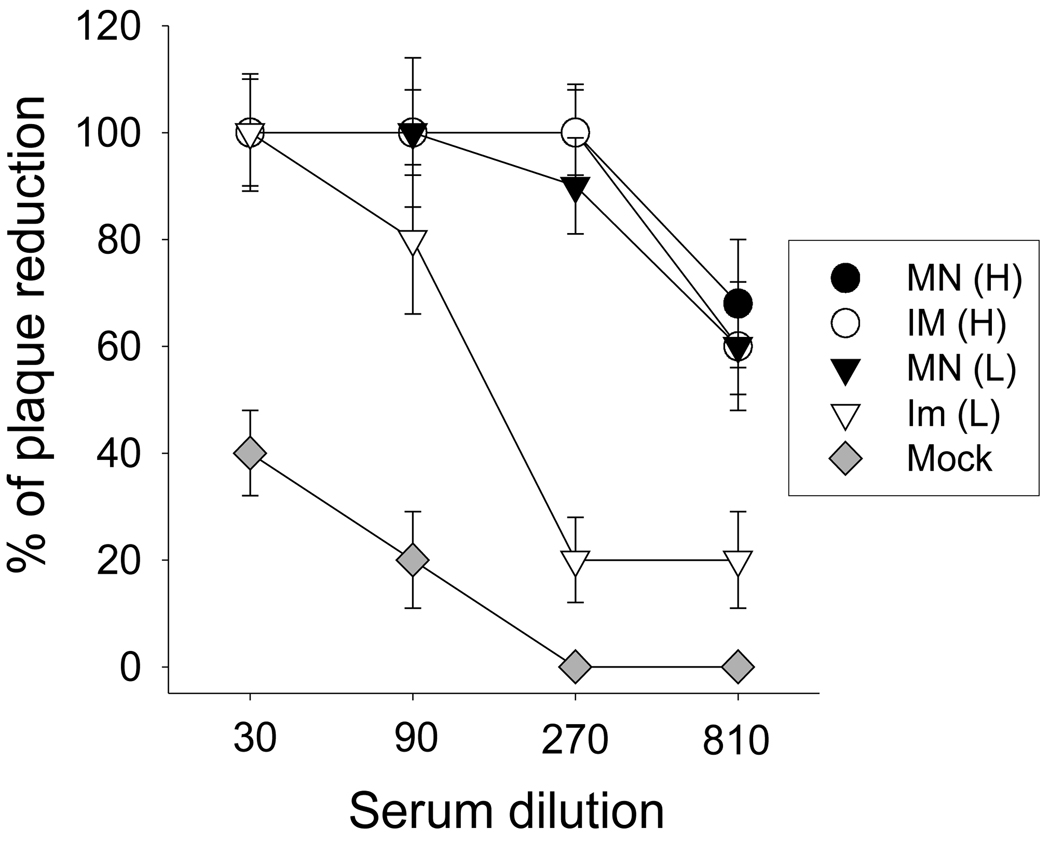
As an additional important serological assay, we determined neutralizing antibodies against A/PR/8/34 virus at day 4 post-challenge. In the high-dose groups, microneedle and IM vaccination showed similarly high levels of recall neutralizing activities (Fig. 5). Microneedle vaccination at the low dose (MN(L)) showed similarly high neutralizing activities. In contrast, the low-dose IM group exhibited a much weaker response. These results indicate that microneedle vaccination in the skin induced virus neutralizing antibodies with significant dose sparing compared to IM immunization.
Fig. 5.
Neutralizing activities against A/PR8 after challenge. Serum samples collected at day 4 after challenge were used to determine neutralizing activities (n=4). Neutralizing titers were expressed as the percentage of plaque reduction compared to that of control. Groups of mice were the same as described in Fig 2.
3.6. Lung viral titers and inflammatory cytokines
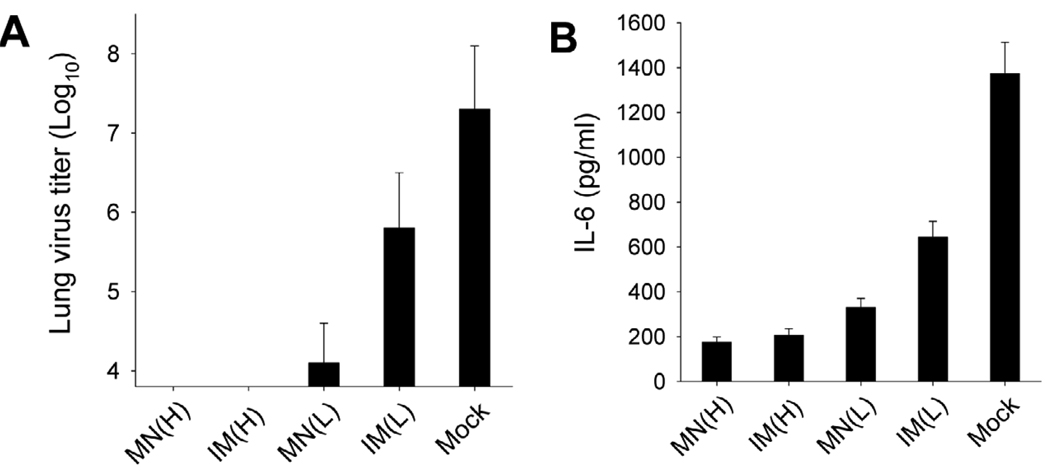
Lung viral titers and inflammatory cytokines at day 4 post challenge provide insights into the efficacy of vaccines in controlling viral replication. Reduced lung viral titers and inflammatory cytokines indicate an effective immune response that clears the lung of virus and reduces inflammation. At high VLP dose, lung viral titers were below the limit of detection when given by either route (Fig. 6A). Microneedle vaccination at low dose reduced lung viral titers by 1580-fold compared to the mock-immunized negative control group. In contrast, IM vaccination at low dose was much less effective, reducing lung viral titers by only 32-fold compared to the negative control.
Fig. 6.
Pulmonary responses after viral challenge. (A) Lung virus titer and (B) lung cytokine IL-6. Lung samples from individual mice in each group (n=4) were collected on day 4 after challenge with a lethal dose of mouse-adapted A/PR/8/34. Each lung sample was diluted to 1 ml with DMEM medium to determine lung virus titers and cytokine IL-6. Groups of mice are as described in the legend of Fig. 2. ANOVA showed no significant differences among groups MN(H), IM(H) and MN(L). A significant difference was found between groups MN(L) and IM(L) (p<0.01).
Cytokine IL-6 is an indicator of lung inflammation due to viral replication. The amount of IL-6 of the microneedle group immunized with high dose VLPs was at low levels similar to those of IM immunization (Fig. 6B). After low dose immunization, the level of IL-6 in the microneedle group was slightly higher than the high-dose comparators, but still significantly lower than that after low-dose IM immunization. Altogether, these results show improved vaccine efficacy by microneedle vaccination with low doses of VLP vaccine compared to those in the corresponding IM group.
3.7. Antibody responses in lung and bone marrow
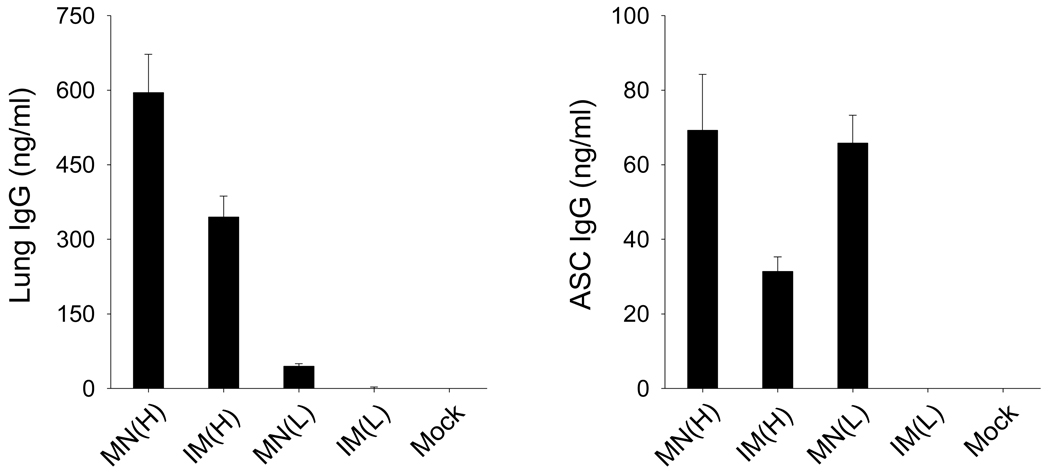
Rapid increases in virus-specific antibodies in lungs post challenge are expected to play an important role in controlling viral replication, since the lung is a major organ for influenza virus replication. On day 4 post challenge, antibody responses were determined in lung extracts (Fig. 7A). The high-dose groups showed significantly greater levels of IgG antibodies in lungs than the low dose groups, demonstrating noticeable dosage effects on increasing levels of IgG antibodies specific to virus. In addition, higher levels of virus-specific IgG antibodies were induced by microneedle vaccination than IM vaccination at both high dose and low dose.
Fig. 7.
Recall antibody responses. (A) Lung IgG and (B) antibody secreting cells (ASC) IgG from bone marrow. Lung and bone marrow samples were collected at day 4 after challenge (n=4). Lung sample IgG or ASC IgG responses were determined by ELISA using A/PR8 coated ELISA plate. Groups are described as in the legend of Fig. 2. Statistical analysis of lung IgG showed that significant differences were found among groups (p<0.0001) using ANOVA. ANOVA analysis of ASC IgG showed that a significant difference (p<0.001) was found between IM(H) and MN(L), and between MN(H) and IM(H) (p<0.001). No significant difference was found between MN(H) and MN(L) (p>0.05).
Long-lived antibody-secreting cells reside in the bone marrow, contributing to the long-term maintenance of serum antibodies. Bone marrow cells collected at day 4 post challenge were cultured for 2 days on plates coated with inactivated A/PR/8/34 virus and then IgG antibodies bound to the plate were determined (Fig. 7B). With both low and high doses of VLP vaccines, higher levels of antibodies were observed in mice immunized using microneedles than those induced by the corresponding IM immunizations. Overall, these results indicate that microneedle vaccination in the skin can induce more effective recall antibody responses than conventional IM immunization.
4. Discussion
Intradermal vaccination has been demonstrated to have dose-sparing effects, which can reduce the cost of vaccines and make it possible to vaccinate more of the population during vaccine shortages. In this study, we utilized solid microneedles coated with influenza VLPs as a means to deliver vaccine to the skin and determined dosage effects in comparison with IM immunization. With a low dose of influenza VLPs, microneedle vaccination induced significantly higher levels of antibody responses as well as improved protection and survival rates compared to corresponding IM immunization. Microneedle vaccination in the skin with low or high doses of influenza VLPs induced increased levels of antibody responses in lungs and bone marrow early post challenge compared to those induced by IM immunization. Overall, this study shows that microneedle delivery of influenza VLP vaccine can be an advantageous approach to enable dose sparing that maintains vaccine efficacy.
In previous studies, the dose sparing effects of ID vaccine delivery appeared to give inconsistent results. Some reports of dose sparing studies demonstrated that lower doses of influenza vaccines via ID delivery induced serological responses equivalent to those obtained by the standard IM dose [21–26]. Auewarakul et al. reported a different result from studies above, demonstrating that ID administration of one fifth lower dose of influenza vaccines induced significantly lower immune responses compared with those from the standard dose of IM [45]. Another clinical trial vaccinating the elderly volunteers (>60years) demonstrated that full-dose ID injection induced significantly higher immune responses including HAI titers and seroconversion rates than full-dose IM injection [20] and two low doses of ID injection showed superior immunity to subcutaneous (SC) injection in infants (<1year) [46].
Most of these studies did not include an equivalent low-dose IM control group. A well-controlled subsequent study investigated the role of different routes of vaccination in inducing immune responses using equal doses by ID and IM immunizations, and concluded that ID delivery was not superior to IM immunization for inducing antibodies in healthy young adults [27]. However, human subjects are heterogenous and some healthy individuals with previous exposure to influenza virus respond better to low antigen doses. Thus, the pre-existing immunity to influenza viruses may influence the outcome of results. It has been difficult to inject vaccines intradermally into the skin of small animal models using needles and syringes. In this regard, studying the detailed immunological aspects after vaccine delivery to the skin is significant and facilitated by using microneedles. Our study demonstrates that microneedle vaccination at a low dose showed superior to IM immunization with the same low dose based on protection following lethal challenge with influenza virus, whereas, a high dose of microneedle vaccination induced similar protective immune responses as IM immunization. Therefore, this study suggests that the superior protection to IM immunization by delivering vaccines to the skin is a dose-dependent phenomenon and that dose sparing effects by ID delivery are likely to be obtained at low vaccine doses.
Microneedle vaccination was less sensitive to dose variations, such that a three-fold reduction in dose from 1 µg to 0.3 µg had either no significant effect or only modest effects on immune responses. In contrast, IM injection was much more responsive to differences in doses, where a three-fold reduction in dose consistently and substantially reduced immunogenicity and protective efficacy. In a previous study using a rat model, immune responses from ID injection of whole inactivated influenza virus over the range of 0.01 µg to 1 µg doses were less dependent on different doses [47]. In contrast, immune responses to IM immunization were more strongly responsive depending on doses injected. Thus, study of laboratory animal models offers some advantages for understanding these immune responses.
It is likely that delivery of vaccines to the skin allows effective targeting to the Langerhans and dermal dendritic cells. In addition, the dermal layer in the skin contains the superficial plexus with branches that drain vertically into well-developed larger lymphatic vessels that access draining lymph nodes [48]. Intradermal delivery of simian human immunodeficiency virus VLP vaccines was recently shown to involve more lymph nodes for an extended period of time leading to larger numbers of germinal center B cells compared to the IM route [49]. Systemically injected soluble antigens passively enter lymph nodes through the afferent lymphatic or blood vessels [50]. Similarly, it is speculated that VLP vaccine antigens delivered IM passively enter the lymphatic vessels to gain access to the follicles of lymphoid organs where many B and T cells reside. Passive transport may require higher doses of vaccines for effective induction of immune responses. In contrast, delivery to the skin may access lymph nodes with lower antigen doses. Therefore, vaccine antigens delivered to the skin are likely to be more immunogenic than IM injection when limited antigens are available or particularly in the immunologically compromised elderly adults [20]. To better understand the underlying mechanisms by which vaccines delivered via microneedle skin vaccination and IM immunization induce differential immune responses, further studies remain to be performed.
In addition to the immunologic advantages of microneedle vaccination in the skin, immunization using a microneedle patch can also offer important logistic advantages compared to conventional hypodermic injection. Microneedles should relieve the pain and apprehension felt by many patients when receiving hypodermic injections [51, 52], and thereby increase patient compliance. The possibility of self-vaccination with microneedle patches could simplify and thereby increase vaccination coverage even more. The small package size of a microneedle patch can also simplify storage, transportation and disposal, as well as reduce the risk of needle-stick injury and needle reuse [53]. Because the cost of a microneedle vaccine is expected to be dominated by the cost of the antigen and its sterile processing (i.e., the microneedles themselves should cost just pennies in mass production), microneedle vaccines are not expected to cost more to manufacture than conventional vaccines given by hypodermic injection. Moreover, the microneedle coating process can be extremely efficient when large numbers of microneedles are coated, meaning that there should be relatively little loss of vaccine during manufacturing.
5. Conclusions
This study provides data in support of the hypothesis that low-dose influenza VLP vaccination via the skin is more immunogenic than low-dose IM vaccination and similarly immunogenic as high-dose IM vaccination. High-dose microneedle vaccination produced immune responses similar to high-dose IM vaccination as assessed by all measures of immune response used in this study, except for recall antibody responses, which were stronger among microneedle-immunized mice. In contrast, low-dose microneedle vaccination produced stronger immune responses than low-dose IM vaccination by measures of primary immune responses and recall immunity. Most importantly, low-dose microneedle was equivalent to high-dose IM vaccination in six of the nine measures of immune response, including HAI and survival post-challenge. We conclude that skin immunization using microneedles enabled dose sparing of influenza VLP vaccine, which may enable an improved vaccination strategy for influenza and other vaccines.
Acknowledgments
This project was supported by NIH grants EB006369 (M.R.P.) and AI0680003 (R.W.C.), Southeast Regional Center of Excellence for Emerging Infections and Biodefense grant AI 057157 (R.W.C), and a Georgia Research Alliance Program grant GRA-VAC09-A (S.M.K). We thank Dr. Mark Allen for use of his laser microfabrication facilities and Dr. Vladimir Zarnitsyn for microneedle fabrication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
M.R.P. serves as a consultant and is an inventor on patents licensed to companies developing microneedle-based products. This possible conflict of interest has been disclosed and is being managed by Georgia Tech and Emory University. R.W.C. and S.M.K. have equity in Zetra Biologicals which is developing VLP technology under license from Emory University. The VLP system reported here is different from VLP vaccine products under development and the information in this manuscript is not directly related to those products.
References
- 1.Hoelscher M, Gangappa S, Zhong WM, Jayashankar L, Sambhara S. Vaccines against epidemic and pandemic influenza. Expert Opin. Drug Deliv. 2008;5:1139–1157. doi: 10.1517/17425247.5.10.1139. [DOI] [PubMed] [Google Scholar]
- 2.Beigel J, Bray M. Current and future antiviral therapy of severe seasonal and avian influenza. Antivir. Res. 2008;78:91–102. doi: 10.1016/j.antiviral.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansson BE, Brett IC. Changing perspective on immunization against influenza. Vaccine. 2007;25:3062–3065. doi: 10.1016/j.vaccine.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 4.Stephenson I, Nicholson KG, Wood JM, Zambon MC, Katz JM. Confronting the avian influenza threat: vaccine development for a potential pandemic. Lancet Infect. Dis. 2004;4:499–509. doi: 10.1016/S1473-3099(04)01105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pattenden LK, Middelberg APJ, Niebert M, Lipin DI. Towards the preparative and largescale precision manufacture of virus-like particles. Trends Biotechnol. 2005;23:523–529. doi: 10.1016/j.tibtech.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Noad R, Roy P. Virus-like particles as immunogens. Trends Microbiol. 2003;11:438–444. doi: 10.1016/s0966-842x(03)00208-7. [DOI] [PubMed] [Google Scholar]
- 7.Bright RA, Carter DM, Daniluk S, Toapanta FR, Ahmad A, Gavrilov V, Massare M, Pushko P, Mytle N, Rowe T, Smith G, Ross TM. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine. 2007;25:3871–3878. doi: 10.1016/j.vaccine.2007.01.106. [DOI] [PubMed] [Google Scholar]
- 8.Haynes JR, Dokken L, Wiley JA, Cawthon AG, Bigger J, Harmsen AG, Richardson C. Influenza-pseudotyped Gag virus-like particle vaccines provide broad protection against highly pathogenic avian influenza challenge. Vaccine. 2009;27:530–541. doi: 10.1016/j.vaccine.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Mahmood K, Bright RA, Mytle N, Carter DM, Crevar C, Achenbach JE, Heaton PM, Tumpey TM, Ross TM. H5N1VLP vaccine induced protection in ferrets against lethal challenge with highly pathogenic H5N1 influenza viruses. Vaccine. 2008;26:5393–5399. doi: 10.1016/j.vaccine.2008.07.084. [DOI] [PubMed] [Google Scholar]
- 10.Pushko P, Tumpey TM, Bu F, Knell J, Robinson R, Smith G. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine. 2005;23:5751–5759. doi: 10.1016/j.vaccine.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 11.Pushko P, Tumpey TM, Van Hoeven N, Belser JA, Robinson R, Nathan M, Smith G, Wright DC, Bright RA. Evaluation of influenza virus-like particles and Novasorne adjuvant as candidate vaccine for avian influenza. Vaccine. 2007;25:4283–4290. doi: 10.1016/j.vaccine.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 12.Quan FS, Steinhauer D, Huang C, Ross TM, Compans RW, Kang S-M. A bivalent influenza VLP vaccine confers complete inhibition of virus replication in lungs. Vaccine. 2008;26:3352–3361. doi: 10.1016/j.vaccine.2008.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross TM, Mahmood K, Crevar CJ, Schneider-Ohrum K, Heaton PM, Bright RA. A Trivalent virus-like particle vaccine elicits protective immune responses against seasonal influenza strains in mice and ferrets. PLoS ONE. 2009;4:e6032. doi: 10.1371/journal.pone.0006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quan FS, Huang CZ, Compans RW, Kang SM. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J. Virol. 2007;81:3514–3524. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valladeau J, Saeland S. Cutaneous dendritic cells. Sem. Immunol. 2005;17:273–283. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 16.WHO. Rabies vaccines. WHO position paper. Weekly Epidemiological Record. 2007;82:425–435. [PubMed]
- 17.Jacobs N, Chen RAJ, Gubser C, Najarro P, Smith GL. Intradermal immune response after infection with Vaccinia virus. J. Gen. Virol. 2006;87:1157–1161. doi: 10.1099/vir.0.81556-0. [DOI] [PubMed] [Google Scholar]
- 18.Warrell MJ, Riddell A, Yu LM, Phipps J, Diggle L, Bourhy H, Deeks JJ, Fooks AR, Audry L, Brookes SM, Meslin FX, Moxon R, Pollard AJ, Warrell DA. A Simplified 4-Site Economical Intradermal Post-Exposure Rabies Vaccine Regimen: A Randomised Controlled Comparison with Standard Methods. PLoS Negl. Trop. Dis. 2008;2:e224. doi: 10.1371/journal.pntd.0000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnou R, Icardi G, De Decker M, Ambrozaitis A, Kazek MP, Weber F, Van Damme P. Intradermal influenza vaccine for older adults: A randomized controlled multicenter phase III study. Vaccine. 2009;27:7304–7312. doi: 10.1016/j.vaccine.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 20.Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, McKeirnan M, Salem H, Mills G, Reid J, Weber F, Saville M. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: A randomized controlled trial. J. Infect. Dis. 2008;198:650–658. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- 21.Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, Van Hoecke C, Howe BJ, Dubin G. Serum antibody responses after intradermal vaccination against influenza. New Engl. J. Med. 2004;351:2286–2294. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- 22.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. New Engl. J. Med. 2004;351:2295–2301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 23.Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27:454–459. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 24.Chiu SS, Peiris JSM, Chan KH, Wong WHS, Lau YL. Immunogenicity and safety of intradermal influenza immunization at a reduced dose in healthy children. Pediatrics. 2007;119:1076–1082. doi: 10.1542/peds.2006-3176. [DOI] [PubMed] [Google Scholar]
- 25.Beran J, Ambrozaitis A, Laiskonis A, Mickuviene N, Bacart P, Calozet Y, Demanet E, Heijmans S, Van Belle P, Weber F, Salamand C. Intradermal influenza vaccination of healthy adults using a new microinjection system: a 3-year randomised controlled safety and immunogenicity trial. BMC Med. 2009;7:13. doi: 10.1186/1741-7015-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jo YM, Song JY, Hwang IS, Lee J, Oh SC, Kim JS, Kim SR, Kim WJ, Cheong HJ. Dose sparing strategy with intradermal influenza vaccination in patients with solid cancer. J. Med. Virol. 2009;81:722–727. doi: 10.1002/jmv.21186. [DOI] [PubMed] [Google Scholar]
- 27.Belshe RB, Newman FK, Wilkins K, Graham IL, Babusis E, Ewell M, Frey SE. Comparative immunogenicity of trivalent influenza vaccine administered by intradermal or intramuscular route in healthy adults. Vaccine. 2007;25:6755–6763. doi: 10.1016/j.vaccine.2007.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laurent PE, Bonnet S, Alchas P, Regolini P, Mikszta JA, Pettis R, Harvey NG. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine. 2007;25:8833–8842. doi: 10.1016/j.vaccine.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin. J. Pain. 2008;24:585–594. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J. Control. Release. 2010;142:187–195. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Enhanced memory responses to H1N1 influenza vaccination in the skin using vaccine coated-microneedles. J. Infect. Dis. 2010;201:190–198. doi: 10.1086/649228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Improved influenza vaccination in the skin using vaccine coated-microneedles. Vaccine. 2009;27:6932–6938. doi: 10.1016/j.vaccine.2009.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quan FS, Kim YC, Yoo DG, Compans RW, Prausnitz MR, Kang SM. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS ONE. 2009;4:e7152. doi: 10.1371/journal.pone.0007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koutsonanos DG, Martin MdP, Zarnitsyn VG, Sullivan SP, Compans RW, Prausnitz MR, Skountzou I. Transdermal influenza immunization with vaccine-coated microneedle arrays. PLoS ONE. 2009;4:e4773. doi: 10.1371/journal.pone.0004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu QY, Zarnitsyn VG, Ye L, Wen ZY, Gao YL, Pan L, Skountzou I, Gill HS, Prausnitz MR, Yang CL, Compans RW. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc. Natl. Acad. Sci. U. S. A. 2009;106:7968–7973. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matriano JA, Cormier M, Johnson J, Young WA, Buttery M, Nyam K, Daddona PE. Macroflux microprojection array patch technology: A new and efficient approach for intracutaneous immunization. Pharm. Res. 2002;19:63–70. doi: 10.1023/a:1013607400040. [DOI] [PubMed] [Google Scholar]
- 37.Widera G, Johnson J, Kim L, Libiran L, Nyam K, Daddona PE, Cormier M. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24:1653–1664. doi: 10.1016/j.vaccine.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 38.Andrianov AK, DeCollibus DP, Gillis HA, Kha HH, Marin A, Prausnitz MR, Babiuk LA, Townsend H, Mutwiri G. Poly di(carboxylatophenoxy)phosphazene is a potent adjuvant for intradermal immunization. Proc. Natl. Acad. Sci. U. S. A. 2009;106:18936–18941. doi: 10.1073/pnas.0908842106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen XF, Prow TW, Crichton ML, Jenkins DWK, Roberts MS, Frazer IH, Fernando GJP, Kendall MAF. Dry-coated microprojection array patches for targeted delivery of immunotherapeutics to the skin. J. Control. Release. 2009;139:212–220. doi: 10.1016/j.jconrel.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 40.Ding Z, Van Riet E, Romeijn S, Kersten GFA, Jiskoot W, Bouwstra JA. Immune modulation by adjuvants combined with diphtheria toxoid administered topically in BALB/c mice after microneedle array pretreatment. Pharm. Res. 2009;26:1635–1643. doi: 10.1007/s11095-009-9874-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding Z, Verbaan FJ, Bivas-Benita M, Bungener L, Huckriede A, van den Berg DJ, Kersten G, Bouwstra JA. Microneedle arrays for the transcutaneous immunization of diphtheria and influenza in BALB/c mice. J. Control. Release. 2009;136:71–78. doi: 10.1016/j.jconrel.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 42.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J. Control. Release. 2007;117:227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hierholz JC, Suggs MT. Standardized viral hemagglutination and hemagglutinationinhibition test. I. Standardization of erythrocyte suspensions. Appl. Microbiol. 1969;18:816–823. doi: 10.1128/am.18.5.816-823.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quan FS, Yoo DG, Song JM, Clements JD, Compans RW, Kang SM. Kinetics of immune responses to influenza virus-like particles and dose-dependence of protection with a single vaccination. J. Virol. 2009;83:4489–4497. doi: 10.1128/JVI.02035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Auewarakul P, Kositanont U, Sornsathapornkul P, Tothong P, Kanyok R, Thongcharoen P. Antibody responses after dose-sparing intradermal influenza vaccination. Vaccine. 2007;25:659–663. doi: 10.1016/j.vaccine.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 46.Sugimura T, Ito Y, Tananari Y, Ozaki Y, Maeno Y, Yamaoka T, Kudo Y. Improved antibody responses in infants less than 1 year old using intradermal influenza vaccination. Vaccine. 2008;26:2700–2705. doi: 10.1016/j.vaccine.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 47.Alarcon JB, Hartley AW, Harvey NG, Mikszta JA. Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines. Clin. Vaccine Immunol. 2007;14:375–381. doi: 10.1128/CVI.00387-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skobe M, Detmar M. Structure, function, and molecular control of the skin lymphatic system. J. Invest. Dermatol. Symp. Proc. 2000;5:14–19. doi: 10.1046/j.1087-0024.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 49.Cubas R, Zhang S, Kwon SK, Sevick-Muraca EM, Li M, Chen CY, Yao QZ. Virus-like particle (VLP) lymphatic trafficking and immune response generation after immunization by different routes. J. Immunother. 2009;32:118–128. doi: 10.1097/CJI.0b013e31818f13c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of human skin following needle insertions. Eur. J. Pain. 1999;3:41–49. doi: 10.1053/eujp.1998.0099. [DOI] [PubMed] [Google Scholar]
- 52.Hanas R. Reducing injection pain in children and adolescents with diabetes: a review of indwelling catheters. Pediatr. Diabetes. 2004;5:102–111. doi: 10.1111/j.1399-543X.2004.00048.x. [DOI] [PubMed] [Google Scholar]
- 53.Hauri AM, Armstrong GL, Hutin YJF. The global burden of disease attributable to contaminated injections given in health care settings. Int. J. Std. AIDS. 2004;15:7–16. doi: 10.1258/095646204322637182. [DOI] [PubMed] [Google Scholar]