Abstract
We demonstrate that influenza A virus strains that circulate in humans differ markedly in the ability of their NS1 proteins to block the activation of IRF3 and interferon-β transcription. Strong activation occurs in cells infected with viruses expressing NS1 proteins of seasonal H3N2 and H2N2 viruses, whereas activation is blocked in cells infected with viruses expressing NS1 proteins of some, but not all seasonal H1N1 viruses. The NS1 proteins of the 2009 H1N1 and H5N1 viruses also block these activations. The difference in this NS1 function is mediated largely by the C-terminal region of the effector domain, which contains the only amino acid (K or E at position 196) that covaries with the functional difference. Further, we show that TRIM25 binds the NS1 protein whether or not IRF3 activation is blocked, demonstrating that binding of TRIM25 by the NS1 protein does not necessarily lead to the blocking of IRF3 activation.
Keywords: Influenza A virus, NS1 protein, IRF3 activation, IFN-β transcription, TRIM25
Introduction
Influenza A viruses cause a highly contagious respiratory disease that results in approximately 36,000 deaths annually in the United States (Thompson et al., 2003). The annual (seasonal) viruses currently circulating in humans are comprised of two subtypes with antigenically distinct surface hemagglutinin (H) and neuraminidase (N) surface proteins, H1N1 and H3N2. Influenza A viruses are also responsible for the world-wide pandemics that usually result in high mortality rates (Wright, 2001). The most severe pandemic occurred in 1918, which was caused by a H1N1 virus. A pandemic in 1957 replaced H1N1 viruses with H2N2 viruses, followed by a 1968 pandemic that replaced the H2N2 viruses with the currently circulating H3N2 viruses. The currently circulating H1N1 viruses were reintroduced by an undetermined mechanism in 1977. We are now in the midst of a pandemic caused by a virus originating in swine, the 2009 H1N1 virus or “swine flu” (Garten et al., 2009). Because the H1 subtype HA of “swine flu” differs substantially from recent H1 HAs of seasonal influenza A viruses, most of the human population lacks immunological protection against “swine flu.” Fortunately, this virus has so far caused only a relatively mild disease. Consequently, all these H1N1, H2N2 and H3N2 viruses can efficiently circulate in the human population. In contrast, H5N1 viruses (“bird flu”), which are extremely virulent in humans, have not yet acquired the ability for efficient human-to-human transmission (WHO, 2010).
Influenza A virus contains eight negative-stranded RNA genomic segments (Lamb, 2001). The smallest segment encodes the NS1 protein, a multi-functional nonstructural protein (Hale et al., 2008; Zhao, 2010). Most NS1 proteins are 230-237 amino acids long, although smaller forms are also found. The NS1 protein is comprised of two functional domains: N-terminal (amino acids 1-73) RNA-binding domain, which binds double-stranded RNA; and C-terminal (amino acids 74-230/237) effector domain, which binds several host proteins. An important role of the NS1 protein is to counter host cell antiviral responses. A major cellular antiviral response is the synthesis of interferon-α/β (IFN-α/β), which in turn activates the transcription of an array of genes encoding proteins that establish an antiviral state (Haller, Kochs, and Weber, 2006; Randall and Goodbourn, 2008). Two NS1 protein-mediated countermeasures against the production of IFN have been described. One countermeasure results from the specific binding by the NS1 effector domain of the 30-kDa subunit of the cellular cleavage and polyadenylation specificity factor (CPSF30), a protein that is required for the 3’ end processing of cellular pre-mRNAs (Nemeroff et al., 1998). As a consequence of the sequestration of CPSF30 by the NS1 protein, most of the IFN-β pre-mRNA synthesized in infected cells is not processed in the nucleus to form mature IFN-β mRNA in the cytoplasm (Das, 2008; Kim, 2002; Noah, 2003; Twu et al., 2007; Twu, 2006). In a second countermeasure the NS1 protein blocks the activation of the IRF3 transcription factor, as well as the activation of NF-κB, thereby blocking the activation of IFN-β transcription and hence the synthesis of IFN-β pre-mRNA (Gack et al., 2009; Mibayashi et al., 2007; Opitz et al., 2006; Pichlmair et al., 2006; Talon, 2000; Wang, 2000). Almost all the studies describing this countermeasure have used the laboratory-generated H1N1 influenza A/PR/8/34 (PR8) virus. In contrast to the results with this virus, it was reported that IRF3 and IFN-β transcription are efficiently activated in cells infected by the H3N2 influenza A/Udorn/72 virus (Ud) (Das, 2008; Kim, 2002; Noah, 2003), which circulated in humans in 1972, demonstrating that the NS1 protein encoded by this virus does not block these activations. In addition, two other studies reported that the NS1 proteins of different influenza A virus strains vary in their ability to block IRF3 activation (Hayman et al., 2006; Kochs et al., 2007) (see Discussion).
To resolve this issue, we determined whether IRF3 and IFN-β transcription are activated in human cells after infection with viruses that express NS1 proteins encoded by various naturally occurring influenza A viruses, specifically including those viruses that have efficiently circulated in humans. We first had to establish a reliable assay to measure the transcription of the IFN-β gene. Because of the NS1 protein-mediated inhibition of the 3’ end processing of cellular pre-mRNAs (Das, 2008; Kim, 2002; Nemeroff et al., 1998; Noah, 2003), IFN-β transcription cannot be assayed by determining the levels of mature, polyadenylated IFN-β mRNA. For the same reason IFN-β transcription cannot be assayed using reporter assays, i.e., using transfected plasmids in which a reporter protein is expressed under the control of a RNA polymerase II-driven IFN-β promoter. In addition, runoff transcription assays (Ausubel, 1994) using nuclei isolated from infected cells are problematic because the large subunit of the host RNA polymerase II undergoes degradation in influenza A virus-infected cells (Vreede et al., 2010). Here we describe another approach to assay the transcription of the IFN-β gene: quantitative RT-PCR measurement of the amount of the transcription product, i.e., IFN-β pre-mRNA that is produced during infection. Specifically, we measure the amount of IFN-β pre-mRNA that contains approximately 100 bases downstream from the poly(A) addition site. Our results show that this IFN-β pre-mRNA species is quite stable, which is consistent with the current understanding of nuclear pre-mRNA processing and degradation (Houseley and Tollervey, 2009) (see Results).
Using this assay for IFN-β transcription and immunoblots to measure IRF3 activation, we show that the ability of the NS1 protein to block the activation of IRF3 and IFN-β transcription differs markedly between HN subtypes. Thus, IRF3 and IFN-β transcription are efficiently activated in cells infected with viruses expressing the NS1 proteins encoded by H3N2 and H2N2 viruses, demonstrating that blocking these activations by the NS1 protein is not required for the efficient circulation of influenza A viruses in humans. In contrast, the activation of IRF3 and IFN-β transcription is blocked in cells infected with viruses expressing the NS1 proteins encoded by some, but not all H1N1 viruses. Unexpectedly, we found that the IRF3 and IFN-β transcription phenotype of the H1N1 NS1 protein fluctuated over several time periods. For example, whereas the H1N1 NS1 proteins since 1991 block activation of IRF-3 and IFN-β transcription in infected cells, many of the H1N1 proteins in the time periods of 1940-1957 and 1977-1990 do not block these activations. The NS1 proteins of 2009 H1N1 (“swine flu”) and H5N1 viruses, like some of the seasonal H1N1 viruses, block these activations in infected cells.
Further, we show that this striking difference in the ability of NS1 proteins to block the activation of IRF3 and IFN-β transcription is mediated largely by the NS1 effector domain, and that the C-terminal region of the effector domain (amino acids 183-230/237) is responsible for a large part of the functional difference. This C-terminal region contains the only amino acid that covaries with the functional difference: amino acid 196 is E in the NS1 proteins that block activation of IRF-3 and IFN-β transcription, whereas it is K in the NS1 proteins that do not block these activations. Our results suggest that the C-terminal region of the effector domain of a subset of NS1 proteins contains a binding site for one or more proteins, with the amino acid at position 196 playing an important role in this binding, and that binding of such a protein by this subset of NS1 proteins results in the blocking of IRF3 activation.
It has been proposed that the binding of the NS1 protein to the E3 ubiquitin ligase TRIM25 is responsible for the blocking of IRF3 activation by the NS1 protein (Gack et al., 2009). In influenza A virus-infected cells, IRF3 activation, as well as NF-kB activation, are initiated by the recognition of viral RNAs by RIG-I (Loo et al., 2008), whose C-terminal CARD domain then interacts with the CARD domain of MAVS, the next component of the signaling pathway (Kawai et al., 2005; Meylan et al., 2005; Seth et al., 2005; Xu et al., 2005). The RIG-I CARD domain needs to be activated for this interaction. This activation was attributed to the ubiquitination of the CARD domain by the TRIM25 E3 ligase (Gack et al., 2008; Gack et al., 2007), which had previously been shown to be required for IRF3 activation (Gack et al., 2007). Because the NS1 protein was shown to bind TRIM25 in infected cells, it was proposed that sequesteration of TRIM25 by the NS1 protein caused the inhibition of the activation of the RIG-I CARD domain and hence inhibition of IRF3 activation (Gack et al., 2009). TRIM25 was reported to bind to the NS1 proteins encoded by both H1N1 and H3N2 viruses in infected cells. We confirmed the latter result, and showed that in infected cells TRIM25 binds not only to a H1N1 NS1 protein that mediates the blocking of IRF3 activation, but also to a H3N2 NS1 protein that does not block IRF3 activation. Consequently, binding of TRIM25 by the NS1 protein does not necessarily lead to the blocking of IRF3 activation, and TRIM25 does not bind selectively to the subset of NS1 proteins that block IRF3 activation. Accordingly, we propose that the blocking of IRF3 activation by a subset of NS1 proteins results from the specific binding of one or more proteins other than TRIM25 to the C-terminal region of the effector domain of these NS1 proteins.
Results
The H3N2 influenza A/Udorn/72 virus does not block the activation of IRF3 and IFN-β transcription in infected mammalian cells
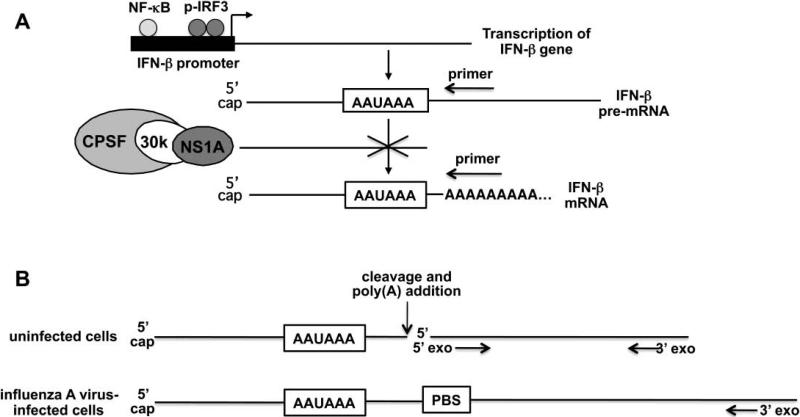
The steps in the production of mature IFN-β mRNA in influenza A virus-infected cells are shown in Figure 1A. Activation of the transcription of the IFN-β gene during virus infection requires the activation of two transcription factors, IRF3 and NF-kB (Maniatis, 1998). We assayed IRF3 activation using immunoblots probed with antibody directed against serine 396-phosphorylated (activated) IRF3. We have found that this immunoblot assay is more quantitative than the assay for the formation of phosphorylation-dependent IRF3 dimers that we previously used (Das, 2008; Kim, 2002).
Figure 1.
The assay for the activation of the transcription of IFN-β transcription in influenza A virus-infected cells. A. The steps in the production of mature IFN-β mRNA in influenza A virus-infected cells. The different oligonucleotide primers used to generate DNA copies specific for either IFN-β pre-mRNA or mature IFN-β mRNA are shown as arrows above the RNA regions complementary to the two primers. B. Comparison of the degradation of pre-mRNAs in uninfected and influenza A virus-infected cells. See text for details. PBS=primer binding site.
Because the NS1 protein of almost all influenza A viruses sequester the cellular CPSF30 3’ end processing factor away from its binding site on the pre-mRNA (the AAUAAA sequence), very little of the IFN-β pre-mRNA is processed to form mature IFN-β mRNA (Das, 2008; Kim, 2002; Nemeroff et al., 1998; Noah, 2003) (Figure 1A). Consequently, the production of IFN-β pre-mRNA, rather than the production of mature IFN-β mRNA, reflects the level of IFN-β transcription. Accordingly, we assayed the transcription of the IFN-β gene by quantitative RTPCR measurement of the amount of IFN-β pre-mRNA that accumulates in infected cells. By using an oligonucleotide primer complementary to a sequence a short distance (less than 100 bases) downstream from the poly (A) addition site for the reverse transcription step, only the pre-mRNA and not the mature mRNA would be copied into DNA (Figure 1A).
Current knowledge of nuclear RNA processing and degradation of cellular pre-mRNA sequences predicts that the IFN-β pre-mRNA species that we assay could be quite stable (Houseley and Tollervey, 2009) (Figure 1B). Termination of polymerase II-directed transcription occurs at sites far downstream of the poly(A) addition site. In uninfected cells the pre-mRNA sequences downstream from the poly(A) addition site are degraded by the combined action of a 5’ exonuclease and a complex of 3’ exonucleases (the nuclear exosome). The 5’ exonuclease initiates degradation at the 5’ end generated by the cleavage at the poly(A) addition site, and catches up with the elongating polymerase, thereby causing the termination of transcription. The 3’ exonucleases initiate degradation at the distant site(s) of termination. In contrast, in influenza A virus-infected cells the NS1 protein blocks cleavage at the poly(A) addition site (Nemeroff et al., 1998), so that degradation of the pre-mRNA sequences downstream from the poly(A) addition site can only be carried out by the 3’ exonucleases. In addition, transcription termination mediated by the 5’ exonuclease does not occur. Consequently, it is likely that a long stretch of pre-mRNA sequences would need to be degraded before reaching the sequence in the pre-mRNA that binds to the oligonucleotide primer used for the quantitative RT-PCR analysis (PBS, primer binding site).
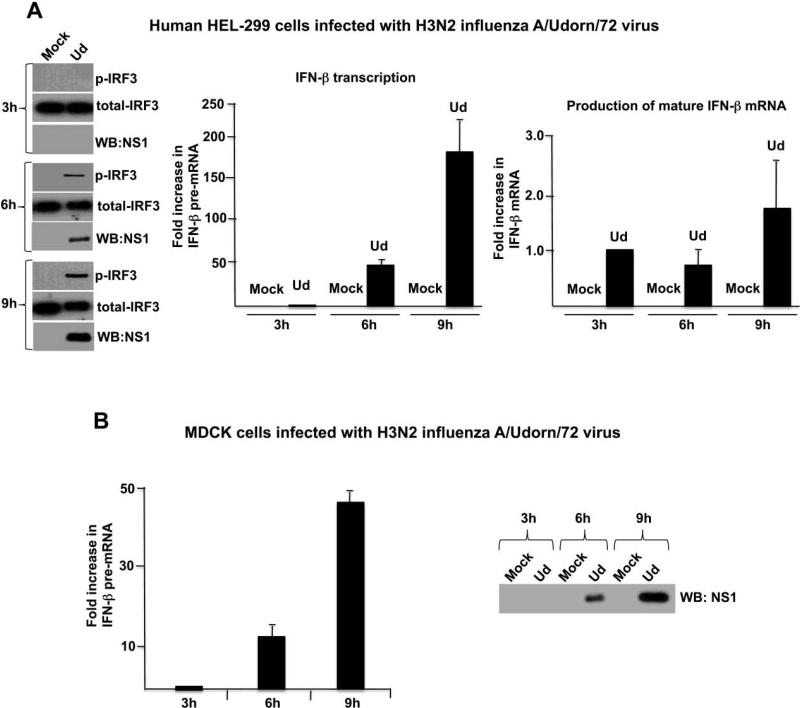
We first used these assays to measure the activation of IRF3 and IFN-β transcription in human embryonic lung (HEL-299) cells infected by the H3N2 influenza A/Udorn/72 (Ud) virus (Figure 2A). Activated (serine 396-phosphorylated) IRF3 was apparent at 6 hours post infection, and increased further by 9 hours post infection. In addition, as shown by the large increase in the amount of IFN-β pre-mRNA relative to that at 3 hours post infection, the Ud virus strongly induced transcription of the IFN-β gene in HEL-299 cells. Because increasing amounts of the NS1 protein were produced from 3 to 9 hours postinfection, we conclude that the Ud NS1 protein does not block the activation of either IRF3 or IFN-β transcription. The increase in IFN-β pre-mRNA from 3 to 9 hours post infection in this experiment, which was carried out in triplicate, was approximately 190-fold. The increase observed in subsequent Ud virus infections has varied from 50-to-190-fold (see below) because the increase is based on the low level of IFN-β pre-mRNA that is produced at 3 hours postinfection, and this level, as might be expected, varies between experiments. The production of increasing amounts of IFN-β pre-mRNA during infection suggests that this pre-mRNA is quite stable, which is consistent with the prediction shown in Figure 1B. Because this prediction is based on the absence of cleavage at the poly(A) addition site, we can conclude that the Ud NS1 protein effectively inhibits this cleavage, as previously documented (Das, 2008; Kim, 2002; Nemeroff et al., 1998; Noah, 2003).
Figure 2.
The H3N2 influenza A/Udorn/72 virus does not block the activation of IRF3 and IFN-β transcription in infected mammalian cells. A. The time course of NS1 protein production, IRF3 activation, IFN-β pre-mRNA synthesis, and production of mature IFN-β mRNA during Ud virus infection of HEL-299 cells. The amounts of the NS1 protein and activated IRF3 at 6 hours postinfection were approximately 25% and 35%, respectively, of the amounts at 9 hours postinfection. B. The time course of NS1 protein production and IFN-β pre-mRNA synthesis during Ud infection of MDCK cells. The amount of the NS1 protein at 6 hours postinfection was approximately 25% of the amount at 9 hours postinfection.
In addition, we used an oligo dT primer in the reverse transcription step to determine the amount of mature, polyadenylated IFN-β mRNA that escapes the NS1 protein-mediated inhibition of pre-mRNA processing (Figure 2A). In contrast to the large increase in IFN-β pre-mRNA from 3 to 9 hours postinfection, the amount of mature IFN-β mRNA increased only a small amount (~2-3-fold) relative to the amount at 3 hours postinfection, demonstrating the effectiveness of the NS1 protein-mediated inhibition of 3’ end processing. Because little NS1 protein was produced at 3 hours postinfection, it is likely that the production of mature IFN-β mRNA at 3 hours postinfection was caused at least in part by incomplete inhibition of the processing of IFN-β pre-mRNA due to a low level of the NS1 protein at this time of infection.
Because MDCK (canine kidney) cells are used for the majority of influenza virus experiments by many investigators, we determined whether the Ud virus induces the production of IFN-β pre-mRNA in these cells. As shown in Figure 2B, Ud virus induces a large (~50-fold) increase in IFN-β pre-mRNA from 3 to 9 hours after infection of MDCK cells, demonstrating that the H3N2 Ud virus does not block the activation of IFN-β transcription in MDCK cells as well as in HEL-299 cells.
A H5N1 virus and the 2009 H1N1 virus block the activation of IRF3 and IFN-α transcription in HEL-299 cells
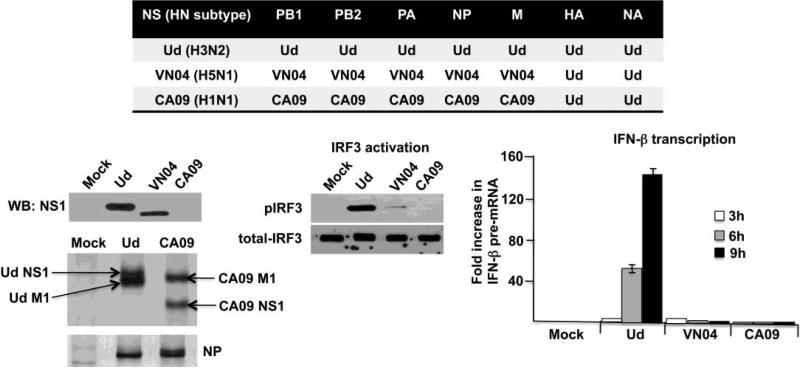
In contrast to the H3N2 Ud virus, infection of HEL-299 cells with either a H5N1 virus (influenza A/Vietnam/1203/04; VN04) or the 2009 H1N1 virus (influenza A/California/09; CA09)did not activate IRF3 or IFN-β transcription (Figure 3). For biosafety reasons, the viruses denoted as VN04 and CA09 contained the Ud HA and NA genes. Whereas the H3N2 Ud virus activated IRF3 and induced a large increase in IFN-β pre-mRNA by 9 hours postinfection, little or no activated IRF-3 or IFN-β pre-mRNA was detected at 9 hours after infection with the H5N1 VN04 or 2009 H1N1 CA09 virus. An immunoblot probed with antibody directed against the Ud NS1 protein showed that the amount of the NS1 protein synthesized in cells infected by the Ud virus was approximately 3 times more than that synthesized in cells infected by the VN04 virus. Nonetheless, the amount of the NS1 protein synthesized in the VN04 virus-infected cells was sufficient to block activation of IRF3 and IFN-β transcription. Because the Ud NS1 antibody only weakly detected the CA09 NS1 protein, cells infected with either the Ud or CA09 virus were labeled with 35S-labeled methionine and cysteine from 6 to 9 hours postinfection, and the amounts of the viral proteins synthesized were analyzed by denaturing gel electrophoresis. This analysis showed that similar amounts of not only the NS1 protein, but also the M1 and NP proteins, were synthesized in cells infected by the Ud and CA09 virus. These results show that the NS1 protein produced during infection by the CA09 virus, like the NS1 protein produced by the VN04 virus, blocks the activation of IRF3 and IFN-β transcription, in contrast to the Ud NS1 protein that does not block these activations. The same results for the VN04 and CA09 viruses were obtained in MDCK cells (data not shown).
Figure 3.
A H5N1 virus and the 2009 H1N1 virus block the activation of IRF3 and IFN-β transcription in HEL-299 cells. Comparison of the time course of IFN-β pre-mRNA synthesis during Ud, VN04 and CA09 virus infections, and comparison of IRF3 activation at 9 hours after infection with these three viruses. Quantitation of the pIRF3 immunoblot using ImageJ software (NIH) showed that the level of pIRF3 in VN04-infected cells was approximately 1% of that in Ud-infected cells. The amount of the NS1 protein produced at 9 hours by these three viruses was determined by an immunoblot using Ud NS1 antibody (Ud and VN04), and by 35S-methionine and cysteine labeling of infected cells from 6 to 9 hours postinfection (Ud and CA09). Two regions of the gel analysis of the 35S-labeled proteins are shown: the NS1/M1 region; and the NP region. The identity of the labeled protein denoted as the Ud NS1 protein was verified by an immunoblot probed with the Ud NS1 antibody. The Ud NS1 protein is larger (237 amino acids) than the CA09 NS1 protein (219 amino acids).
The different effects of the HN virus subtypes on the activation of IRF3 and IFN-β transcription is mediated largely by the effector domain of the encoded NS1 protein
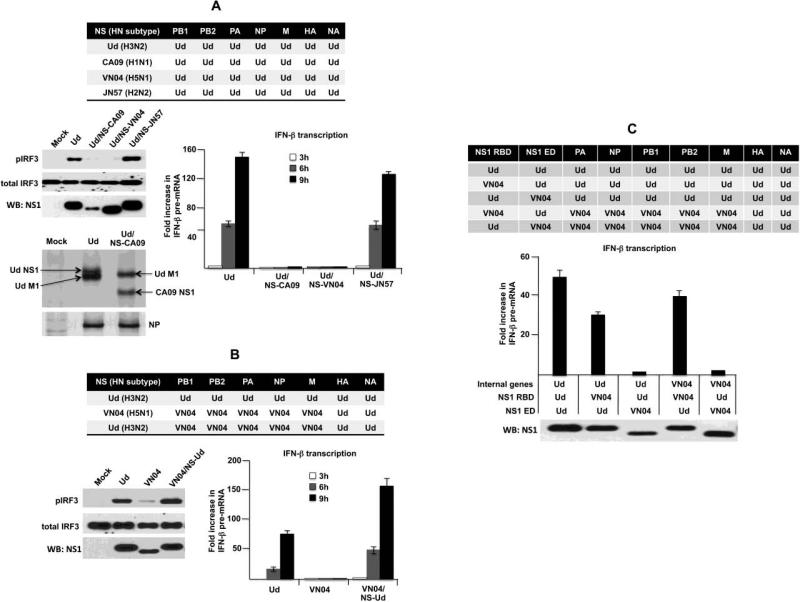
To verify that the effects on the activation of IRF3 and IFN-β are mediated by the NS1 protein, we generated a series of chimeric Ud viruses in which the H3N2 Ud NS gene was replaced by the NS gene from other influenza A virus subtypes (Figure 4A). Replacement of the Ud NS gene with the NS gene from either the 2009 H1N1 CA09 or H5N1 VN04 virus yielded viruses that blocked the activation of IRF3 and IFN-β transcription, demonstrating that the NS1 protein encoded by either of these two HN subtypes was sufficient to enable the Ud virus to block these two processes. In contrast, replacement of the H3N2 gene with the NS gene from the H2N2 influenza A/Japan/57 (JN57) virus yielded a virus that retained the ability to activate IRF3 and IFN-β transcription, demonstrating that the H2N2 NS1 protein shares this phenotype with the H3N2 Ud NS1 protein. An immunoblot probed with antibody directed against the Ud NS1 protein showed that similar amounts of the Ud, VN04 and NS-JN NS1 proteins were synthesized, and a 35S-methionine and cysteine labeling experiment showed that a similar amount of the CA09 NS1 protein was synthesized. These results demonstrate that the NS1 proteins encoded by different influenza A virus HN subtypes differ in their ability to block the activation of IRF3 and IFN-β transcription.
Figure 4.
The different effects of the HN virus subtypes on the activation of IRF3 and IFN-β transcription is mediated largely by the effector domain of the encoded NS1 protein. A. Comparison of the time course of IFNβ pre-mRNA synthesis during infection with the recombinant Ud viruses encoding the indicated NS1 protein, and comparison of IRF3 activation at 9 hours after infection with the indicated Ud recombinants. The amount of the NS1 protein produced at 9 hours by these viruses was determined by an immunoblot using Ud NS1 antibody and by 35S-methionine and cysteine labeling of infected cells from 6 to 9 hours postinfection (Ud and Ud/NS-CA09). Two regions of the gel analysis of the 35S-labeled proteins are shown: the NS1/M1 region; and the NP region. The identity of the labeled protein denoted as the Ud NS1 protein was verified by an immunoblot probed with the Ud NS1 antibody. B. Comparison of the time course of IFN-β pre-mRNA synthesis, and comparison of IRF3 activation at 9 hours after infection with the Ud virus, and a VN04 virus encoding either its own (VN04) NS1 protein or the Ud NS1 protein. The amount of the NS1 protein produced at 9 hours by these three viruses was determined by an immunoblot using Ud NS1 antibody. C. Comparison of IFN-β pre-mRNA synthesis at 9 hours after infection by Ud recombinants encoding the Ud NS1 protein or a NS1 protein that contained either the VN04 RBD fused to the Ud ED, or the Ud RBD fused to the VN04 ED; or after infection with a VN04 virus that contained either the VN04 RBD fused to the Ud ED, or the Ud RBD fused to the VN04 ED. The amount of the NS1 protein produced at 9 hours by these viruses was determined by an immunoblot using Ud NS1 antibody.
In a reverse experiment, we determined whether the H3N2 Ud NS1 protein was sufficient to confer IRF3 activation to the H5N1 VN04 virus, whose NS1 protein blocks IRF3 activation. We generated a chimeric VN04 virus in which the H5N1 VN04 NS gene was replaced by the H3N2 Ud NS gene (Figure 4B). This chimeric virus, like the H3N2 Ud virus itself, effectively activated IRF3 and IFN-β transcription. These results confirm that the NS1 protein is primarily responsible for determining whether different influenza A virus HN subtypes either activate IRF3 and IFN-β transcription or block these activations.
To determine the contribution of the two major domains of the NS1 protein, the RNA-binding domain (RBD) and effector domain (ED), to the IFN-β transcription phenotype, we generated the recombinant viruses shown in Figure 4C. One set of recombinants was comprised of a Ud virus in which the Ud NS gene (encoding the full-length NS1 protein) was replaced with a NS gene encoding a NS1 protein that contained either the VN04 RBD fused to the Ud ED, or the Ud RBD fused to the VN04 ED. The latter, but not the former NS1 chimeric protein enabled the Ud virus to block IFN-β transcription. Consequently, the ED of the VN04 NS1 protein was sufficient to change the IRF3 activation phenotype of the Ud virus. Similarly, VN04 recombinant viruses that express chimeric VN04-Ud NS1 proteins showed that the Ud ED, but not the Ud RBD, enabled the VN04 virus to turn on IFN-β transcription. We conclude that the ED of the encoded NS1 protein is largely responsible for determining whether the virus blocks the activation of IRF3 and IFN-β transcription in infected cells.
The ability of the NS1 protein to block the activation of IRF-3 and IFN-β transcription differs between HN subtypes of seasonal influenza A viruses
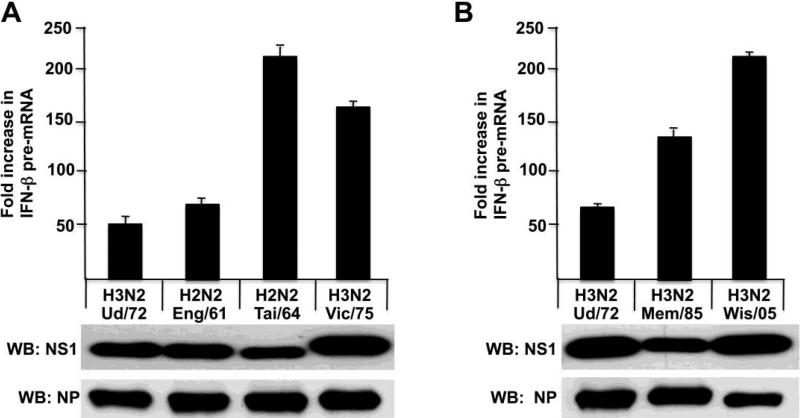
As shown above, one H3N2 NS1 protein and one H2N2 NS1 protein did not block the activation of IRF3 and IFN-β transcription. To determine whether this phenotype is shared by other H3N2 and H2N2 NS1 proteins, we determined whether IFN-β transcription is activated by H2N2 and H3N2 viruses isolated from humans at various times during their circulation in the human population (Figure 5). Because of biosafety concerns, we did not assay H2N2 viruses themselves, but instead assayed chimeric H3N2 Ud viruses in which the H3N2 NS gene was replaced by a H2N2 NS gene. Immunoblots probed with antibody directed against the Ud NS1 protein showed that similar amounts of the NS1 protein were synthesized in the cells infected by these viruses. In addition, immunoblots showed that a similar amount of the viral NP protein was produced. H3N2 viruses isolated from 1972 through 2005 activated IFN-β transcription, demonstrating that the NS1 proteins encoded by all these H3N2 do not block the activation of IRF3 and IFN-β transcription. The A/Wisconsin/67/2005 virus was the H3N2 vaccine strain in 2005. In addition, the NS1 protein of the H2N2 1961 and 1964 strains, like that of the 1957 strain (Figure 4A), did not block these activations (Figure 5A). The apparent differences in the increase in IFN-β pre-mRNA between these H3N2 and H2N2 strains reflects relatively small differences in the 3 hour value of IFN-β pre-mRNA. We conclude that blocking these two host antiviral responses has not been required for the efficient circulation of H2N2 and H3N2 viruses in the human population.
Figure 5.
H3N2 and H2N2 viruses isolated from humans at various times during their circulation in the human population do not block the activation of IRF-3 and IFN-β transcription. IFN-β pre-mRNA synthesis was measured at 3 and 9 hours after infection with the indicated H3N2 and H2N2 viruses. The viruses denoted as H2N2 viruses are actually Ud virus recombinants in which the Ud NS gene was replaced by the NS gene from the indicated H2N2 virus strain. The experiments in panels A and B were done on different days, explaining the different values for the increase in IFN-β pre-mRNA synthesis by the Ud virus. The amounts of the NS1 and NP proteins produced at 9 hours by these viruses were determined by immunoblots probed with either Ud NS1 antibody or NP antibody, respectively.
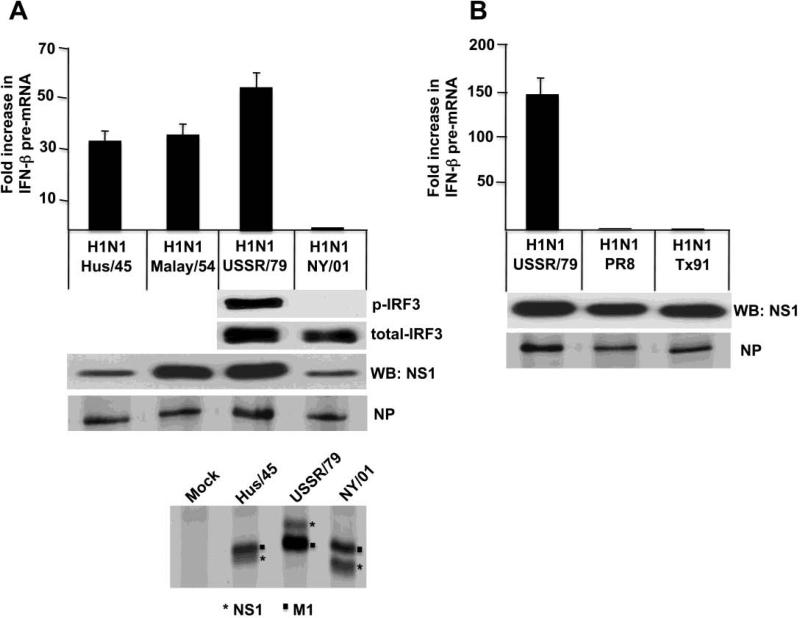
The NS1 protein of the 2009 H1N1 virus (CA09) blocked the activation of IRF3 and IFN-β transcription (Figures 3 and 4A). To determine whether this phenotype is shared by the NS1 proteins of seasonal H1N1 viruses prior to 2009, we determined whether IFN-β transcription is activated by seasonal H1N1 viruses isolated from humans at various times during their circulation in the human population (Figure 6). Immunoblots probed with antibody directed against the Ud NS1 protein showed that similar amounts of the NS1 protein were synthesized in the cells infected by four of these H1N1 viruses, A/Malaysia/54 (Malay/54), A/USSR/46/79 (USSR/79), A/PR8/8/34 (PR8) and A/Texas/36/91 (Tx91). However, the Ud NS1 antibody poorly detected the NS1 proteins of two of the H1N1 viruses, A/AA/Huston/45 Hus/45) and A/New York/312/01 (NY/01) (Figure 6A). Based on a35S-methionine and cysteine labeling experiment, the amounts of the NS1 protein synthesized in cells infected by the latter two viruses were similar to the amount of the NS1 protein synthesized in cells infected by the USSR/79 virus. Unexpectedly, we found that the IFN-β transcription phenotype of the H1N1 NS1 protein fluctuated over several time periods. As shown by the level of IFN-β pre-mRNA in infected cells, the NS1 protein encoded by a 1934 H1N1 virus (PR8) and the NS1 proteins encoded by two H1N1 viruses isolated since1991 (Tx91 and NY/01) blocked the activation of IFN-β transcription (Figure 6 A&B). In contrast, the NS1 proteins encoded by two H1N1 viruses (Hus/45 and Malay/54) isolated just prior to the replacement of H1N1 viruses by H2N2 viruses in 1957 did not block activation of IFN-β transcription, as was also the case for the NS1 protein of a H1N1 virus (USSR/79) isolated soon after the reappearance of H1N1 viruses in 1977. The dramatic difference in IFN-β pre-mRNA synthesis between the 1979 and 2001 H1N1 viruses is mirrored by a similar difference in the activation of IRF3 (Figure 6A). We conclude that blocking the activation of IRF3 and IFN-β transcription was not required for the efficient circulation of H1N1 viruses in the human population during a certain period of time. However, the basis for the fluctuations in the ability of H1N1 NS1 proteins to block IFN-β transcription remains to be determined (see Discussion).
Figure 6.
The IRF3 and IFN-β transcription phenotype of H1N1viruses fluctuated over several time periods. IFN-β pre-mRNA synthesis was measured at 3 and 9 hours after infection with the indicated H1N1 viruses, and IRF3 activation at 9 hours was assayed after infection with the USSR/79 and NY/01 viruses. The amount of the NS1 protein produced by these H1N1 viruses was determined by immunoblots probed with the Ud NS1 antibody. The amount of the NS1 protein synthesized in cells infected by the Hus/45, USSR/79 and NY/01 viruses was analyzed by 35S-methionine and cysteine labeling of infected cells from 6 to 9 hours postinfection. The NS1/M1 protein region of the gel analysis of the 35S-labeled proteins is shown. The identity of the labeled proteins denoted as the Hus/45 and USSR/79 NS1 protein was verified by an immunoblot probed with the Ud NS1 antibody; the NY/01 NS1 protein was not detected in this immunoblot. A separate 35S labeling experiment was carried out using all the H1N1 viruses to quantitate the amounts of the NP protein synthesized in cells infected by these viruses. The experiments in panels A and B were done on different days, explaining the different values for the increase in IFN-β pre-mRNA synthesis by the USSR/79 virus.
The role of the C-terminal region of the effector domain and the amino acid at position 196 in imparting the IRF-3 and IFN-β transcription phenotype
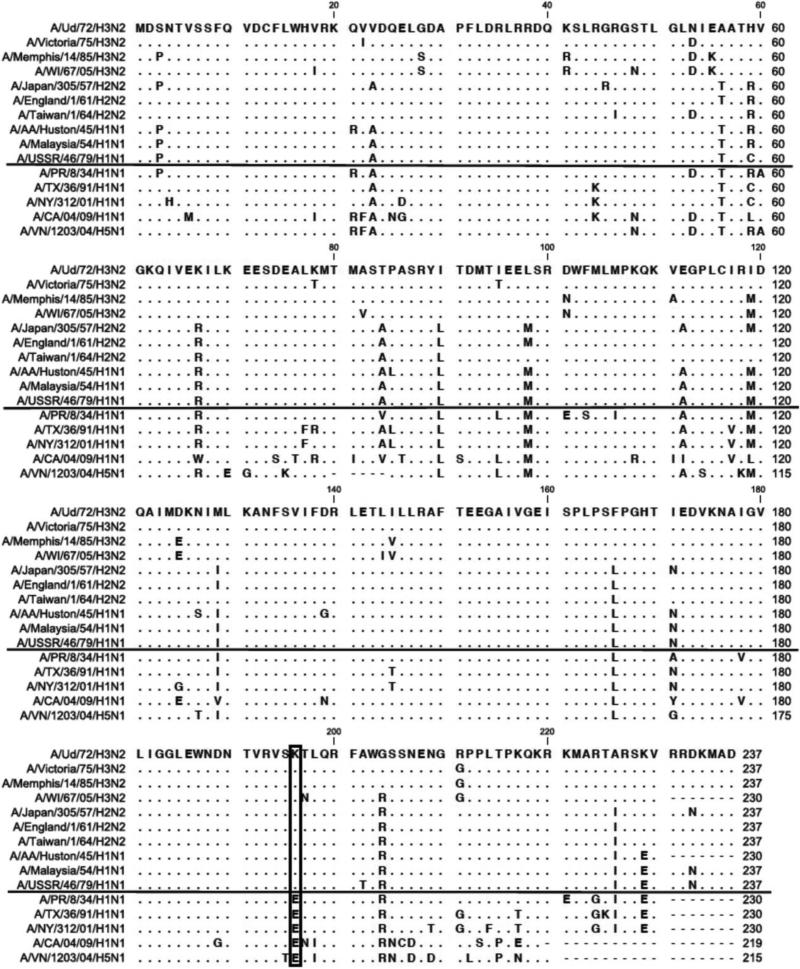
Alignment of the sequences of the NS1 proteins of the viruses that we assayed shows that the identity of only one amino acid, namely the amino acid at position 196 in the effector domain, closely correlated with the IRF3 and IFN-β transcription phenotype of the NS1 proteins that we assayed (Figure 7). All the NS1 proteins that do not block the activation of IRF3 and IFN-β transcription contain K at this position, whereas all the NS1 proteins that block these activations contain E at this position. In fact, K at position 196 in the NS1 protein predominated in all H3N2 and H2N2 viruses (Table 1), indicating that almost all H3N2 and H2N2 viruses probably activate IRF3 and IFN-β transcription, as was the case for the H3N2 and H2N2 viruses that we assayed (Figure 5). Conversely, the NS1 proteins of all the 2009 H1N1 and H5N1 viruses contain E at position 196 (Table 1), indicating that all these viruses probably block the activation of IRF3 and IFN-β transcription, as was the case for the 2009 H1N1 and H5N1 virus strains that we assayed (Figures 3 and 4). The predominant amino acid at position 196 in the NS1 proteins of H1N1 viruses fluctuated over several time periods (Table 1), and the fluctuation mirrored the results of our IFN-β transcription and IRF3 activation assays (Figure 6). Specifically, consistent with these assays, E predominated in the NS1 proteins during the 1918-1939, and the 1991-2008 time periods, whereas K predominated during the 1940-1957, and the 1977-1990 time periods (Table 1).
Figure 7.
Alignment of the sequences of the NS1 proteins of viruses that were assayed for their ability to block the activation of IRF3 and IFN-β transcription. The virus strains above the solid line do not block activation of IRF3 and IFN-β transcription, and the virus strains below the solid line block these activations. The identity of the amino acid at position 196 (boxed) in the NS1 protein correlates with the IRF3 and IFN-β transcription phenotype of the virus: K in strains that do not block activation; and E in strains that block activation.
Table 1.
| NS1 protein | IRF3 activation | ||||
|---|---|---|---|---|---|
| HN subtype1 | Years | 196E | 196K | Tested viruses2 | |
| H3N2 | 1968-2009 | 3 | 1935 | A/Udorn/1972 | + |
| A/Victoria/1975 | + | ||||
| A/Memphis/14/1985 | + | ||||
| A/Wisconsin/67/2005 | + | ||||
| H2N2 | 1957-1968 | 2 | 91 | A/Japan/305/1957 | + |
| A/England/1/1961 | + | ||||
| A/Taiwan/1/1964 | + | ||||
| H1N1 | 1918-1939 | 11 | 0 | A/PR/8/1934 | - |
| H1N1 | 1940-1957 | 3 | 25 | A/AA/Huston/1945 | + |
| A/Malaysia/1954 | + | ||||
| H1N1 | 1977-1990 | 11 | 86 | A/USSR/46/1979 | + |
| H1N1 | 1991-2008 | 574 | 15 | A/Texas/36/1991 | - |
| A/New York/312/2001 | - | ||||
| H1N1 | 2009 | 1183 | 0 | A/California/04/2009 | - |
| H5N1 | 1998-2009 | 165 | 0 | A/Vietnam/1203/04 | - |
The NS gene comes from the denoted HN virus subtype.
For the H3N2 subtypes and the H1N1 subtypes (except for the 2009 strain) the indicated viruses themselves were used. For biosafety reasons, the 2009 H1N1 and H5N1 viruses contained Ud HA and NA genes, and the H2N2 viruses were Ud virus recombinants in which the Ud NS gene was replaced by the NS gene from the indicated H2N2 virus strain. The tested viruses encode a NS1 protein containing the amino acid (K or E) at position 196 that predominated during the indicated time period.
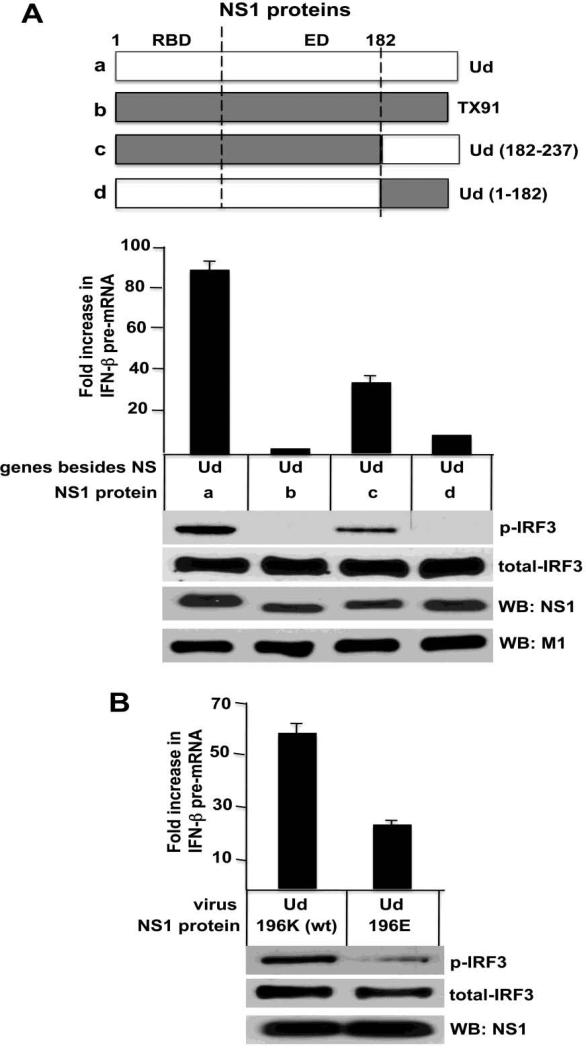
Based on this correlation, we reasoned that a C-terminal fragment of the effector domain containing amino acid 196 would make a large contribution to the IRF3 and IFN-β transcription phenotype of the NS1 protein. We tested the region of the effector domain from amino acid 183 to the C-terminus. We generated recombinant Ud viruses in which the Ud NS1 protein was replaced with a NS1 protein that contained either the entirety or part of the NS1 protein of the H1N1 influenza ATexas/36/1991 (TX91) (Figure 8A, Top). Replacement of the entire Ud NS1 protein with the TX91 NS1 protein leads to complete inhibition of the activation of IRF3 and IFN-β transcription (Figure 8A), confirming the results of Figure 3. Restoring the Ub NS1 C-terminal region (amino acids 183-237) substantially restored the activation of IRF3 and IFN-β transcription: the level of activated IRF3 and IFN-β transcription was 50% and 40%, respectively, of that observed with the full-length Ud NS1 protein. In contrast, only a minimal effect on these activations resulted from restoration of the larger region of the Ud NS1 protein comprising amino acids 1-182, which encompasses its RBD and the majority of its effector domain (amino acids 74-182): activated IRF3 was not detected, and the level of IFN-β transcription was increased only about 5-10%. Similar levels of the NS1 and M1 proteins were produced by the four viruses. We conclude that the C-terminal region of the effector domain is responsible for imparting a large part of the IRF3 and IFN-β transcription phenotype.
Figure 8.
The role of the C-terminal region of the effector domain and the amino acid at position 196 in imparting the IRF-3 and IFN-β transcription phenotype. A. IFN-β pre-mRNA synthesis was measured at 3 and 9 hours after infection, and IRF3 activation was assayed at 9 hours after infection with the Ud virus recombinants expressing the indicated NS1 proteins. The amounts of the NS1 and M1 proteins produced at 9 hours by these viruses were determined by immunoblots. B. IFN-β pre-mRNA synthesis was measured at 3 and 9 hours after infection, and IRF3 activation was assayed at 9 hours after infection with either the wild-type (wt) Ud NS1 protein or a Ud virus expressing a Ud virus containing a K-to-E mutation at position 196.
To determine whether the identity of the amino acid at position 196 by itself affects the IRF3 and IFN-β transcription phenotype, we generated a Ud virus that encodes a NS1 protein in which the K at position 196 was changed to E (Figure 8B). This Ud mutant virus activated IRF3 much less efficiently (20-25%) compared the wt virus, and the level of IFN-β transcription was reduced to about 35% of that of the wild-type virus. These results indicate that the amino acid at position 196 of the NS1 protein plays a significant role in determining the IRF3 and IFN-β transcription phenotype.
The NS1 protein, whether or not it inhibits IRF3 activation, binds TRIM25 in infected cells
A reasonable interpretation of the above results is that the C-terminal region of a subset of NS1 proteins contains a binding site for one or more proteins, with the amino acid at position 196 playing an important role in this binding. Our working hypothesis is that binding of such a protein by a subset of NS1 proteins results in the blocking of IRF3 activation, whereas the NS1 proteins that do not block IRF3 activation do not bind this protein.
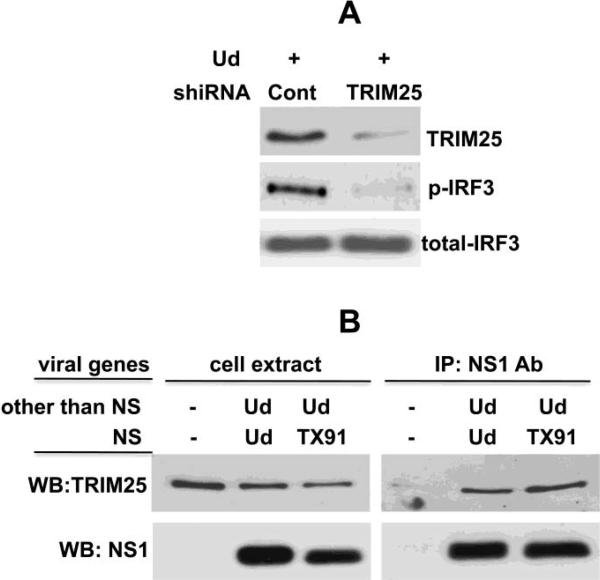
As discussed in the Introduction, it has been proposed that the binding of TRIM25 by the NS1 protein is responsible for the inhibition of IRF3 activation by the NS1 protein (Gack et al., 2009). TRIM25 has been shown to be required for IRF3 activation via the RIG-I pathway (Gack et al., 2007), which we confirmed by a shiRNA knockdown experiment (Figure 9A). Consequently, if a NS1 protein sequestered TRIM25 away from its required function in the RIG-I pathway, this would provide an explanation for the ability of a NS1 protein to inhibit IRF3 activation (Gack et al., 2009). However, TRIM25 was reported to bind to H3N2 NS1 proteins as well as H1N1 NS1 proteins (Gack et al., 2009). If this is indeed the case, then the binding of TRIM25 by the NS1 protein does not necessarily lead to the blocking of IRF3 activation. In fact, as shown in Figure 9B, we confirmed the results of Gack et al., 2009: both the H3N2 Ud NS1 protein, which does not block IRF3 activation, and the Tx91 NS1 protein, which blocks IRF3 activation, bind endogenous TRIM25 to similar extents in infected cells. We conclude that TRIM25 binding by the NS1 protein does not necessarily lead to the blocking of IRF3 activation, and that TRIM25 does not bind selectively to the subset of NS1 proteins that block IRF3 activation.
Figure 9.
The NS1 protein, whether or not it blocks IRF3 activation, binds TRIM25 in infected cells. A. TRIM25 is required for IRF3 activation. HEL-299 cells were transfected with plasmids expressing either a control shiRNA or a TRIM25-specific shiRNA (Gack et al., 2007) for 24 hours, followed by infection with Ud virus for 9 hours. Aliquots of the cell extracts were analyzed by immunoblots probed with either anti-TRIM25 antibody, or antibody against phosphorylated or total IRF3. B. Both the H3N2 Ud and the H1N1 Tx91 NS1 proteins bind TRIM25. At 9 hours after infection with the Ud virus expressing the indicated NS1 protein (Ud, Tx91), an aliquot of the cell extracts was immunoprecipitated with anti-Ud NS1 antibody, followed by immunoblots probed with anti-TRIM25 antibody.
Discussion
Here we demonstrate that only a subset of the seasonal influenza A viruses that have circulated in humans encode NS1 proteins that block the activation of IRF3 and the transcription of the IFN-β gene in infected cells. Indeed, we show that the ability of the NS1 protein to block these activations differs between HN virus subtypes. Viruses expressing the NS1 proteins encoded by seasonal H3N2 and H2N2 viruses do not block the activation of IRF3 and IFN-β transcription. These results confirm our previous reports that demonstrated that IRF3 and IFN-β transcription are activated in cells infected with the H3N2 Ud virus (Das, 2008; Kim, 2002; Noah, 2003), and show that H2N2 viruses share this property. In contrast to H3N2 and H2N2 viruses, viruses expressing the NS1 proteins encoded by some, but not all seasonal H1N1 viruses block these activations. Actually the IRF3 and IFN-β transcription phenotype of the H1N1 NS1 protein fluctuated over several time periods. The NS1 proteins of H1N1 viruses prior to 1940, as exemplified by the 1934 PR8 virus, block IRF3 and IFN-β transcription, whereas this was most likely not the case for a substantial number of the H1N1 NS1 proteins during the period (1940-1957) just prior to the replacement of H1N1 viruses by H2N2 viruses. We presume that a H1N1 NS gene encoding the latter type of NS1 protein was passed on to the H2N2 pandemic virus, and subsequently passed on to the ensuing H3N2 pandemic virus. This H3N2 NS1 protein phenotype, the inability to block the activation of IRF3 and IFN-β transcription, has been preserved up to the present time. In contrast, the same NS1 protein phenotype, which was apparently encoded by a substantial number of H1N1 viruses after the reappearance of H1N1 viruses in 1977, was not preserved. Instead, sometime in the early 1990s H1N1 NS1 proteins acquired the ability to block the activation of IRF3 and IFN-β transcription.
These findings raise an intriguing question. It seems logical that it would be beneficial for influenza A viruses to counter a major cellular antiviral response such as the activation of IRF3 and IFN-β transcription, and that consequently influenza A viruses that lack the ability to effectively block these activations would be selected against. Contrary to these expectations, H3N2 influenza A viruses continue to circulate in humans even though the NS1 proteins encoded by these viruses do not block the activation of IRF3 and IFN-β transcription. One possibility is that H3N2 NS1 proteins bind the cellular CPSF30 protein with very high affinity in infected cells, so that the resulting very effective inhibition of the 3’ end processing of IFN-β pre-mRNA is a sufficient countermeasure against the synthesis of IFN-β. In fact, this NS1 function would also be expected to effectively inhibit the synthesis of other antiviral proteins that are encoded by genes whose transcription is activated during infection. As a consequence, there might not be significant selective pressure against H3N2 viruses that encode NS1 proteins that lack the ability to block the activation of IRF3 and NF-kB and hence IFN-β transcription. If this hypothesis also applied to H1N1 viruses, it would be predicted that the fluctuations in the IRF3 and IFN-β phenotype of the NS1 proteins of H1N1 viruses would reflect fluctuations in the affinity of H1N1 NS1 proteins for CPSF30 in infected cells. In other words, this hypothesis predicts that the NS1 proteins encoded by many of the H1N1 viruses in the periods of 1940-1957 and 1977-1990 bind CPSF30 with very high affinity in infected cells and do not block IRF3 activation, whereas the NS1 proteins encoded by H1N1 viruses since the early 1990s lost the ability to bind CPSF30 with very high affinity in infected cells and as a consequence gained the ability to block IRF3 activation. Determining the affinity of a particular NS1 protein for CPSF30 in infected cells is complicated by the fact the NS1-CPSF30 complex in infected cells is part of a macromolecular complex that includes the viral polymerase complex (PB1, PB2, PA and NP) which stabilizes the NS1 protein-CPSF30 interaction (Kuo, 2009).
By generating a series of viruses expressing chimeric NS1 proteins, we showed that that the difference in the ability of NS1 proteins to block the activation of IRF3 and IFN-β transcription is mediated largely by the NS1 effector domain, and that no detectable role is played by the N-terminal RNA-binding domain. Further, our results provide strong evidence that the C-terminal region of the effector domain is largely responsible for conferring the IRF3 and IFN-β transcription phenotype. Whereas replacing the H3N2 Ud NS1 protein with the H1N1 Tx91 NS1 protein caused the resulting Ud virus to lose the ability to activate IRF3 and IFN-β transcription, a chimeric NS1 protein in which only the C-terminal region of the Tx91 effector domain was replaced with the corresponding region of the Ud effector domain enabled the resulting recombinant Ud virus to activate IRF3 and IFN-β transcription to a substantial degree. Interestingly, the identity of an amino acid in this region of the effector domain, at position 196, closely correlates with the IRF3 and IFN-β transcription phenotype of the NS1 protein. The NS1 proteins that did not block the activation of IRF3 and IFN-β transcription contain K at this position, whereas the NS1 proteins that blocked these activations contain E at this position. In fact, K at position 196 in the NS1 protein predominated in all H3N2 and H2N2 viruses, and the fluctuation over several time periods in the identity of the predominant amino acid at position 196 in the NS1 proteins of H1N1 viruses mirrored the results of our IFN-β transcription and IRF3 activation assays. Consistent with the important role of this amino acid, a K-to-E mutation at this position in the NS1 protein of the H3N2 Ud virus substantially reduced the activation of IRF3 and IFN-β transcription during Ud virus infection.
For these experiments we assayed IRF3 activation using immunoblots probed with an antibody directed against phosphorylated (activated) IRF3 and assayed the transcription of the IFN-β gene during infection by measuring the amounts of the primary transcripts of the IFN-β gene, i.e., IFN-β pre-mRNA, at various times after infection. Specifically, in the latter assay we used quantitative RT-PCR to measure the amount of the IFN-β pre-mRNA species that contains 100 bases downstream of the polyA addition site. This assay demonstrates that influenza A virus strains that have circulated in humans fall into two classes: virus strains that block the production of detectable IFN-β pre-mRNA, and virus strains that instead induce the production of substantial amounts of IFN-β pre-mRNA that continue to accumulate during at least 9 hours after infection. Our finding that the amount of the IFN-β pre-mRNA species that we assayed progressively increased during infection by the latter class of viruses indicated that this pre-mRNA species is quite stable. Consequently, the level of this IFN-β pre-mRNA species likely mirrors the level of IFN-β transcription. We showed that this assay works in human HEL-299 cells and in MDCK cells, indicating that in both of these cell lines it takes a long time for the 3’ exonucleases in the nuclear exosome to degrade the IFN-β pre-mRNA sequences that are more than 100 bases downstream from the polyA addition site (diagramed in Figure 1B). It remains to be determined whether this is the case in all cell lines.
Two previous studies used other assays to determine whether different influenza A virus strains vary in their effects on IRF3 activation (Hayman et al., 2006; Koch et al., 2007). In one study, IRF3 activation was assayed by determining whether IRF3 was imported into the nucleus in infected cells, as analyzed by immunofluorescence (Hayman et al., 2006). In addition, activation of NF-κB was measured by electrophoretic mobility shift assays using nuclear extracts from infected cells. These investigators found that the H3N2 A/Sydney/5/97 virus strain activates IRF3 and NF-κB, in agreement with our results showing that IRF3 and IFN-β transcription are activated in cells infected with H3N2 viruses. However, these investigators did not detect activation of IRF3 and NF-κB in cells infected with two other H3N2 viruses, A/Vic/3/75 and A/England/492/95. In contrast, our assays showed that the A/Vic/3/75 virus does activate IRF3 and IFN-β transcription, as was also the case for three other H3N2 viruses that we assayed. We anticipate that our assays will also show that the H3N2 A/England/492/95 virus activates IRF3 and IFN-β transcription, particularly as its NS1 protein has K at position 196, which we have shown is important for the activation of IRF3 and IFN-β transcription by the H3N2 A/Udorn/72 virus. As confirmation, in future experiments we will use our assays to test additional H3N2 viruses, including H3N2 A/England/492/95, for their ability to activate IRF3 and IFN-β transcription. In the second previous study, investigators analyzed three H1N1 viruses, PR8, Tx91, and A/Bm/1/18 (Kochs et al., 2007). Instead of directly measuring IRF3 activation in cells infected by these three H1N1 viruses, these investigators determined whether the large amount of the NS1 protein that accumulated after 12 hours of infection by these influenza A viruses inhibited the IRF3 dimerization induced by a subsequent 6 hour superinfection by Sendai virus. However, it was not established that the effect of such accumulated NS1 protein on subsequent Sendai virus-induced IRF3 activation accurately mirrored the actions of the NS1 protein on IRF3 activation induced by the H1N1 influenza A viruses themselves at earlier times of infection. In fact, our results provide strong evidence that this assay does not measure IRF3 activation in influenza A virus-infected cells. These investigators reported that the PR8 NS1 protein, but not the Tx91 A/Bm/1/18 virus NS1 proteins, blocked subsequent Sendai virus-induced IRF3 activation, and interpreted these results as demonstrating that the PR8 NS1 protein, but not the Tx91 and A/Bm/1/18 NS1 proteins, blocked IRF3 activation in influenza A virus-infected cells. In contrast, our direct assays established that the Tx91 NS1 protein, as well the PR8 NS1 protein, block the activation of IRF3 and IFN-β transcription in influenza A virus-infected cells (Figures 6 and 8). Thus, our direct assays show that the NS1 proteins of these two H1N1 viruses do not differ in their ability to block these activations. We also found that the H1N1 A/Bm/1/18 NS1 protein shares the same ability (unpublished experiments).
As discussed above, our results show that the C-terminal region of the effector domain of the NS1 protein is largely responsible for conferring the IRF3 and IFN-β transcription phenotype of influenza A viruses. These results suggest that the C-terminal region of the effector domain contains a binding site for one or more proteins, with the amino acid at position 196 playing an important role in this binding. One possibility is that the binding of such a protein by the subset of NS1 proteins with E at position 196 results in the inhibition of IRF3 activation. We determined whether the E3 ubiquitin ligase TRIM25 might serve this function, as it has been proposed that the binding of TRIM25 by the NS1 protein is responsible for the inhibition of IRF3 activation by the NS1 protein (Gack et al., 2009). It was proposed that the NS1 protein sequesters TRIM25 away from its function in the RIG-I pathway, which was presumed to be ubiquitination of the C-terminal CARD domain of RIG-I (Gack et al., 2009). However, a recent study has provided evidence that the RIG-I CARD domain is not activated by TRIM25-mediated ubiquitination but rather is activated by binding free (unanchored) K63-linked ubiquitin chains that are already present in the cells (Zeng et al., 2010). Presumably TRIM25 is the E3 ligase involved in the synthesis of these ubiquitin chains prior to infection. In any case, our results demonstrate that TRIM25 binding by the NS1 protein does not necessarily lead to the inhibition of IRF3 activation, and that TRIM25 does not bind selectively to the subset of NS1 proteins that block IRF3 activation. Both the H3N2 Ud NS1 protein, which does not block IRF3 activation, and the Tx91 NS1 protein, which blocks IRF3 activation, bind endogenous TRIM25 in infected cells. These results suggest that TRIM25 is not required during infection, but rather may be required prior to infection, perhaps to synthesize the polyubiquitin chains described by Zeng et al., 2010. Consequently, the role of the binding of TRIM25 binding by the NS1 protein in infected cells is not clear. Accordingly, we propose that the selective binding of proteins other than TRIM25 to a subset of NS1 proteins is responsible for blocking IRF3 activation.
Materials and Methods
Cells and viruses
HEL-299 and MDCK cells were maintained in DMEM containing 10% fetal bovine serum. All influenza A virus stocks were grown in 10-day fertilized eggs, and virus titers were determined by plaque assays in MDCK cells. Cells were infected with the indicated virus at a multiplicity of 2 plaque-forming units/cell, and the cells were incubated in DMEM containing 0.5μg/mL N-acetylated trypsin in the absence of serum. The following influenza A viruses were provided by Richard J. Webby: H1N1 viruses A/AA/Huston/45, A/USSR/46/79, A/Texas/36/91 (TX91); and H3N2 viruses A/Memphis/14/85, A/Wisconsin/67/05. Jeffrey K. Taubenberger provided the H1N1 viruses A/Malaysia/54 and A /New York/312/2001. The H3N2 influenza A/Victoria/3/75 virus was purchased from ATCC. Recombinant influenza A viruses were generated using plasmid-based reverse genetics, as previously described (Takeda, 2002; Twu, 2006). The negative-strand viral genomic RNAs are encoded in eight pHH21 plasmids under the control of a polymerase I promoter. The pHH21 plasmids encoding the eight Ud genes were provided by Robert A. Lamb; the pHH21 plasmids encoding the PB1, PB2, PA, NP, M and NS genes of H5N1 A/Vietnam/1203/04 (VN04), and the pHH21 plasmid encoding the NS gene of H2N2 A/Japan/305/57 (JN57) were provided by Ruben O. Donis; and the pHH21 plasmid encoding the NS gene of TX91 was provided by Terence M. Tumpey. To prepare the pHH21 plasmids encoding the PB1, PB2, PA, NP, M and NS genes of the 2009 H1N1 A/California/04/09 (CA09), viral RNA provided by Ruben O. Donis was copied into double-stranded DNA using RT-PCR and inserted into the BsmBI site of the pHH21 plasmid. The pHH21 plasmids encoding the H2N2 NS genes of A/England/1/61 and A/Taiwan/1/64 were prepared in the same way using viral RNA provided by Richard J. Webby. To prepare the pHH21 plasmid encoding a NS1 protein in which the RNA-binding domain (amino acids 1-73) is encoded by Ud and the effector domain is encoded by VN04, and vice versa, the DNA encoding the RNA-binding and effector domain sequences were ligated using appropriate primers and standard procedures. Because the sequence encoding the NS1 RNA-binding domain encodes only the first 9 amino acids of the overlapping reading frame of the NS2 protein and these amino acids are common to the Ud and VN04 NS2 proteins, the NS gene encoding the VN04 NS1 protein effector domain encodes the VN04 NS2 protein, and the NS gene encoding the Ud NS1 effector domain encodes the Ud NS2 protein. A similar procedure was used to prepare the pHH21 plasmid encoding a NS1 protein in which the first 182 amino acids is encoded by Tx91 and the C-terminal sequences of the effector domain are encoded by Ud, and vice versa. The resulting NS genes encode either the Ud or Tx91 NS2 protein, respectively, with a single amino acid change (L or M) at position 14. The indicated K-to-E mutation was introduced into the NS1 protein reading frame using standard oligonucleotide mutagenesis methods; this mutation did not change the sequence of the NS2 protein. All eight genomic RNAs of all the recombinant viruses were sequenced. Based on plaque sizes, none of the recombinant viruses were attenuated. All experiments using viruses containing any VN04, CA09, or H2N2 genes were carried out in a BSL3 laboratory using BSL3-enhanced procedures. We were restricted from using recombinant viruses that contain the HA and NA genes of these viruses.
Measurement of IFN-β pre-mRNA and mRNA using quantitative RT-PCR
RNA was isolated from infected cells at the indicated times after infection by using the TRIZOL reagent. To measure the level of IFN-β pre-mRNA in infected HEL-299 cells, 1 μg of total RNA, which corresponds to equal cell equivalents, was reverse transcribed using a 20–mer oligonucleotide (5'-CAACTAATAGGTACTTGG CA-3') complementary to a sequence 76-95 bases downstream from the poly(A) addition site of human IFN-β pre-mRNA. To measure the level of IFN-β pre-mRNA in infected MDCK cells, the RNA was reverse transcribed using the previously described 23-mer oligonucleotide complementary to a sequence 75-98 bases downstream from the poly(A) addition site of canine IFN-β pre-mRNA (Das, 2008). Where indicated, the RNA from infected HEL-299 cells was reverse transcribed using oligo dT to measure the level of mature IFN-β mRNA. The amounts of both IFN-β pre-mRNA and IFN-β mRNA were determined using the TaqMan Gene Expression Assay (Applied Biosystems) with 5’ and 3’ primers complementary to internal sequences shared by the pre-mRNA and mRNA (forward 5'-CAGTCTGCACCTGAAAA ATATTATG-3'; reverse 5'-GATTTCCACTCTGACTATGGTCCA GG-3') and a TaqMan MGB (minor groove binder) internal probe. Real-time PCR analysis was carried out using the Perkin-Elmer/Applied Biosystems 7900HT sequence detector (Twu et al., 2007). The quantitative PCR reactions for each sample was performed in triplicate. Error bars are shown in each Figure. The CT values of samples collected at times after 3 hours postinfection (usually at 9 hours postinfection) were normalized to the CT value at 3 hours postinfection. In several experiments we determined whether an additional normalization was needed: both the 3-hour and 9 hour CT values were first normalized to the CT value of β-actin mRNA, and then the resulting 9 hour CT value was normalized to the resulting 3 hour CT value. Essentially the same fold difference between the 9 hour and 3 hour samples was obtained with and without this additional normalization.
Assay for the activation of endogenous IRF3 in influenza A virus-infected cells
At the indicated times after infection, cells were suspended in the PhosphoSafe extraction reagent (Novagen). After vortexing, the mixture was maintained at 4º C for 10 minutes, and then centrifuged at 10,000 × g for 5 minutes. An aliquot of the supernatant was subjected to SDS-polyacrylamide gel electrophoresis, followed by immunoblots using either antibody against activated (S396-phosphorylated) IRF3 (Cell Signaling) or against total IRF3 (provided by Michael David) (Navarro, 1998). The levels of activated and total IRF3 were quantitated using ImageJ software (NIH) as described previously (Hsiang, 2009).
Assays for the interaction of the NS1 protein with endogenous TRIM25 in infected cells
At 8 hours after infection with the Ud virus expressing the indicated NS1 protein (Ud or Tx91), cells were suspended in a buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% NP-40, and a mixture of protease and phosphatase inhibitors. Cell extracts were immunoprecipitated with antibody against the Ud NS1 protein, followed by immunoblots probed with anti-TRIM25 antibody (BD Biosciences).
Quantitating the amount of the NS1 protein synthesized in infected cells
At the indicated times after infection by an influenza A virus expressing a H3N2, H2N2, or H5N1 VN04 NS1 protein, aliquots of cell extracts were analyzed by immunoblots probed with the rabbit antibody that had been generated against the H3N2 Ud NS1 protein that was expressed in bacteria. In immunoblots this antibody efficiently detects H3N2, H2N2 NS1 proteins and the H5N1 VN04 NS1 protein. This NS1 antibody detects only some H1N1 NS1 proteins efficiently, so that immunoblots could only be used for quantitating the NS1 protein in cells infected by viruses expressing one of these H1N1 NS1 proteins. The levels of the NS1 proteins were quantitated using ImageJ software (NIH) as described previously (Hsiang, 2009). To measure the production of other H1N1 NS1 proteins, cells at 6 hours after infection with a virus expressing such a H1N1 NS1 protein were washed twice with methionine-and cysteine-free DMEM, and were then incubated for 3 hours in this DMEM containing a mixture of (35S)methionine and (35S)cysteine. Cells were washed twice with PBS and lysed in Laemmli sample buffer. An aliquot was loaded onto 12% SDS-polyacrylamide gels for analysis by autoradiography. The levels of other viral proteins were quantitated as described in the Figure legends.
Acknowledgments
This investigation was supported by NIH Grants AI-11772 and AI11772-36S1 to RMK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current protocols in molecular biology. John Wiley & Sons: New York: 1994. [Google Scholar]
- Das K, Ma L-C, Xiao R, Aramini J, Marklund J, Kuo R-L, Arnold E, Krug RM, Montelione GT. Structural basis for suppression by influenza A virus of a host antiviral response. Proc. Natl. Acad. Sci. USA. 2008;105:13093–13098. doi: 10.1073/pnas.0805213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack MU, Albrecht RA, Urano T, Inn KS, Huang IC, Carnero E, Farzan M, Inoue S, Jung JU, Garcia-Sastre A. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe. 2009;5:439–449. doi: 10.1016/j.chom.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack MU, Kirchhofer A, Shin YC, Inn KS, Liang C, Cui S, Myong S, Ha T, Hopfner KP, Jung JU. Roles of RIG-I N-terminal tandem CARD and splice variant in TRIM25-mediated antiviral signal transduction. Proc Natl Acad Sci U S A. 2008;105:16743–16748. doi: 10.1073/pnas.0804947105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S, Jung JU. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier RA, Pappas C, Alpuche-Aranda CM, Lopez-Gatell H, Olivera H, Lopez I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD, Jr., Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM, Smith DJ, Klimov AI, Cox NJ. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale BG, Randall RE, Ortin J, Jackson D. The multifunctional NS1 protein of influenza A viruses. J Gen Virol. 2008;89:2359–2376. doi: 10.1099/vir.0.2008/004606-0. [DOI] [PubMed] [Google Scholar]
- Haller O, Kochs G, Weber F. The interferon response circuit: induction and suppression by pathogenic viruses. Virology. 2006;344:119–130. doi: 10.1016/j.virol.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman A, Comely S, Lackenby A, Murphy S, McCauley J, Goodbourn S, Barclay W. Variation in the ability of human influenza A viruses to induce and inhibit the IFN-beta pathway. Virology. 2006;347:52–64. doi: 10.1016/j.virol.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Houseley J, Tollervey D. The many pathways of RNA degradation. Cell. 2009;136(4):763–76. doi: 10.1016/j.cell.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Hsiang T-Y, Zhao C, Krug RM. Interferon-induced ISG15 conjugation inhibits influenza A virus gene expression and replication in human cells. J. Virology. 2009;83:5971–5977. doi: 10.1128/JVI.01667-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Latham AG, Krug RM. Human influenza viruses activate an interferon-independent transcription of cellular antiviral genes: Outcome with influenza A virus is unique. Proc Natl Acad Sci U S A. 2002;99:10096–10101. doi: 10.1073/pnas.152327499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochs G, Garcia-Sastre A, Martinez-Sobrido L. Multiple anti-interferon actions of the influenza A virus NS1 protein. J Virol. 2007;81:7011–7021. doi: 10.1128/JVI.02581-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo R-L, Krug RM. The influenza A virus polymerase is an integral component of the CPSF30-NS1A protein complex in infected cells. Journal of Virology. 2009;83:1611–1616. doi: 10.1128/JVI.01491-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RA, Krug RM. Orthomyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. 4th edition Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 1487–1532. [Google Scholar]
- Loo YM, Fornek J, Crochet N, Bajwa G, Perwitasari O, Martinez-Sobrido L, Akira S, Gill MA, Garcia-Sastre A, Katze MG, Gale M., Jr. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol. 2008;82:335–345. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T, Falvo JV, Kim TH, Kim TK, Lin CH, Parekh BS, Wathelet MG. Structure and function of the interferon-beta enhanceosome. Cold Spring Harb Symp Quant Biol. 1998;63:609–620. doi: 10.1101/sqb.1998.63.609. [DOI] [PubMed] [Google Scholar]
- Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- Mibayashi M, Martinez-Sobrido L, Loo YM, Cardenas WB, Gale M, Jr., Garcia-Sastre A. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J Virol. 2007;81:514–524. doi: 10.1128/JVI.01265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro L, Mowen K, Rodems S, Weaver B, Reich N, Spector D, David M. Cytomegalovirus activates interferon immediate-early response gene expression and an interferon regulatory factor 3-containing interferon- stimulated response element-binding complex. Mol Cell Biol. 1998;18:3796–3802. doi: 10.1128/mcb.18.7.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff ME, Barabino SM, Li Y, Keller W, Krug RM. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3'end formation of cellular pre-mRNAs. Mol Cell. 1998;1:991–1000. doi: 10.1016/s1097-2765(00)80099-4. [DOI] [PubMed] [Google Scholar]
- Noah DL, Twu KY, Krug RM. Cellular antiviral responses against influenza A virus are countered at the posttranscriptional level by the viral NS1A protein via its binding to a cellular protein required for the 3' end processing of cellular pre-mRNAS. Virol. 2003;307:386–395. doi: 10.1016/s0042-6822(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Opitz B, Rejaibi A, Dauber B, Eckhard J, Vinzing M, Schmeck B, Hippenstiel S, Suttorp N, Wolff T. IFNbeta induction by influenza A virus is mediated by RIG-I which is regulated by the viral NS1 protein. Cell Microbiol. 2006;9:930–938. doi: 10.1111/j.1462-5822.2006.00841.x. [DOI] [PubMed] [Google Scholar]
- Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, Reis e Sousa C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5'-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Takeda M, Pekosz A, Shuck K, Pinto LH, Lamb RA. Influenza a virus M2 ion channel activity is essential for efficient replication in tissue culture. J Virol. 2002;76:1391–1399. doi: 10.1128/JVI.76.3.1391-1399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talon J, Horvath CM, Polley R, Basler CF, Muster T, Palese P, Garcia-Sastre A. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J Virol. 2000;74:7989–7996. doi: 10.1128/jvi.74.17.7989-7996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- Twu KY, Kuo RL, Marklund J, Krug RM. The H5N1 influenza virus NS genes selected after 1998 enhance virus replication in mammalian cells. J Virol. 2007;81:8112–8121. doi: 10.1128/JVI.00006-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twu KY, Noah DL, Rao P, Kuo R-L, Krug RM. The CPSF30 binding site on the NS1A protein of influenza A virus is a potential antiviral target. J. Virol. 2006;80:3957–3965. doi: 10.1128/JVI.80.8.3957-3965.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreede FT, Chan AY, Sharps J, Fodor E. Mechanisms and functional implications of the degradation of host RNA polymerase II in influenza virus infected cells. Virology. 2010;396:125–34. doi: 10.1016/j.virol.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li M, Zheng H, Muster T, Palese P, Beg AA, Garcia-Sastre A. Influenza A virus NS1 protein prevents activation of NF-kappaB and induction of alpha/beta interferon. J Virol. 2000;74:11566–11573. doi: 10.1128/jvi.74.24.11566-11573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Avian influenza. 2010 www.who.int/csr/disease/avian_influenza/en/
- Wright PF, Webster RG. Orthomyxoviruses. In: Knipe DM, Howley PM, editors. Fields Virology, 4th edition. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 1533–1579. [Google Scholar]
- Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Zeng W, Sun L, Jiang X, Chen X, Hou F, Adhikari A, Xu M, Chen ZJ. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell. 2010;141:315–330. doi: 10.1016/j.cell.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Kuo R-L, Krug RM. The NS1 protein of influenza A virus. In: Wang Q, Tao YZ, editors. Influenza Molecular Virology. Caister Academic Press; Norfolk, UK: 2010. pp. 1–14. [Google Scholar]