Abstract
We recently reported that the oligosomatostatin receptor agonist, ODT8-SST increases food intake in rats via the somatostatin2 receptor (sst2). We characterized ingestive behavior following intracerebroventricular (icv) injection of a selective sst2 agonist in freely fed mice during the light phase. The sst2 agonist (0.01, 0.03, 0.1, 0.3 or 1µg/mouse) injected icv under short inhalation anesthesia dose-dependently increased cumulative light phase food intake over 4h compared to vehicle with a 3.1-times increase at 1µg/mouse (p<0.05). Likewise, the sst2,3,5 agonist octreotide (0.3 or 1µg/mouse) dose-dependently increased 4-h food intake, whereas selective sst1 or sst4 agonists at 1µg/mouse did not. In vehicle-treated mice, high fat diet increased caloric intake/4h by 2.8-times compared to regular diet (p<0.05) and values were further increased 1.4-times/4h by the sst2 agonist. Automated continuous assessment of food intake established a 6.6-times higher food intake during the dark phase due to increased number of meals, meal size, meal duration and rate of ingestion compared to non-treated mice during the light phase. During the first 4h post icv sst2 agonist injection, mice had a 57% increase in number of meals with a 60% higher rate of ingestion, and a 61% reduction in inter-meal intervals, whereas meal sizes were not altered compared to vehicle. These data indicate that activation of brain sst2 receptors potently stimulates ingestive behavior under basal or high fat diet-stimulated conditions in mice. The shortened inter-meal interval suggests an inhibitory effect of the sst2 agonist on “satiety”, whereas “satiation” is not altered as indicated by normal meal size.
Keywords: brain, CCK8-S, ingestive behavior, mice, octreotide, satiation, satiety, somatostatin receptors
1. Introduction
Somatostatin-14 was isolated from 490,000 ovine hypothalami on the basis of its ability to inhibit growth hormone (GH) secretion from dispersed pituitary cells in culture [1]. Subsequently, as shown for various other hypothalamic releasing or inhibitory factors, somatostatin was found to be widely distributed in the brain and to exert a number of GH-independent effects on neuroendocrine, cognitive behavioral, autonomic and visceral functions [2–4]. The diverse biological actions of somatostatin are mediated by the interplay with five distinct plasma membrane G-protein coupled receptor subtypes, namely sst1 through sst5 [5]. Somatostatin displays nanomolar affinity to sst2 including the splicing variants sst2a and sst2b [6, 7] and to sst3–5, whereas it has lower affinity to sst1 [5, 8]. We previously reported that octreotide (SMS 201–995), a broadly used stable oligosomatostatin receptor agonist that binds to three of the five receptor subtypes, namely sst2, sst3 and sst5 with highest affinity for sst2 [5, 7, 9] and ODT8-SST, a stable pan-activator of sst receptors [10] act in the brain to promote digestive function as shown by the stimulation of gastric acid secretion and gastric emptying [11–13]. Recently, we extended these observations by showing that ODT8-SST injected intracerebroventricularly (icv) acts in the brain to increase light phase food intake in rats via an sst2 mediated mechanism as indicated by the complete blockade using an sst2 antagonist [13]. Likewise the newly developed selective sst2 peptide agonist, des-AA1,4–6,11–13-[DPhe2,Aph7(Cbm),DTrp8]-Cbm-SST-Thr-NH2 [9] injected icv in ad libitum fed rats induces a centrally-mediated, long-lasting stimulation of light phase food intake which was prevented by the icv injection of a selective sst2 antagonist [14]. Moreover, the sst2 antagonist injected icv before the onset of the dark photoperiod reduced dark phase feeding [14] when rats have their maximum food intake [15]. Taken together, these data point towards a role of brain sst2 receptor activation in the regulation of food intake in rats. This is further supported by the more prominent distribution of the sst2 than other sst receptor subtypes in the hypothalamus [16, 17], a brain area involved in the control of food intake [18].
So far, the central actions of somatostatin or sst2 agonist to influence food intake have been mainly derived from studies performed in rats [13, 14, 19, 20]. A few recent reports extended the central action of somatostatin to stimulate food intake to chicks [21] and mice using the pan-sst1–5 agonist, ODT8-SST [13]. In addition, while meal pattern analysis is of primary importance to assess mechanisms regulating eating behavior [22], the underlying food intake microstructure induced by activation of brain sst2 receptors is still unknown.
In the present study we first assessed whether icv injection of the selective sst2 peptide agonist (IC50: 7.5–20 nM) [9] stimulates the light phase food intake in freely fed mice. To ascertain the specificity of the response toward sst2 receptors, we investigated the influence of the stable sst2/sst3/sst5 agonist, octreotide [23] and the recently developed sst1 or sst4 peptide agonists [24, 25]. Then, we monitored changes in meal pattern induced by the selective sst2 peptide agonist injected icv in ad libitum fed mice. This was performed using a recently developed automated episodic food intake monitoring system for mice (BioDAQ, Research Diets, Inc., New Brunswick, NJ). We first validated this new system under our conditions by microstructure analysis of the food consumption in the dark and light phase and in response to intraperitoneal (ip) injection of sulfated cholecystokinin (CCK)8-S known to curtail the food intake response to a fast in mice [26].
2. Materials and methods
2.1. Animals
Adult male C57Bl/6 mice (6–8 weeks of age, Harlan Laboratories) were housed 4/cage under controlled illumination (6:00 AM to 6:00 PM) and temperature (21–23°C). Animals had free access to standard rodent diet (Prolab RMH 2500; LabDiet, PMI Nutrition, Brentwood, MO) and tap water. Protocols were approved by the Veterans Administration Institutional Animal Care and Use Committee (# 05-058-02). All experiments were started between 9:00 and 10:00 AM.
2.2. Peptides
The sst1 agonist, des-AA1,4–6,10,12,13-[DTyr2,D-Agl(NMe,2naphtoyl)8,IAmp9]-SRIF-Thr-NH2 MW: 1238.5, compound #25 in [24], sst2 agonist, des-AA1,4–6,11–13-[dPhe2,Aph7(Cbm),dTrp8]-Cbm-SRIF-Thr-NH2, MW: 1132.5, compound #3 in [9] and sst4 agonist, des-AA1,2,4,5,12,13-[Aph7]-Cbm-SRIF, MW: 1137.4, compound #15 in [25] (Clayton Foundation Laboratories, Salk Institute, La Jolla, CA) were synthesized as we previously described and purity was characterized by high pressure liquid chromatography, capillary zone electrophoresis and mass spectrometry [9]. The sst2/sst3/sst5 agonist, octreotide (MW 1019.3, Bachem America, Inc., Torrance, CA) and selective sst agonists were kept in powder form at −80 °C and dissolved in pyrogen-free distilled water immediately before administration. No selective sst3 or sst5 peptide agonists have been developed yet and therefore these could not be tested. Sulfated CCK-8 (CCK8-S; Bachem) was dissolved in saline and the stock solution stored at −80 °C. Immediately before the start of the experiments, CCK8-S was diluted in sterile saline (0.9% NaCl; Hospira, Inc., Lake Forest, IL) to reach a dose of 10 µg/kg body weight (= 9 nmol) in 100 µl.
2.3. Intracerebroventricular injection
Injection into the lateral brain ventricle (5 µl) was performed acutely under short isoflurane anesthesia (2–3 min, 4.5% vapor concentration in oxygen; VSS, Rockmart, GA) as in our previous studies [27]. The injection site was localized at the apex of the equal triangle between the eyes and the back of the head, and cleaned with Povidone-Iodine 10% (Aplicare Inc., Meriden, CT). The skull was punctured manually at the point of least resistance with a 30-gauge needle equipped with a polyethylene tube leaving 4–4.5 mm of the needle tip exposed and attached to a Hamilton syringe. On average, mice completely recovered from anesthesia within 5 min. Accuracy of injections was assessed in our previous studies by injecting cresyl violet dye icv under similar conditions in 50 mice [27]. Moreover, behavioral response in mice such as increased grooming after icv injection of the sst2 agonist as observed in rats [14] indicated that accuracy of the icv injection was 100%.
2.4. Manual food intake monitoring
Ad libitum fed naïve mice that had been trained for single housing on grids three times for 4, 8, and 24 h respectively during the week before the experiment were injected icv with sst2 agonist (0.01, 0.03, 0.1, 0.3 or 1 µg/mouse = 0.01, 0.03, 0.1, 0.3 or 1 nmol) or vehicle (pyrogen-free distilled water). Afterwards, mice were housed singly on a grid and food intake (g/25g bw) of standard diet was measured at 1, 2, 4, 6, 9 and 24 h post injection. Doses used were based on our previous dose response studies in rats [14]. In separate studies, mice fed ad libitum were injected with octreotide (0.3 or 1µg/mouse = 0.3 or 1 nmol), sst1 or sst4 agonists (0.3 or 1 µg/mouse = 0.3 or 0.9 nmol) or vehicle and food intake of standard diet was assessed at 1, 2 and 4 h post injection. Doses of the selective peptide sst agonists were based on the effective doses of the sst2 agonist. In another experiment, sst2 agonist (1 µg/mouse) or vehicle was injected icv and mice had access to either standard diet (calories from protein 29%, fat 12% and carbohydrates 59%, 3.4 kcal/g diet, Prolab RMH 2500; LabDiet) or high fat diet (calories from protein 20%, fat 45% and carbohydrates 35%, 4.7 kcal/g diet, formula D12451, Research Diets, Inc., Jules Lane, New Brunswick, NJ). Food intake was monitored at 1, 2, 4, 6, 9, and 24 h post injection and calculated as the amount of ingested calories/mouse. All icv injections were performed in the light phase between 9:00 and 9:30 AM. In all experiments, food intake was determined by measuring the difference between the pre-weighed chow and the weight of chow and spillage at the end of each monitoring period. Experiments were repeated in a crossover design in the same batch of mice for each treatment.
2.5. Automated food intake monitoring
The microstructure analysis of feeding behavior was conducted using the BioDAQ episodic Food Intake Monitor for mice (BioDAQ, Research Diets, Inc., New Brunswick, NJ), which allows continuous monitoring of meal patterns in undisturbed mice with minimal human interference. The system consists of a low spill food hopper placed on an electronic balance mounted together on the animals’ home cage. Mice were habituated for one week to single housing and feeding with purified standard rodent diet (AIN-93M, Research Diets, Inc.) through a food hopper in regular housing cages with environmental enrichment and bedding material. This diet was chosen because it causes less spillage than the Prolab diet (Prolab RMH 2500; LabDiet) and therefore reduces the risk of the “bridging phenomenon” that occurs when a pile of retained food spillage underneath the gate can cause erroneous measurements. Water was provided ad libitum from regular water bottles. Mice usually habituated to the new environment within 3–4 days and showed normal food intake and regular body weight gain.
In the validation experiments, ad libitum fed mice were left undisturbed and feeding microstructure was monitored for 24 h and analyzed separately during the light (6:00 AM – 6:00 PM) and dark phase (6:00 PM – 6:00 AM). We also monitored the feeding microstructure for 2 h in response to ip injection (100 µl) of vehicle (pyrogen-free saline) or CCK8-S (10 µg/kg body weight) in overnight food but not water deprived mice. The CCK8-S dose was selected from our previous studies in mice performed under similar conditions except the food intake was assessed manually [26]. In separate groups of freely fed mice, sst2 agonist (1 µg/mouse) or vehicle (distilled water) was injected icv during the light phase and microstructure of food ingestion was recorded for 24 h. Experiments were repeated in a crossover design in the same batch of mice for each treatment.
The BioDAQ system weighs the hopper with food (± 0.01g) second by second and algorithmically detects 'not eating' as weight stable and 'eating' as weight unstable. Feeding bouts (changes in stable weight before and after a bout) are recorded as feeding bout vectors with a start time, duration, and amount consumed. Bouts are separated by an inter-bout interval (IBI), and meals consist of one or more bouts separated by an inter-meal interval (IMI). IBI, IMI and minimum meal amount are user definable. In this trial, we used the BioDAQ with an IBI of 5 sec, IMI of 5 min, and minimum meal amount of 0.02 g. This entails that food intake was considered as one meal when the feeding bouts occurred within 5 min of the previous response and their sum was equal to or greater than 0.02 g; if bouts of feeding were longer than 5 min apart, they were considered as a new meal. Meal parameters included the number of meals, meal size, meal duration, rate of ingestion, inter-meal interval (time difference between the end of one meal and the initiation of the next meal) and total time spent eating (time in min or % that mice spent in feeding bouts or meals). These parameters were calculated by the software provided by the manufacturer (BioDAQ Monitoring Software 2.2.02). Immediately following collection, data can be viewed in either Data Viewer or Excel for analysis. Body weight of the mice was assessed before and 24 h after injection.
2.6. Statistical analysis
Data are expressed as mean ± SEM and were analyzed by one-way analysis of variance (ANOVA) followed by Tukey post hoc test or two-way ANOVA followed by Holm-Sidak method. Differences between groups were considered significant when p < 0.05.
3. Results
3.1. The sst2 agonist injected into the lateral brain ventricle increases the light phase food intake in ad libitum fed mice
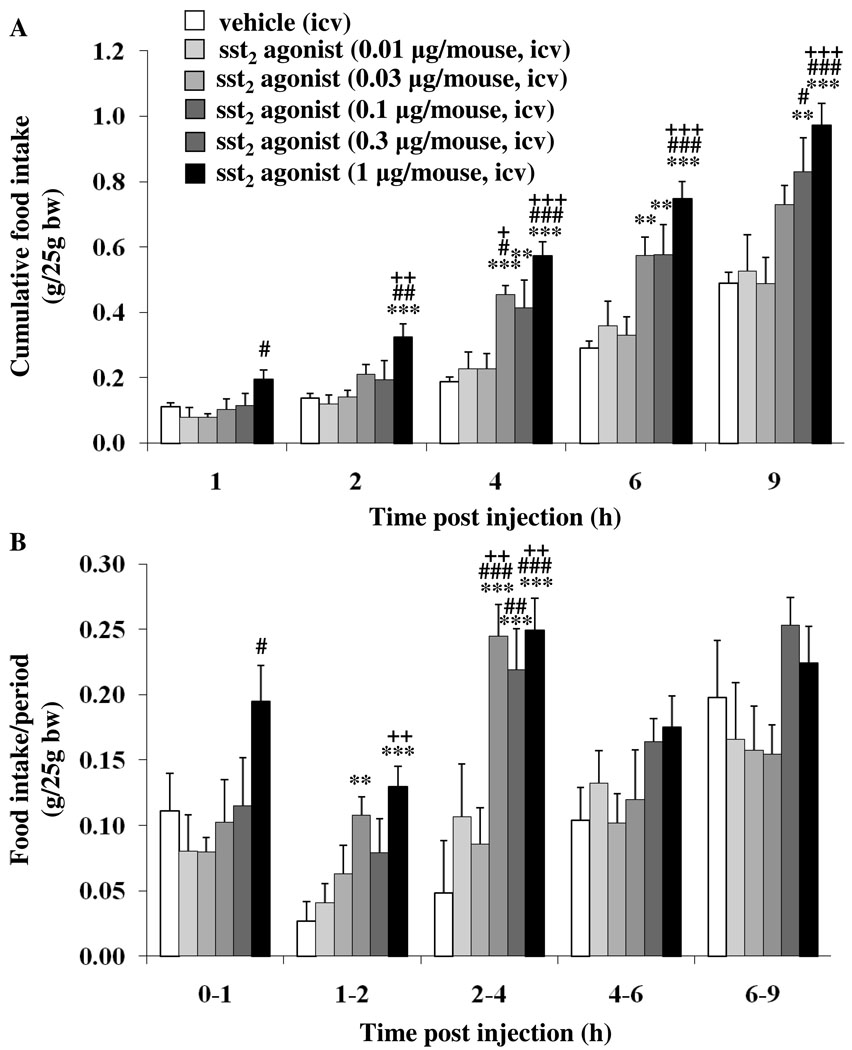
In ad libitum fed mice, icv injection of sst2 agonist (0.01, 0.03, 0.1, 0.3 and 1 µg/mouse) under short inhalation anesthesia induced a dose-related increase in light phase food intake. The significant increase in the cumulative food intake induced by the sst2 agonist (0.1 µg/mouse) versus vehicle was observed at 4 h (0.45 ± 0.03 vs. 0.19 ± 0.02 g/25g bw, p < 0.001) and 6 h (0.57 ± 0.06 vs. 0.29 ± 0.02 g/25g bw, p < 0.01), whereas the 9-h cumulative food intake was no longer altered (Fig. 1A). The sst2 agonist at 0.3 µg/mouse had a longer duration of action as the increased cumulative food intake was observed not only at 4 h (0.41 ± 0.09 g/25g bw, p < 0.01) and 6 h (0.58 ± 0.09 g/25g bw, p < 0.01) but also at 9 h post injection compared to vehicle (0.83 ± 0.10 vs. 0.49 ± 0.03 g/25g bw, p < 0.01; Fig. 1A). At 1 µg/mouse, the sst2 agonist injected icv had an earlier onset of action with a trend to increase at 1 h which became significant at 2 h post injection (0.32 ± 0.04 vs. 0.14 ± 0.01 g/25g bw, p < 0.001) and was maintained throughout the 9-h experimental period compared to vehicle (p < 0.001; Fig. 1A). The 9-h cumulative food intake was 17% higher after 1 µg/mouse than following 0.3 µg/mouse (Fig. 1A). Two-way ANOVA showed a significant influence of dose (F(5,210) = 40.6, p < 0.001), time (F(4,210) = 125.5, p < 0.001) and dose × time (F(20,210) = 3.0, p < 0.001). When food intake was expressed as food intake/period, the increase reached significance at the 2nd h post injection at 0.1 and 1 µg/mouse and was observed for the subsequent 2 h at 0.1, 0.3 and 1 µg/mouse (p < 0.001) and no longer thereafter (Fig. 1B) including the 4–6, 6–9 and 9–24 h periods. Based on these data the highest dose of 1 µg/mouse icv was used in all subsequent studies and emphasis was put on the first 4 h post injection.
Fig. 1.
Dose-related stimulatory effect of sst2 agonist injected into the lateral brain ventricle on light phase food intake in ad libitum fed mice. The sst2 agonist (0.01, 0.03, 0.1, 0.3 or 1 µg/mouse) or vehicle was injected icv during the light phase in ad libitum fed non-cannulated mice under short anesthesia, food intake monitored and expressed as cumulative (A) or food intake/period (B). Each bar represents the mean ± SEM of 6–15 mice/group. ** p < 0.01, *** p < 0.001 vs. vehicle. + p < 0.05, ++ p < 0.01, +++ p < 0.001 vs. sst2 agonist 0.01 µg. # p < 0.05, ## p < 0.01, ### p < 0.001 vs. sst2 agonist 0.03 µg.
We then assessed whether the orexigenic effect of the sst2 agonist icv can also be observed under stimulated conditions using a palatable diet. Access to high fat diet compared to a standard rodent diet significantly increased caloric intake in icv vehicle injected mice during the 24-h measurement period (p < 0.05, Table 1). When mice were given access to high fat diet post icv injection, the sst2 agonist induces a further 1.7-, 1.5-, 1.4- and 1.2-times increase in caloric intake at 1, 2, 4 and 6 h post injection, respectively, compared to vehicle (Table 1). Two-way ANOVA indicated a significantly different food intake between diet groups at all time points (e.g. 4 h: F(1,28) = 27.8, p < 0.001). Treatment showed a significant effect at 4 h (F(1,28) = 4.7, p < 0.05) and 6 h (F(1,28) = 4.4, p < 0.05) post injection.
Table 1.
Cumulative food intake (kcal/mouse) in response to intracerebroventricular injection of sst2 agonist (1 µg/mouse) or vehicle in mice fed either regular or high fat diet post injection during the light phase
| Treatment | ||||
|---|---|---|---|---|
| Time post injection (h)a |
Regular diet/ vehicle |
High fat diet/ vehicle |
Regular diet/ sst2 agonist |
High fat diet/ sst2 agonist |
| 1 | 0.53 ± 0.14 | 2.06 ± 0.22 | 0.69 ± 0.10 | 3.41 ± 0.87**,## |
| 2 | 0.76 ± 0.14 | 2.58 ± 0.26 | 1.10 ± 0.11 | 3.93 ± 0.91**,## |
| 4 | 1.33 ± 0.24 | 3.61 ± 0.32* | 2.12 ± 0.16* | 4.90 ± 0.86**,## |
| 6 | 1.81 ± 0.30 | 4.91 ± 0.42**, # | 2.94 ± 0.19** | 5.89 ± 0.84**,## |
| 9 | 2.78 ± 0.45 | 6.89 ± 0.41**, # | 3.87 ± 0.25 | 7.64 ± 1.16**,## |
| 24 | 14.43 ± 0.63 | 23.97 ± 0.74**, ## | 12.76 ± 0.60 | 22.12 ± 1.21**,## |
Mice fed ad libitum with standard chow were injected icv at time point 0 h (between 9:00 and 9:30 AM) and exposed to standard or high fat diet.
Values are mean ± SEM of n=8 mice/group.
p < 0.05,
p < 0.01 vs. regular diet/vehicle;
p < 0.05,
p < 0.01 vs. regular diet/sst2 agonist
3.2. Octreotide injected into the lateral brain ventricle increases the light phase food intake, whereas selective sst1 and sst4 agonists have no effect in ad libitum fed mice
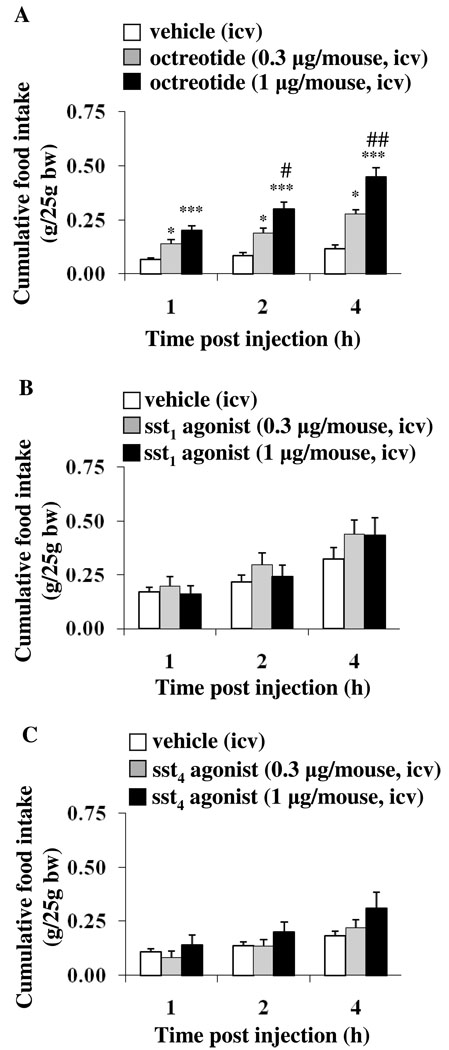
To assess the selectivity of the response to the sst2 agonist, we used the ss2/sst3/sst5 agonist, octreotide (0.3 or 1 µg/mouse, icv) which induced a 2.1- and 3.1-times increase, respectively, of the light phase food intake as monitored 1 h post icv injection in ad libitum fed mice (Fig. 2A). The cumulative food intake was further increased at 2 h (2.2- and 3.4-times respectively) and 4 h (2.4- and 3.8-times respectively) post icv octreotide injection (Fig. 2A). In contrast, icv injection (0.3 or 1 µg/mouse) of the selective sst1 (Fig. 2B) or sst4 agonists (Fig. 2C) under the same conditions did not influence the light phase food intake at 1, 2, and 4 h post injection compared to icv vehicle (p > 0.05).
Fig. 2.
Octreotide increases light phase food intake whereas the selective sst1 or sst4 agonists injected intracerebroventricularly do not. Ad libitum fed mice were icv injected with octreotide (A), sst1 (B), and sst4 agonists (C) or vehicle and cumulative food intake was assessed for 4 h post injection. Each bar represents the mean ± SEM of 6–11 mice/group, * p < 0.05, *** p < 0.001 vs. vehicle. # p < 0.05, ## p < 0.01 vs. octreotide 0.3 µg.
3.3. Microstructure of light and dark phase food ingestion in undisturbed freely fed mice
Automated food intake monitoring for a period of 24 h and separate analysis of the light and dark phase showed a 6.6-times higher food intake during the dark phase in non-treated undisturbed mice. Microstructural analysis indicated that the nocturnal compared with the light phase pattern of food consumption was associated with an increased number of meals (3.9-times), meal size (1.5-times), meal duration (2.4-times) and rate of ingestion (2.2-times), whereas inter-meal intervals were reduced 7.1-times (p < 0.05; Table 2).
Table 2.
Food intake microstructure of undisturbed mice during the light and dark phase
| Parameters | Light phase | Dark phase |
|---|---|---|
| (6:00 AM to 6:00 PM) | (6:00 PM to 6:00 AM) | |
| Food intake (g/25g bw) | 0.36 ± 0.06 | 2.39 ± 0.05*** |
| Number of meals | 4.00 ± 0.53 | 15.75 ± 0.88*** |
| Meal size (g) | 0.11 ± 0.02 | 0.17 ± 0.01* |
| Meal time total (h) | 0.82 ± 0.23 | 5.75 ± 0.42*** |
| Meal duration (min) | 9.33 ± 1.13 | 22.53 ± 2.18*** |
| Latency to first meal (h) | 3.42 ± 1.49 | 0.45 ± 0.27 |
| Rate of ingestion (mg/min) | 1.88 ± 0.35 | 4.14 ± 0.26*** |
| Inter-meal intervals (h) | 2.57 ± 0.45 | 0.36 ± 0.03*** |
Values are mean ± SEM of n=8 mice.
p < 0.05 and
p < 0.001 vs. light phase
3.4. The sst2 agonist injected into the lateral brain ventricle alters the light phase feeding microstructure of ad libitum fed mice
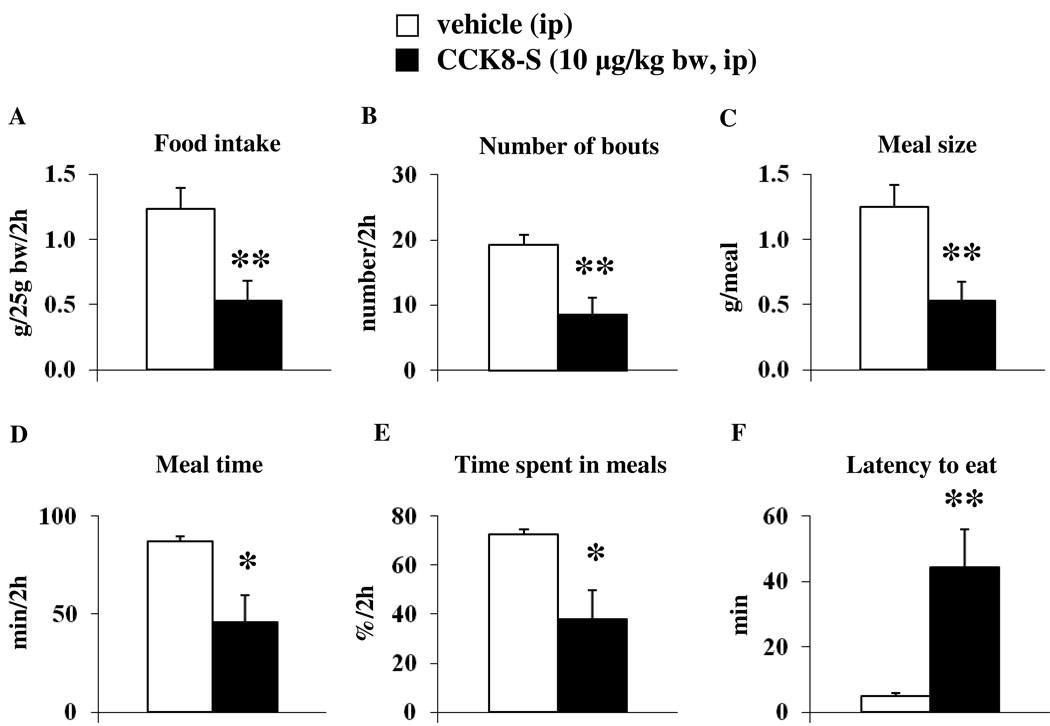
We first tested the meal microstructure in response to peripheral injection of CCK8-S under our conditions of automated recording of standard chow food intake. Injection of the control peptide, CCK8-S (10 µg/kg, ip) in overnight fasted mice reduced food intake during the first 30 min of the 2-h re-feeding period (0.18 ± 0.12 vs. 1.06 ± 0.14 g/25g bw, p < 0.001) and the cumulative 2-h food intake compared to ip injection of vehicle (0.53 ± 0.15 vs. 1.23 ± 0.16 g/25g bw, p < 0.01; Fig. 3A). This was associated with a decreased number of feeding bouts (8.57 ± 2.56 vs. 19.29 ± 1.55 bouts/2h, p < 0.01; Fig. 3B) and a reduced meal size (0.53 ± 0.15 vs. 1.25 ± 0.17 g/meal, p < 0.01; Fig. 3C) compared to vehicle. Mice injected with CCK8-S spent significantly less time eating compared to vehicle (45.74 ± 14.10 vs. 87.01 ± 2.53 min/2h, p < 0.05; Fig. 3D) corresponding to 38.1 ± 11.8 vs. 72.5 ± 2.1 % of the 2-h re-feeding period (p < 0.05; Fig. 3E). The latency to start eating was significantly increased following CCK8-S compared to vehicle (44.41 ± 11.61 vs. 4.99 ± 0.74 min, p < 0.01; Fig. 3F) and the rate of ingestion tended to be decreased but did not reach statistical significance (2.41 ± 1.32 vs. 4.70 ± 1.81 mg/min, p > 0.05).
Fig. 3.
CCK8-S injected ip decreases food intake by decreasing meal size and increasing the latency to eat as assessed by an automated episodic food intake monitoring device. Mice were fasted overnight and injected with CCK8-S (10 µg/kg bw in 100 µl saline) or vehicle (saline) during the light phase. Afterwards, the food intake pattern during the 2-h re-feeding period including food intake (A), number of bouts (B), meal size (C), meal time (D), % time spent in meals (E) and latency to eat (F) was assessed using an automated episodic food intake monitoring device. Each bar represents the mean ± SEM of 7 mice/group. * p < 0.05 and ** p < 0.01 vs. vehicle.
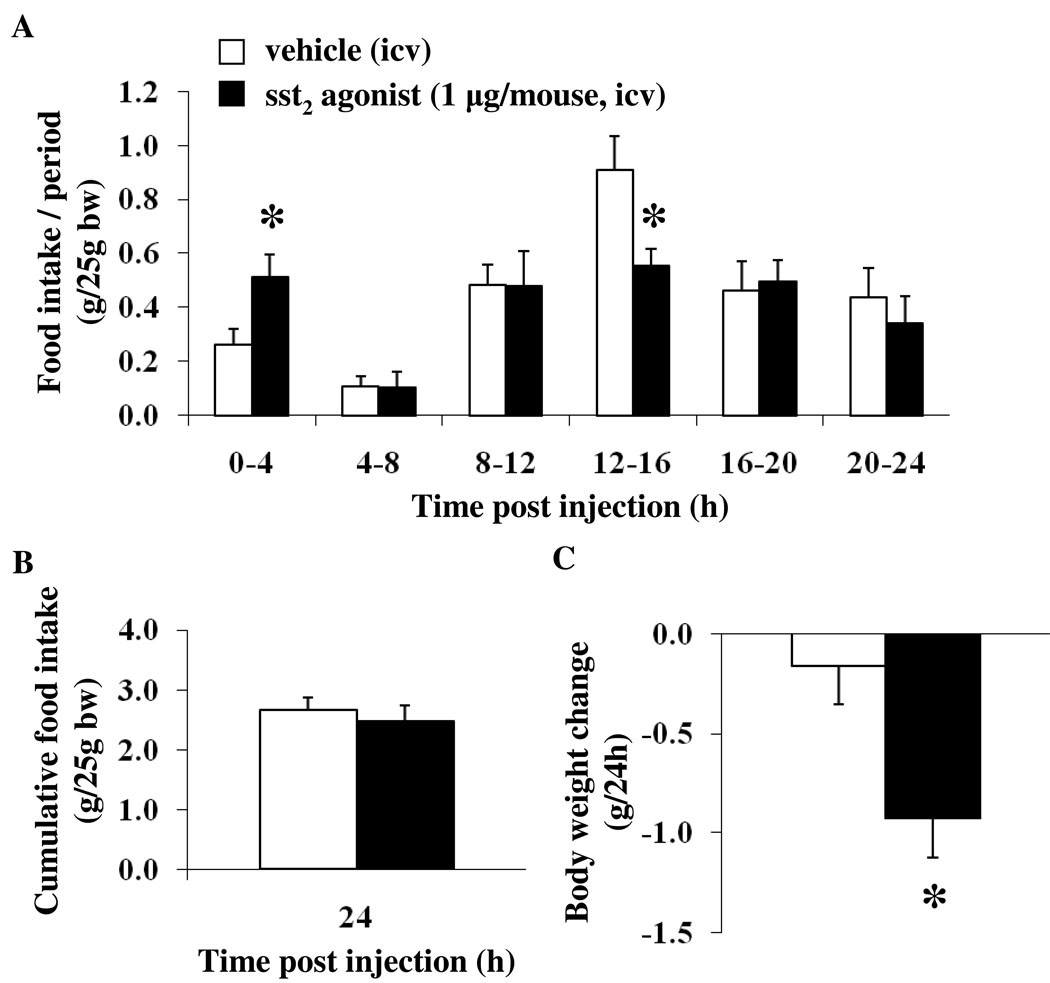
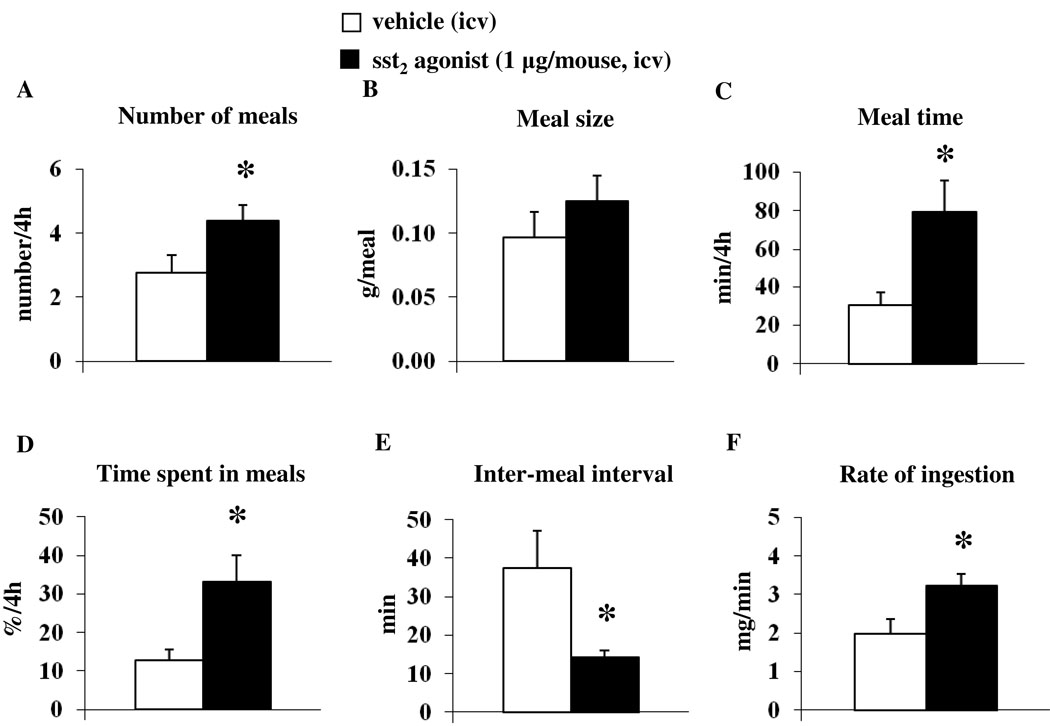
Automated assessment of ingestive behavior in the light phase after icv injection of sst2 agonist confirmed the stimulation of food intake during the first 4 h post injection compared to values after icv vehicle (0.51 ± 0.08 vs. 0.26 ± 0.06 g/25g bw, p < 0.05 after automated assessment and 0.56 ± 0.06 vs. 0.17 ± 0.01 g/25g bw, p < 0.01 after manual assessment), whereas in the subsequent 12–16 h period post injection during the dark phase, food intake was significantly reduced (0.55 ± 0.06 vs. 0.91 ± 0.13 g/25g bw, p < 0.05; Fig. 4A). Although the cumulative 24-h food intake was not altered after icv sst2 agonist (2.48 ± 0.25 vs. 2.66 ± 0.21 g/25g bw, p < 0.05; Fig. 4B), the body weight was significantly reduced compared to that of vehicle-treated mice (−0.93 ± 0.20 vs. −0.16 ± 0.19 g, p < 0.05; Fig. 4C). Meal pattern analysis of the first 4-h period post injection showed that the sst2 agonist increased the number of meals (4.38 ± 0.50 vs. 2.75 ± 0.56 meals/4h, p < 0.05; Fig. 5A), whereas the meal size was not significantly altered compared to that of vehicle (0.13 ± 0.02 vs. 0.10 ± 0.02 g/meal, p > 0.05; Fig. 5B). Mice treated icv with the sst2 agonist spent significantly more time eating compared to vehicle (79.29 ± 16.61 vs. 30.56 ± 6.50 min/4h, p < 0.05; Fig. 5C) corresponding to 33.0 ± 6.9 vs. 12.7 ± 2.7 % of the first 4 h (p < 0.05; Fig. 5D). The latency to the first meal tended to be reduced following sst2 agonist injection versus vehicle icv, although this did not reach statistical significance (10.83 ± 1.45 vs. 23.72 ± 6.21 min, p = 0.08). The inter-meal intervals were significantly reduced in the sst2 agonist group (14.39 ± 1.66 vs. 37.37 ± 9.80 min, p < 0.05; Fig. 5E), and the rate of ingestion was increased compared to values in the vehicle group (3.23 ± 0.31 vs. 1.97 ± 0.40 mg/min, p < 0.05; Fig. 5F).
Fig. 4.
The sst2 agonist injected icv increases light phase food intake during the first 4-h period while decreasing food intake during the dark phase as assessed by automated monitoring of ingestive behavior. The sst2 agonist (1 µg/mouse) or vehicle was injected icv during the light phase in ad libitum fed non-cannulated mice under short anesthesia and food intake/period assessed using automated episodic food intake monitoring (A). The cumulative 24-h food intake was not changed (B) whereas the body weight was decreased at 24 h post injection (C). Each bar represents the mean ± SEM of 8 mice/group. * p < 0.05 vs. vehicle.
Fig. 5.
The sst2 agonist injected into the lateral brain ventricle alters the food intake microstructure during the first 4-h period post injection. The sst2 agonist (1 µg/mouse) or vehicle was icv injected in ad libitum fed mice during the light phase and feeding microstructure including number of meals (A), meal size (B), meal time (C), % time spent in meals (D), inter-meal interval (E) and rate of ingestion (F) was assessed using an automated episodic food intake monitoring device. Each bar represents the mean ± SEM of 8 mice/group. * p < 0.05 vs. vehicle.
4. Discussion
In the present study, we provide the first evidence in ad libitum fed mice that a selective sst2 agonist des-AA1,4–6,11–13-[DPhe2,Aph7(Cbm),DTrp8]-Cbm-SST-Thr-NH2 [9] injected icv under short anesthesia stimulates the light phase food intake when rodents have a low drive to eat [15]. The peptide action was dose-related, occurred within 2 h and was long acting as shown by 1.5–1.7- and 2.0-times higher cumulative food intake still observed at 9 h post icv injection at 0.1, 0.3 and 1 µg, respectively, compared to icv injection of vehicle. The characteristics of peptide action (dose-related, onset and duration) in mice are similar to those reported in rats when the sst2 agonist was injected icv through a chronic icv cannula [14] showing that the sst2 agonist exerts an orexigenic effect in both rodent species.
Previous and present findings indicate that the icv sst2 agonist action is mediated by activation of brain sst2 receptors. We reported that the sst2 agonist-induced food intake in the light phase is completely prevented by pretreatment with the sst2 antagonist in rats [14]. In the present study, icv injection of sst1 or sst4 agonists tested at the two highest effective nmol doses of sst2 agonist had no effect on light phase food intake in mice as reported in rats [13]. However, it cannot be ruled out that higher doses may have resulted in an alteration of food intake. By contrast, the orexigenic effect can be induced by the icv injection of stable oligosomatostatin agonists such as octreotide (SMS 201–995) (present study) which displays high affinity to sst2 receptors [5, 7, 9] or the pan-activator of sst receptors, ODT8-SST injected icv at a similar nanomolar dose in mice [13]. Of interest, however, was the observation that the sst2/sst3/sst5 agonist octreotide and also ODT8-SST increased food intake in mice is occurring within the first hour post icv injection which differs from the sst2 agonist (present study) [13]. Since the sst2 agonist and octreotide (present study) and previously ODT8-SST [13] were injected icv under similar conditions of administration and dose (0.9 – 1 nmol), these differences may indicate the involvement of additional receptors in the early phase such as the sst3 and sst5, and/or the combined action on multiple sst receptors or differential diffusion capacity of the peptides. For instance, icv injection of corticotropin releasing factor (CRF) induced early suppression of food intake that is mediated by the activation of the CRF1 receptor, whereas the later phase involves an activation of CRF2 [28]. The centrally mediated action of icv injected sst2 is supported by our previous demonstration that neither peripheral injection of ODT8-SST in mice at 0.12 to 0.4 mg/kg [13] nor the sst2 agonist in rats influenced the light phase food intake in ad libitum fed animals [14]. In addition, other studies showed that somatostatin injected ip at a dose of 10 µg/kg bw reduces the dark phase food intake in rats [29]. The wide distribution of sst2 receptors in the mouse brain including prominent expression in food intake regulatory centers such as the arcuate and paraventricular nucleus of the hypothalamus [30] also provides neuroanatomical support for a central orexigenic action of sst2 agonists.
The sst2 mediated stimulation of food intake was not only observed under basal conditions in mice fed standard rodent diet but also under already stimulated conditions when mice had access to palatable high fat food. High fat diets (also called Western type diets) have been reported to increase caloric intake 1.3-times in 24 h and consecutively body weight in mice [31]. Similarly, in the present study mice injected icv with vehicle and acutely exposed to high fat diet (calories from protein 20%, fat 45% and carbohydrates 35%, 4.7 kcal/g diet) increased the light phase caloric intake 2.8-times during the first 4h and the 24-h cumulative food intake 1.7-times compared to values in mice fed a standard diet (calories from protein 29%, fat 12% and carbohydrates 59%, 3.4 kcal/g diet). In the icv sst2 agonist injected mice, the cumulative caloric intake/4 h was further enhanced 1.4-times compared to values in vehicle-treated mice with access to high fat diet. The relatively lower response to sst2 agonist in mice fed high fat compared to standard diet may represent a ceiling effect linked with the capacity of the stomach. It has recently been shown that galanin plays an important role in the preference for high fat diets as indicated by a fat-avoiding phenotype of galanin knockout (KO) mice [32]. These KO mice consumed significantly fewer calories from a high fat diet and gained less body weight compared to their wild type littermates. When different types of macronutrients were available, galanin KO mice also chose 3-times less fat than wild type which was reversed by icv treatment with galanin [32]. An interaction between brain somatostatin receptor activation and galanin resulting in increased consumption of high fat diets is supported by the localization of galanin neurons in the arcuate nucleus that express the sst2 receptor [33, 34] and the activation of arcuate neurons by icv injection of the sst2 agonist in rats [35]. However, sst2 KO mice ingest the same amount of food as their wild type littermates when fed a high fat diet [36] as observed for other orexigenic peptide receptors through which somatostatin agonists act centrally to stimulate food intake, namely the neuropeptide Y (NPY)-Y1 signaling system [13]. Y1 deficient mice consumed the same amount of food as their wild type littermates [37] pointing towards the existence of compensatory pathways under these conditions.
Although food intake was strongly stimulated during the light phase, there was a decrease during the dark phase in sst2 injected mice resulting in a similar 24-h cumulative food intake in both treatment groups. This may be due to increased satiety signaling following food ingestion during the light phase that reduces the drive to eat during the dark phase but could also be related to the loss of efficacy of the peptide sst2 agonist after 9 h. Despite the similar 24-h cumulative food intake, the body weight was significantly decreased by 3.4% at 24 h post icv sst2 agonist injection when compared to vehicle. In our previous study in rats we observed a sustained increase in ambulatory and fine movement (grooming, washing and licking) behavior following icv sst2 agonist [14] that may contribute to the decrease in body weight as grooming behavior was also observed in the present study, although it was not quantified. In addition, the pan-sst1–5 agonist, ODT8-SST injected icv also reduced body weight at 24 h post injection in rats, which was associated with an increased energy expenditure [13]. Based on these findings, the observed reduction in body weight could be due to an increase in physical activity as reflected in heightened locomotor activity associated with increased energy expenditure. These possible underlying mechanisms have to be further investigated. In addition, there is evidence that, although sst2 KO and wild type mice showed a similar weight gain after 14 weeks of high fat diet, sst2 KO mice had significantly less epididymal white and intrascapular brown adipose tissue than wild type mice [36] giving rise to a role of the sst2 receptor in the regulation of body composition.
To get insight into changes in microstructure of eating induced by the brain activation of sst2 receptors, we used a novel automated episodic food intake monitoring device that allows continuous recording of standard rodent chow in undisturbed mice. We first assessed the dark and light phase variations of food intake microstructure under spontaneous feeding conditions. As expected, during the dark compared to the light phase, we observed a higher food intake which was associated with an increased number of meals, meal size, meal duration and rate of ingestion and a reduction of inter-meal intervals. Likewise, other studies in mice showed that the higher food intake during the dark phase is mainly achieved by an increased number of meals [38] as also reflected by a shortened inter-meal interval and only to a smaller extent by altering meal size. Initial studies investigating the food intake pattern in undisturbed mice used the beam breaker method in combination with feeding recording of powder food intake [39]. More physiologically, solid food has been used in recent studies in form of micropellets that were delivered through a hole which was controlled by photobeams [38, 40]. The measurement system applied in the present study allows the use of standard rodent diet which is continuously weighed every second to calculate food intake as recently used in rats [41–43]. After assessing the 24-h food intake microstructure in non-treated mice, we validated the responsiveness of our monitoring system to a peptide well established to reduce food intake during the re-feeding period after an overnight fast in mice [26]. CCK8-S injected ip (10 µg/kg bw) reduced the 2-h re-feeding food intake by 56% as described before when food intake was assessed manually [26]. Microstructure analysis showed a 2.2-times reduction in the number of feeding bouts, a 2.4-times decrease in meal size indicating the induction of “satiation” (mechanisms causing meal termination [44]) and an 8.9-times increase in the latency to start eating providing the first characterization of meal pattern alteration in fasted lean mice injected ip with CCK8-S and re-fed with regular chow. Previous studies reported a decrease in meal size of liquid food intake in lean mice [45] and an increased latency to eat in obese ob/ob mice [46] following 8 or 18 µg/kg CCK-8 in mice fasted for 1.5–4.5 h or 23 h, respectively. In line with these findings, CCK1 receptor knockout mice fed a high fat diet consumed larger meals and had a decreased latency to start eating following a 6-h fast [47].
Using the automated episodic food intake monitoring system, sst2 agonist injected icv was found to increase food intake during the first 4 h post injection with values similar to those obtained by manual assessment. Microstructural analysis indicated that the orexigenic effect is due to an increase in the number of meals which were ingested at an increased rate and occurred with shortened inter-meal intervals, whereas meal size was unaltered. This pattern points towards reduced “satiety” (mechanisms causing later onset of the next meal after one completed meal [44]) as reflected in shortened inter-meal intervals but unaltered “satiation” (mechanisms causing meal termination [44]) as indicated by a normal meal size. In contrast, systemic administration of ghrelin stimulates food intake by increasing meal size in mice [38] indicating that the sst2 orexigenic response most likely does not involve ghrelin signaling. These finding are in keeping with the observation that the icv injection of the pan-somatostatin agonist, ODT8-SST, which induces an orexigenic effect via the sst2 receptor, increases ghrelin levels with a 3-h delayed onset, whereas food intake was already stimulated during the first hour in rats [13]. However, leptin tends to reduce meal frequency and decreases meal duration after direct brain injection [48]. Previous reports indicate that somatostatin reduces hypothalamic leptin signaling as assessed by STAT3-phosphorylation and nuclear STAT3 translocation [48]. Similarly, a selective sst2 agonist, L-779 976 icv reduced STAT3-phosphorylation in the ventromedial hypothalamus and the lateral hypothalamic area associated with a reversal of the leptin-induced reduction of 24-h meal frequency in rats [48]. Based on these data it can be speculated that this negative interaction between activation of sst2 receptors and leptin may play a role in the sst2 agonist-induced changes in quantity and organization of spontaneous food intake. Such a downstream interaction with the leptin signaling system would also be in line with the delayed onset of the sst2 orexigenic effect. Further studies are warranted to corroborate this assumption.
In summary, the present study established that icv injection of the sst2 agonist induces a dose-related, delayed in onset and sustained stimulation of the light phase ingestive behavior under basal and stimulated conditions by palatable high fat food in mice fed ad libitum. In contrast, under the same conditions sst1 or sst4 agonists had no effect while the stable sst2/sst3/sst5 agonist octreotide also increased food intake supportive of the key role of activation of brain sst2 receptors in the orexigenic response. Automated continuous assessment of standard chow intake in undisturbed mice demonstrated that the orexigenic response to brain sst2 activation involves an increase in the number of meals, rate of ingestion and reduction of the inter-meal interval. The feeding microstructure is indicative of brain sst2 receptor activation influencing brain circuitries involved in “satiety”, whereas “satiation” is not altered as indicated by normal meal size.
Acknowledgments
This work was supported by German Research Foundation Grants STE 1765/1-1 (A.S.), GO 1718/1-1 (M.G.), Veterans Administration Research Career Scientist Award, R01 NIH DK-33061, Center Grant DK-41301 (Animal Core and Supplement Grant) (Y.T). J. R. is the Dr. Frederik Paulsen Chair in Neurosciences Professor. We are grateful to Mrs. Honghui Liang for her excellent technical support, and we thank Dr. Douglas Compton (BioDAQ, Research Diets, Inc.) for his technical advice and Ms. Eugenia Hu for reviewing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
A.S., M.G., L.W., P.K., H.M. and Y.T. have nothing to disclose. J.R. is Founder of Sentia Medical Sciences, Inc. No conflicts of interest exist.
References
- 1.Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- 2.Brown M, Taché Y, Rivier J, Pittman Q. Peptides and regulation of body temperature. Adv Biochem Psychopharmacol. 1981;28:397–407. [PubMed] [Google Scholar]
- 3.Guillemin R. Hypothalamic hormones a.k.a. hypothalamic releasing factors. J Endocrinol. 2005;184:11–28. doi: 10.1677/joe.1.05883. [DOI] [PubMed] [Google Scholar]
- 4.Viollet C, Lepousez G, Loudes C, Videau C, Simon A, Epelbaum J. Somatostatinergic systems in brain: networks and functions. Mol Cell Endocrinol. 2008;286:75–87. doi: 10.1016/j.mce.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 6.Vanetti M, Kouba M, Wang X, Vogt G, Hollt V. Cloning and expression of a novel mouse somatostatin receptor (SSTR2B) FEBS Lett. 1992;311:290–294. doi: 10.1016/0014-5793(92)81122-3. [DOI] [PubMed] [Google Scholar]
- 7.Hoyer D, Bell GI, Berelowitz M, Epelbaum J, Feniuk W, Humphrey PP, O'Carroll AM, Patel YC, Schonbrunn A, Taylor JE, Reisine T. Classification and nomenclature of somatostatin receptors. Trends Pharmacol Sci. 1995;16:86–88. doi: 10.1016/s0165-6147(00)88988-9. [DOI] [PubMed] [Google Scholar]
- 8.Leroux P, Bucharles C, Bologna E, Vaudry H. des-AA-1,2,5[D-Trp8, IAmp9]somatostatin-14 allows the identification of native rat somatostatin sst1 receptor subtype. Eur J Pharmacol. 1997;337:333–336. doi: 10.1016/s0014-2999(97)01282-x. [DOI] [PubMed] [Google Scholar]
- 9.Grace CR, Erchegyi J, Koerber SC, Reubi JC, Rivier J, Riek R. Novel sst2-selective somatostatin agonists. Three-dimensional consensus structure by NMR. J Med Chem. 2006;49:4487–4496. doi: 10.1021/jm060363v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erchegyi J, Grace CR, Samant M, Cescato R, Piccand V, Riek R, Reubi JC, Rivier JE. Ring size of somatostatin analogues (ODT-8) modulates receptor selectivity and binding affinity. J Med Chem. 2008;51:2668–2675. doi: 10.1021/jm701444y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoneda M, Raybould H, Taché Y. Central action of somatostatin analog, SMS 201–995, to stimulate gastric acid secretion in rats. Peptides. 1991;12:401–406. doi: 10.1016/0196-9781(91)90076-2. [DOI] [PubMed] [Google Scholar]
- 12.Martinez V, Rivier J, Coy D, Taché Y. Intracisternal injection of somatostatin receptor 5-preferring agonists induces a vagal cholinergic stimulation of gastric emptying in rats. J Pharmacol Exp Ther. 2000;293:1099–1105. [PubMed] [Google Scholar]
- 13.Stengel A, Coskun T, Goebel M, Wang L, Craft L, Alsina-Fernandez J, Rivier J, Taché Y. Central injection of the stable somatostatin analog, ODT8-SST induces a somatostatin2 receptor mediated orexigenic effect: role of neuropeptide Y and opioid signaling pathways in rats. Endocrinology. 2010:4224–4235. doi: 10.1210/en.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, Taché Y. Selective central activation of somatostatin2 receptor increases food intake, grooming behavior and rectal temperature in rats. J Physiol Pharmacol. 2010;61:399–407. [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenwasser AM, Boulos Z, Terman M. Circadian organization of food intake and meal patterns in the rat. Physiol Behav. 1981;27:33–39. doi: 10.1016/0031-9384(81)90296-1. [DOI] [PubMed] [Google Scholar]
- 16.Csaba Z, Simon A, Helboe L, Epelbaum J, Dournaud P. Targeting sst2A receptor-expressing cells in the rat hypothalamus through in vivo agonist stimulation: neuroanatomical evidence for a major role of this subtype in mediating somatostatin functions. Endocrinology. 2003;144:1564–1573. doi: 10.1210/en.2002-221090. [DOI] [PubMed] [Google Scholar]
- 17.Stepanyan Z, Kocharyan A, Pyrski M, Hubschle T, Watson AM, Schulz S, Meyerhof W. Leptin-target neurones of the rat hypothalamus express somatostatin receptors. J Neuroendocrinol. 2003;15:822–830. doi: 10.1046/j.1365-2826.2003.01077.x. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 19.Danguir J. Food intake in rats is increased by intracerebroventricular infusion of the somatostatin analogue SMS 201–995 and is decreased by somatostatin antiserum. Peptides. 1988;9:211–213. doi: 10.1016/0196-9781(88)90030-7. [DOI] [PubMed] [Google Scholar]
- 20.Feifel D, Vaccarino FJ. Central somatostatin: a re-examination of its effects on feeding. Brain Res. 1990;535:189–194. doi: 10.1016/0006-8993(90)91600-l. [DOI] [PubMed] [Google Scholar]
- 21.Tachibana T, Cline MA, Sugahara K, Ueda H, Hiramatsu K. Central administration of somatostatin stimulates feeding behavior in chicks. Gen Comp Endocrinol. 2009;161:354–359. doi: 10.1016/j.ygcen.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Geary N. A new way of looking at eating. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1444–R1446. doi: 10.1152/ajpregu.00066.2005. [DOI] [PubMed] [Google Scholar]
- 23.Lamberts SW, van der Lely AJ, de Herder WW, Hofland LJ. Octreotide. N Engl J Med. 1996;334:246–254. doi: 10.1056/NEJM199601253340408. [DOI] [PubMed] [Google Scholar]
- 24.Erchegyi J, Cescato R, Grace CR, Waser B, Piccand V, Hoyer D, Riek R, Rivier JE, Reubi JC. Novel, potent, and radio-iodinatable somatostatin receptor 1 (sst1) selective analogues. J Med Chem. 2009;52:2733–2746. doi: 10.1021/jm801314f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erchegyi J, Waser B, Schaer JC, Cescato R, Brazeau JF, Rivier J, Reubi JC. Novel sst(4)-selective somatostatin (SRIF) agonists. 3. Analogues amenable to radiolabeling. J Med Chem. 2003;46:5597–5605. doi: 10.1021/jm030245x. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Martinez V, Rivier J, Taché Y. Peripheral urocortin inhibits gastric emptying and food intake in mice: differential role of CRF receptor 2. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1401–R1410. doi: 10.1152/ajpregu.2001.281.5.R1401. [DOI] [PubMed] [Google Scholar]
- 27.Martinez V, Wang L, Rivier J, Grigoriadis D, Taché Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol. 2004;556:221–234. doi: 10.1113/jphysiol.2003.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine AS, Morley JE. Peripherally administered somatostatin reduces feeding by a vagal mediated mechanism. Pharmacol Biochem Behav. 1982;16:897–902. doi: 10.1016/0091-3057(82)90041-7. [DOI] [PubMed] [Google Scholar]
- 30.Fehlmann D, Langenegger D, Schuepbach E, Siehler S, Feuerbach D, Hoyer D. Distribution and characterisation of somatostatin receptor mRNA and binding sites in the brain and periphery. J Physiol Paris. 2000;94:265–281. doi: 10.1016/s0928-4257(00)00208-4. [DOI] [PubMed] [Google Scholar]
- 31.Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffe-Scrive M. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism. 1995;44:645–651. doi: 10.1016/0026-0495(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 32.Adams AC, Clapham JC, Wynick D, Speakman JR. Feeding behaviour in galanin knockout mice supports a role of galanin in fat intake and preference. J Neuroendocrinol. 2008;20:199–206. doi: 10.1111/j.1365-2826.2007.01638.x. [DOI] [PubMed] [Google Scholar]
- 33.Lanneau C, Peineau S, Petit F, Epelbaum J, Gardette R. Somatostatin modulation of excitatory synaptic transmission between periventricular and arcuate hypothalamic nuclei in vitro. J Neurophysiol. 2000;84:1464–1474. doi: 10.1152/jn.2000.84.3.1464. [DOI] [PubMed] [Google Scholar]
- 34.Larm JA, Gundlach AL. Galanin-like peptide (GALP) mRNA expression is restricted to arcuate nucleus of hypothalamus in adult male rat brain. Neuroendocrinology. 2000;72:67–71. doi: 10.1159/000054573. [DOI] [PubMed] [Google Scholar]
- 35.Goebel M, Stengel A, Wang L, Coskun T, Rivier J, Taché Y. Pattern of Fos expression in the brain induced by selective activation of somatostatin receptor 2 in rats. Brain Res. 2010;1351:150–164. doi: 10.1016/j.brainres.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh V, Grötzinger C, Nowak KW, Zacharias S, Goncz E, Pless G, Sauer IM, Eichhorn I, Pfeiffer-Guglielmi B, Hamprecht B, Wiedenmann B, Plöckinger U, Strowski MZ. Somatostatin receptor subtype-2-deficient mice with diet-induced obesity have hyperglycemia, nonfasting hyperglucagonemia, and decreased hepatic glycogen deposition. Endocrinology. 2007;148:3887–3899. doi: 10.1210/en.2006-1659. [DOI] [PubMed] [Google Scholar]
- 37.Kushi A, Sasai H, Koizumi H, Takeda N, Yokoyama M, Nakamura M. Obesity and mild hyperinsulinemia found in neuropeptide Y-Y1 receptor-deficient mice. Proc Natl Acad Sci U S A. 1998;95:15659–15664. doi: 10.1073/pnas.95.26.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tabarin A, Diz-Chaves Y, Consoli D, Monsaingeon M, Bale TL, Culler MD, Datta R, Drago F, Vale WW, Koob GF, Zorrilla EP, Contarino A. Role of the corticotropin-releasing factor receptor type 2 in the control of food intake in mice: a meal pattern analysis. Eur J Neurosci. 2007;26:2303–2314. doi: 10.1111/j.1460-9568.2007.05856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gannon KS, Smith JC, Henderson R, Hendrick P. A system for studying the microstructure of ingestive behavior in mice. Physiol Behav. 1992;51:515–521. doi: 10.1016/0031-9384(92)90173-y. [DOI] [PubMed] [Google Scholar]
- 40.Chi MM, Powley TL. c-Kit mutant mouse behavioral phenotype: altered meal patterns and CCK sensitivity but normal daily food intake and body weight. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1170–R1183. doi: 10.1152/ajpregu.00015.2003. [DOI] [PubMed] [Google Scholar]
- 41.Farley C, Cook JA, Spar BD, Austin TM, Kowalski TJ. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obes Res. 2003;11:845–851. doi: 10.1038/oby.2003.116. [DOI] [PubMed] [Google Scholar]
- 42.Kowalski TJ, Farley C, Cohen-Williams ME, Varty G, Spar BD. Melanin-concentrating hormone-1 receptor antagonism decreases feeding by reducing meal size. Eur J Pharmacol. 2004;497:41–47. doi: 10.1016/j.ejphar.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 43.Dunn-Meynell AA, Sanders NM, Compton D, Becker TC, Eiki J, Zhang BB, Levin BE. Relationship among brain and blood glucose levels and spontaneous and glucoprivic feeding. J Neurosci. 2009;29:7015–7022. doi: 10.1523/JNEUROSCI.0334-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fekete EM, Inoue K, Zhao Y, Rivier JE, Vale WW, Szucs A, Koob GF, Zorrilla EP. Delayed satiety-like actions and altered feeding microstructure by a selective type 2 corticotropin-releasing factor agonist in rats: intra-hypothalamic urocortin 3 administration reduces food intake by prolonging the post-meal interval. Neuropsychopharmacology. 2007;32:1052–1068. doi: 10.1038/sj.npp.1301214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strohmayer AJ, Smith GP. Cholecystokinin inhibits food intake in genetically obese (C57BL/6j-ob) mice. Peptides. 1981;2:39–43. doi: 10.1016/s0196-9781(81)80009-5. [DOI] [PubMed] [Google Scholar]
- 46.Batt RA. Decreased food intake in response to cholecystokinin (pancreozymin) in wild-type and obese mice (genotype ob/ob) Int J Obes. 1983;7:25–29. [PubMed] [Google Scholar]
- 47.Donovan MJ, Paulino G, Raybould HE. CCK(1) receptor is essential for normal meal patterning in mice fed high fat diet. Physiol Behav. 2007;92:969–974. doi: 10.1016/j.physbeh.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stepanyan Z, Kocharyan A, Behrens M, Koebnick C, Pyrski M, Meyerhof W. Somatostatin, a negative-regulator of central leptin action in the rat hypothalamus. J Neurochem. 2007;100:468–478. doi: 10.1111/j.1471-4159.2006.04219.x. [DOI] [PubMed] [Google Scholar]