Abstract
Background
BRCA1 or BRCA2 (BRCA1/2) mutated ovarian carcinomas may originate in the fallopian tube. We investigated alterations in BRCA1/2 tubal epithelium to define the molecular pathogenesis of these carcinomas.
Methods
Tubal epithelium was evaluated from 31 BRCA1/2 mutation carriers with gynecologic carcinomas (BRCA CA), 89 mutation carriers undergoing risk-reducing salpingo-oophorectomy (RRSO), and 87 controls. Ki-67 expression and p53 foci (≥10/12 consecutive staining cells) were scored by two investigators blinded to case designation. p27 and p21 expression was evaluated within p53 foci. Loss of heterozygosity at the BRCA1/2 mutation site was evaluated in microdissected p53 foci and tubal neoplasms.
Results
Background tubal proliferation as measured by Ki-67 staining was increased in BRCA1 RRSO (p=0.005) compared with controls. Women with BRCA1/2 mutations were found to have more p53 foci per tubal segment than controls (p=0.02). p27 was decreased in 12/28 p53 foci from women with BRCA1 mutations and 0/16 from controls (p=0.002). There was no loss of the wildtype BRCA1/2 allele in 5 tested p53 foci. Tubal neoplasia lost the wildtype allele in 6/6 cases (p=0.002).
Conclusions
These observations suggest a model of tubal carcinogenesis in women with BRCA1/2 mutations. Increased proliferation occurs globally in at-risk tubal epithelium. A TP53 mutation with clonal proliferation and loss of p27 occurs prior to neoplastic proliferation. Loss of the wildtype BRCA1/2 allele occurs with neoplastic proliferation and prior to invasion.
Keywords: fallopian tube, BRCA1, BRCA2, p53, p27, Ki-67, ovarian cancer
Introduction
The majority of epithelial ovarian carcinomas are of high-grade serous or undifferentiated histology and present with established metastatic disease. Early detection strategies have been hindered by an inability to define the molecular progression of a clear precursor lesion.
Approximately 10–15% of ovarian carcinomas occur in women with inherited mutations in BRCA1 and BRCA21, 2, who have an estimated 20–50% lifetime risk of ovarian carcinoma2, 4. Multiple studies have examined the prophylactically removed ovaries of these women in attempts to find precursor lesions in ovarian epithelium. The majority of recent studies with pathologists blinded to patient status using both standard light microscopy and immunohistochemistry have been unable to identify a reproducible precursor (preneoplastic or non-invasive neoplastic) lesion in ovarian epithelium from high-risk women5–7.
Recently, a number of studies have considered the role of the fallopian tube in the pathogenesis of high-grade serous carcinomas of the ovary and peritoneum. Occult carcinomas identified at the time of risk-reducing salpingo-oophorectomy (RRSO) in women with BRCA1/2 mutations are found in 4.4–38% of high-risk women undergoing RRSO, with 57–100% of lesions found in the fallopian tube8–15. These findings support the hypothesis that most hereditary carcinomas of the ovary and peritoneum are seeded by neoplastic cells from the fallopian tube.
Carcinomas in BRCA1/2 mutation carriers have typically lost the wildtype BRCA1/2 allele16–18. Loss of heterozygosity for BRCA1/2 has also been described in high-grade intraepithelial neoplasia in the fallopian tubes of mutation carriers19, indicating that loss of the wildtype allele is an early step in BRCA1/2 related carcinogenesis.
High-grade serous pelvic carcinomas have a high rate of TP53 mutations (50–80%)20–25. Fallopian tubes from women with and without BRCA1/2 mutations have recently been found to contain clusters of epithelial cells with immunostaining for p53, called “p53 signatures”26, 27. These foci of p53 positive cells have been shown to contain TP53 mutations26 and have been hypothesized to represent precursor lesions to high-grade serous carcinomas26–30. Interestingly, fallopian tubes from control women have been found to have a similar rate of p53 foci as BRCA1/2 mutation carriers, despite their much lower risk of ovarian carcinoma26, 27. If p53 foci are the site of later neoplastic proliferation, there should be other molecular alterations that distinguish these foci in normal and high risk women.
The purpose of this study was to characterize the molecular events that differentiate fallopian tube epithelium in women with and without BRCA1/2 mutations. We examined expression of p53 and Ki-67 (a protein used as a marker for cellular proliferation)31 in histologically normal fallopian tube epithelium from normal-risk controls, women with BRCA1/2 mutations who have undergone risk-reducing salpingo-oophorectomy (RRSO), and women with BRCA1/2 mutations who have developed overt gynecologic carcinomas. Furthermore, we characterized proliferation and expression of regulators of the cell cycle (Ki-67, p27, and p21) within p53 foci in tubal epithelium to define molecular alterations that could impact neoplastic potential in the p53 foci of high-risk women. We examined cases of tubal neoplasia and non-neoplastic p53 foci for loss of heterozygosity at the known BRCA1/2 mutation to define the molecular progression of these lesions. From these data we are able to add significant detail to a model for the molecular pathogenesis of hereditary ovarian carcinoma.
Methods
Formalin-fixed, paraffin embedded specimens and clinical information were obtained through the University of Washington Gynecologic Oncology Tissue Bank, as approved by the Human Subjects Committee of the Institutional Review Board. One block from each fallopian tube was chosen that included distal fallopian tube. Tubal sections were obtained from 31 women with BRCA1 (23) and BRCA2 (8) associated ovarian, primary peritoneal, or tubal carcinoma (BRCA CA) and 89 women with BRCA1 (56) and BRCA2 (33) mutations that had undergone RRSO. Controls consisted of 61 women undergoing benign gynecological surgery and 26 women who underwent salpingo-oophorectomy with negative testing for BRCA1/2 mutations (including full DNA sequencing and comprehensive rearrangement testing). Fallopian tubes from all groups except BRCA CA were completely serially sectioned as part of prospective studies specifically examining the fallopian tube. Clinical data included age, presence of BRCA1/2 mutations, type of malignancy, stage, grade, and histology.
Immunohistochemistry
Paraffin sections were deparaffinized, rehydrated, and endogenous peroxidases blocked. Heat-mediated antigen retrieval was performed in a citrate buffer (Antigen Unmasking Solution, Vector Labs). Slides were treated with mouse monoclonal antibodies (Dako; Copenhagen, Denmark) against p53 (DO-7, diluted 1:500), Ki-67 (MIB-1, diluted 1:100), p27 (Anti-Kip1, 1:500, Transduction Labs), and p21 (WAF1, 1:100, Cal-Biochem). After secondary antibody with horseradish peroxidase (anti-mouse, Vector labs), sections were stained in DAB and counterstained with hematoxylin. Negative and positive controls were assessed for each run.
Slides were scored by two independent observers blinded to case designation for the number of p53 foci. p53 foci were defined in this study as at least 10 of 12 consecutive cells staining strongly positive for p53 according to the definition of Shaw et al27. For Ki-67, positive epithelial cells were scored (0=none, 1=1%, 2=2–4%, 3=5–15%, 4=>15%). If bilateral tubes were assessed, the highest score was recorded for the case. Immunostain findings are reported only for histologically normal fallopian tube, excluding p53 or Ki-67 staining of intra-epithelial neoplasia or carcinoma.
Sections immediately adjacent to p53 foci were scored for Ki67, p27 and p21 expression as increased, decreased or similar to surrounding tubal epithelium. Discrepancies in the identification of p53 foci, or in p27, p21, or Ki-67 within p53 foci were resolved by group review. Overall Ki-67 scores were analyzed separately for each observer, with p values reported for observer 1 and observer 2. Since the Ki-67 scores corresponded to unequal percentage values, they could not be accurately combined between the two observers.
DNA analysis
Loss of heterozygosity was analyzed in microdissected epithelium using DNA sequencing at the known BRCA1/2 mutation site in neoplastic tubal epithelium and in non-neoplastic p53 foci. Tubal epithelium was obtained by laser-capture microdissection with a Veritus system (Arcturus, Mountain View, CA) from adjacent formalin-fixed sections. For each case normal epithelium from another tubal section was also analyzed. Lymphocyte DNA samples from the same patients were used as controls. DNA was extracted using the PicoPure DNA extraction kit (Arcturus) and genomic DNA was amplified by polymerase chain reaction, using primers specific to the patient’s known mutation in BRCA1 or BRCA2. PCR products were purified and sequenced with BigDye Terminator v3.1 (Applied Biosystems, Foster City, CA) using ABI 3100 Genetic Analyzer (Applied Biosystems). Sequences at the mutation site were analyzed for relative ratios of wildtype and mutant sequences. All PCR and sequencing reactions were performed at least twice for each microdissected sample.
Statistical analyses
Statistical analyses were performed using Prism or Instat software (Graphpad, Inc, San Diego, CA), and Stata IC version 10.1. Comparisons of continuous variables were assessed using the Student’s T test for two variables. The Mann-Whitney T test was used for nonparametric data. All p values were two-sided. Contingency tables were made for comparison of categorical variables and p values were derived using Fishers Exact test or Chi Square. Comparisons of independent and dependent variables were assessed using Spearman correlation.
Results
Patient and specimen characteristics
Among the 31 women with BRCA1/2 associated overt carcinomas, there were 23 ovarian carcinomas, 5 primary peritoneal carcinomas, and 3 fallopian tube carcinomas. Of the ovarian carcinomas 20 were stage III-IV, and three were stage I (two endometrioid, one serous). Histologically, the ovarian carcinomas were primarily serous (14) or undifferentiated carcinoma (6), and three were endometrioid. The primary peritoneal carcinomas were all stage III-IV, with four serous carcinomas and one poorly differentiated adenocarcinoma. Of the fallopian tube carcinomas, one was stage IC, one IIC, and one unstaged, and all were of serous histology.
Of the 89 women with BRCA1/2 mutations undergoing RRSO, 9 women had occult gynecologic neoplasms (6 high-grade intraepithelial neoplasms in the fallopian tube, 2 microinvasive stage IA tubal carcinomas, and one IA grade 1 endometrioid endometrial carcinoma). The women with occult neoplasia on RRSO were included with BRCA CA in subsequent analyses. Many of these cases have been previously reported12. The 26 women undergoing salpingo-oophorectomy with negative genetic testing had no cases of occult neoplasia.
The 61 women who had salpingo-oophorectomy for benign indications had the following pathologic diagnoses: benign ovarian lesions (23), uterine leiomyomas (14), endometriosis (10), cervical intraepithelial neoplasia (1), chronic PID (2), adenomyosis (1), cervicitis (2), and endometrial polyp (1). Seven cases had normal pathology.
Clinical characteristics of the study groups are delineated in Table 1. Women with BRCA1/2 associated carcinomas (including occult neoplasia) were significantly older than women with BRCA1/2 mutations undergoing RRSO; with a median age of 49.5 years (range 39–66), compared to 45 years (range 31–69) for BRCA1/2 RRSO (p=0.0002, Mann-Whitney t-test). None of these groups were significantly different in age from controls. The number of fallopian tube sections reviewed per case differed across the groups. The BRCA1/2 carcinoma cases (including occult neoplasia) had significantly fewer fallopian tube sections per case than the combined BRCA1/2 RRSO (p<0.0001, Mann-Whitney t-test), and controls (p=0.004). Controls had fewer fallopian tube sections/case than BRCA1/2 RRSO, p=0.008).
Table 1.
Case Characteristics
|
BRCA1/2 with overt carcinoma |
BRCA1/2 RRSO with occult neoplasia |
BRCA1/2 RRSO with negative pathology |
Controls | |||||
|---|---|---|---|---|---|---|---|---|
| Case type |
BRCA1 | BRCA2 | BRCA1 | BRCA2 | BRCA1 | BRCA2 | Benign | Negative genetic testing |
| Total # cases |
23 | 8 | 7 | 2 | 49 | 31 | 61 | 26 |
| Median age* (range) |
49 (39–66) |
58 (47–76) |
46 (39–62) |
55.5 (46–65) |
44 (31–69) |
46 (35–69) |
48 (25–84) |
48 (33–61) |
| Median # tubal sections/ case** (range) |
4.0 (2–13) |
4.5 (2–10) |
7.5 (3–19) |
9 (5–13) |
8.0 (3–19) |
9.0 (1–18) |
6.0 (2–15) |
8.0 (3–15) |
| Bilateral tubal sections available |
14/23 (60.9%) |
6/8 (75.0%) |
4/7 (57.1%) |
2/2 (100%) |
41/49 (83.7%) |
28/31 (90.3%) |
40/61 (65.6%) |
22/26 (84.6%) |
BRCA1/2 CA including occult older than BRCA1/2 RRSO (median age 49.5 vs. 45, p=0.0002).
BRCA1/2 CA including occult with fewer tubal sections/case than BRCA1/2 RRSO (p<0.0001), and controls (p=0.004). Controls with fewer tubal sections/case than BRCA1/2 RRSO, p=0.008).
Immunohistochemistry
p53 foci in tubal epithelium
p53 foci were present in cases from all risk groups (26.4–47.5%, Table 2). Fallopian tube specimens with p53 foci had more fallopian tube sections examined compared with those that did not have foci (median of 9 vs. 7 sections, p=0.003, Mann-Whitney t-test), suggesting that the amount of tubal epithelium reviewed influences how often p53 foci are found. As there were different numbers of tubal sections available amongst the groups (Table 1), it was difficult to compare case positivity based solely on the percentage with any identified p53 foci. For this reason, the number of p53 foci was also calculated relative to the total number of stained fallopian tube sections for each patient (Table 2). By this measure, p53 foci were more frequent in all BRCA1/2 mutation carriers compared with controls (p=0.02, Mann-Whitney t-test), and in mutation carriers with carcinomas (including occult neoplasias) compared with controls (p=0.006). There was no difference in frequency of p53 foci between BRCA1 and BRCA2 (p=0.83), between BRCA CA and BRCA1/2 RRSO (p=0.08), or between BRCA1/2 RRSO and controls (p=0.1). There was no difference in age between women with and without p53 foci (p=0.4) across all groups or within any particular group.
Table 2.
p53 foci
|
BRCA1/2 with overt carcinoma |
BRCA1/2 RRSO with occult neoplasia |
BRCA1/2 RRSO with negative pathology |
Controls | |||||
|---|---|---|---|---|---|---|---|---|
| Case type | BRCA1 | BRCA2 | BRCA1 | BRCA2 | BRCA1 | BRCA2 | Benign | Negative genetic testing |
|
Cases with p53 foci |
9/23 (39.1%) |
5/8 (62.5%) |
4/7 (57.1%) |
1/2 (50.0%) |
20/49 (40.8%) |
11/31 (35.5%) |
16/61 (26.2%) |
7/26 (26.9%) |
|
Mean #p53 foci/tubal segs/case* (combined) |
0.102 | 0.231 | 0.135 | 0.115 | 0.065 | 0.062 | 0.042 | 0.059 |
| 0.135 | 0.130 | 0.064 | 0.054 | |||||
| 0.134 | ||||||||
| 0.087 | ||||||||
BRCA1/2 mutation carriers had a significantly higher frequency of p53 foci than controls (p=0.02).
p27, p21, and Ki-67 within p53 foci
Results for protein expression of p27 and p21 (cell cycle inhibitors), and Ki-67 (a marker of cellular proliferation) within p53 foci are shown in Table 3 and Figure 1. An attempt was made to examine all p53 foci, however the particular fold of fallopian tube epithelium containing the focus was not always present in adjacent sections or adjacent sections were not always available.
Table 3.
Alterations in p27, p21, and Ki-67 in p53 foci.
|
BRCA1/2 with carcinoma and occult neoplasia |
BRCA1/2 RRSO with negative pathology |
Controls | ||||
|---|---|---|---|---|---|---|
| BRCA1 | BRCA2 | BRCA1 | BRCA2 | Benign | NGT** | |
| p27↓ | 4/9* | 1/11 | 8/19* | 2/12 | 0/8* | 0/8* |
| p21↓ | 1/8 | 0/12 | 1/19 | 1/12 | 1/7 | 2/8 |
| Ki-67↑ | 3/12 | 0/13 | 2/22 | 0/13 | 1/15 | 1/8 |
p27 was decreased in 12/28 p53 foci from BRCA1 mutation carriers, but never in controls, p=0.002. p27 was increased in 1 BRCA1 RRSO, 1 BRCA2 RRSO, 1 Benign, and 1 NGT. p21 was increased in 1 Benign.
NGT (negative genetic testing)
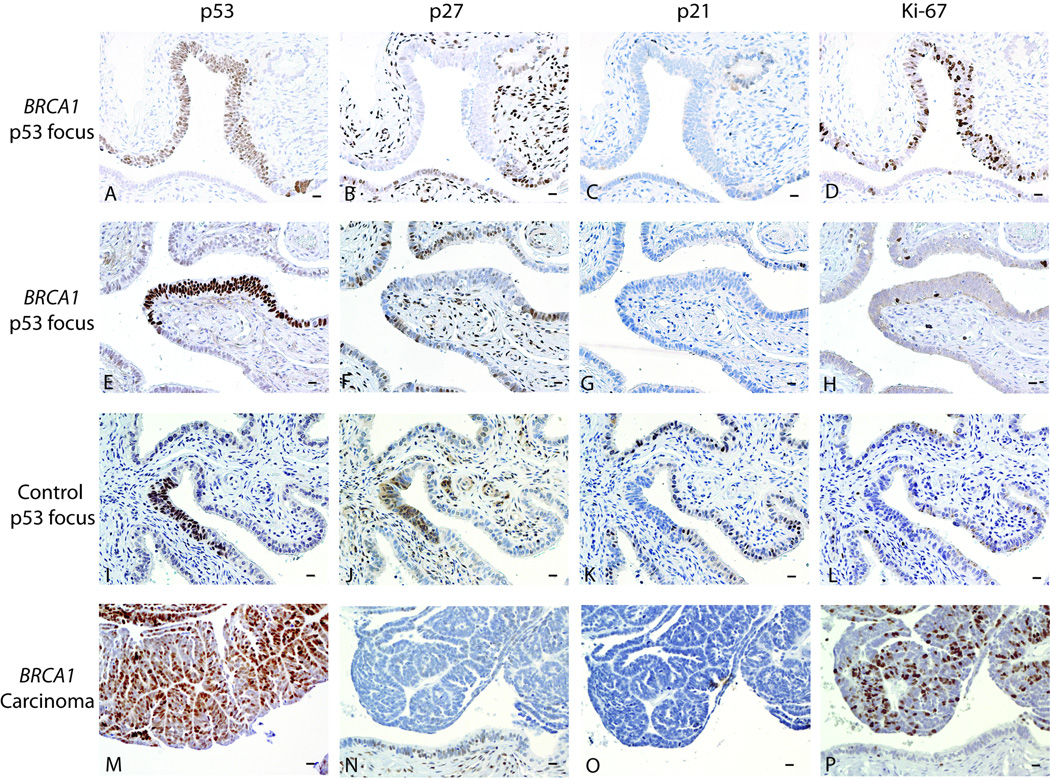
Figure 1. Examples of p53 foci and staining for p27, p21, and Ki-67 within foci and carcinoma.
Bars in right lower corners represent 10 µm. A–D. 62 year old woman with BRCA1 mutation who underwent RRSO with normal pathological findings. A. Large p53 focus. B. Decreased p27 expression within the focus. C. p21 expression is unchanged in the p53 focus compared to nearby epithelial staining. D. Ki-67 expression is increased in the p53 focus relative to nearby epithelial staining. E–H. 48 year old woman with BRCA1 mutation who underwent RRSO with normal pathological findings. E. p53 focus. F. Decreased p27 expression within the focus. G. p21 expression is unchanged in the p53 focus compared to nearby epithelial staining. H. Ki-67 expression is unchanged compared to nearby epithelial staining. I–L. Fallopian tube cross-sections from a 39 year old woman with endometriosis (benign control). I. p53 focus. J. p27 expression is not altered within the p53 focus relative to nearby tubal epithelium. K. p21 expression is decreased in the p53 focus. L. Ki-67 expression is similar in the p53 focus and nearby tubal epithelium. M–P. Microinvasive tubal carcinoma from a BRCA1 mutation carrier. M. Carcinoma stains positive for p53. N. p27 appears decreased relative to nearby benign epithelium. O. p21 staining is patchy and unchanged from surrounding benign epithelium. P. Ki-67 stain is diffusely positive in carcinoma.
p27 was decreased in 12/28 (42.9%) p53 foci from BRCA1 mutation carriers compared to 0/16 control foci (p=0.002, Fisher’s exact). These 44 foci were from 40 women, with 11/24 (45.8%) BRCA1 mutation carriers having at least one p53 focus with decreased p27 compared with 0/16 control women (p=0.001). p27 expression was decreased in 3/23 (13%) p53 foci from BRCA2 mutation carriers, fewer than in BRCA1 p53 foci (p=0.03) and non-significantly more than control foci (p=0.26). The 23 p53 foci from BRCA2 mutation carriers were from 14 women, with 3/14 (21.4%) having at least one p53 focus with decreased p27, compared with 11/24 BRCA1 mutation carriers (p=0.18), and 0/16 controls (p=0.09).
p21 expression was most commonly unchanged within foci (59/66) and was similarly expressed in p53 foci from all groups.
Ki-67 expression was increased in p53 foci in 5/34 foci from BRCA1, 0/26 from BRCA2, and 2/23 from controls. The percentage of p53 foci with increased Ki-67 expression was not significantly different between study groups.
Ki-67 staining in tubal epithelium
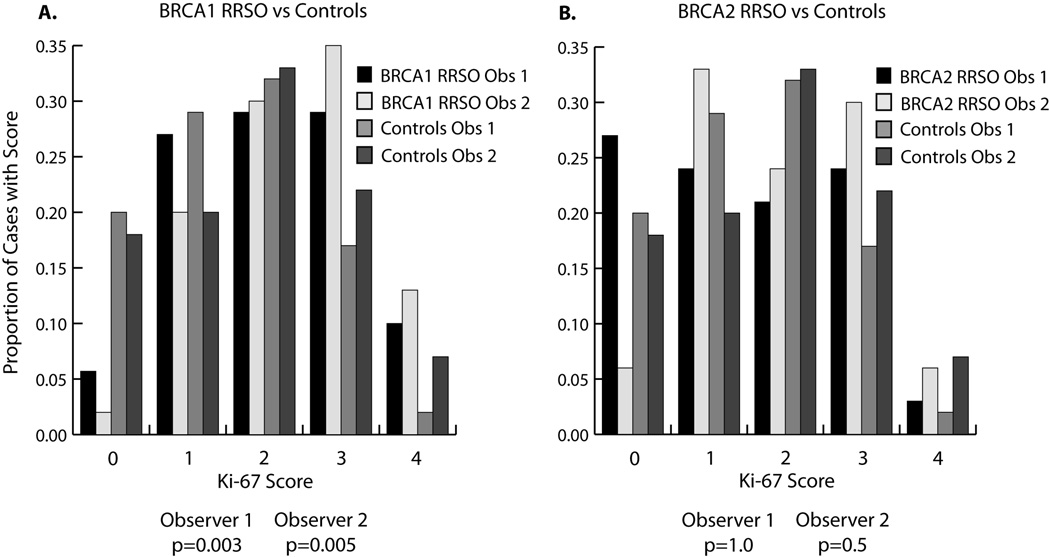
Overall Ki-67 expression in tubal epithelium was analyzed separately for each of the two observers, who were blinded to case designation. Tubal Ki-67 expression was significantly greater in women with BRCA1 mutations undergoing RRSO than controls [p=0.003 (observer1), p=0.005 (observer 2), chi square test for trend] (Figure 2). BRCA1 RRSO also had higher Ki-67 expression than BRCA2 RRSO [p=0.03 (observer 1), p=0.08 (observer 2)]. In contrast, BRCA2 RRSO did not have different Ki-67 expression compared to controls [p=1.0 (observer 1), p=0.5 (observer 2)] (Figure 2). Within the subgroup of BRCA1 RRSO, those with p53 foci had higher Ki-67 expression than those without p53 foci [p=0.02 (observer 1), p=0.04 (observer 2), chi square test for trend]. In controls and BRCA2 RRSO, there was no difference in Ki-67 expression between those with and without p53 foci. Amongst controls, age had a highly significant inverse correlation with Ki-67 score [r= −0.35, p=0.001 (observer1); r= −0.32, p=0.002 (observer 2), Spearman correlation]. This inverse correlation was still present, but less significant, in BRCA1/2 RRSO cases [r= −0.24, 0.02 (observer 1); r=−0.20, p=0.06 (observer 2)]. Ages were similar between BRCA1 RRSO and controls and do not explain the differences seen in Ki-67 expression.
Figure 2. Distribution of Ki-67 scores in BRCA1 RRSO and BRCA2 RRSO vs. controls.
A. Proportion of cases with each Ki-67 score (0–4), comparing BRCA1 RRSO with controls. BRCA1 RRSO had significantly higher Ki-67 scores than controls [p=0.003 (observer 1), p=0.005 (observer 2), chi square test for trend]. B. Ki-67 scores in BRCA2 RRSO were not significantly different from controls.
DNA analysis within p53 foci and intraepithelial neoplasia
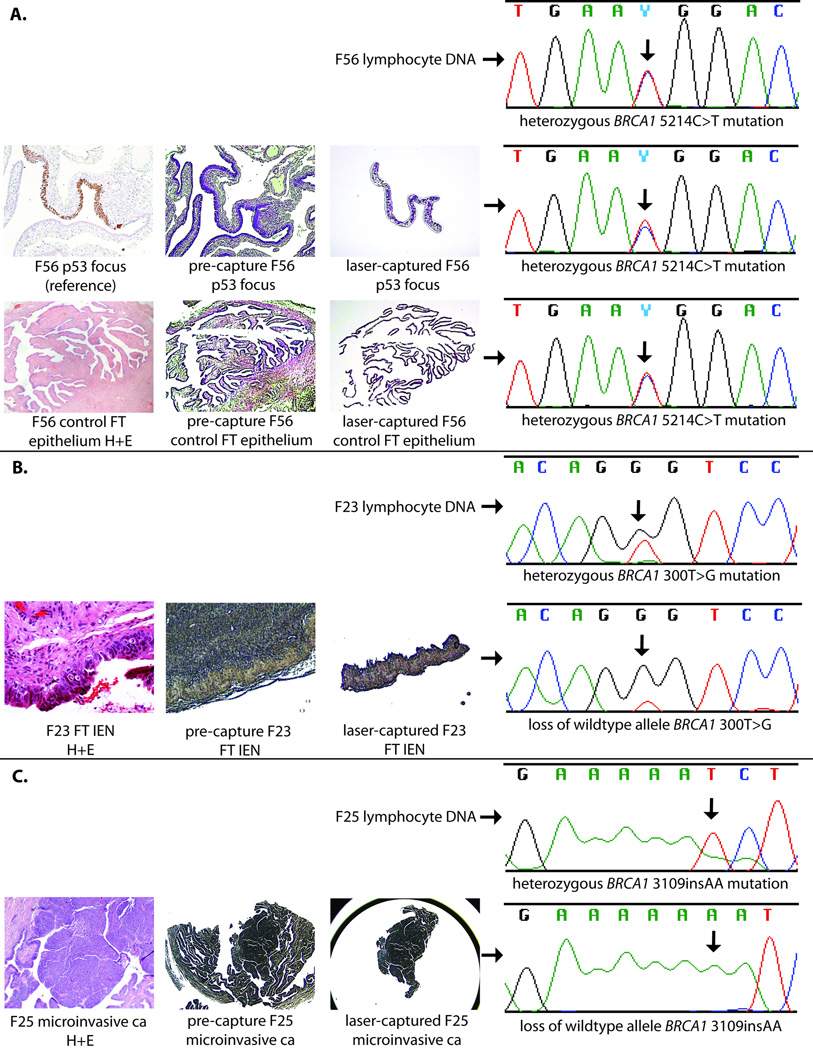
Nine p53 foci and paired tubal epithelium from the same patient distant from the p53 foci were isolated by laser capture microdissection of adjacent sections and DNA was extracted. For five of these nine p53 foci, DNA samples were successfully PCR amplified and sequenced for the known BRCA1 or BRCA2 mutation and compared to germline DNA as well as tubal epithelium from the same patient without p53 staining. All five of these p53 foci had demonstrated decreased p27 protein expression. The mutations for these cases included four in BRCA1: 2576delC, 5214C>T, 1224G>A, and 1246delA, and one in BRCA2: 5358del4. There was no evidence of loss of heterozygosity at the mutation site in any sample. Similarly, microdissected fallopian tube epithelium from the same cases in areas without p53 staining consistently showed heterozygosity for the known mutation. In contrast, 6/6 intraepithelial or microinvasive neoplasms from the fallopian tubes of BRCA1 mutation carriers had loss of the wildtype allele (p=0.002, Fisher’s exact). Examples of sequencing data for p53 foci and tubal intraepithelial neoplasia are shown in Figure 3.
Figure 3. Sequencing results from p53 foci and tubal neoplasia.
Laser-capture photos and hematoxylin and eosin (H+E) slides included for reference. A. Sequencing results for lymphocyte DNA, p53 focus, and control benign fallopian tube (FT) epithelium from patient F56. All specimens show heterozygous mutation in BRCA1 5214C>T. B. Sequencing results for lymphocyte DNA and fallopian tube intraepithelial neoplasia (FT IEN) from patient F23. FT IEN has lost the wildtype allele, showing predominantly BRCA1 300T>G sequence. C. Sequencing results from lymphocyte DNA and microinvasive FT carcinoma from patient F25. The carcinoma has lost the wildtype allele.
Discussion
The relatively high rate of p53 foci in tubal epithelium of normal risk women in this study, and others26, 27, compared to the relative infrequency of ovarian carcinoma in these women suggests that other alterations are necessary if these foci serve as potential sites of malignant transformation. In order to define differences in neoplastic potential between p53 foci in high and normal risk women, we examined protein expression of the cell-cycle inhibitors p27 and p21, and the proliferation marker Ki-67 within p53 foci. p27 expression was frequently decreased in p53 foci from BRCA1 mutation carriers (and some BRCA2 mutation carriers), but was never decreased in control foci (p=0.002). These data are the first to demonstrate a difference between p53 foci in high risk women and those occurring in women unlikely to develop ovarian carcinoma. Consequently, these data support the neoplastic potential of a significant number of the p53 foci that arise in tubal epithelium of BRCA1 mutation carriers. Conversely, retention of normal cell cycle checkpoints in p53 foci in normal risk women may explain the rarity of neoplastic progression of these lesions in women at normal risk for ovarian or tubal carcinoma.
Alterations in TP53 have been suggested to be a prerequisite to BRCA1 associated carcinogenesis21, 32. TP53 mutations have been identified in a small number of tested p53 foci (from women with and without BRCA1/2 mutations), and in one case the identical TP53 mutation was found in a co-existing intraepithelial neoplasia (IEN, also called tubal intraepithelial carcinoma)26. Similarly, Kindelberger and colleagues identified the same TP53 mutation in tubal IEN and co-existent sporadic pelvic serous carcinomas30. These data and ours suggest that alterations of TP53 are not only important for neoplastic progression but may precede a histologically identifiable neoplasm in at risk epithelium. Interestingly, a lower percentage of p53 foci in BRCA2 mutation carriers have loss of p27 compared to BRCA1 mutation carriers. If loss of p27 contributes to neoplastic transformation of p53 foci, then the decreased rate of p27 loss in BRCA2 p53 foci could be related to the lower lifetime risk of ovarian carcinoma in women with BRCA2 compared to BRCA1 mutations. Alternatively, the lower rate of p27 loss may indicate that alterations in CDKN1B (encoding p27) plays a less prominent role in BRCA2 compared to BRCA1 tubal carcinogenesis.
CDKN1B acts as a tumor suppressor by negatively regulating the transition from G0 to S phase via inhibition of cyclin E-CDK233. Studies in human breast cancer cell lines demonstrate that BRCA1 is a transcriptional activator of the CDKN1B promoter34, 35. Breast cancers in women with BRCA1/2 mutations characteristically have decreased p27 expression compared to sporadic carcinomas and normal breast tissue36, 37.
BRCA1/2 carcinomas typically have lost the wildtype allele16–18. Similarly, we demonstrated loss of the wildtype allele in all six early tubal neoplasms in BRCA1 mutation carriers. However, in five non-neoplastic p53 foci we did not find evidence for loss of heterozygosity of BRCA1/2. Thus, TP53 mutations and decreased p27 expression appear to occur before loss of the wildtype allele in the pathogenesis of BRCA1 tubal carcinomas. Haploinsufficiency of BRCA1/2 in combination with a TP53 mutation may have contributed to the loss of p27 in p53 foci of non-neoplastic tubal epithelium. The decrease in p27 expression and resultant loss of cell cycle inhibition could then result in increased cell proliferation and neoplastic potential. The six cases with early tubal neoplasia all had loss of the wildtype allele. Therefore, both TP53 mutation and p27 loss precede loss of the wildtype allele, which is probably the rate limiting step for neoplastic transformation. By the time loss of the wildtype BRCA1/2 allele occurs, neoplastic proliferation is histologically evident.
Ki-67 was elevated in a minority of p53 foci from both normal risk women and BRCA1/2 mutation carriers. These data are similar to findings recently reported by Shaw and colleagues27. Most pathologically recognized intraepithelial neoplasia (in situ or early invasive carcinoma) has both increased Ki-67 and p5326. However, co-expression of Ki-67 and p53 does not define IEN in the absence of severe cytological or architectural atypia. Conversely, Shaw and colleagues demonstrated that 21% of high grade IEN will not over-express p53 and one of the tubal carcinomas also lacked p53 expression27. Therefore it is important to define clear histopathological criteria for diagnosing tubal neoplasms that does not rely on the p53 and Ki-67 expression pattern. It is critical to avoid both under and over-diagnosing tubal IEN as some patients receive chemotherapy for these lesions8, 38.
Ki-67 expression was globally increased in tubal epithelium from women with BRCA1 mutations undergoing RRSO compared to normal risk women. Similarly, Piek and colleagues found a higher proportion of Ki-67 expressing tubal epithelial cells in morphologically normal tissue removed for risk-reduction when compared with controls in a small series of 12 high-risk women that included 7 with confirmed BRCA1 mutations39. Burga and colleagues recently demonstrated that human mammary epithelial cells heterozygous for a BRCA1 mutation have a higher proliferative rate in cell culture compared to wildtype mammary cells40. Together, these data suggest that haploinsufficiency of BRCA1 influences proliferation in breast and tubal epithelium, those tissues most at risk for malignant transformation in BRCA1 mutation carriers. In our series, those women with BRCA1 mutations undergoing RRSO who had p53 foci had more tubal epithelial proliferation compared to those without foci, suggesting that the conditions of increased proliferation could contribute to the formation of p53 foci. In normal risk women, we identified a highly significant inverse correlation of tubal epithelial proliferation and age. Interestingly, the relationship of decreasing proliferation with advancing age was less prominent in the tubal epithelium of high-risk women. We speculate that the increased proliferation in tubal epithelium in BRCA1 mutation carriers, particularly as they age, sets the stage for neoplastic transformation.
We observed a significant increase in the frequency of p53 foci in those with BRCA1/2 mutations compared to controls, particularly in BRCA1/2 mutation carriers with overt or occult gynecologic carcinoma. The percentage of women with any p53 foci is consistent with data from Christopher Crum’s group at Brigham and Women’s Hospital that first described the existence of p53 foci in tubal epithelium26, and higher than the rate found by Shaw et al27. However, neither Shaw and colleagues nor Crum and colleagues found a difference in the number of p53 foci between cases and controls, and neither evaluated BRCA1/2 mutation carriers with overt carcinomas. Because p53 foci are a relatively rare event, the identification of p53 foci is likely to depend on the volume of tubal epithelium evaluated. In order to account for variable tubal epithelial volumes between cases, we analyzed the number of p53 foci per number of tubal sections evaluated. Fewer tubal sections were available in our cases from women with overt carcinoma, but the number of p53 foci/tubal section was highest in these cases (Tables 1 and 2). We had a sufficient number of women undergoing RRSO to analyze the data separately for women with BRCA1 and BRCA2 mutations, and the rate of p53 foci was similar for tubal epithelium with mutations in either gene (Table 2). While the patients with BRCA1/2 related carcinomas tended to be older, there was no significant difference in age between those with and without p53 foci, a finding which has been confirmed by Saleemudin et al41.
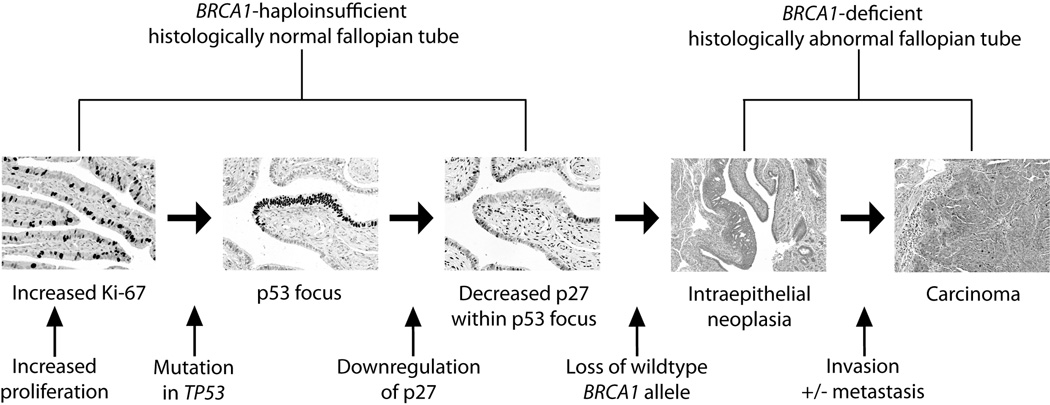
Previous authors have suggested a model for BRCA1/2 tubal carcinogenesis based on the progression of p53 foci to overt neoplasia42. Our data allows us to expand this model and add potential molecular details (Figure 4). We propose that haploinsufficiency of BRCA1 leads to increased tubal epithelial proliferation (as evidenced by increased Ki-67 expression). This tubal proliferation does not decrease appropriately with advancing age (as is the case with normal-risk women). These conditions support the clonal expansion of tubal cells with random TP53 mutations leading to the formation of p53 foci in BRCA1 haploinsufficient epithelium. Decrease of p27 expression occurs in cells that are TP53 mutant and BRCA1 haploinsufficient leading to loss of cell-cycle inhibition and increased neoplastic potential. Finally, loss of heterozygosity for BRCA1 occurs at the time of development of histologically identifiable intraepithelial neoplasia but prior to invasion.
Figure 4. Proposed model for the molecular pathogenesis of hereditary ovarian carcinoma.
Haploinsufficiency of BRCA1 in mutation carriers leads to increased tubal epithelial proliferation, as demonstrated by increased Ki-67 staining. Increased proliferation could increase the likelihood of TP53 mutations, leading to p53 foci. Loss of cell-cycle inhibition through downregulation of p27, followed by loss of DNA repair by loss of the wildtype BRCA1 allele then leads to neoplastic proliferation. Tubal neoplasia can then become invasive, and seed the ovary and peritoneal cavity.
Acknowledgments
This study was supported by the Yvonne Betson Trust and R01CA131965 (to EMS).
Footnotes
There are no financial disclosures from any author.
References
- 1.Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807–2816. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- 2.Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68:700–710. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford D, Easton DF, Bishop DT, Narod SA, Goldgar DE. Risks of cancer in BRCA-1 mutation carriers. Breast cancer linkage consortium. Lancet. 1994;343:692–695. doi: 10.1016/s0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- 4.King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 5.Barakat RR, Federici MG, Saigo PE, Robson ME, Offit K, Boyd J. Absence of premalignant histologic, molecular, or cell biological alterations in prophylactic oophorectomy specimens from BRCA1 heterozygotes. Cancer. 2000;89:383–390. doi: 10.1002/1097-0142(20000715)89:2<383::aid-cncr25>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 6.Cai KQ, Klein-Szanto A, Karthik D, et al. Age-dependent morphological alterations of human ovaries from populations with and without BRCA mutations. Gynecol Oncol. 2006;103:719–728. doi: 10.1016/j.ygyno.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 7.Piek JM, Verheijen RH, Menko FH, et al. Expression of differentiation and proliferation related proteins in epithelium of prophylactically removed ovaries from women with a hereditary female adnexal cancer predisposition. Histopathology. 2003;43:26–32. doi: 10.1046/j.1365-2559.2003.01654.x. [DOI] [PubMed] [Google Scholar]
- 8.Carcangiu ML, Peissel B, Pasini B, Spatti G, Radice P, Manoukian S. Incidental carcinomas in prophylactic specimens in BRCA1 and BRCA2 germ-line mutation carriers, with emphasis on fallopian tube lesions: report of 6 cases and review of the literature. Am J Surg Pathol. 2006 Oct;30(10):1222–1230. doi: 10.1097/01.pas.0000202161.80739.ac. [DOI] [PubMed] [Google Scholar]
- 9.Callahan MJ, Crum CP, Medeiros F, Kindelberger DW, Elvin JA, Garber JE, Feltmate CM, Berkowitz RS, Muto MG. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol. 2007;25(25):3985–3990. doi: 10.1200/JCO.2007.12.2622. [DOI] [PubMed] [Google Scholar]
- 10.Colgan TJ, Murphy J, Cole DE, Narod S, Rosen B. Occult carcinoma in prophylactic oophorectomy specimens: prevalence and association with BRCA germline mutation status. Am J Surg Pathol. 2001;25:1283–1289. doi: 10.1097/00000478-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Finch A, Shaw P, Rosen B, Murphy J, Narod SA, Colgan TJ. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol. 2006;100:58–64. doi: 10.1016/j.ygyno.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 12.Lamb JD, Garcia RL, Goff BA, Paley PJ, Swisher EM. Predictors of occult neoplasia in women undergoing risk-reducing salpingo-oophorectomy. Am J Obstet Gynecol. 2006;194:1702–1709. doi: 10.1016/j.ajog.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Leeper K, Garcia R, Swisher E, Goff B, Greer B, Paley P. Pathologic findings in prophylactic oophorectomy specimens in high-risk women. Gynecol Oncol. 2002;87:52–56. doi: 10.1006/gyno.2002.6779. [DOI] [PubMed] [Google Scholar]
- 14.Medeiros F, Muto MG, Lee Y, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Path. 2006;30:230–236. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 15.Powell CB, Kenley E, Chen L, et al. Risk-reducing salpingo-oophorectomy in BRCA mutation carriers: role of serial sectioning in the detection of occult malignancy. J Clin Oncol. 2005;23:127–132. doi: 10.1200/JCO.2005.04.109. [DOI] [PubMed] [Google Scholar]
- 16.Neuhausen SL, Marshall CJ. Loss of heterozygosity in familial tumors from three BRCA1-linked kindreds. Cancer Res. 1994;54:6069–6072. [PubMed] [Google Scholar]
- 17.Collins N, McManus R, Wooster R, et al. Consistent loss of the wild type allele in breast cancers from a family linked to the BRCA2 gene on chromosome 13q12–13. Oncogene. 1995;10:1673–1675. [PubMed] [Google Scholar]
- 18.Gudmundsson J, Johannesdottir G, Bergthorsson JT, et al. Different tumor types from BRCA2 carriers show wild-type chromosome deletions on 13q12–13. Cancer Res. 1995;55:4830–4832. [PubMed] [Google Scholar]
- 19.Piek JM, van Diest PJ, Zweemer RP, et al. Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–456. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 20.Kmet LM, Cook LS, Magliocco AM. A review of p53 expression and mutation in human benign, low malignant potential, and invasive epithelial ovarian tumors. Cancer. 2003;97:389–404. doi: 10.1002/cncr.11064. [DOI] [PubMed] [Google Scholar]
- 21.Schuijer M, Berns EM. Is TP53 dysfunction required for BRCA1-associated carcinogenesis? Mol Cell Endocrinol. 1999;155:143–152. doi: 10.1016/s0303-7207(99)00117-3. [DOI] [PubMed] [Google Scholar]
- 22.Schuijer M, Berns EM. TP53 and ovarian cancer. Hum Mutat. 2003;21:285–291. doi: 10.1002/humu.10181. [DOI] [PubMed] [Google Scholar]
- 23.Singer G, Stohr R, Cope L, et al. Patterns of p53 mutations separate ovarian serous borderline tumors and low- and high-grade carcinomas and provide support for a new model of ovarian carcinogenesis. Am J Surg Pathol. 2005;29:218–224. doi: 10.1097/01.pas.0000146025.91953.8d. [DOI] [PubMed] [Google Scholar]
- 24.Swisher EM, Wollan M, Mahtani SM, et al. Tumor-specific p53 sequences in blood and peritoneal fluid of women with epithelial ovarian cancer. Am J Obstet Gynecol. 2005;193:662–667. doi: 10.1016/j.ajog.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 25.Rosen AC, Ausch C, Klein M, Graf AH, Metzenbauer M, Philipp K, Reiner A. p53 expression in fallopian tube carcinomas. Cancer Lett. 2000;156:107. doi: 10.1016/s0304-3835(00)00335-9. [DOI] [PubMed] [Google Scholar]
- 26.Lee Y, Miron A, Drapkin R, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 27.Shaw PA, Rouzbahman M, Pizer ES, Pintilie M, Begley H. Candidate serous cancer precursors in fallopian tube epithelium of BRCA1/2 mutation carriers. Mod Pathol. 2009:1–6. doi: 10.1038/modpathol.2009.89. Epub 2009 Jun 19. [DOI] [PubMed] [Google Scholar]
- 28.Folkins AK, Jarboe EA, Saleemuddin A. A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol Oncol. 2008;109:168–173. doi: 10.1016/j.ygyno.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crum CP, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- 30.Kindelberger DW, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 31.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 32.Brodie SG, Deng CX. BRCA1-associated tumorigenesis: what have we learned from knockout mice? Trends Genet. 2001;17:S18–S22. doi: 10.1016/s0168-9525(01)02451-9. [DOI] [PubMed] [Google Scholar]
- 33.Sherr DJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 34.Williamson EA, Dadmanesh F, Koeffler HP. BRCA1 transactivates the cyclin-dependent kinase inhibitor p27 (Kip1) Oncogene. 2002;21(20):3199–3206. doi: 10.1038/sj.onc.1205461. [DOI] [PubMed] [Google Scholar]
- 35.Williamson EA, Wolf I, O’Kelly J, Bose S, Tanosaki S, Koeffler HP. BRCA1 and FOXA1 proteins coregulate the expression of the cell cycle-dependent kinase inhibitor p27Kip1. Oncogene. 2006;25:1391–1399. doi: 10.1038/sj.onc.1209170. [DOI] [PubMed] [Google Scholar]
- 36.Chappuis PO, Kapusta L, Begin LR, et al. Germline BRCA1/2 mutations and p27Kip1 protein levels independently predict outcome after breast cancer. J Clin Oncol. 2000;18(24):4045–4052. doi: 10.1200/JCO.2000.18.24.4045. [DOI] [PubMed] [Google Scholar]
- 37.Palacios J, Honrado E, Osorio A, et al. Phenotypic characterization of BRCA1 and BRCA2 tumors based in a tissue microarray study with 37 immunohistochemical markers. Breast Cancer Res Treat. 2005;90:5–14. doi: 10.1007/s10549-004-1536-0. [DOI] [PubMed] [Google Scholar]
- 38.Agoff SN, Garcia RL, Goff BA, Swisher EM. Follow-up of in situ and early-stage fallopian tube carcinoma in patients undergoing prophylactic surgery for proven or suspected BRCA-1 or BRCA-2 mutations. Am J Surg Pathol. 2004;28(8):1112–1114. doi: 10.1097/01.pas.0000131554.05732.cd. [DOI] [PubMed] [Google Scholar]
- 39.Piek JM, van Diest PJ, Zweemer RP, et al. Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–456. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 40.Burga LN, Tung NM, Troyan SL, et al. Altered proliferation and differentiation properties of primary mammary epithelial cells from BRCA1 mutation carriers. Cancer Res. 2009;69(4):1273–1278. doi: 10.1158/0008-5472.CAN-08-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saleemuddin A, Folkins AK, Garrett L, et al. Risk factors for a serous cancer precursor (“p53 signature”) in women with inherited BRCA mutations. Gynecol Oncol. 2008;111:226–232. doi: 10.1016/j.ygyno.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crum CP, Drapkin R, Miron A, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]