Abstract
Syngeneic graft-versus-host disease (SGVHD) develops following lethal irradiation, reconstitution with syngeneic bone marrow (BM) and treatment with a 21 day course of the immunosuppressive agent cyclosporine A (CsA). Clinical symptoms of SGVHD appear 2-3 weeks post CsA with inflammation occurring in the colon and liver. Previously we have demonstrated that CD4+ T cells and a T helper cell type 1 cytokine response (TH1) are involved in the development of SGVHD associated intestinal inflammation. Studies have recently discovered an additional T cell lineage that produces IL-17 and is termed TH17. It has been suggested that inflammatory bowel disease is a result of a TH17 response rather than a TH1 response. This study was designed to investigate TH17 involvement in SGVHD-associated colitis. Following induction of SGVHD, the levels of TH17 and TH1 cytokine mRNA and protein were measured in control and SGVHD animals. In vivo cytokine neutralization was performed to determine the role of the prototypic TH17 cytokine, IL-17, in the disease process. We found that during CsA-induced murine SGVHD there was an increase in both TH17 and TH1 mRNA and cytokines within the colons of diseased mice. The administration of an anti-mouse IL-17A mAb did not alter the course of disease. However, neutralization of IL-17A resulted in an increased production of IL-17F, a related family member, with an overlapping range of effector activities. These results demonstrate that in the pathophysiology of SGVHD, there is a redundancy in the TH17 effector molecules that mediate the development of SGVHD.
Keywords: IL-17, CD4+ T cells, transplantation, cyclosporine A, mucosal inflammation
1. INTRODUCTION
Cyclosporine A is a T cell-specific [1] immunosuppressive agent which has been used both clinically [2] and experimentally [3] to prevent the rejection of solid organs and GVHD following bone marrow transplantation (BMT). Syngeneic GVHD develops in mice following lethal irradiation, reconstitution with syngeneic BM, and treatment with a short course of CsA [4, 5]. CsA-induced SGVHD was first described in the rat [6] and later in the mouse [7] and was termed SGVHD due to the similar pathology between it and allogeneic GVHD. Clinical symptoms of murine SGVHD include weight loss and diarrhea, with the major sites of pathology being the colon and liver [4, 5, 8, 9].
CD4+ T cell-mediated immunity has been characterized by the production of specific cytokine profiles. Until recently effector CD4+ T cells have been subdivided into two distinct populations. TH1 T cells are potent activators of cell mediated immunity characterized by the production of the cytokines IL-2 and IFN-γ [10]. On the other hand, TH2 CD4+ T cells modulate antibody responses and have been shown to produce the cytokines IL-4, IL-5 and IL-13. Recent studies have described an additional CD4+ T cell population that produced the cytokine IL-17 (also known as IL-17A) and were termed TH17 [11]. The prototypic TH17 cytokine, IL-17 has been shown to consist of six family members, IL17A-F [12]. Of these IL-17A and IL-17F have the highest homology and have overlapping activity. The development of TH17 cells has been found to be dependent on the production of IL-23 [13], a heterodimeric cytokine that is composed of a unique IL-23p19 subunit and a p40 (IL-12p40) subunit that it shares with IL-12 and is secreted by activated dendritic cells. In vivo, IL-23 has been shown to aid in the expansion of IL-17 producing T cells [14, 15] and maintaining TH17 effector function [16]. Studies have suggested that the cytokines IL-6 and TGF-β, are responsible for the induction of differentiation of naïve cells into TH17 cells [17-20].
Evidence is now emerging that TH17 cells play a central role in the pathogenesis of various inflammatory disorders [21-24] including inflammatory bowel diseases (IBD) [25-27]. Other inflammatory diseases such as rheumatoid arthritis [24], collagen-induced arthritis and experimental autoimmune encephalomyelitis [23] have demonstrated beneficial effects of IL-17 neutralization. Conversely, others have reported that in DSS induced colitis or in a transfer model of colitis that removal of IL-17 was found to increase the severity of the inflammation and progression of the disease and had a protective role [28, 29]. Similar results were observed when IL-17 deficient T cells were utilized to induce allogeneic GVHD [30]. These results suggest that TH17 cells and IL-17 have both pro and anti inflammatory functions that are dependent on the model system in which they are studied.
Recent studies by Bryson et al, have demonstrated that the development of murine SGVHD is dependent on CD4+ T cells [31, 32]. Furthermore, the development of murine SGVHD was associated with the production of IFN-γ, IL-12 and TNF-α cytokines [33, 34]. Neutralization of IL-12p40 or TNF-α resulted in the inhibition of disease [33, 34]. Due to the shared IL-12p40 subunit between IL-23 and IL-12 the question was raised as to whether SGVHD mediated colon inflammation is a result of a TH1, TH17 or a mixed response involving both populations of cells. The current study was designed to analyze TH17 immunity during the development of SGVHD. The results demonstrated an increase in TH17 and TH1 cytokines in colon and the periphery of SGVHD mice. In vivo neutralization of the prototypic TH17 cytokine, IL-17, failed to alter the course of SGVHD. However, as IL-17A was removed from the CsA-treated animals, increased production of the related IL-17F was observed. As these cytokines have overlapping function, the results suggest that a redundancy likely exists in the role of IL-17 family members in the development of murine SGVHD.
2. METHODS
2.1 Mice
C3H/HeN mice were purchased from Harlan (Indianapolis, IN) at 20–21 days of age and were used within 1 week of arrival. All mice were housed in sterile micro-isolator cages (Lab Products, Maywood, NJ) and fed autoclaved food and acidified water ad libitum. Animal protocols were reviewed and approved by the Animal Care and Use Committee at the University of Kentucky.
2.2 Induction of SGVHD
Bone marrow was isolated from the femurs and tibias of donor mice. The donor BM suspensions were prepared in RPMI 1640 (Cellgro, Herndon, VA) supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin. The resulting cell suspensions were depleted of Thy-1+ cells as previously described [9]. Recipient mice were lethally irradiated with 900 cGy in a Mark I 137Cs irradiator (J.L. Shepherd and Associates, Glendale, CA). The animals were reconstituted i.v. with 5 × 106 T cell-depleted BM (ATBM) 4–6 h after conditioning. Beginning on the day of transplantation, the mice were treated daily for 21 days with either, 15 mg/kg/day CsA (purchased through the Division of Laboratory Animal Resources) in the diluent olive oil (Sigma-Aldrich, St. Louis, MO) or diluent alone. Following cessation of CsA therapy, the animals were weighed three times per week and observed for clinical symptoms of SGVHD (weight loss, diarrhea). Animals that developed weight loss for three consecutive weighings, developed diarrhea or succumbed to SGVHD were considered positive for the induction of disease.
2.3 Real Time Reverse transcriptase (RT) Polymerase Chain Reaction (PCR)
Total colon RNA was isolated using Trizol reagent (Invitrogen, Grand Island, NY). 1μg of RNA from each group was reversed transcribed into cDNA using the Promega reverse transcription system (Madison, WI). 2.5 μl of cDNA was combined with 10μl of master mix (0.5 U Platinum Taq (Invitrogen), 0.2 nM of each dNTP, 0.2 mM PCR buffer (Idaho technology Inc, Salt Lake City, UT), 1X SYBR Green (Molecular probes, Eugene, OR) and 1μM of primer. Primers for GAPDH, IL-12p40, IFN-γ, TNF-α, IL-17, IL-6, CCL2 [35], IL-23p19[36], IL-17F [37], CCL5 [38] and CCL7 [39] were purchased from Integrated DNA Technologies (Coralville, IA). Real time RT-PCR was performed on a Roche Lightcycler (Roche Diagnostics, Indianapolis, IN). PCR conditions were as follows: 30 seconds at 95°C, followed by 50 cycles of 6 seconds at 95°C, 10 seconds at 60°C and 15 seconds at 72°C. The conditions for CCL7 were identical to that described abovedwith the exception that the analing temperature was 55°C. The levels of IL-12, IFN-γ, TNF-α, IL-17, IL-6, IL-23p19, CCL2, CCL5 and CCL7 were normalized to GAPDH calculated by the comparative ΔΔCT method.
2.4 Histological Analysis of SGVHD
Colon samples were obtained at times when clinical symptoms of SGVHD were observed, and placed into 10% buffered formalin. Tissues were then embedded into paraffin, and 4–6 μm sections were cut and mounted onto a glass side. All slides were stained with a standard H&E protocol and were graded blind without the knowledge of treatment group according to a previously published grading scale [40].
2.5 Cytokine Analysis of Serum and Colon Explant Cultures
To monitor cytokine protein production in the colon, 0.1 g of colon was taken from each mouse at the time of tissue retrieval. The tissue was then washed 4 times in PBS, placed into 1 ml of 10% complete RPMI growth media (10% FBS, penicillin, streptomycin, 5×10−5 M 2-ME) that was supplemented with 50 ug/ml gentamicin and 0.25 ug/ml amphotericin B, then placed in a 37°C 5% CO2 incubator for 24 hours. The supernatant was removed and analyzed for various cytokines. Cardiac bleeds were performed at the time of tissue collection. Blood was allowed to coagulate for 24 h at 4°C and the serum was obtained by centrifugation. Analysis of serum and colon explant supernatant cytokines was performed using the Luminex xMAP system and the “Beadlyte mouse 21-plex cytokine detection system” (Upstate, Temecula, CA) or via an IL-17-specific ELISA kit as per manufacturer’s instructions (IL-17A ELISA Ready-SET-Go; eBioscience, San Diego, CA).
2.6 Detection of Intracellular Cytokine Production
Isolated lymphoid cells from the spleen or mesenteric lymph nodes (MLN) were placed in 10% complete RPMI growth media and stimulated with anti-mouse CD3 ascites for 8 hours at 37°C. 2 μM monensin (eBioscience) was added 4 hours before the cells were placed in staining buffer (PBS containing 1% FCS, 0.1% NaN3). To reduce nonspecific staining, cells were incubated with anti-CD16/CD32 (Fc Block; BD PharMingen, San Diego, CA). 1 × 106 cells were then stained with fluorochrome-conjugated mAb against CD4 (Caltag Burlingame CA). Intracellular staining for IL-17, IFN-γ and TNF-α was performed using Intracellular Cytokine Staining Kit (eBioscience, San Diego, CA) according to manufacturer’s directions. The cells were analyzed using a BD Biosciences FACSCalibur flow cytometer (San Jose, CA).
2.7 In Vivo Neutralization of IL-17
C3H/HeN mice were induced for SGVHD as described. Beginning on day 21 post-BMT recipient mice were treated with rat anti-mouse IL-17A (M210) (A kind gift from Amgen, Seattle WA) or rat IgG (Jackson Immuno Research Laboratories Inc) through 2 weeks post CsA. Mice received either, 200μg rat anti-mouse IL-17 mAb (according to manufacturer’s recommendations) or 200μg of control rat IgG i.p. for either 3 days and then every other day for 2 weeks, for 7 days and then every other day for 2 weeks or for 14 consecutive days.
2.8 Statistical Analysis
Statistical differences between groups were determined using the Student’s t test, Fisher’s exact test (induction) or log rank test. Differences ≤ 0.05 were considered statistically different.
3. RESULTS
3.1 SGVHD mice demonstrate both TH1 and TH17 cytokine responses
Previous studies have shown that during murine SGVHD there was an induction of TH1 cytokines (IFN-γ and IL-12p40) in the colons of diseased animals [33, 34]. TH17 associated cytokines (IL-17, IL-23p19, IL-6 and TNF-α) have been shown to be increased in inflammatory disorders including colitis. As neutralization of IL-12p40 significantly altered the development of SGVHD, and is a shared subunit of IL-23, we sought to determine whether TH17 cytokines were increased in the development of this inducible disease.
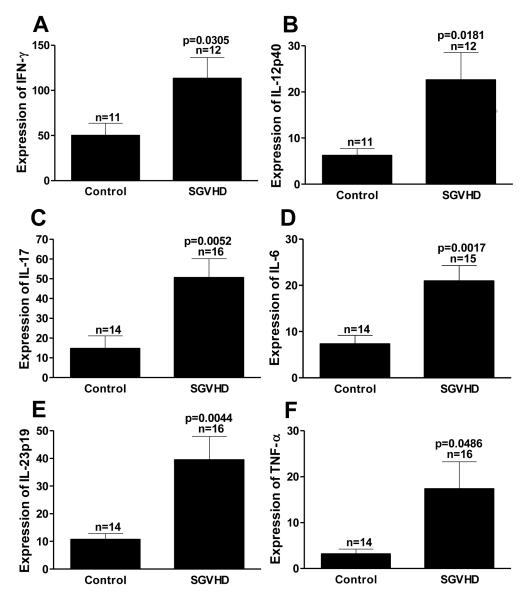
To determine if TH17 immunity was induced during the induction of murine SGVHD, C3H/HeN mice were lethally irradiated and reconstituted with syngeneic ATBM then treated for 21 days with CsA. Colon samples were obtained (2-3 weeks post CsA) from control and SGVHD mice, mRNA was extracted and real time RT-PCR performed to determine the mRNA expression levels of TH1 and TH17 associated cytokines. As expected, based on previous studies [33, 41], TH1 associated cytokine (IFN-γ, IL-12p40) mRNA expression was increased significantly (p≤0.05) (Fig. 1 A,B). In addition to TH1 immunity, mRNA expression of the TH17 associated cytokines IL-17, IL-6, TNF-α and IL-23p19 (Fig. 1 C-F) was also significantly elevated. We have shown that there is an increased CD4+ T cell infiltration into the colon during CsA therapy which was critical to disease induction [32]. To determine if TH1 and TH17 cytokine production was altered during the SGVHD induction period d0 to d21 post-BMT, colonic mRNA expression levels were determined by real time RT-PCR. At day 21 post-BMT colonic mRNA analysis showed that the TH17 associated cytokines IL-17, IL-6 and TNF-α were significantly increased in mice treated with CsA. Analysis of the TH1 associated cytokines at the same time point demonstrated an increased level of expression, although they were not significantly increased in comparison to control animals (data not shown).
Figure 1.
SGVHD mice showed increased colon mRNA expression level of TH1 and TH17 associated cytokines. Lethally irradiated C3H/HeN mice recipient mice were transplanted with aged matched ATBM cells (5 × 106) followed by a 21 day course of either 15mg/kg CsA in the diluent olive oil or the diluent alone. At the time of disease (d35-44) mice were euthanized and their colons removed. Colonic mRNA was then extracted and cDNA prepared. Samples were analyzed using real time RT-PCR for the primers IFN-γ, TNF-α, IL-12p40, IL-23p19, IL-6, and IL-17. Expression of these genes was normalized to GAPDH using the ΔΔCT method. Represents pooled data from 3 experiments. n = number of samples analyzed within each group.
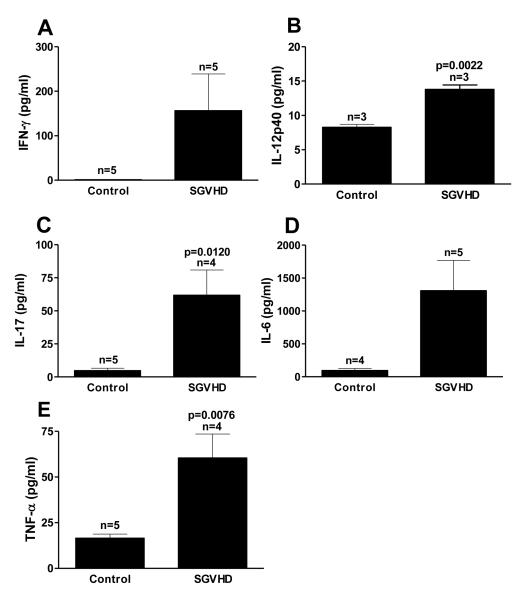
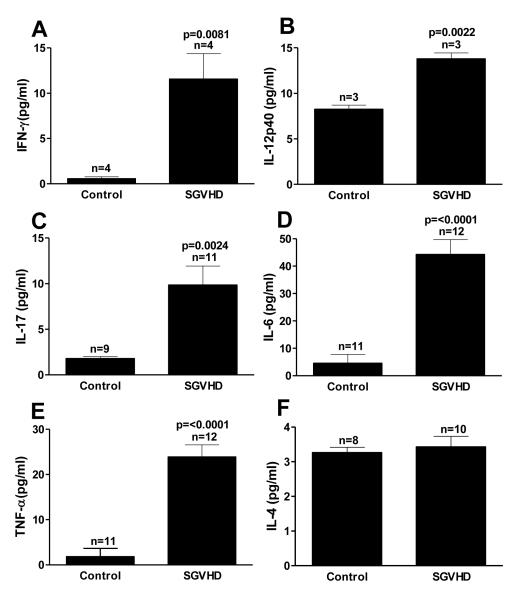
To determine if increased levels of TH1 and TH17 mRNA translated into increased production of TH1- and TH17-associated cytokines in the colons and systemically, colon explants and blood serum samples were taken from control and SGVHD mice and the cytokine levels determined. Culture supernatants obtained from the colons of diseased animals demonstrated a significant increase in the TH1 cytokines IFN-γ, and IL-12p40 compared to those isolated from control mice (Fig. 2 A, B). Significant increases in the production of IL-17, TNF-α and IL-6 were also observed (Fig. 2 C-E). Similarly, an increase in TH1 and TH17 cytokines were observed in diseased compared to control serum samples (Fig. 3) with significant increases seen in IFN-γ, IL-12p40, IL-17, IL-6 and TNF-α. We have previously shown that TH2 immunity was not increased in the SGVHD animal [34]. In line with those results, the TH2 cytokine IL-4, was not increased in the serum of diseased animals (Fig. 3F) suggesting that there was not a general increase in all TH immune responses during SGVHD. Thus, an enhanced production of TH1 and TH17 associated cytokines was observed at the mRNA and protein level in SGVHD versus control BMT animals.
Figure 2.
Increased production of TH1 and TH17 associated cytokines in colon explants of SGVHD mice. Colon tissue explants were cultured for 24 hours as described in the methods. The media was removed and the concentration of the cytokines IFN-γ (A), IL-12p40 (B), IL-17 (C), IL-6 (D) and TNF-α (E) was determined the Luminex 100™ system. Data is representative of 3 experiments. n = number of samples analyzed within each group.
Figure 3.
Increased TH1 and TH17 associated cytokines in serum of SGVHD diseased mice. To determine systemic concentrations of cytokines, blood was obtained by cardiac puncture at the time of active disease and the serum was isolated. The concentration of the cytokines IFN-γ (A), IL-12p40 (B), IL-17 (C), IL-6 (D),TNF-α (E) and IL-4 (F) in the isolated serum was determined using the Luminex 100™ system. Data is representative of 2 experiments. n = number of samples analyzed within each group.
3.2 Analysis of T cells from SGVHD mice for TH1 and TH17 T cell populations
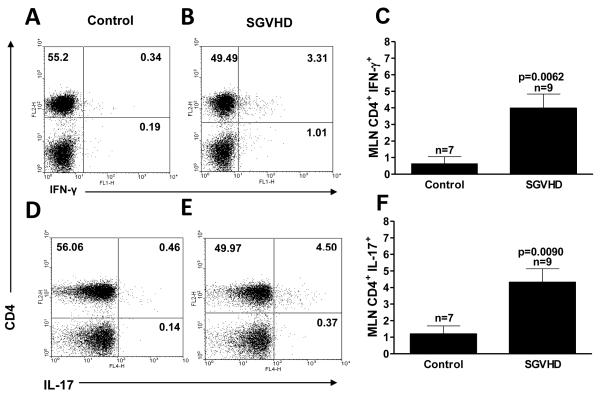
We have previously demonstrated that CD4+ T cells were responsible for the development of SGVHD [31, 32]. Given these findings, studies were undertaken to determine if CD4+ T cells producing TH1 and TH17 cytokines could be detected. Spleen and MLN cells were isolated from control BMT and SGVHD animals and analyzed by intracellular staining for IFN-γand IL-17 production (Fig. 4). In the spleen there was a significant (p≤0.05) increase in the CD4+ T cells producing IFN-γ(data not shown) in comparison to control mice. Cells isolated from the MLN of SGVHD mice demonstrated a significant increase in CD4+ T cells producing the cytokines IL-17 (Fig 4 E vs D), IFN-γ(Fig. 4B vs A) and TNF-α (data not shown) compared to lymphoid cells isolated from control animals (Fig 4 C, F). These findings demonstrate that TH1 and TH17 T cell populations were present in the peripheral lymphoid tissues during active disease.
Figure 4.
CD4+ T cells from SGVHD mice produced increased levels of TH1 and TH17 associated cytokines. 107 MLN cells from control and SGVHD animals were stimulated with10 μg/ml of αCD3 for 8 hours. During the last 4 hours of incubation 2 μM of monensin was added to block secretion of the cytokines from the cells. The cells were harvested, stained with anti-CD4 antibody followed by intracellular cytokines as described. Stained cells were analyzed by flow cytometry for 2 color analysis of CD4, IFN-γ, IL-17 and TNF-α. Results depicted in A, B and D, E is representative of 3 experiments. Data presented in C and F represents pooled samples from 2 experiments. n = number of samples analyzed within each group.
3.3 In vivo neutralization of IL-17A did not alter the induction of SGVHD
Data presented above demonstrated that TH1 and TH17 T cells and cytokines were elevated in tissues from SGVHD mice. Studies were undertaken to evaluate the role of the prototypic TH17 cytokine, IL-17 (IL-17A), in the development of SGVHD. Beginning on d21 post-BMT, groups of control and CsA treated mice received either, rat anti-mouse IL-17A mAb or control rat IgG i.p. for 3 days and then every other day for 2 weeks. This initial treatment protocol was based upon that utilized for studies analyzing the role of IL-12 and TNF-α in the SGVHD model [33, 34]. Based on a failure to alter clinical symptoms it was apparent that the administration of anti-mouse IL-17A mAb did not inhibit the induction of SGVHD (data not shown).
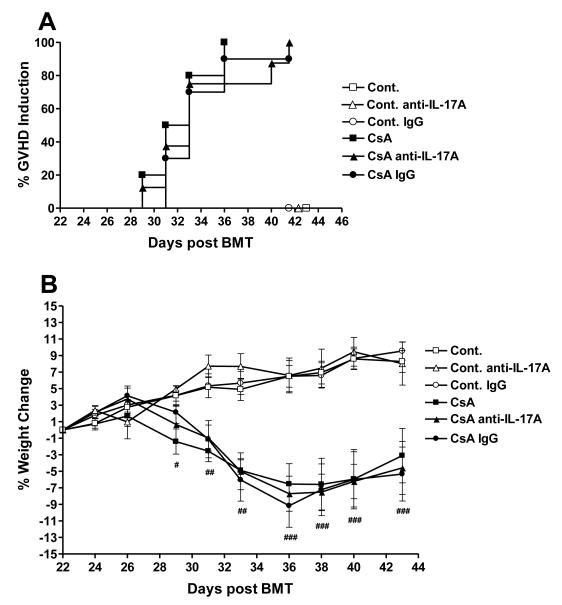
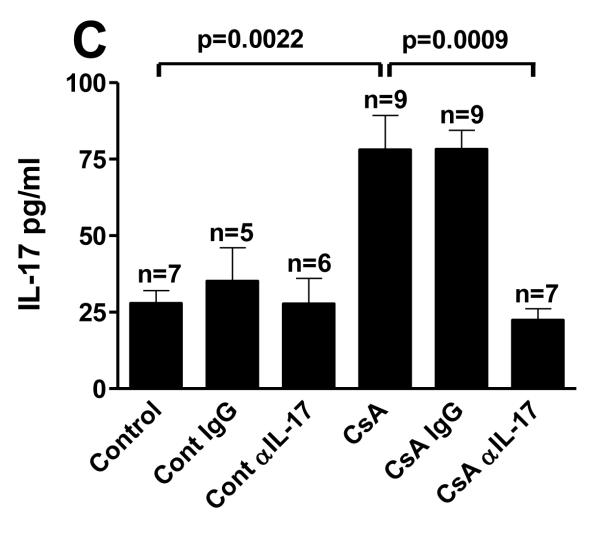
As anti-IL-17A mAb failed to alter SGVHD development using the initial treatment protocol, additional studies were performed in which the frequency of anti-IL-17A mAb therapy was increased throughout the post-CsA treatment period. Transplant control and CsA treated mice were injected with anti- IL-17A mAb or rat IgG for either 7 days beginning on day 21 post-BMT, then every other day for 1 week or for 14 consecutive days (data not shown). Increasing the frequency, and hence the amount of anti-IL-17A, did not alter the course of disease induction. The time course for induction (Fig. 5A), and disease-associated weight loss (Fig. 5B), was similar between the CsA treated and anti-IL-17A treated CsA animals. A significant difference was observed between control and CsA alone (p≤0.05) but there was no significant difference between CsA and CsA-anti-IL-17A-treated groups. This data demonstrated that the administration of anti-mouse IL-17A mAb did not prevent the onset or development of SGVHD. Since treatment with anti-IL-17A did not alter the induction of SGVHD it was possible that the mAb therapy failed to neutralize IL-17 in vivo. Given this possibility, the levels of IL-17A present in the serum isolated from control and SGVHD mice treated with the M210 anti-IL-17A antibody was measured. As shown in Figure 5C, in vivo therapy with the anti-IL-17A mAb significantly reduced the levels of circulating IL-17A in both control BMT and SGVHD mice two weeks after cessation of CsA therapy demonstrating that the antibody therapy was effective in limiting the levels of IL-17A in the treated animals.
Figure 5.
Anti-IL-17A therapy did not prevent the development of CsA-induced SGVHD. C3H/HeN mice were lethally irradiated (900cGy) and reconstituted with 5 × 106 ATBM from syngeneic aged-matched mice and treated for 21 days with 15mg/kg CsA. Control BMT or CsA-treated mice were then injected with 200μg of anti- IL-17 or control antibody for 7 days beginning 21 days post-BMT then every other day for 1 week. Mice were weighed individually 3 times a week and observed for symptoms of SGVHD (weight loss, diarrhea or mortality). (A) Induction of SGVHD was significantly different between BMT controls and and CsA-treated groups as determined by the log rank test (p≤0.05). (B) Percentage weight change from start of antibody treatment. Representative of 2 experiments. # p≤0.05, Control BMT (n=14) vs CsA (n=10); ## p≤0.05, Control BMT vs CsA, Control BMT anti-IL17 (n=6) vs CsA anti-IL-17A (n=8); ### p≤0.05, Control BMT vs CsA, Control anti-IL-17A vs CsA anti-IL-17A, Control Ig (n=6) vs CsA IgG (n=8). CsA vs CsA anti-IL-17A, p>0.05 at all time points. (C) In vivo anti-IL-17A therapy after CsA therapy neutralized serum levels of IL-17. Mice were induced for the development of SGVHD as described. Beginning on the last day of CsA therapy, mice were treated with 200μg/injection of anti-IL-17A mAb or control rat IgG for 7 consecutive days then every other day for an additional week. The mice were bled and the prepared serum analyzed for IL-17 by ELISA. Pooled data from 2 experiments. n = number of samples analyzed within each group.
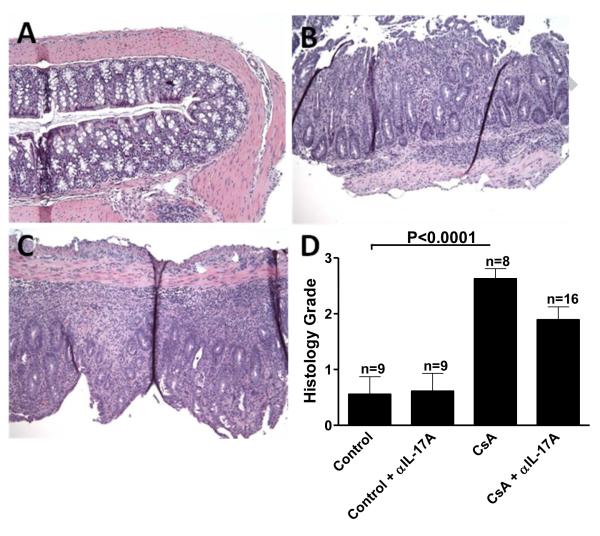
While the induction of SGVHD, based on clinical symptoms (weight loss, diarrhea), was not altered, it was important to determine if IL-17 neutralization resulted in changes in the scope or intensity of tissue pathology associated with disease. Colon or liver tissue samples were analyzed and graded for SGVHD pathology. Colon sections from the BMT control animals had normal colon architecture with no significant difference in the histology grades (Fig 6D) between BMT controls (Fig. 6A) or control animals treated with either anti-IL-17AmAb or control Ab (data not shown). Tissue sections from control SGVHD mice (Fig. 6B) or CsA-treated, control Ab-treated animals (data not shown) presented with dense inflammatory cell infiltration of the colonic mucosa of diseased animals. A slightly reduced pathology grade (Fig. 6D) that approached significance (p=0.052) was observed in the tissues from CsA-treated animals given anti-IL-17A (Fig. 6C) versus CsA treated SGVHD mice (Fig. 6). Similarly, neutralization of IL-17A also reduced the pathology grade in the liver of CsA-treated animals compared to SGVHD animals (p=0.04)(data not shown). Thus, anti-IL-17A treatment, while moderately reducing the pathology associated with the target tissues, did not significantly alter the induction of clinical symptoms of murine SGVHD.
Figure 6.
Anti-mouse IL-17A mAb did not alter SGVHD colon pathology. To monitor the pathology that developed in anti-mouse IL-17 treated SGVHD mice, colon tissue samples were removed from BMT control or SGVHD animals that were treated with anti-IL-17A mAb or control Ig. Colon tissue was graded for SGVHD pathology as previously described [40]. (A) BMT control samples had normal colon architecture with no significant difference between controls, or controls treated with either control Ab or anti-IL-17A mAb. Colon pathology grade (D) from SGVHD mice was significantly different from BMT control mice showing dense inflammatory cell infiltration of the colonic mucosa of diseased animals (B). There was no significant difference in the histology grade between SGVHD and SGVHD animals treated with anti-IL-17A mAb (C) or CsA treated control Ig mice (histology not shown). n= 4 to 7 mice per group from 2 experiments.
3.4 Neutralization of IL-17 resulted in increased production of IL-17F
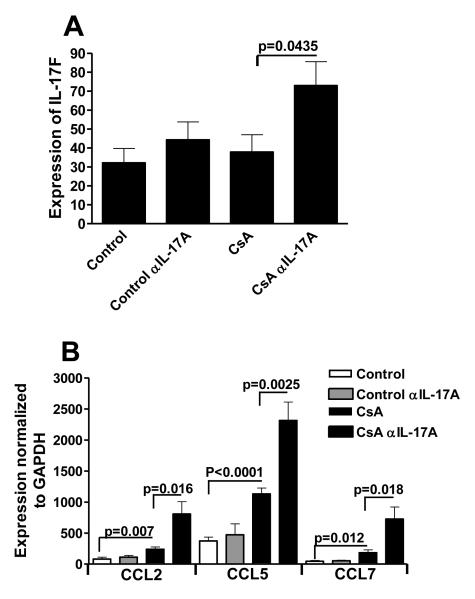
It has been shown that IL-17A negatively regulates the induction of IL-17F [42, 43]. As these cytokines have a high degree of homology and function, studies were initiated to monitor serum levels of IL-17F protein in control and anti-IL-17-treated animals. While the amount of IL-17F in the serum of anti-IL-17-treated CsA-treated animals was increased compared to those treated with CsA-alone, the levels were at the minimal limits of detection of the ELISA assay (~3pg/ml)(data not shown). Given these low levels of detectable IL-17F in the serum of transplanted animals, studies were performed to monitor changes in the mRNA for IL-17F in the colons of of SGVHD and anti-IL-17A-treated CsA-treated animals. As shown in Figure 7A, following neutralization of IL-17A, significantly increased mRNA levels of IL-17F was observed. In addition, IL-17F has been shown to induce the production of several mediators including CCL2,CCL5, and CCL7 [42]. In association with increased mRNA for IL-17F, increased production of mRNA for the chemokines CCL2, CCL5, and CCL7 was also observed (Fig. 7B). These studies demonstrated that treatment of mice with mAb against IL-17A in the immediate post-CsA treatment period effectively neutralized IL-17A and led to significantly reduced circulating levels of the cytokine and measurable downstream effects resulting in increased production of IL-17F in the colon of the CsA-induced antibody-treated animals.
Figure 7.
Neutralization of IL-17 resulted in increased mRNA for IL-17F and downstream mediators. Animals were induced for SGVHD as described. The animals were treated with anti-IL-17A mAb or control rat IgG for 14 days. Colon tissue was removed from these animals and was analyzed by real time PCR for the presence of IL-17F (A), CCL2, CCL5 and CCL7 (B). Expression of these genes was normalized to GAPDH using the ΔΔCT method. Represents pooled data from 2 experiments. n =7.
4. DISCUSSION
Many studies have been conducted with the emphasis on identifying the involvement of particular T helper cell populations and associated cytokine production in the development of chronic inflammation with the objective of identifying therapeutic targets for future treatments. Before the discovery of the role that TH17 T cells played in inflammation we reported that the development of SGVHD-associated colon inflammation involved TH1 immunity and was inhibited by in vivo neutralization of IL-12p40 [33]. It has now been shown that IL-23, a cytokine responsible for maintaining the effector function of TH17 T cells, shares the IL-12 p40 subunit with IL-12 [14, 44]. Therefore it was unclear whether anti-IL-12p40 therapy blocked a TH1 or TH17 immune response during inhibition of SGVHD. The current study was designed to determine if TH17 immunity was elevated and if the prototypic TH17 cytokine, IL-17 (IL-17A), participated in, or regulated the development of, murine SGVHD. Although TH17-associated cytokines were significantly increased systemically and in the colon of SGVHD mice, neutralization of IL-17 failed to prevent the development of clinical symptoms and pathology associated with murine SGVHD. However, concurrent with a decrease in IL-17 in the mAb-treated animals was an increase in the production of IL-17F, a related IL-17 cytokine family member with similar function. These findings suggest that TH17 immunity was increased and that a redundancy in the effector function of IL-17 family members likely exists in the SGVHD animal.
It has been shown that CD4+ T cells and TH1 associated cytokines were associated with the development of murine SGVHD [32-34] and CD4+ T cells from SGVHD mice could adoptively transfer disease into irradiated secondary recipients [31]. Recently a unique T cell subset, termed TH 17, has been associated with the production of the cytokines IL-17, TNF-α and IL-6. The effector function of TH17 cells is IL-23 dependent and since IL-23 and IL-12 share the same IL-12p40 subunit, the question has been raised as to whether the inflammatory disorders which have previously been termed TH1 have TH17 involvement. Before the understanding of the shared IL-12p40 subunit we had demonstrated that IL-12p40 neutralization inhibited the development of SGVHD and that SGVHD was mediated by a TH1 immune response [33, 34]. To understand the role of IL-17 and TH17 T cells in murine SGVHD the level of TH17 T cells and associated cytokines were determined. It was shown that CD4+ TH17 T cells (IL-17 producing) were increased in the periphery of SGVHD animals. Similarly, a significant increase in TH17-associated cytokines (IL-17, IL-6, IL-23 and TNF-α) was also present in the colons and periphery of diseased animals. However, consistent with previous studies in the SGVHD model, TH1-associated immunity was also significantly elevated as well [33, 34]. To determine the role of the prototypic TH17 cytokine, IL-17 (IL-17A), in the development of SGVHD, in vivo neutralization studies were performed. Mice that had been treated with CsA and anti-mouse IL-17A mAb developed clinical symptoms (weight loss and diarrhea) at a similar rate to animals treated with CsA alone or CsA and control Ab, with tissue pathology at a slightly reduced level. It has been shown that IL-17 negatively regulated the production of IL-17F [43]. Furthermore, it was demonstrated in an IBD model that IL-17 and IL-17F had redundant function; depletion of both cytokines was required to inhibit disease suggesting that these cytokines had a redundant and pathologic role in colonic inflammation [45]. Concomitant with the decrease in IL-17 was an increase in the related IL-17F and chemokines that have been shown to be regulated by IL-17F [42].
It has been shown in several different animal models of inflammation that both TH17 and TH1 immunity are present, including allogeneic GVHD [30, 46, 47] and autoimmune models [48, 49]. The relative contribution of TH17 and TH1 immunity varied in these models. Research by Lohr et. al. using a model of systemic autoimmune disease similar to GVHD, inferred that the TH17 response occurred early, by day 10 after initiation of disease, that gave way to a TH1 immune response [48]. Eliminating the TH1 response through the use of effector cells from IFN-γ−/− or T-bet −/− mice did not ameliorate disease. These findings indicated that in the absence of a TH1 response, TH17 immunity became the driving force for disease development. Analysis of SGVHD colon mRNA expression levels at 21 days post-BMT demonstrated that there was a significant increase in the TH17 associated cytokines IL-17, IL-6 and TNF-α in CsA treated mice. TH1 associated cytokines IFN-γand IL-12p40 were elevated, but not significantly different from control mice at this time. It could be inferred from these results, along with research into the systemic autoimmune disease [48], that the colitis observed in murine SGVHD may not be a result of a single immune response but encompassing both TH17 and TH1 CD4+ T cells.
The actual role of IL-17 in the pathophysiology of intestinal inflammation remains uncertain. It has been suggested that IL-17 is important in the regulation of tight junctions between epithelial cells in the colon [50]. Through the use of IL-17−/− donor animals, T cell production of IL-17 appears not to be a requirement for colonic inflammation in the T cell transfer model of colitis [50, 51]. Similarly, IL-17 neutralization had no effect on the generation of colitis following the transfer of naïve CD4+ T cells into Rag−/− recipients [52]. Neutralization of both IL-6 and IL-17 did abolish the development of colon inflammation suggesting that neutralization of IL-6 has a beneficial effect on TH17-induced colitis. Alternatively, the administration of anti-IL-17A mAb in the T cell independent DSS colitis model enhanced disease [28]. When allogeneic GVHD was analyzed, both TH17 and TH1 have been shown to participate in the disease process. Removal of IFN-γenhanced allogeneic GVHD [53, 54]. However, it was also demonstrated that TH17 cells, through the production of IL-17, modulated development of TH1 immunity during GVHD [30, 47]. Similar to the DSS colitis model, in the absence of IL-17, enhanced TH1-mediated disease occurred, suggesting that IL-17 played an anti-inflammatory role in the initiation and progression of inflammation. IL-17 appeared not to play a protective role in the development of SGVHD as the disease course and pathology was not enhanced in anti-IL-17-treated animals. In fact, the tissue pathology associated with the development of SGVHD was slightly reduced in the anti-IL-17A-treated mice.
The present study was designed to determine the role of TH17 cells and the prototypic TH17 cytokine, IL-17, in the induction of SGVHD. The observed findings in the SGVHD model demonstrated that while significant increases in TH17 immunity was observed in diseased animals, there was no alteration in the induction of SGVHD or severity of colitis following treatment with anti-IL-17A mAb. Given the redundancy in the production of IL-17/IL-17F in the SGVHD model, a protective role for IL-17 was not observed. Future studies will be required to determine the levels of regulation between TH17 and TH1 immunity and the role of IL-17 family members in this inducible model of chronic inflammation.
5. ACKNOWLEDGEMENTS
This work supported by National Institutes of Health Grant P01 CA092372 (JSB).
ABBREVIATIONS
- BMT
Bone Marrow Transplantation
- CsA
Cyclosporine A
- GVHD
Graft-versus-host disease
- IBD
inflammatory bowel disease
- MLN
mesenteric lymph nodes
- RT PCR
Real Time Reverse transcriptase Polymerase Chain Reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. REFERENCES
- [1].Lillehoj H, Malek T, Shevach E. Differential effects of cyclosporin-A on the expression of T and B lymphocyte activation antigens. Journal of Immunology. 1984;133:244–250. [PubMed] [Google Scholar]
- [2].Kahan B. Cyclosporine. New England Journal of Medicine. 1989;21:1725–1738. doi: 10.1056/NEJM198912213212507. [DOI] [PubMed] [Google Scholar]
- [3].Green C. Experimental transplantation and cyclosporine. Transplantation. 1988;46:402–406. doi: 10.1097/00007890-198808001-00001. [DOI] [PubMed] [Google Scholar]
- [4].Bryson JS, Jennings CD, Caywood BE, Kaplan AM. Induction of a syngeneic graft-versus-host disease-like syndrome in DBA/2 mice. Transplantation. 1989;48:1042–7. doi: 10.1097/00007890-198912000-00030. [DOI] [PubMed] [Google Scholar]
- [5].Bryson JS, Jennings CD, Caywood BE, Kaplan AM. Strain specificity in the induction of syngeneic graft-versus-host disease in mice. Transplantation. 1991;51:911–3. [PubMed] [Google Scholar]
- [6].Glazier A, Tutschka PJ, Farmer ER, Santos GW. Graft-versus-host disease in cyclosporin A-treated rats after syngeneic and autologous bone marrow reconstitution. J Exp Med. 1983;158:1–8. doi: 10.1084/jem.158.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cheney RT, Sprent J. Capacity of cyclosporine to induce auto-graft-versus-host disease and impair intrathymic T cell differentiation. Transplantation Proceedings. 1985;17:528–30. [Google Scholar]
- [8].Bucy RP, Xu XY, Li J, Huang G. Cyclosporin A-induced autoimmune disease in mice. J Immunol. 1993;151:1039–50. [PubMed] [Google Scholar]
- [9].Bryson JS, Jennings CD, Caywood BE, Kaplan AM. Thy1+ bone marrow cells regulate the induction of murine syngeneic graft-versus-host disease. Transplantation. 1993;56:941–5. doi: 10.1097/00007890-199310000-00031. [DOI] [PubMed] [Google Scholar]
- [10].Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–31. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- [11].Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–4. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- [12].Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–76. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- [13].Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006;24:519–40. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- [14].Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, Shellito JE, Schurr JR, Bagby GJ, Nelson S, Kolls JK. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol. 2003;170:4432–6. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–6. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- [17].Veldhoen M, Stockinger B. TGFbeta1, a “Jack of all trades”: the link with pro-inflammatory IL-17-producing T cells. Trends Immunol. 2006;27:358–61. doi: 10.1016/j.it.2006.06.001. [DOI] [PubMed] [Google Scholar]
- [18].Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- [19].Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- [20].Reiner SL. Development in motion: helper T cells at work. Cell. 2007;129:33–6. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- [21].Chabaud M, Fossiez F, Taupin JL, Miossec P. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J Immunol. 1998;161:409–14. [PubMed] [Google Scholar]
- [22].Linden A. Role of interleukin-17 and the neutrophil in asthma. Int Arch Allergy Immunol. 2001;126:179–84. doi: 10.1159/000049511. [DOI] [PubMed] [Google Scholar]
- [23].Rohn TA, Jennings GT, Hernandez M, Grest P, Beck M, Zou Y, Kopf M, Bachmann MF. Vaccination against IL-17 suppresses autoimmune arthritis and encephalomyelitis. Eur J Immunol. 2006;36:2857–67. doi: 10.1002/eji.200636658. [DOI] [PubMed] [Google Scholar]
- [24].Koenders MI, Lubberts E, Oppers-Walgreen B, van den Bersselaar L, Helsen MM, Di Padova FE, Boots AM, Gram H, Joosten LA, van den Berg WB. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am J Pathol. 2005;167:141–9. doi: 10.1016/S0002-9440(10)62961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:382–8. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- [26].Holtta V, Klemetti P, Sipponen T, Westerholm-Ormio M, Kociubinski G, Salo H, Rasanen L, Kolho KL, Farkkila M, Savilahti E, Vaarala O. IL-23/IL-17 immunity as a hallmark of Crohn’s disease. Inflamm Bowel Dis. 2008;14:1175–1184. doi: 10.1002/ibd.20475. [DOI] [PubMed] [Google Scholar]
- [27].Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]
- [29].O’Connor W, Jr., Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–9. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yi T, Zhao D, Lin CL, Zhang C, Chen Y, Todorov I, LeBon T, Kandeel F, Forman S, Zeng D. Absence of donor Th17 leads to augmented Th1 differentiation and exacerbated acute graft-versus-host disease. Blood. 2008;112:2101–10. doi: 10.1182/blood-2007-12-126987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bryson JS, Jennings CD, Brandon JA, Perez J, Caywood BE, Kaplan AM. Adoptive transfer of murine syngeneic graft-vs.-host disease by CD4+ T cells. J Leukoc Biol. 2007;82:1393–400. doi: 10.1189/jlb.0307183. [DOI] [PubMed] [Google Scholar]
- [32].Bryson JS, Zhang L, Goes SW, Jennings CD, Caywood BE, Carlson SL, Kaplan AM. CD4+ T cells mediate murine syngeneic graft-versus-host disease-associated colitis. J Immunol. 2004;172:679–87. doi: 10.4049/jimmunol.172.1.679. [DOI] [PubMed] [Google Scholar]
- [33].Flanagan DL, Gross R, Jennings CD, Caywood BE, Goes S, Kaplan AM, Bryson JS. Induction of syngeneic graft-versus-host disease in LPS hyporesponsive C3H/HeJ mice. J Leukoc Biol. 2001;70:873–80. [PubMed] [Google Scholar]
- [34].Flanagan DL, Jennings CD, Bryson JS. Th1 cytokines and NK cells participate in the development of murine syngeneic graft-versus-host disease. J Immunol. 1999;163:1170–7. [PubMed] [Google Scholar]
- [35].Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods. 2001;25:386–401. doi: 10.1006/meth.2001.1261. [DOI] [PubMed] [Google Scholar]
- [36].Cho ML, Kang JW, Moon YM, Nam HJ, Jhun JY, Heo SB, Jin HT, Min SY, Ju JH, Park KS, Cho YG, Yoon CH, Park SH, Sung YC, Kim HY. STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J Immunol. 2006;176:5652–61. doi: 10.4049/jimmunol.176.9.5652. [DOI] [PubMed] [Google Scholar]
- [37].Yamaguchi Y, Fujio K, Shoda H, Okamoto A, Tsuno NH, Takahashi K, Yamamoto K. IL-17B and IL-17C are associated with TNF-alpha production and contribute to the exacerbation of inflammatory arthritis. J Immunol. 2007;179:7128–36. doi: 10.4049/jimmunol.179.10.7128. [DOI] [PubMed] [Google Scholar]
- [38].Varona R, Cadenas V, Gomez L, Martinez AC, Marquez G. CCR6 regulates CD4+ T-cell-mediated acute graft-versus-host disease responses. Blood. 2005;106:18–26. doi: 10.1182/blood-2004-08-2996. [DOI] [PubMed] [Google Scholar]
- [39].Miyazaki D, Nakamura T, Ohbayashi M, Kuo CH, Komatsu N, Yakura K, Tominaga T, Inoue Y, Higashi H, Murata M, Takeda S, Fukushima A, Liu FT, Rothenberg ME, Ono SJ. Ablation of type I hypersensitivity in experimental allergic conjunctivitis by eotaxin-1/CCR3 blockade. Int Immunol. 2009;21:187–201. doi: 10.1093/intimm/dxn137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bryson JS, Jennings CD, Lowery DM, Carlson SL, Pflugh DL, Caywood BE, Kaplan AM. Rejection of an MHC class II negative tumor following induction of murine syngeneic graft-versus-host disease. Bone Marrow Transplant. 1999;23:363–72. doi: 10.1038/sj.bmt.1701557. [DOI] [PubMed] [Google Scholar]
- [41].Flanagan DM, Jennings CD, Goes SW, Caywood BE, Gross R, Kaplan AM, Bryson JS. Nitric oxide participates in the intestinal pathology associated with murine syngeneic graft-versus-host disease. J Leukoc Biol. 2002;72:762–8. [PubMed] [Google Scholar]
- [42].Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z, Dong C. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–75. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].von Vietinghoff S, Ley K. IL-17A controls IL-17F production and maintains blood neutrophil counts in mice. J Immunol. 2009;183:865–73. doi: 10.4049/jimmunol.0804080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Becker C, Dornhoff H, Neufert C, Fantini MC, Wirtz S, Huebner S, Nikolaev A, Lehr HA, Murphy AJ, Valenzuela DM, Yancopoulos GD, Galle PR, Karow M, Neurath MF. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis. J Immunol. 2006;177:2760–4. doi: 10.4049/jimmunol.177.5.2760. [DOI] [PubMed] [Google Scholar]
- [45].Leppkes M, Becker C, Ivanov, Hirth S, Wirtz S, Neufert C, Pouly S, Murphy AJ, Valenzuela DM, Yancopoulos GD, Becher B, Littman DR, Neurath MF. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136:257–67. doi: 10.1053/j.gastro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- [46].Chen X, Vodanovic-Jankovic S, Johnson B, Keller M, Komorowski R, Drobyski WR. Absence of regulatory T-cell control of TH1 and TH17 cells is responsible for the autoimmune-mediated pathology in chronic graft-versus-host disease. Blood. 2007;110:3804–13. doi: 10.1182/blood-2007-05-091074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kappel LW, Goldberg GL, King CG, Suh DY, Smith OM, Ligh C, Holland AM, Grubin J, Mark NM, Liu C, Iwakura Y, Heller G, van den Brink MR. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood. 2009;113:945–52. doi: 10.1182/blood-2008-08-172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med. 2006;203:2785–91. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kelchtermans H, Schurgers E, Geboes L, Mitera T, Van Damme J, Van Snick J, Uyttenhove C, Matthys P. Effector mechanisms of interleukin-17 in collagen-induced arthritis in the absence of interferon-gamma and counteraction by interferon-gamma. Arthritis Res Ther. 2009;11:R122. doi: 10.1186/ar2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kinugasa T, Sakaguchi T, Gu X, Reinecker HC. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001–11. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- [51].Noguchi D, Wakita D, Tajima M, Ashino S, Iwakura Y, Zhang Y, Chamoto K, Kitamura H, Nishimura T. Blocking of IL-6 signaling pathway prevents CD4+ T cell-mediated colitis in a T(h)17-independent manner. Int Immunol. 2007;19:1431–40. doi: 10.1093/intimm/dxm114. [DOI] [PubMed] [Google Scholar]
- [52].Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–6. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Murphy WJ, Welniak LA, Taub DD, Wiltrout RH, Taylor PA, Vallera DA, Kopf M, Young H, Longo DL, Blazar BR. Differential effects of the absence of interferon-gamma and IL-4 in acute graft-versus-host disease after allogeneic bone marrow transplantation in mice. J Clin Invest. 1998;102:1742–8. doi: 10.1172/JCI3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yang YG, Dey BR, Sergio JJ, Pearson DA, Sykes M. Donor-derived interferon gamma is required for inhibition of acute graft-versus-host disease by interleukin 12. J Clin Invest. 1998;102:2126–35. doi: 10.1172/JCI4992. [DOI] [PMC free article] [PubMed] [Google Scholar]