Abstract
People at risk of falling exhibit increased gait variability, which may predict future falls. However, the causal mechanisms underlying these correlations are not well known. Increased neuronal noise associated with aging likely leads to increased gait variability, which could in turn lead to increased fall risk. This paper presents a model of how changes in neuromuscular noise independently affect gait variability and probability of falling, and aims to determine the extent to which changes in gait variability directly predict fall risk. We used a dynamic walking model that incorporates a lateral step controller to maintain lateral stability. Noise was applied to this controller to approximate neuromuscular noise in humans. Noise amplitude was varied between low amplitudes that did not induce falls and high amplitudes for which the model always fell. With increasing noise amplitude, the model fell more often and after fewer steps. Gait variability increased with noise amplitude and predicted increased probability of falling. Importantly, these relationships were not linear. At either low gait variability or very high gait variability, small increases in noise and variability affected probability of falling very little. Conversely, at intermediate noise and/or variability levels, the same small increases resulted in large increases in probability of falling. Our results validate the idea that age-related increases in neuromuscular noise likely play a direct contributing role in increasing fall risk. However, neuromuscular noise remains only one of many important factors that need to be considered. These findings have important implications for fall prevention research and practice.
Keywords: dynamic walking, gait, neuromuscular noise, variability, falling, elderly
1. Introduction
The risk of falling while walking increases with age (Tinetti et al., 1988) and people with high fall risk exhibit increased gait variability (Maki, 1997; Hausdorff et al., 2001; Owings and Grabiner, 2004; Richardson et al., 2005). Increased gait variability may predict future falls (Maki, 1997; Hausdorff et al., 2001; Brach et al., 2010). Recently, Brach et al. (2010) estimated the clinically relevant changes in gait variability that predicted impaired walking mobility in older adults. However, it remains unclear if this increased variability directly causes these falls. Different studies have yielded conflicting results as to which gait variability measures best predict fall risk (Owings and Grabiner, 2004; Brach et al., 2005; Moe-Nilssen and Helbostad, 2005). Furthermore, there are no theoretical reasons why such correlations should exist. Our group previously showed that gait variability was not correlated with local dynamic stability during walking (Dingwell et al., 2001; Dingwell and Marin, 2006). Therefore, the underlying causal mechanisms for correlations between gait variability and fall risk remain undetermined.
Neuronal noise increases with ageing (Shaffer and Harrison, 2007), due to many factors. With age, the number of motor units decreases and their size increases (Johnson and Duberley, 1998; Fling et al., 2009; Ling et al., 2009), creating more discretized force generation. Muscle spindle and proprioceptor function deteriorates, thereby degrading sensory information accuracy (Shaffer and Harrison, 2007). Nerve conduction velocities decrease (Kim et al., 2007), increasing time delays. These age-related increases in neuronal noise likely lead to increased kinematic gait variability. The resulting consequences, however, are not immediately obvious because kinematic variability reflects the summed effects of both small perturbations experienced while walking and the neural control actions taken to respond to them. While these control actions may reduce variability of certain gait variables, they can also increase variability in other variables (Cusumano and Cesari, 2006; Dingwell et al., 2010). Thus, increased gait variability could indicate either increased or possibly decreased fall risk, depending on which variable is being examined. Thus, a clearer understanding of how these factors interrelate is required.
The relationships between neuromuscular noise amplitude, gait variability and probability of falling are difficult to investigate experimentally. Neuromuscular or control noise cannot easily be directly manipulated in humans. Also, experiments requiring the many perturbations needed to assess probability of falling are prohibitive. However, these experimental limitations can be overcome using simulations. Requirements for such a model are that it incorporates neuromuscular noise and walks dynamically (i.e. can walk and/or fall over without tracking some pre-specified trajectory). Su and Dingwell (2007) and Byl and Tedrake (2009) applied external perturbations to dynamic walking models by having them walk over stochastically uneven terrain and investigated their variability and probability of falling, respectively. Variability and probability of falling both increased when the magnitudes of the imposed perturbations increased. These studies did not, however, investigate neuronal control. Dean and Kuo (2007) showed that noisier neural control associated with aging increased step variability. They did not, however, vary neuronal noise amplitudes systematically, nor did they directly quantify fall risk.
This study determined how neuromuscular noise amplitude affects gait variability and probability of falling, and the extent to which changes in gait variability might predict fall risk. We hypothesized that with increasing neuromuscular noise amplitude: 1) probability of falling would increase, 2) the number of steps taken before falling would decrease and 3) kinematic variability would increase. We also hypothesized that 4) gait variability would significantly predict fall risk in our model. Fall risk might increase smoothly and monotonically with increased gait variability. Conversely, perhaps a hard threshold exists such that slow, smooth changes in variability yield sharp changes in fall risk, making variability a poor predictor of fall risk.
2. Methods
We replicated a 3D dynamic walking model described by Kuo (1999). The model is described briefly below. Additional details are provided in the Supplement. Although simple, this model exhibits a substantially human-like gait. It was implemented in Matlab (Mathworks, R2008a) and modified to simulate multiple consecutive steps and incorporate controller noise. The model had two rigid legs with semi-circular feet attached by a variable splay angle (φ) to the pelvis (Fig. 1, Table 1). The model’s configuration was defined by a stance leg angle (θSt), swing leg angle (θ Sw), and roll angle (θRoll). Thus, the primary state variables of the model were:
| (1) |
Figure 1.
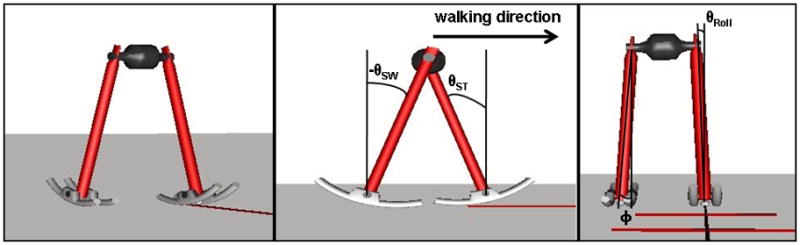
Graphical representation of the 3D dynamic walking model. The figure on the left is a frontal-lateral view. The middle figure is a lateral view with the front foot as the stance foot and the angles of the swing leg (θSw), stance leg (θSt) and walking direction indicated. The right figure is a frontal view with the leg splay angle (φ) and lateral roll angle (θRoll) indicated. The slope of the ground is ignored in this figure.
Table 1.
Model parameters, their definitions, and values or ranges (as applicable).
| Term | Definition | Value/range |
|---|---|---|
| δcont | Noise term added to the lateral step controller at an individual step | −24×10−5 to 24×10−5 |
| ω | Angular velocity if the lateral step adjustment were achieved not instantaneously but over the full stride time and by the most direct path | |
| IL | Moment of inertia of the swing limb | 0.0696 |
| jnoise | Maximum noise amplitude within each noise level | −24×10−5 to 24×10−5 |
| L | Leg length | 1 |
| φ | Splay angle | |
| R | Foot radius | 0.3L |
| W | Length of pelvis segment | 0.3L |
Including θRoll enabled lateral movement of the model, creating lateral instability that required active control (Kuo, 1999). A controller was therefore implemented to make lateral step adjustments that approximated the accumulated control action over a complete step; i.e. φ was adjusted at each ground contact to return the state variables as close as possible to their limit cycle values.
Initial conditions on the stable limit cycle were estimated from Kuo (1999) and further optimized for a moderate walking speed (0.94 m/s for L = 1 m). A single ‘noise free’ simulation was performed and the state variables (Eq. 1) at foot strike from this trial were used by the controller as a reference.
Noise was applied to the lateral step controller, comparable to the neuromotor output noise present in humans. After the lateral step controller determined the optimal change in φ required, small errors (δcont) were added to each change in φ. Sequential values of δcont were chosen as uniformly distributed random numbers with maximum amplitude ± jnoise. Each perturbed φ produced deviations in all six state variables. Thus, kinematic variability in the model outputs resulted from the direct interaction of both the controller’s corrections for prior deviations and the applied noise.
We ran multiple sets of simulations where jnoise was varied between small amplitudes that did not make the model fall over and large amplitudes for which the model always fell over. For each jnoise, 100 walking trials were simulated. Each simulation was run until either the model fell over or until it walked 125 consecutive steps. This reflects walking behavior in humans, where 90.5% of walking bouts are less than 100 steps (Orendurff et al., 2008). For each jnoise, the probability of falling (%Fall) was computed as the percentage of the 100 trials where the model fell after ≤ 125 steps. The average number of steps to falling (STF) was calculated across all 100 trials. STF was set to 125 steps when the model did not fall over.
The minimum “energy input” required for the lateral step adjustment was proportional to Econt:
| (2) |
where IL was the moment of inertia of the swing limb and ω was the average angular velocity that would have been required to enact the lateral adjustment over the full stride and by the most direct path:
| (3) |
with tn the time at stride n, and tn−1 the time at the previous stride. This provided a relative measure of the effort required to enact control and maintain dynamic stability.
Step length (SL) and step width (SW) were calculated at the end of each step as the distance between the feet in the sagittal and frontal planes, respectively. Step time (ST) was calculated as the time between each foot contact. Step variability was calculated as the standard deviations of step length (SDSL), step width (SDSW) and step time (SDST).
Kinematic state variability was calculated by extracting the data for each individual stride (based on the instant of foot contact), time-normalizing each state variable to 0–100% stride, calculating the standard deviation at each percent and computing the mean of these standard deviations (Dingwell and Marin, 2006):
| (4) |
Where 〈·〉denotes the average over all values of i and q ∈ {θRoll, θSt, θSw, θ̇Roll;, θ̇St, θ̇Sw}. These variables were combined to calculate MSD(θTot), computed as the length of the vector containing all six individual MSD measures.
To exclude potential fall dynamics, the last five steps of each simulation were excluded in the variability and Econt calculations. Since the number of trials where the model did not fall over varied for the different noise amplitudes, average gait variability and Econt data were calculated for the first 20 trials for which the model walked ≥55 steps. Trials where the model fell before ≥55 steps were excluded, as using too few steps could yield inaccurate results. Excluding these trials had minimal or no effect on our final outcomes. Because we imposed uniformly distributed random noise, the model would behave similar to the trials where the model did not fall over. Only the fall dynamics that were excluded from the analysis would differ.
Correlations between step variability and kinematic state variability and %FALL or STF were assumed to be sigmoidal based on visual inspection:
| (5) |
with c = 100 for correlations with %FALL and c = 125 for correlations with STF. Sigmoidal functions (Eq. 5) were fit using ‘lsqcurvefit’ in Matlab. Correlation coefficients (r) between fits and data were calculated using ‘corrcoef’. Variance accounted for (r2) and statistical significance (p) were reported for each relationship.
3. Results
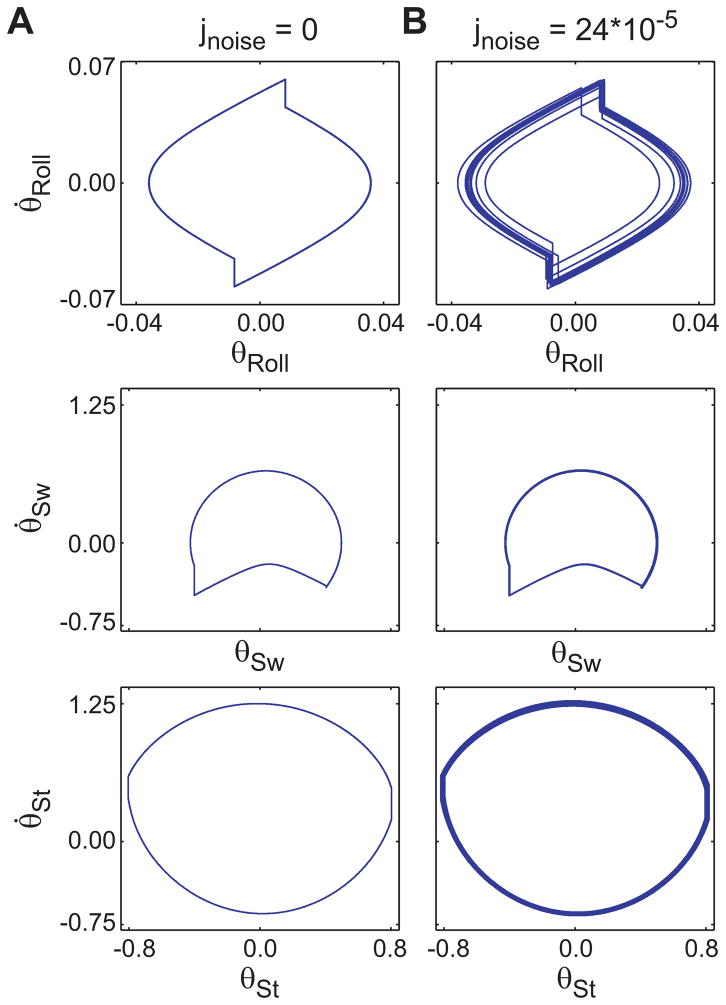
Phase portraits for trials with controller noise (e.g. Fig. 2b) exhibited greater movement variations than the ‘noise free’ trial (Fig. 2a). The maximum noise level where the model could still walk 125 steps at least once was jnoise = 24×10−5 (%FALL = 99%; Fig. 3a).
Figure 2.
Phase portraits for the six state variables, ([θRoll, θ̇Roll;], [θSw, θ̇Sw], [θSt,, θ̇St]) of the walking model both (a) without noise and (b) with jnoise = 24×10−5. All three phase planes became more variable when noise was applied to the controller. Note that the scales for the top two plots are different from those of the bottom four plots. The [θRoll, θ̇Roll;] phase plane appeared to exhibit the largest response to the added noise. However, the scale of this graph is smaller and the overall increase in variability was of similar magnitude as for the other state variables (Fig. 5).
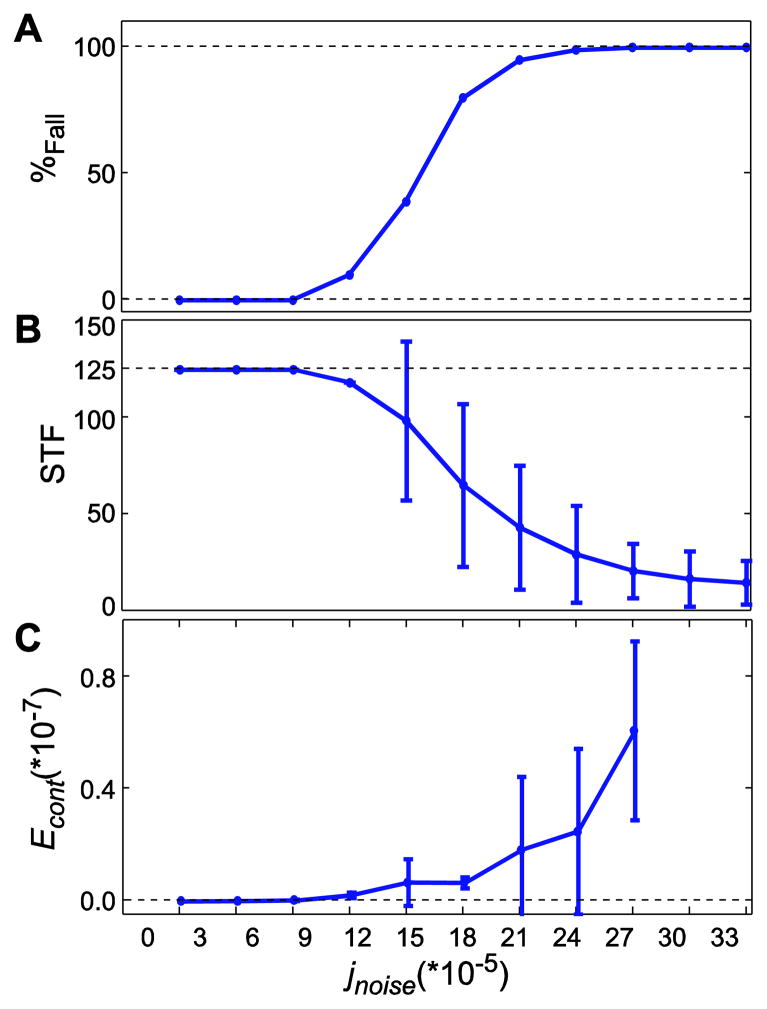
Figure 3.
(A) Probability of falling (%Fall), (B) average number of steps taken before falling (STF), and (C) minimum mechanical work required by the controller (Econt), each plotted against noise amplitude (jnoise). In B and C, error bars indicate between-trial ±1 standard deviations. Both %FALL and STF were calculated up to jnoise = 33×10−5. Econt was only calculated up to jnoise = 27×10−5 because at least 55 steps were required to calculate Econt and the model fell over sooner in most cases for the higher jnoise values. As jnoise was increased, the model fell over more often (A) and after fewer steps (B), and the amount of work the controller did to try to prevent those falls increased approximately exponentially (C).
Probability of falling increased from 0% at jnoise≤9×10−5 to 100% at jnoise≥27×10−5 (Fig. 3a). The number of steps to falling (STF) decreased for jnoise > 9×10−5 and continued decreasing, even after %Fall reached 100% (Fig. 3b). This confirmed our first two hypotheses that increased neuronal noise amplitudes (jnoise) would make the model fall more often (increased %Fall) and sooner (fewer STF). The equivalent energy required by the controller (Econt) also increased with jnoise and began increasing faster at jnoise > 9×10−5, where %Fall > 0% (Fig. 3c).
Step variability and kinematic state variability both increased with controller noise amplitude (Figs. 4–5). At low noise amplitudes, small increases in jnoise produced small or no increases in step variability. At intermediate jnoise (near where the model first started falling over), the same small increases in jnoise resulted in large increases in step variability. At high jnoise values, further increases had little or no effect as step variability approached its maximum limit. This partly confirmed our third hypothesis that kinematic gait variability would increase with increased neuronal noise amplitudes (jnoise).
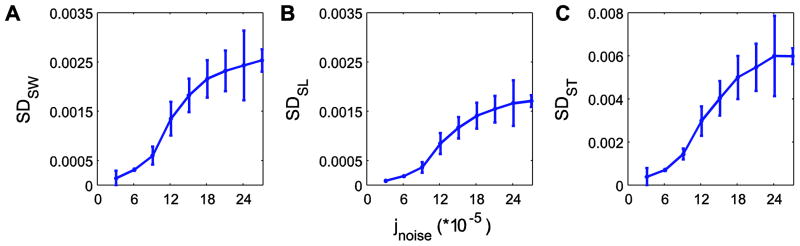
Figure 4.
Mean within-trial variability for (A) step width (SDSW), (B) step length (SDSL) and (C) step time (SDST), each plotted against noise amplitude (jnoise). Error bars indicate between-trial ±1 standard deviations. For the highest noise level (jnoise = 27×10−5), additional simulations beyond the first 100 were performed to obtain 20 trials of at least 55 steps, as the model fell over after fewer steps at this noise level. For all measures, step variability increased most rapidly at intermediate jnoise levels and exhibited smaller incremental changes at the lowest and highest jnoise levels, where %Fall (Fig. 3A) was either very low or very high.
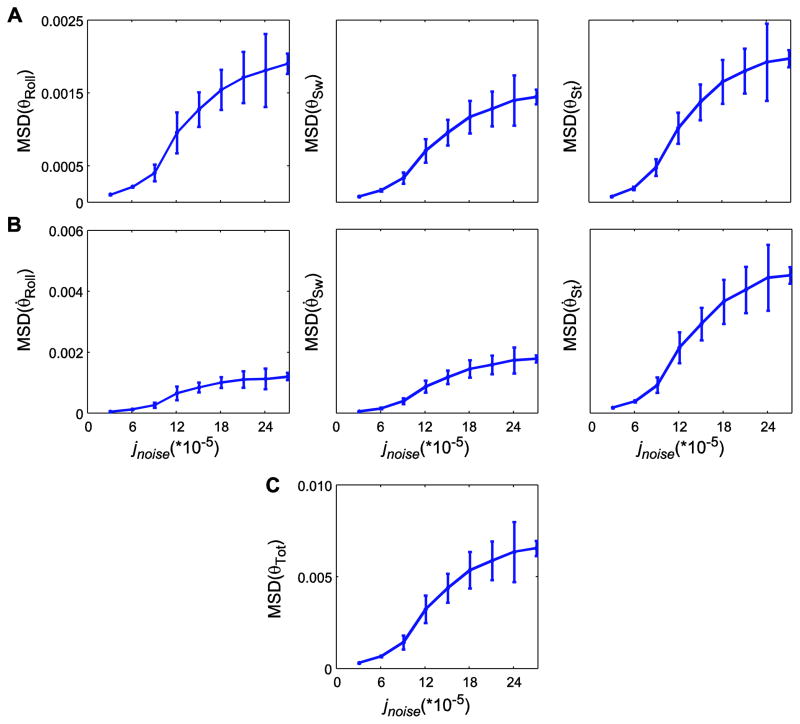
Figure 5.
Within-trial mean standard deviations (MSD) for kinematic state variables for (A) angular displacements (θRoll, θSw, and θSt), (B) angular velocities (θ̇Roll;, θ̇Sw, and θ̇St), and (C) total kinematic state variability (MSDθtot), each plotted against noise amplitude (jnoise). Error bars indicate between-trial ±1 standard deviations. For the highest noise level (jnoise = 27×10−5), additional simulations beyond the first 100 were performed to obtain 20 trials of at least 55 steps, as the model fell over after fewer steps at this noise level. For all kinematic states, MSD increased most rapidly at intermediate jnoise levels and exhibited smaller incremental changes at the lowest and highest jnoise levels, where %Fall (Fig. 3A) was either very low or very high.
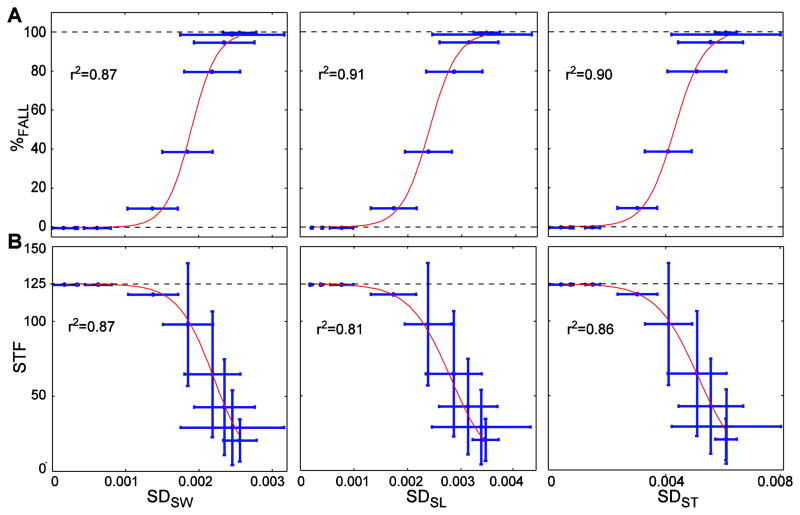
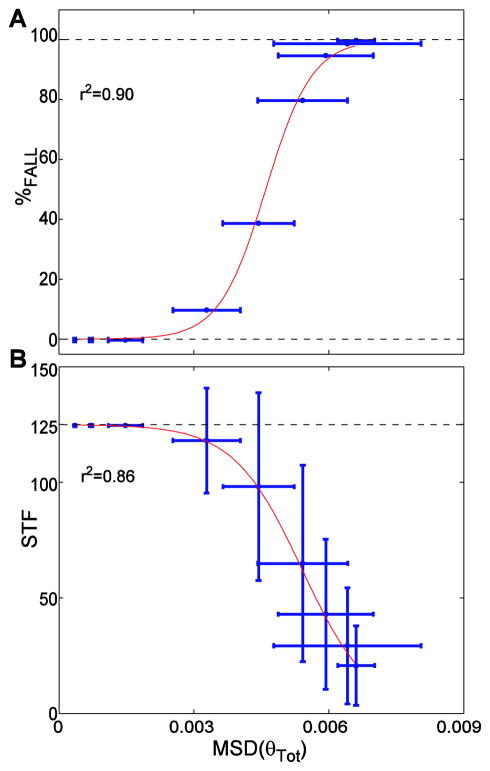
Increases in step variability or kinematic state variability predicted increases in probability of falling and decreases in STF (Figs. 6–7 and Supplement). Importantly, these relationships were not linear. Similar to the trends in Figs. 4–5, increases in kinematic variability yielded maximal increases in %FALL at intermediate variability levels, but induced minimal changes in %FALL when initial variability was either low or high. Sigmoidal fits of these variability measures against both %FALL and STF were all highly statistically significant (0.81 ≤ r2 ≤ 0.91; p ≤ 8.9 × 10−4) (Figs. 6–7 and Supplement). This partly confirmed our final hypothesis that increased gait variability would significantly predict increased fall risk.
Figure 6.
Mean within-trial step variability (Fig. 4) versus (A) probability of falling (%FALL) and (B) average number of steps taken before falling (STF). Data points are average values. Red lines are the sigmoidal functions fitted to these average data. Horizontal error bars indicate between-trial ±1 standard deviations of the variability. Vertical error bars indicate between-trial ±1 standard deviations of STF. The r2 values are shown for each sigmoidal fit. All correlations were highly significant (r2≥0.81; p≤8.9×10−4).
Figure 7.
Total within-trial kinematic state variability (MSDθtot; Fig. 5C) versus (A) probability of falling (%FALL) and (B) the average number of steps before falling (STF). Horizontal error bars indicate between-trial ±1 standard deviations of the variability. Vertical error bars indicate between-trial ±1 standard deviations of STF. The data points are the average values and red lines are the sigmoidal functions fitted to the average data. The r2 values are shown for each sigmoidal fit. Both correlations were highly significant (r2≥ 0.86; p≤ 2.9×10−4).
4. Discussion
Identifying the causal mechanisms that increase fall risk in the elderly is critical to targeting appropriate interventions. However, individual mechanisms cannot be identified using longitudinal or cross-sectional studies because most potential contributing factors (neuronal noise, strength, reflexes, cognition, etc.) change simultaneously with aging. Likewise, the contributions of individual mechanisms are difficult to identify using experimental intervention studies because most of these factors cannot be directly manipulated. Simulations are therefore essential because they allow us to isolate the effects of individual candidate mechanisms as potential underlying causes of increased fall risk. This study used dynamic walking simulations to determine how neuromuscular noise contributes to gait variability and probability of falling. When noise of increasing amplitude was applied to the model’s controller, kinematic variability increased (Figs. 4–5) and the model fell over more often (Fig. 3A) and after fewer steps (Figs. 3B). Changes in kinematic variability predicted risk of falling in a sigmoidal way (Figs. 6–7).
The ‘noise free’ simulation was consistent with the original Kuo (1999) model. Our model walked at a non-dimensionalized velocity of 0.30 and step width of 0.40 at a slope of 4%, comparable to Kuo’s (1999) Figs. 6b and 7a. The phase portrait of θRoll (Fig. 2) was qualitatively similar to Kuo’s (1999) Fig. 5b, which was at a different slope and therefore of different magnitude. Our results also confirmed previous 2D dynamic walking model findings that gait variability increased with controller noise amplitude (Gates et al., 2007) or an equivalent amplitude of an uneven walking surface (Su and Dingwell, 2007).
Likewise, our STF results (Fig. 3B) were qualitatively comparable to the “mean first passage times” (MFPT) of Byl and Tedrake (2009) for their 2D rimless wheel model that walked across irregular surfaces of different amplitudes. Those authors applied truncated Gaussian noise and used statistical mechanics methods to predict how many steps their model would take, on average, before falling (i.e., MFPT). Their MFPTs reached much higher values (upwards of ~1015 consecutive steps) than our STF measures. This was partly because we terminated our simulations after 125 steps, but also probably partly due to the fact that our 3D model was inherently locally unstable (in the lateral direction), while their 2D model was inherently locally stable. However, both studies yielded similar trends: i.e., approximately exponentially decreasing STF (Fig. 3B) or MFPT (Byl and Tedrake, 2009) with increasing noise input.
We applied uniformly distributed random noise to our controller. Other noise distributions could have been used, including any that remained bounded but were not uniform (Byl and Tedrake, 2009). However, the exact distributions of actual physiological noise are not known. Similar distributions to the one used here (i.e., still bounded, but with different shapes), might change the quantitative results slightly, but would not affect the qualitative trends observed.
Had we run longer simulations, we expect that at very low jnoise, %Fall would still be ~0%, but STF would continue to increase approximately exponentially. Our variability measures were all extracted from sequences of walking steps in which the model did not fall down (i.e. prior to the fall). We therefore expect these measures would change little or not at all had we computed them from longer walking sequences. Thus, longer simulations might change the relationships between variability and fall risk in small quantitative ways, but would not change their qualitative behavior.
The most important implication of the sigmoidal relationships found between kinematic variability and fall risk (Figs. 6–7) is that specific increases in gait variability may not always lead to increased fall risk, as recently suggested (Brach et al., 2010). At low initial noise (and/or variability) levels, added noise was easily counteracted by the controller to maintain low fall risk (Fig. 3A) with very little additional control effort (Fig. 3C). Converesely, at high initial noise (and/or variability) levels, the model had already exceeded its capacity to prevent falls (Fig. 3A), regardless of any additional control effort applied (Fig. 3C). It was only at intermediate noise levels that increases in simulated neuromuscular noise significantly influenced model kinematic variability (Figs. 4–5), which in turn increased probability of falling (Figs. 6–7).
We can potentially translate these findings to humans by considering the general cases of young adults, healthy older adults and frequent fallers. Young adults likely exhibit low variability, so for them small increases in variability would only negligibly increase their fall risk. The intermediate gait variability conditions likely represent moderately healthy older adults who exhibit increased variability (Kang and Dingwell, 2008) but not necessarily increased fall risk. For them, similar subsequent increases in variability could result in significantly increased fall risk, consistent with Brach et al. (2010). Frequent fallers likely exhibit high variability already, but additional increases may not further increase their already high fall risk. Brach et al. (2010) only examined community-dwelling elderly, who likely fall into the intermediate category. Therefore, their recommendations may not apply to either young healthy subjects or to frequent fallers, due to the constraints identified in the present modeling work.
Our dynamic walking model was sufficiently complex to address our stated objectives. However, it did differ from humans in several ways. For example, it did not have knees and did not incorporate a double support phase. One potential limitation was that controller noise was added only at the instant of ground contact, and not continuously. These discrete perturbations however reflected the cumulative result of all the continuous-time perturbations applied throughout the swing phase. Neuromuscular noise in humans is also signal-dependent (Harris and Wolpert, 1998), but we did not incorporate this into our model. While such modifications would have made the model more complex, they would not likely have altered the qualitative outcomes presented here.
Factors like walking speed (Pavol et al., 2001; Kang and Dingwell, 2008), muscular strength (i.e. the maximum capacity of the controller to generate a response) (Pijnappels et al., 2008), and/or response times (i.e., reflexes) (van den Bogert et al., 2002) likely also influence fall risk in humans. Because our purpose here was to determine the isolated contributions of neuromuscular noise, we did not incorporate or vary these other factors. Incorporating these elements would not likely change the shapes of the curves shown in Figs. 3–7, but would instead shift the range of jnoise values that exhibited the greatest sensitivity to increases in fall risk. For example, adding reflexes would allow the model to withstand larger noise amplitudes and thus exhibit greater gait variability and increased STF (Byl and Tedrake, 2009), but the model would still exhibit the same qualitative behavior. Thus, factors beyond physiological noise are also important to consider when determining if a given incremental increase in gait variability will significantly alter fall risk or not. Future modeling efforts will be essential to identify the individual contributions of each of these other factors and to determine how they interact with each other.
Although our model differed from humans, our results support and/or extend previous experimental studies. When humans were subjected to continuous random mechanical or visual perturbations that were qualitatively comparable to the controller noise we added to our model, step width and step length variability similarly increased (McAndrew et al., 2010). Likewise, step width variability decreased when humans were externally stabilized (Donelan et al., 2004), which would be comparable to reducing the controller noise in our model. Also, increases in step and kinematic variability in humans predicted a greater probability of falling (Owings and Grabiner, 2004; Brach et al., 2005; Moe-Nilssen and Helbostad, 2005), a finding similar to our simulation results at intermediate variability levels.
In our simulations, all step and kinematic variability measures were good predictors of fall risk at intermediate variability levels. This contrasts somewhat with the sometimes contradictory findings in humans regarding which variability measures may best predict fall risk (Owings and Grabiner, 2004; Brach et al., 2005; Moe-Nilssen and Helbostad, 2005). Some of these descrepancies might be related to our finding that increases in fall risk with increased gait variability depend greatly on the initial level of gait variability. This could easily alter how and/or when certain specific increases in different gait variability measures (Brach et al., 2010) either do or do not predict risk of falling. Therefore, the specific relationship between gait variability and fall risk will likely depend on each person’s physical characteristics (e.g., muscle strength, reflexes, etc.) and likely also situational factors (e.g., walking speed, lighting, obstacles, etc.). The individual contributions of these additional factors remain largely unknown and will require further study.
The ultimate potential of the findings of our study is that they demonstrate how neuronal noise and variability relate to fall risk. Our results validate the idea that age-related increases in neuromuscular noise likely play a direct contributing role in increasing fall risk, even though other potentially contributing factors also need to be considered.
Supplementary Material
Acknowledgments
Funding for this project was provided by Grant #1-R21-EB007638-01A1 from the National Institutes of Health.
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest associated with this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brach JS, Berlin JE, VanSwearingen JM, Newman AB, Studenski SA. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. Journal of NeuroEngineering and Rehabilitation. 2005;2 (1):21. doi: 10.1186/1743-0003-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brach JS, Perera S, Studenski S, Katz M, Hall C, Verghese J. Meaningful change in measures of gait variability in older adults. Gait & Posture. 2010;31 (2):175–179. doi: 10.1016/j.gaitpost.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byl K, Tedrake R. Metastable walking machines. The International Journal of Robotics Research. 2009;28 (8):1040–1064. [Google Scholar]
- 4.Cusumano JP, Cesari P. Body-goal variability mapping in an aiming task. Biological Cybernetics. 2006;94 (5):367–379. doi: 10.1007/s00422-006-0052-1. [DOI] [PubMed] [Google Scholar]
- 5.Dean JC, Alexander NB, Kuo AD. The effect of lateral stabilization on walking in young and old adults. IEEE Transactions on Biomedical Engineering. 2007;54 (11):1919–1926. doi: 10.1109/TBME.2007.901031. [DOI] [PubMed] [Google Scholar]
- 6.Dingwell JB, Cusumano JP, Sternad D, Cavanagh PR. Local dynamic stability versus kinematic variability of continuous overground and treadmill walking. ASME Journal of Biomechanical Engineering. 2001;123 (1):27–32. doi: 10.1115/1.1336798. [DOI] [PubMed] [Google Scholar]
- 7.Dingwell JB, John J, Cusumano JP. Do humans optimally exploit redundancy to control step variability in walking? PLoS Computational Biology. 2010;6(7):e1000856. doi: 10.1371/journal.pcbi.1000856. http://dx.doi.org/10.1371/journal.pcbi.1000856. [DOI] [PMC free article] [PubMed]
- 8.Dingwell JB, Marin LC. Kinematic variability and local dynamic stability of upper body motions when walking at different speeds. Journal of Biomechanics. 2006;39 (3):444–452. doi: 10.1016/j.jbiomech.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Donelan JM, Shipman DW, Kram R, Kuo AD. Mechanical and metabolic requirements for active lateral stabilization in human walking. Journal of Biomechanics. 2004;37 (6):827–835. doi: 10.1016/j.jbiomech.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Fling BW, Knight CA, Kamen G. Relationships between motor unit size and recruitment threshold in older adults: Implications for size principle. Experimental Brain Research. 2009;197 (2):125–133. doi: 10.1007/s00221-009-1898-y. [DOI] [PubMed] [Google Scholar]
- 11.Gates DH, Su JL, Dingwell JB. Possible biomechanical origins of the long-range correlations in stride intervals of walking. Physica A: Statistical and Theoretical Physics. 2007;380 (1):259–270. doi: 10.1016/j.physa.2007.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature. 1998;394 (6695):780–784. doi: 10.1038/29528. [DOI] [PubMed] [Google Scholar]
- 13.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: A 1-year prospective study. Archives of Physical Medicine and Rehabilitation. 2001;82 (8):1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 14.Johnson IP, Duberley RM. Motoneuron survival and expression of neuropeptides and neurotrophic factor receptors following axotomy in adult and ageing rats. Neuroscience. 1998;84 (1):141–150. doi: 10.1016/s0306-4522(97)00500-9. [DOI] [PubMed] [Google Scholar]
- 15.Kang HG, Dingwell JB. Separating the effects of age and walking speed on gait variability. Gait & Posture. 2008;27 (4):572–577. doi: 10.1016/j.gaitpost.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Kim GH, Suzuki S, Kanda K. Age-related physiological and morphological changes of muscle spindles in rats. Journal of Physiology. 2007;582:525–538. doi: 10.1113/jphysiol.2007.130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo AD. Stabilization of lateral motion in passive dynamic walking. The International Journal of Robotics Research. 1999;18 (9):917–930. [Google Scholar]
- 18.Ling SM, Conwit RA, Ferrucci L, Metter EJ. Age-associated changes in motor unit physiology: Observations from the baltimore longitudinal study of aging. Archives of Physical Medicine and Rehabilitation. 2009;90 (7):1237–1240. doi: 10.1016/j.apmr.2008.09.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maki BE. Gait changes in older adults: Predictors of falls or indicators of fear? Journal of the American Geriatric Society. 1997;45 (3):313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 20.McAndrew PM, Dingwell JB, Wilken JM. Walking variability during continuous pseudo-random oscillations of the support surface and visual field. Journal of Biomechanics. 2010;43 (8):1470–1475. doi: 10.1016/j.jbiomech.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moe-Nilssen R, Helbostad JL. Interstride trunk acceleration variability but not step width variability can differentiate between fit and frail older adults. Gait & Posture. 2005;21 (2):164–170. doi: 10.1016/j.gaitpost.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Orendurff MS, Schoen JA, Bernatz GC, Segal AD, Glenn K. How humans walk: Bout duration, steps per bout, and rest duration. Journal of Rehabilitation Research and Development. 2008;45 (7):1077–1090. doi: 10.1682/jrrd.2007.11.0197. [DOI] [PubMed] [Google Scholar]
- 23.Owings TM, Grabiner MD. Step width variability, but not step length variability or step time variability, discriminates gait of healthy young and older adults during treadmill locomotion. Journal of Biomechanics. 2004;37:935–938. doi: 10.1016/j.jbiomech.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Pavol MJ, Owings TM, Foley KT, Grabiner MD. Mechanisms leading to a fall from an induced trip in healthy older adults. Journals of Gerontology Series A-Biological Sciences and Medical Sciences. 2001;56 (7):M428–M437. doi: 10.1093/gerona/56.7.m428. [DOI] [PubMed] [Google Scholar]
- 25.Pijnappels M, van der Burg J, Reeves ND, van Dieën JH. Identification of elderly fallers by muscle strength measures. European Journal of Applied Physiology. 2008;102 (5):585–592. doi: 10.1007/s00421-007-0613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson JK, Thies SB, DeMott TK, Ashton-Miller JA. Gait analysis in a challenging environment differentiates between fallers and nonfallers among older patients with peripheral neuropathy. Archives of Physical Medicine and Rehabilitation. 2005;86 (8):1539–1544. doi: 10.1016/j.apmr.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 27.Shaffer SW, Harrison AL. Ageing of the somatosensory system: A translative perspective. Physical Therapy. 2007;87 (2):193–207. doi: 10.2522/ptj.20060083. [DOI] [PubMed] [Google Scholar]
- 28.Su JL, Dingwell JB. Dynamic stability of passive dynamic walking on an irregular surface. ASME Journal of Biomechanical Engineering. 2007;129 (6):802–810. doi: 10.1115/1.2800760. [DOI] [PubMed] [Google Scholar]
- 29.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. The New England Journal of Medicine. 1988;319 (26):1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 30.van den Bogert AJ, Pavol MJ, Grabiner MD. Response time is more important than walking speed for the ability of older adults to avoid a fall after a trip. Journal of Biomechanics. 2002;35 (2):199–205. doi: 10.1016/s0021-9290(01)00198-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.