Dear Sirs,
Hemophilia A is a bleeding disorder caused by the deficiency of Factor VIII (FVIII)(1). In circulation, FVIII binds to its carrier protein vWF with high affinity (Kd < 0.5nM) and this strong non-covalent interaction is critical for the normal half life and circulating plasma concentration of FVIII (2-3). The complex protects FVIII from proteolytic degradation by proteases including activated protein C and prevents cellular uptake by LRP (2). The non-covalent interaction of vWF with FVIII involves amino acids 1672-1689 within the A3 domain and 2303-2332 of the C2 domain of FVIII (3-4). LRP binds to FVIII via A2, A3 and C2 domains (5-6). The monoclonal antibody ESH4, directed against residues 2303-2332 of the C2 domain of FVIII, inhibits binding to both vWF and LRP (5). This implies that vWF and LRP binding sites are in close proximity and/or overlap. This same region (2303-2332) also mediates the interaction of FVIII with phosphatidylserine (PS) on platelet membranes (7). Recent evidences also suggest the participation of C1 domain of FVIII in lipid binding (8-9). FVIII also binds to other anionic lipids including phosphatidylinositol (PI), but the specificity and affinity is highest for PS compared to other anionic lipids (10). Based on these studies it is interesting to note that within C2 domain the binding site for three macromolecular interactions of vWF, LRP and phospholipids either overlap or in close proximity (Figure 1a). We have demonstrated previously that FVIII-PS and FVIII-PI complex reduced inhibitor development (11-12), suggesting therapeutic potential of FVIII-lipid complex. However, the catabolism of FVIII-lipid complex is not completely understood and could be complicated due to overlapping or proximal binding sites and comparable binding affinities in nM range for both phospholipid and vWF. Further phospholipid binding can sterically block macromolecular interaction involving C1 and C2 domain of FVIII. In order to delineate the role of vWF on catabolism of FVIII-lipid complex, pharmacokinetic studies of FVIII in the presence and in the absence of PS and PI, in Hemophilia A (HA) and vWF−/− mice were carried out. Our data suggests that FVIII-lipid complex showed an improved pharmacokinetic profile in both HA mice as well as in vWF−/−mice. All animal handling was performed in accordance with the guidelines of institutional animal care and use committees (IACUC) at the University at Buffalo.
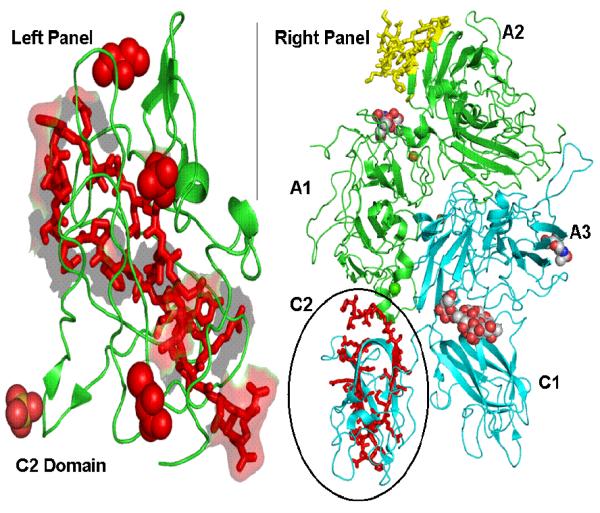
Figure 1. Schematic representation of macromolecular binding sites of FVIII and pharmacokinetic profile of FVIII and FVIII-lipid complex in HA and vWF−/− mice.
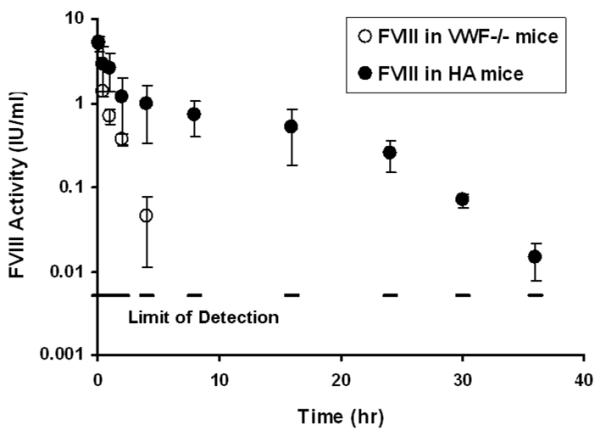
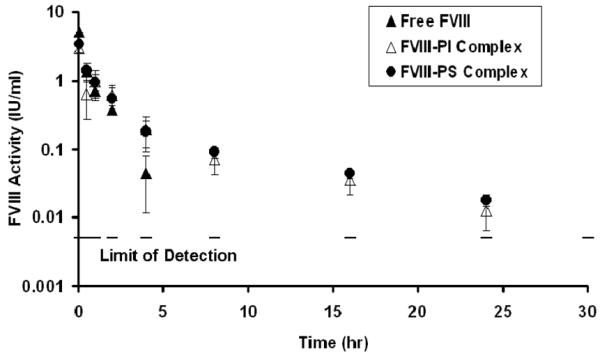
(A) Right panel shows macromolecular binding sites on FVIII structure as red and yellow sticks, where as, left panel shows speculated overlap in macromolecular binding site in C2 domain as red sticks. This figure was regenerated using polyview 3D (http://polyview.cchmc.org/polyview3d.html) based on the structure of B domain deleted FVIII by Ngo and co-workers (22) and structure of C2 domain of human FVIII resolved at 1.5 A° by Pratt and coworkers (23). [PDB codes: left panel: 1D7P, for right panel: 3CDZ]. (B) Plasma activity time profiles of FVIII in HA mice and vWF−/− mice, following i.v. administration. FVIII was administered at a dose of 200 IU/kg. The symbols are mean values and error bars represent the standard deviation. Saline treated vWF−/− mice were used as control to determine baseline plasma FVIII levels in this animal model (n=6). This baseline level was subtracted from activity measured in all time points. (C) Plasma activity time profiles of FVIII, FVIII-PI and FVIII-PS complex in vWF−/− mice. FVIII, FVIII-PI and FVIII-PS were administered as i.v. injections at a dose of 200 IU/kg. The symbols are mean values and error bars represent the standard deviation.
FVIII-PI and FVIII-PS complexes were prepared as described previously (11). Pharmacokinetic studies and analysis were carried out as described previously (13-14). Statistical Analysis was conducted using Minitab 15 (Minitab Inc, State College, PA). Statistical differences (p<0.05) were tested using the Student independent t-test and one-way ANOVA followed by Dunnet’s post-hoc multiple comparison test. Systemic exposures were compared using Bailer-Satterwaite method(15).
In order to establish the role of vWF in FVIII catabolism and to obtain relevant pharmacokinetic parameters, pharmacokinetic studies of FVIII were carried out in vWF−/− mice and compared with that obtained in HA mice (Figure 1b). FVIII was cleared much faster (~6 fold) in the absence of vWF. In vWF−/− mice FVIII levels could be detected for only 4 hrs post administration, whereas in HA mice plasma levels of FVIII could be measured up to 36 hrs post injection. Pharmacokinetic parameters are summarized in Table 1. Analysis revealed a significant reduction in systemic exposure of FVIII in the absence of vWF; The area under the activity curve (AUAC) for FVIII in the absence of vWF was nearly 6 fold lower compared to the AUAC of FVIII in presence of vWF (p<0.05) and half-life was ~4 fold lower than in HA mice. These observations are consistent with previous studies that vWF is required for the plasma survival (16). This also explains why patients with von Willebrand disease type 3, exhibit a secondary deficiency of FVIII and have reduced half-life of intravenously (i.v.) administered FVIII (17).
Table 1.
Pharmacokinetic parameters determined by noncompartmental analysis using WinNonlin 5.2 (Pharsight, Cary, NC) following i.v. administration of 200 IU/kg FVIII, FVIII-PS, and FVIII-PI in vWF−/− mice and HA mice
| FVIII Formulation |
Mouse Model |
Terminal half life (hr) |
AUAC (IU.hr/ml) |
CL (ml/hr) |
Mean Residence Time (hr) |
|---|---|---|---|---|---|
| Free FVIII | vWF−/− | 0.74 | 3.40 | 1.46 | 0.73 |
| Free FVIII | HA | 2.90 | 20.37 | 0.25 | 8.81 |
| FVIII-PS Complex |
vWF−/− | 6.93 | 4.95 | 1.00 | 4.61 |
| FVIII-PI Complex |
vWF−/− | 6.51 | 4.35 | 1.14 | 4.20 |
| FVIII-PI complex |
HA | 7.41 | 31.03 | 0.16 | 13.73 |
Plasma activity profiles of FVIII and FVIII-lipid complex demonstrated that FVIII-lipid complex showed improved plasma circulation time of FVIII in vWF−/− mice (Figure 1c). It is striking to note that in FVIII-lipid treated animals plasma FVIII activity was detectable till 24 hr post injection compared to free FVIII which was not detectable after 4 hr in the absence of vWF. Non compartmental analysis of the profiles (Table 1) demonstrates that the terminal half life of FVIII-PI showed a ~8 fold increase with associated improvement in MRT and CL. Similar changes were observed for FVIII-PS complex. These results suggest that phospholipid binding improve plasma survival of FVIII, possibly by redistribution of bound species based upon relative reactant concentrations. This is consistent with overlapping binding and its relative affinities. The phospholipids bind FVIII with a high affinity (~6± 2 nM)(18). This binding is comparable to FVIII-LRP (~18±6 nM) (16), but not as strong as FVIII-vWF (0.5 nM) (7, 17-18).
If the benefits observed in vWF−/− mice come exclusively from the reduction of LRP binding, the effect of phospholipids may be lost in the presence of normal vWF concentrations. In order to investigate the effects of phospholipids in the presence of vWF, catabolism studies were carried out for FVIII and FVIII-lipid complex in HA mice (Table 1). Plasma terminal half-life demonstrated prominent difference between the profiles of free FVIII and FVIII-PI and was nearly doubled. The activity was reproducibly detectable for only 36 hr post-injection following administration with FVIII, whereas FVIII activity was detectable up to 42 hr following administration with FVIII-PI complexes. The mean residence time of FVIII-PI complex was also prolonged as compared to free FVIII. Thus association of FVIII with PI prolonged the circulation time and improved plasma survival for FVIII not only in the absence but also in the presence of vWF. The improved profile observed for FVIII-PI complex in HA mice (in the presence of vWF) compared to vWF−/− suggest that both vWF and phospholipids contribute synergistically to the plasma survival of FVIII. We speculate that protection by vWF of FVIII released from FVIII-PI complex prevents LRP mediated clearance of FVIII. In addition, phospholipid binding also likely interfere with equilibrium between free FVIII and vWF-FVIII complex by reducing the generation of free FVIII from FVIII-vWF complex and clearance of vWF-FVIII complex by Kupfer cells(19-20) as PI can resist RES uptake (21).
In conclusion our findings demonstrate that phospholipid binding reduces catabolism of FVIII resulting in increased plasma circulation time both vWF−/− mice and HA mice, which may be mediated by macromolecular interactions. These interactions may be utilized to design therapeutic preparations that are long acting in Hemophilia A and vWF deficient patients.
ACKNOWLEDGEMENTS
This work was supported by a grant from the National Institutes of Health (R01 HL-70227) to SVB. We are grateful to Drs. Kazazian and Sarkar of the University of Pennsylvania for providing the initial FVIII knockout mice model, and to Dr. Bernstein of Western New York Hemophilia Foundation, for providing albumin-free recombinant Factor VIII (Advate). Dipak S. Pisal received pre-doctoral fellowship from Pfizer Inc.
Abbreviations
The abbreviations used are:
- FVIII
Factor VIII
- vWF
von Willebrand Factor
- LRP
low density lipoprotein receptor related protein
- PI
phosphatidylinositol
- PS
phosphatidylserine
- RES
reticuloendothelial system
- AUAC
area under the activity curve
- MRT
mean residence time
- t½
circulation half-life
- CL
clearance
- i.v.
intravenous
- vWF−/−
vWF knockout
- HA
Hemophilia A
References
- 1.Klinge J, Ananyeva NM, Hauser CA, et al. Hemophilia A--from basic science to clinical practice. Semin Thromb Hemost. 2002 Jun;28(3):309–22. doi: 10.1055/s-2002-32667. [DOI] [PubMed] [Google Scholar]
- 2.Lenting PJ, VANS CJ, Denis CV. Clearance mechanisms of von Willebrand factor and factor VIII. J Thromb Haemost. 2007 Jul;5(7):1353–60. doi: 10.1111/j.1538-7836.2007.02572.x. [DOI] [PubMed] [Google Scholar]
- 3.Saenko EL, Scandella D. The acidic region of the factor VIII light chain and the C2 domain together form the high affinity binding site for von willebrand factor. J Biol Chem. 1997 Jul 18;272(29):18007–14. doi: 10.1074/jbc.272.29.18007. [DOI] [PubMed] [Google Scholar]
- 4.Saenko EL, Shima M, Rajalakshmi KJ, et al. A role for the C2 domain of factor VIII in binding to von Willebrand factor. J Biol Chem. 1994 Apr 15;269(15):11601–5. [PubMed] [Google Scholar]
- 5.Lenting PJ, Neels JG, van den Berg BM, et al. The light chain of factor VIII comprises a binding site for low density lipoprotein receptor-related protein. J Biol Chem. 1999 Aug 20;274(34):23734–9. doi: 10.1074/jbc.274.34.23734. [DOI] [PubMed] [Google Scholar]
- 6.Saenko EL, Yakhyaev AV, Mikhailenko I, et al. Role of the low density lipoprotein-related protein receptor in mediation of factor VIII catabolism. J Biol Chem. 1999 Dec 31;274(53):37685–92. doi: 10.1074/jbc.274.53.37685. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert GE, Kaufman RJ, Arena AA, et al. Four hydrophobic amino acids of the factor VIII C2 domain are constituents of both the membrane-binding and von Willebrand factor-binding motifs. J Biol Chem. 2002 Feb 22;277(8):6374–81. doi: 10.1074/jbc.M104732200. [DOI] [PubMed] [Google Scholar]
- 8.Meems H, Meijer AB, Cullinan DB, et al. Factor VIII C1 domain residues Lys 2092 and Phe 2093 contribute to membrane binding and cofactor activity. Blood. 2009 Oct 29;114(18):3938–46. doi: 10.1182/blood-2009-01-197707. [DOI] [PubMed] [Google Scholar]
- 9.Hsu TC, Pratt KP, Thompson AR. The factor VIII C1 domain contributes to platelet binding. Blood. 2008 Jan 1;111(1):200–8. doi: 10.1182/blood-2007-01-068957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kemball-Cook G, Barrowcliffe TW. Interaction of factor VIII with phospholipids: role of composition and negative charge. Thromb Res. 1992 Jul 1;67(1):57–71. doi: 10.1016/0049-3848(92)90258-c. [DOI] [PubMed] [Google Scholar]
- 11.Ramani K, Miclea RD, Purohit VS, et al. Phosphatidylserine containing liposomes reduce immunogenicity of recombinant human factor VIII (rFVIII) in a murine model of hemophilia A. J Pharm Sci. 2008 Apr;97(4):1386–98. doi: 10.1002/jps.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purohit VS, Ramani K, Sarkar R, et al. Lower inhibitor development in hemophilia A mice following administration of recombinant factor VIII-O-phospho-L-serine complex. J Biol Chem. 2005 May 6;280(18):17593–600. doi: 10.1074/jbc.M500163200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berntorp E, Bjorkman S. The pharmacokinetics of clotting factor therapy. Haemophilia. 2003 Jul;9(4):353–9. doi: 10.1046/j.1365-2516.2003.00762.x. [DOI] [PubMed] [Google Scholar]
- 14.Barrowcliffe TW, Raut S, Sands D, et al. Coagulation and chromogenic assays of factor VIII activity: general aspects, standardization, and recommendations. Semin Thromb Hemost. 2002 Jun;28(3):247–56. doi: 10.1055/s-2002-32658. [DOI] [PubMed] [Google Scholar]
- 15.Nedelman JR, Gibiansky E, Lau DT. Applying Bailer’s method for AUC confidence intervals to sparse sampling. Pharm Res. 1995 Jan;12(1):124–8. doi: 10.1023/a:1016255124336. [DOI] [PubMed] [Google Scholar]
- 16.Bovenschen N, Mertens K, Hu L, et al. LDL receptor cooperates with LDL receptor-related protein in regulating plasma levels of coagulation factor VIII in vivo. Blood. 2005 Aug 1;106(3):906–12. doi: 10.1182/blood-2004-11-4230. [DOI] [PubMed] [Google Scholar]
- 17.Saenko E, Kannicht C, Loster K, et al. Development and applications of surface plasmon resonance-based von Willebrand factor-collagen binding assay. Anal Biochem. 2002 Mar 15;302(2):252–62. doi: 10.1006/abio.2001.5555. [DOI] [PubMed] [Google Scholar]
- 18.Saenko E, Sarafanov A, Greco N, et al. Use of surface plasmon resonance for studies of protein-protein and protein-phospholipid membrane interactions. Application to the binding of factor VIII to von Willebrand factor and to phosphatidylserine-containing membranes. J Chromatogr A. 1999 Aug 6;852(1):59–71. doi: 10.1016/s0021-9673(99)00491-4. [DOI] [PubMed] [Google Scholar]
- 19.Kamps JA, Scherphof GL. Receptor versus non-receptor mediated clearance of liposomes. Adv Drug Deliv Rev. 1998 Jun 8;32(1-2):81–97. doi: 10.1016/s0169-409x(97)00133-6. [DOI] [PubMed] [Google Scholar]
- 20.Denis CV, Christophe OD, Oortwijn BD, et al. Clearance of von Willebrand factor. Thromb Haemost. 2008 Feb;99(2):271–8. doi: 10.1160/TH07-10-0629. [DOI] [PubMed] [Google Scholar]
- 21.Gabizon A, Papahadjopoulos D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6949–53. doi: 10.1073/pnas.85.18.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ngo JC, Huang M, Roth DA, et al. Crystal structure of human factor VIII: implications for the formation of the factor IXa-factor VIIIa complex. Structure. 2008 Apr;16(4):597–606. doi: 10.1016/j.str.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Pratt KP, Shen BW, Takeshima K, et al. Structure of the C2 domain of human factor VIII at 1.5 A resolution. Nature. 1999 Nov 25;402(6760):439–42. doi: 10.1038/46601. [DOI] [PubMed] [Google Scholar]