Abstract
Objective
For radical prostatectomy, the advantages of robotic surgery may facilitate precise dissection and improve functional outcomes. However, patients with larger prostates may still pose increased challenges due to impaired visualization and mobility in the pelvis. For this reason, we undertook a study to better understand relationships between large prostate size and robotic prostatectomy outcomes with respect to intraoperative and pathologic factors.
Methods
Patients undergoing robotic-assisted radical prostatectomy from 2003 to 2008 at our institution were included in this retrospective study. Prostate size was categorized into three groups (<50, 50-100, >100 grams). We compared surgical and quality of life (EPIC scores) outcomes among groups using generalized linear models and chi-square testing.
Results
Patients with the largest prostates had longer operative times (>100 vs. <50 gm, 250 vs. 232 minutes, p<0.01) and more blood loss (>100 vs. <50 gm, 250 vs. 155 mL, p=0.01). Conversely, these patients had fewer positive surgical margins and lower Gleason sums (both p<0.01). Despite worse baseline irritative symptoms (>100 vs. <50 gm, 79.7 vs. 90.0, p<0.001) and sexual function (>100 vs. <50 gm, 38.2 vs. 77.9, p<0.001), these differences resolved at 3 months (p=0.92, p=0.88, respectively). Recovery of continence was relatively sluggish compared to those with the smallest prostates (>100 vs. <50 gm, 44.0, 62.2, p=0.03).
Conclusion
Not surprisingly, larger prostate size was associated with increased operative times and blood loss, although of questionable clinical significance. While these patients appeared to benefit regarding irritative symptoms, continence was delayed. Longer follow-up is needed to further assess recovery.
Keywords: robotic, prostatectomy, prostate size, outcomes
Introduction
Larger prostate size poses treatment challenges among men with prostate cancer. For patients interested in radiation, increasing prostate size limits adequate dose-delivery for brachytherapy and can even require pre-treatment androgen deprivation.1, 2 In surgical patients, larger prostates have reduced mobility in the pelvis and can impair visualization. Invariably, these technical challenges result in greater blood loss, longer operative times, and inevitably, can translate into worse quality of life.3-6 For example, among patients undergoing conventional radical retropubic prostatectomy, those with prostates over 60 grams have worse recovery of sexual function.4 Regardless of a patient's treatment preference, large prostate size has implications for treatment choice and outcomes.
However, the advantages of robotic-assisted radical prostatectomy may mitigate some of the challenges posed by large prostate size. Stereoscopic visualization, magnification and the pressure exerted by the CO2 are associated with less blood loss, better nerve sparing and outstanding functional outcomes.7-10 In light of these advantages, robotics may overcome the obstacles posed by larger prostates thereby broadening the treatment options for such patients.
For this reason, we undertook a study to better understand relationships between large prostate size and robotic prostatectomy outcomes with respect to intraoperative and pathologic factors. Using the Expanded Prostate Cancer Index Composite (EPIC), a validated measure of health-related quality of life,11 we also evaluated the extent to which a robotic approach can mitigate some of the functional disadvantages associated with larger prostate size.
Materials and Methods
Study Population
We retrospectively identified patients undergoing robotic-assisted radical prostatectomy for clinically localized prostate cancer at our institution between June 1, 2003 and September 30, 2008. Clinical characteristics, including demographics and disease-related data, are prospectively collected and entered into our prostate cancer database for each patient undergoing radical prostatectomy. To evaluate relationships between prostate size and surgical outcomes, patients were sorted into three size groups based on the pathologic prostate weight (less than 50 grams, 50-100 grams and over 100 grams). All patients were included in the analysis of demographic, intraoperative and pathologic factors associated with prostate size (n=885). Typically, patients complete a battery of self-administered questionnaires at each visit, including the EPIC Short Form.11 Patients complete the EPIC preoperatively and at various intervals postoperatively, depending on surgeon preference for follow-up. To evaluate relationships between prostate size and health-related quality of life outcomes, a subset of patients with baseline and follow-up EPIC data (n=306) were evaluated. Patients completing the EPIC questionnaire either before or after surgery, but not at both time periods, were excluded from the EPIC analysis. Although patients may not have addressed or answered all questions within each domain, those with complete baseline and postoperative responses in any domain were evaluated.
Outcomes
All outcomes were assessed at the patient level. First, we evaluated several surgical outcomes including blood loss, operative time, margin status and the quality of the nerve sparing (none, unilateral/partial bilateral, bilateral). The last was based on the surgeon assessment at the completion of the procedure. Second, we evaluated early functional recovery (i.e., at 3 months postoperatively) using EPIC's urinary continence, urinary irritative and sexual domains.
Statistical analysis
Our principal independent variable for this study was prostate size, assessed at the patient level. For the purpose of analysis, we used generalized linear models and the Mantel-Haenszel chi-square test for continuous and categorical data, respectively. For the functional outcomes in particular, we fitted parsimonious multivariable regression models adjusting for baseline EPIC score and any marginally significant covariates (i.e., p < 0.1 on bivariate analysis).
All analyses were performed using computerized software (SAS Institute, Cary, NC) and all testing was two-sided. The probability of a Type I error was set at 0.05. The study protocol was approved by the institutional review board.
Results
Larger prostate size was associated with older age and higher baseline PSA levels (Table 1). Patients with the largest prostates, i.e., over 100 gms, accounted for a minority of the study population. Generally, those with the largest prostates were over the age of 60 (p<0.001), had a mean preoperative PSA level of 10.0 ng/mL (p<0.001), and were more likely to harbor clinical stage T1 disease (p=0.03). We observed no statistically significant differences with respect to race (p=0.18), although increasing BMI was associated with increasing prostate size (p=0.001).
Table 1.
Patient characteristics associated with prostate size
| Characteristic | Prostate size (gm) | p-value | ||
|---|---|---|---|---|
| <50 | 50-100 | >100 | ||
| Patients (no.) | 582 | 279 | 24 | |
| Prostate size (gm, mean) | 38.9 | 62.0 | 119.6 | p<0.001 |
| PSA (ng/mL, mean) | 5.8 | 6.6 | 10.0 | p<0.001 |
| Age at surgery (years, mean) | 59.4 | 62.0 | 67.4 | p<0.001 |
| Age at surgery (%) | p<0.001 | |||
| <50 | 10.1 | 3.6 | 0 | |
| 50-59 | 42.8 | 34.8 | 0 | |
| 60-69 | 40.7 | 49.1 | 75.0 | |
| >70 | 6.4 | 12.5 | 25.0 | |
| Race (%) | p=0.18 | |||
| White | 84.7 | 83.1 | 91.7 | |
| Black | 5.0 | 7.9 | 8.3 | |
| Other | 10.3 | 9.0 | 0 | |
| BMI (%) | p=0.001 | |||
| <25 | 19.9 | 15.8 | 12.5 | |
| 25 – 29 | 49.5 | 44.4 | 41.6 | |
| 30 – 34 | 23.9 | 28.3 | 29.2 | |
| >35 | 6.7 | 11.5 | 16.7 | |
| Clinical stage (%) | p=0.03 | |||
| T1 | 76.8 | 84.2 | 87.5 | |
| ≥T2 | 23.2 | 15.8 | 12.5 | |
Among surgical outcome measures, increasing prostate size was associated with greater intraoperative blood loss (>100 vs. <50 gm, 250 vs. 155 mL, p=0.01) and longer operative time (>100 vs. <50 gm, 250 vs. 232 minutes, p<0.01). As illustrated in Table 2, patients with the largest prostates had more favorable pathology with respect to Gleason sum (p<0.01) and margin status (p<0.01). In fact, among patients with prostates over 100 grams, all surgical margins were negative.
Table 2.
Operative and pathologic factors associated with prostate size*
| Characteristic | Prostate size (gm) | p-value | ||
|---|---|---|---|---|
| <50 | 50-100 | >100 | ||
| Estimated blood loss (mL, mean) | 155 | 169 | 250 | p=0.01 |
| OR time (minutes) | 232 | 248 | 250 | p<0.01 |
| Neurovascular bundle sparing (%) | p=0.17 | |||
| Bilateral | 64.2 | 61.7 | 54.2 | |
| Unilateral/partial bilateral | 22.9 | 22.9 | 25.0 | |
| None | 12.9 | 15.4 | 20.8 | |
| Bladder neck sparing (%) | p=0.75 | |||
| Yes | 5.4 | 3.7 | 0.0 | |
| No | 94.6 | 96.3 | 100.0 | |
| Surgical margin (%) | p<0.01 | |||
| Negative | 81.0 | 88.9 | 100.0 | |
| Positive - focal | 15.0 | 9.3 | 0 | |
| Positive- extensive | 4.0 | 1.8 | 0 | |
| Pathologic Gleason sum (%) | p<0.01 | |||
| ≤6 | 22.0 | 36.2 | 47.6 | |
| 7 | 74.0 | 58.6 | 38.1 | |
| ≥8 | 4.0 | 5.2 | 14.3 | |
Based on final pathologic specimen
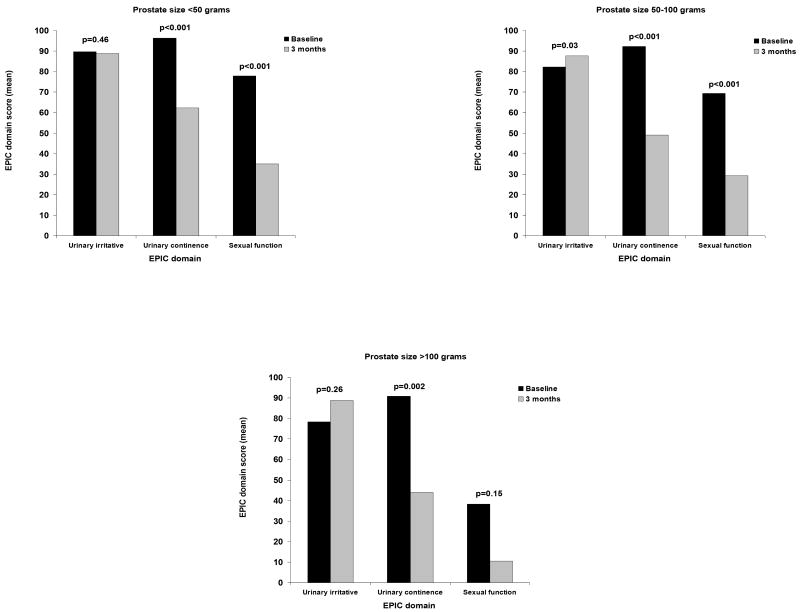
In terms of baseline disease-specific functional status, patients with the largest prostates had more urinary symptoms and worse sexual health prior to surgery (Table 3). For example, relative to patients with the smallest prostates, those with prostates over 100 grams had more irritative symptoms (90.0 vs. 79.7, respectively, p<0.001) and considerably diminished sexual function (77.9 vs. 38.2, respectively, p<0.001) at baseline. As shown in Table 3, recovery of urinary continence by men with prostates less than 50 grams outpaced that for those with prostates larger than 100 grams, even after adjusting for baseline function, age and BMI (62.2 vs. 44.0, respectively, p=0.03). A similar trend was noted for sexual function, although not statistically significant. In contrast, postoperative irritative urinary symptoms improved from baseline among men with the largest prostate glands such that no differences between the groups were evident at 3 months (p=0.92). Figure 1 illustrates the within group comparisons for EPIC urinary and sexual function scores between baseline health and the three month outcomes.
Table 3.
EPIC urinary and sexual function baseline and outcomes following robotic prostatectomy by prostate size†
| EPIC domain | Prostate size (gm) | p-value | ||
|---|---|---|---|---|
| <50 | 50-100 | >100 | ||
| Baseline EPIC urinary irritative subscale, mean | 90.0 | 82.2 | 79.7 | p<0.001 |
| 3 month EPIC urinary irritative, mean* | 88.8 | 87.6 | 88.8 | p=0.92 |
| Baseline EPIC urinary incontinence subscale, mean | 96.0 | 92.0 | 90.9 | p<0.01 |
| 3 month EPIC urinary incontinence subscale, mean** | 62.2 | 49.0 | 44.0 | p=0.03 |
| Baseline EPIC sexual subscale, Mean | 77.9 | 68.5 | 38.2 | p<0.001 |
| 3 month EPIC sexual subscale, mean*** | 35.0 | 29.3 | 10.5 | p=0.88 |
Adjusted for
baseline,
baseline, age at surgery and BMI
baseline, age at surgery and nerve-sparing
Each group of prostate size (<50 grams, 50-100 grams, and >100 grams) corresponded to 204, 80 and 8 study patients for the urinary continence domain, 198, 76 and 6 study patients for the urinary irritative domain, and 196, 79 and 6 study patients for the sexual function domain, respectively.
Figure 1.
EPIC urinary and sexual function baseline and 3 month outcomes following robotic prostatectomy.
Unadjusted EPIC domain scores by prostate size group.
Discussion
Patients electing robotic prostatectomy may have higher expectations for this innovative approach compared to open surgery.12 However, among those with very large prostates, it is unclear if such expectations are warranted given the increased technical challenges. Thus, appropriately setting expectations for patients undergoing robotic prostatectomy may improve their satisfaction and minimize regret with regard to their treatment choice and outcomes.12 In this study, we found that men with the largest prostates had longer operative times and more blood loss, although these were not likely clinically significant. Not surprisingly, these patients had better oncologic outcomes in terms of surgical margins, likely a manifestation of their more favorable disease characteristics and the relative tumor burden within the gland.13 While early recovery of continence appeared relatively sluggish among patients with the largest prostates, irritative urinary symptoms improved by three months after surgery to the point that there were no longer differences between the groups.
Despite the unique technical challenges posed by significantly enlarged prostates, the robotic approach compared favorably to others in terms of perioperative outcomes. Traditionally with the conventional retropubic approach, larger prostate size has been associated with longer operative times and greater blood loss, suggesting a higher degree of technical difficulty.3-6 While we observed a similar trend for the robotic approach to radical prostatectomy, the observed differences in terms of blood loss especially, were less dramatic implying that the benefits of robotics (e.g., pneumoperitoneum and 3-D visualization) are able to mitigate some of the consequences of the procedure. As with those undergoing retropubic prostatectomy, patients with larger prostates tend to have more favorable oncologic outcomes, at least in terms of achieving negative margins.5, 14 This finding may be, in part, due to a variety of factors, including greater lead time bias (i.e., men with larger prostates are more likely to have multiple biopsies prior to detecting their cancer) and decreased prostate cancer density.13, 15, 16
While the long-term implications of the robotic approach on disease-specific quality of life are unknown with respect to prostate size, it is apparent that early recovery of bladder control is diminished among those with prostates larger than 100 grams. One plausible reason relates to the reconstruction of the bladder neck after surgery among all patients with the largest prostates. While the benefits of bladder neck sparing have not been rigorously evaluated, many have documented its benefit of promoting early continence in prospective case series.17-19 A second possible reason for this finding is that the quality of the nerve sparing was poorer among those with the largest prostates.19, 20 In our analysis, we attempted to adjust for this using a qualitative assessment by the surgeon at the time of the procedure. Last, increasing body mass index may be associated with worse continence following radical prostatectomy21 and patients with larger prostates in our study tended to have slightly higher indices. However, differences in continence recovery with respect to prostate size existed even after adjustment for BMI. What is readily apparent from these data is that those with larger prostates experienced improvements in their irritative urinary symptoms after prostatectomy.
These study findings should be interpreted with some caution. They represent the surgical outcomes of a high volume, single institution. However, even high volume surgeons may uncommonly encounter such patients with prostates larger than 100 grams. Thus, systematic efforts to refine the robotic procedure for these patients are warranted, especially since treatment options for these individuals are more limited. Next, our sample size for patients with the largest glands is relatively small thereby limiting the statistical power to detect some potentially meaningful differences. Nonetheless, relative to the historical data describing outcomes for open surgery, the robotic approach appears to mitigate the clinical significance of the increased blood loss for men with larger prostates. Last, and perhaps most importantly, the long term implications of robotics on oncologic and functional outcomes is unclear as it relates to prostate size. In the meantime, it is important to appropriately counsel these patients so that they may understand the short term outcomes of the procedure, particularly the deleterious effects on continence.
Large prostate size increases the complexity of robotic prostatectomy and results in longer operative times and increased blood loss, though the differences appear to relatively small and unlikely of clinical significance. Negative surgical margins appear to be more readily achievable in this population, although early recovery of urinary continence is hindered. Understanding the downstream effects of large prostate size on longer term functional outcomes following robotic prostatectomy will better inform us as to its overall utility in these challenging patients.
Acknowledgments
Dr. Skolarus is supported by an NIH T32 training grant (NIH 2 T32 DK007782-06), the American Urological Association North Central Section, the American Urological Association Foundation Research Scholar Program and the American Cancer Society Postdoctoral Fellowship Program.
Footnotes
Presented at the 27th Annual World Congress of Endourology, 2009, Munich, Germany.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Locke J, Ellis W, Wallner K, et al. Risk factors for acute urinary retention requiring temporary intermittent catheterization after prostate brachytherapy: a prospective study. Int J Radiat Oncol Biol Phys. 2002;52:712. doi: 10.1016/s0360-3016(01)02657-8. [DOI] [PubMed] [Google Scholar]
- 2.Stone NN, Stock RG. Prostate brachytherapy in patients with prostate volumes >/= 50 cm(3): dosimetic analysis of implant quality. Int J Radiat Oncol Biol Phys. 2000;46:1199. doi: 10.1016/s0360-3016(99)00516-7. [DOI] [PubMed] [Google Scholar]
- 3.Hsu EI, Hong EK, Lepor H. Influence of body weight and prostate volume on intraoperative, perioperative, and postoperative outcomes after radical retropubic prostatectomy. Urology. 2003;61:601. doi: 10.1016/s0090-4295(02)02422-6. [DOI] [PubMed] [Google Scholar]
- 4.Hollenbeck BK, Dunn RL, Wei JT, et al. Determinants of long-term sexual health outcome after radical prostatectomy measured by a validated instrument. J Urol. 2003;169:1453. doi: 10.1097/01.ju.0000056737.40872.56. [DOI] [PubMed] [Google Scholar]
- 5.Pettus JA, Masterson T, Sokol A, et al. Prostate size is associated with surgical difficulty but not functional outcome at 1 year after radical prostatectomy. J Urol. 2009;182:949. doi: 10.1016/j.juro.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konety BR, Sadetsky N, Carroll PR. Recovery of urinary continence following radical prostatectomy: the impact of prostate volume--analysis of data from the CaPSURE Database. J Urol. 2007;177:1423. doi: 10.1016/j.juro.2006.11.089. [DOI] [PubMed] [Google Scholar]
- 7.Menon M, Tewari A, Baize B, et al. Prospective comparison of radical retropubic prostatectomy and robot-assisted anatomic prostatectomy: the Vattikuti Urology Institute experience. Urology. 2002;60:864. doi: 10.1016/s0090-4295(02)01881-2. [DOI] [PubMed] [Google Scholar]
- 8.Menon M, Shrivastava A, Kaul S, et al. Vattikuti Institute prostatectomy: contemporary technique and analysis of results. Eur Urol. 2007;51:648. doi: 10.1016/j.eururo.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 9.Ficarra V, Novara G, Artibani W, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55:1037. doi: 10.1016/j.eururo.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 10.Patel VR, Thaly R, Shah K. Robotic radical prostatectomy: outcomes of 500 cases. BJU Int. 2007;99:1109. doi: 10.1111/j.1464-410X.2007.06762.x. [DOI] [PubMed] [Google Scholar]
- 11.Wei JT, Dunn RL, Litwin MS, et al. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899. doi: 10.1016/s0090-4295(00)00858-x. http://roadrunner.cancer.med.umich.edu/epic/ [DOI] [PubMed]
- 12.Schroeck FR, Krupski TL, Sun L, et al. Satisfaction and regret after open retropubic or robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2008;54:785. doi: 10.1016/j.eururo.2008.06.063. [DOI] [PubMed] [Google Scholar]
- 13.Foley CL, Bott SR, Thomas K, et al. A large prostate at radical retropubic prostatectomy does not adversely affect cancer control, continence or potency rates. BJU Int. 2003;92:370. doi: 10.1046/j.1464-410x.2003.04361.x. [DOI] [PubMed] [Google Scholar]
- 14.Freedland SJ, Isaacs WB, Platz EA, et al. Prostate size and risk of high-grade, advanced prostate cancer and biochemical progression after radical prostatectomy: a search database study. J Clin Oncol. 2005;23:7546. doi: 10.1200/JCO.2005.05.525. [DOI] [PubMed] [Google Scholar]
- 15.Uzzo RG, Wei JT, Waldbaum RS, et al. The influence of prostate size on cancer detection. Urology. 1995;46:831. doi: 10.1016/s0090-4295(99)80353-7. [DOI] [PubMed] [Google Scholar]
- 16.D'Amico AV, Whittington R, Malkowicz SB, et al. A prostate gland volume of more than 75 cm3 predicts for a favorable outcome after radical prostatectomy for localized prostate cancer. Urology. 1998;52:631. doi: 10.1016/s0090-4295(98)00228-3. [DOI] [PubMed] [Google Scholar]
- 17.Braslis KG, Petsch M, Lim A, et al. Bladder neck preservation following radical prostatectomy: continence and margins. Eur Urol. 1995;28:202. doi: 10.1159/000475052. [DOI] [PubMed] [Google Scholar]
- 18.Freire MP, Weinberg AC, Lei Y, et al. Anatomic Bladder Neck Preservation During Robotic-Assisted Laparoscopic Radical Prostatectomy: Description of Technique and Outcomes. Eur Urol. 2009 doi: 10.1016/j.eururo.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Cambio AJ, Evans CP. Minimising postoperative incontinence following radical prostatectomy: considerations and evidence. Eur Urol. 2006;50:903. doi: 10.1016/j.eururo.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Bradford TJ, Weizer AZ, Gilbert SM, et al. Is residual neurovascular tissue on prostatectomy specimens associated with surgeon intent at nerve-sparing and postoperative quality of life measures? Urol Oncol. 2008 doi: 10.1016/j.urolonc.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 21.Ahlering TE, Eichel L, Edwards R, et al. Impact of obesity on clinical outcomes in robotic prostatectomy. Urology. 2005;65:740. doi: 10.1016/j.urology.2004.10.061. [DOI] [PubMed] [Google Scholar]