Abstract
The light response in retinal ON bipolar cells is associated with disinhibition of current flow through cation channels recently identified as type 1 members of the melastatin transient receptor potential (TRPM) family. We determined the developmental expression of Trpm1 in the wild type C57BL/6, DBA/2J, DBA2J-Gpnmb mouse retinas and in Pde6brd1 retinas characterized by degeneration of rod photoreceptors. Trpm1 mRNA in wild type retinas was low at birth but exhibited progressive increases in abundance up to early adulthood at postnatal day 21 (P21). Retinal Trpm1 mRNA content did not decrease following loss of photoreceptors. At P21, TRPM1-immunopositive perikarya migrated into the outer nuclear layer. The TRPM1 protein was trafficked to discrete postsynaptic puncta in wild type retinas whereas in adult Pde6brd1 mouse retinas, TRPM1 translocated to bipolar perikarya and bar-like structures in the distal inner nuclear layer. These findings show that expression and localization of the TRPM1 in the mouse retina is plastic, modulated by use-dependence and availability of sustained excitatory input.
Keywords: TRPM1, retina, bipolar cell, gene expression, degeneration
Introduction
Glutamate, released from vertebrate photoreceptors, activates retinal ON bipolar neurons through a sign inverting mechanism mediated by the GRM6 – Goα/Gβ5-nyctalopin-RGS7/11 transduction pathway (Nomura et al., 1994; Dhingra et al., 2002; Rao et al., 2007; Chen et al., 2010; Zhang et al., 2010). While all the steps in the ON bipolar transduction cascade remain to be determined, the main cation channel downstream from the heteromeric G protein has been identified as TRPM1, a member of the melastatin TRP channel family (Morgans et al., 2009; Koike et al., 2010). Mice, equines and humans lacking functional TRPM1 develop congenital stationary night blindness (CSNB2), a multigene disease associated with loss of the ERG b-wave (Bellone et al., 2008; Shen et al., 2009; van Genderen et al., 2009; Nakamura et al., 2010). Genetic elimination of Trpm1 compromised ON bipolar transduction in mice (Koike et al., 2010; Morgans et al., 2009) whereas heterologous expression of mGluR6, Goα and TRPM1 reconstituted a nonselective cation channel that was negatively regulated by glutamate (Koike et al., 2010). Nonetheless, the precise mechanism underlying TRPM1 transduction remains to be determined because a proportion of these channels might be localized to intracellular, not plasmalemmal, compartments (Oancea et al., 2009; Patel and Docampo, 2009). As a first step towards an integrated view of TRPM1 function in the mouse retina, we studied the plasticity of Trpm1 expression during development and degeneration.
A previous microarray study suggested that transcription of the Trpm1 gene in the mouse retina increases transiently at postnatal day 6 (P6) and returns to the baseline at P10 (Kim et al., 2008). Given that the Trpm1 transcript levels peak early in postnatal development when synaptic connections and transmission are not yet fully functional (Kim et al., 2008) yet TRPM1 plays an indispensable function in adult vision (Morgans et al., 2009), we decided to re-examine this process across the entire developmental sequence of the wild type mouse retina. To assess if the gene is affected by the loss of bipolar inputs and outputs, we also studied Trpm1 expression in Pde6brd1 (rd1) and DBA/2J retinas in which rod photoreceptors and retinal ganglion cells underwent degeneration. In the rd1 retina the majority of rod photoreceptors die in the first postnatal week, leading to severe changes in cellular architecture and remodeled neural circuitry in the retina (Carter-Dawson et al., 1978; Jones et al., 2003). The deafferentiation is associated with remodeling of postsynaptic sites on bipolar cells and an increased glutamatergic drive in the inner retina (Strettoi and Pignatelli. 2000; Marc et al., 2007; Stasheff, 2008; Margolis et al., 2008). It is believed that the canonical mGluR6 pathway that defines ON bipolar cells disappears following deafferentiation. Surprisingly, however, our results demonstrate that retinal Trpm1 transcript and protein levels are maintained following major photoreceptor loss. Such maintained expression of Trpm1 and Grm6 genes may preserve the ability of the ON bipolar transduction mechanisms in the deafferented inner retina to respond, under circumstances yet to be determined, to glutamatergic signals.
Materials and Methods
Animals
Breeding pair founders for C57BL6 wild type, DBA/2J, D2.B6-Tyrp1B6GpnmbB6/Sj (hereafter referred to as DBA-Gpnmb) and Pde6brd1 mice were obtained from Jackson Laboratories (Bar Harbor, ME). The wild type and Pde6brd1 animals were on the same background, as were the DBA/2J and DBA-Gpnmb animals.
In situ hybridization
In situ hybridization and probe synthesis were performed as described (Punzo C, 2007; IOVS (48) 849–857). Sp6 RNA polymerase was used to generate the probes. The probes for Trpm1 were generated by sub-cloning part of the coding sequence into pGEMT-Easy (Promega). The forward primer was: CAGGGTCAGAAAGCATGGAT; reverse: CCCAGCCTTGTGTTGATCTT. The identity of the gene was verified by sequence analysis. For paraffin sections, retinas were fixed for 30 min in 4% PF/PBS at RT, washed with PBS and dehydrated to 100% ethanol using a ladder of increased EtOH concentrations before embedding in 50/50 xylene/paraffin (60 deg; 15 min) and 100% paraffin (4 × 30 min at 60 deg C).
Semi-quantitative Real Time PCR
Total RNA from retina was extracted with Trizol and total RNA was converted to cDNA using the SuperScript III First-Strand Synthesis kit from Invitrogen. Real-time PCR was performed on a thermocycler (GeneAmp 5700; ABI, Foster City, CA) using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) reagents according to the manufacturer’s instructions. The probes were: Trpm1 (exons 4–5): Forward: AAAGGAGGATGAGCAGCAGTCGTC; Reverse: ATTCTCCAGAGCG CTCACCATCCT; Grm6 (exons 6–7): Forward: CAGCCATGGAGCCTACTGATGG; Reverse: CACTGCTGGCACTTCCATTGG; CACNA1F: Forward CTGGGCCGAGTG ATGATGATGG; Reverse: TCTGATTGTCCTGCCCTTGAGTTC; Vglut2: Forward GACATAGTGGAACAGGAAAGATGG; Reverse TTTCACAAAACACTGCAAGATA AGCT; Rho: Forward GCCTCAGTCTGCATCCCTCCTCTA; Reverse AAGCGTCCC AGTTTCCATCCATT. Amplification of PCR products was measured by fluorescence associated with binding of double-stranded cDNA to SYBR Green in the reaction mixture. After an initial denaturation step of 50 deg C for 2 min and 95 deg for 10 min, PCR reaction was repeated for 40 cycles at 95 deg for 15 sec, 1 cycle at 58 deg for 30 sec, and 1 cycle at 72 deg C for 30 sec. After amplification, the ratio of gene-of-interest mRNA to glyceraldehyde-3-phosphate dehydrogenase (Gapdh) reference gene was calculated for each sample. A random sample at the earliest age of interest was assigned a value of 1 and other values calculated relative to the sample. Every experiment consisted of samples from at least 3 animals, each gene was studied in 3 – 5 separate experiments. The experiments in Fig. 3A were conducted on separate PCR plates, hence DBA/2J values for Vglut2 are compared to normalized C57BL/6 values for each age. Statistical significance was determined with the two-tailed nonparametric Mann-Whitney test.
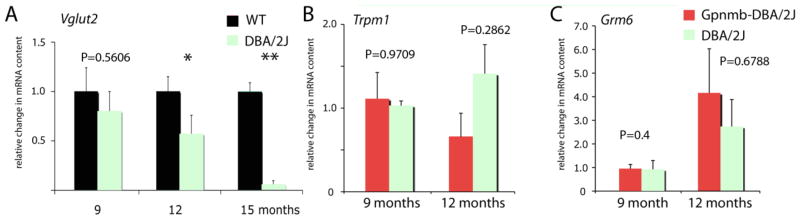
Figure 3.
(A) Vglut2 gene expression in C57BL/6 and DBA/2J retinas shows gradual loss of the RGC marker in the DBA/2J strain; *P<0.05; ** P<0.01. (B & C) Trpm1 and Grm6 mRNA levels in DBA/2J are not statistically different compared to Gpnmb-DBA/2J control retinas.
Immunohistochemistry
Enucleated eyecups with the retinas were immersion-fixed for 1 h in 4% (w/v) paraformaldehyde in phosphate buffer (PB; 0.1M; pH=7.4). The retinas were rinsed two times in PB, cryoprotected in 15 and 30% sucrose overnight at 4° C, mounted in OCT and cryostat sectioned at 16 μm. Retinal sections were washed in PB for 15 min, then permeabilized and blocked in a solution containing 0.5% Triton X-100 and 10% goat serum. The TRPM1 antibody, used at 1:100, has been characterized previously (Koike et al., 2010). Antibodies raised against the synaptic vesicle marker SV2 (K. Buckley; Developmental Studies Hybridoma Bank, Univ. of Iowa) and protein kinase C α subunit (PKCα, clone MC5, Santa Cruz Biotechnology) were used at 1:100. The cone marker peanut agglutinin lectin conjugated to Alexa Fluor 594 nm (PNA; Invitrogen) was used at 1:10 dilution. We utilized the following secondary antibodies: Alexa™ 488 and Alexa™ 594 nm goat anti-mouse or goat anti-rabbit IgG (H+L) conjugates (Invitrogen), diluted at 1:1000. After incubation, sections were washed in PB and covered with Vectashield (Vector, Burlingame, CA). The immunolabeled sections were examined by confocal microscopy (Zeiss LSM 510). In double-labeled experiments, the images were acquired separately from each laser channel (488 Ar or 546 HeNe lines), then recombined. Adjustments of contrast and intensity were made in Photoshop (Adobe, San Jose, CA) and were uniform across the entire image.
Results
The majority of bipolar neurons in the mouse retina develop in the first week after birth (Young, 1985) whereas their synaptic contacts mature between the eye opening and the third postnatal week (P12–P21; Olney, 1968; Fisher, 1979). Using semi-quantitative RT-PCR, Trpm1 mRNA content was analyzed in the postnatal mouse retina to determine whether expression of the Trpm1 gene matches bipolar perikaryal and/or synaptic development. As illustrated in Fig. 1, low levels of Trpm1 mRNA in wild type control retinas were detected in the first week after birth. Trpm1 mRNA levels increased around the time of eye opening (P12–P14; black bars in Fig. 1), reaching adult levels at P21. In situ hybridization analysis showed strong signal with the antisense probe in the distal INL, whereas little expression was detected in other retinal layers (Fig. 1B), indicating that the Trpm1 signal is predominantly confined to bipolar neurons. Increased expression of the Trpm1 gene in the developing mouse retina is consistent with gradual development of bipolar synapses (Fisher, 1979) and excitatory neurotransmission (Tian and Copenhagen, 2001) in the first week after the eye opening.
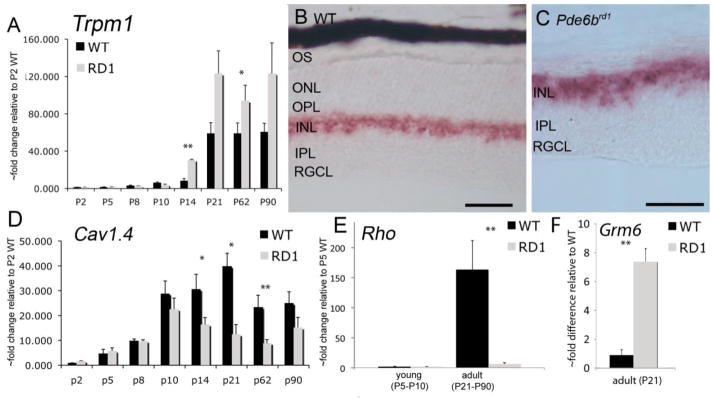
Figure 1.
Trpm1 gene expression in C57BL/6 wild type and Pde6brd1 retina. (A) Real Time PCR (RT-PCR) analysis for Trpm1 in wild type and rd1 retinas at different postnatal ages. RT-PCR signals are plotted relative to the value in a single WT animal at P2 (assigned the value of 1). ISH for Trpm1 in wild type (B) and Pde6brd1 (C) retinas. The reaction product is confined to bipolar perikarya. Scale bar =50 μm. (D – F) RT-PCR analysis for Cav1.4 (CACNA1F), Rho and Grm6 genes in wild type and Pde6brd1 retinas. *P<0.05; **P<0.01 for WT compared to rd1 at each age. Abbreviations: OS, outer segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; RGCL, retinal ganglion cell layer.
Retinal degenerations in mouse models of retinitis pigmentosa are characterized by dendritic outgrowth of deafferented bipolar and horizontal neurons (Strettoi et al., 2003; Jones et al., 2003) and by large-scale rhythmic bursting in postsynaptic neurons triggered by the loss of bipolar input (Stasheff, 2008; Margolis et al., 2008). To determine whether the Trpm1 message is affected by remodeling processes in the outer retina, we analyzed Trpm1 mRNA content in the Pde6brd1 retinitis pigmentosa mouse model in which the large majority of rods degenerate by P21 (Lolley, 1974; Carter-Dawson et al., 1978). The ON bipolar neurons show extensive remodeling response in Pde6brd1 retinas in which they extend dendrites into the ONL in search of glutamatergic inputs (Strettoi and Pignatelli, 2000). While adult Pde6brd1 retinas were associated with near complete loss of rhodopsin and a reduction in the content of Cav1.4 L-type channel transcripts (Fig. 1D & E), prominent Trpm1 mRNA signatures were observed in surviving rd1 bipolar neurons (Fig. 1C) and retinas (Fig. 1A). Expression of the Trpm1 gene in the postnatal rd1 retina was elevated above the wild type at the eye opening (Fig. 1A). Unexpectedly, despite significant photoreceptor loss, Trpm1 mRNA levels in rd1 retinas did not decline at any postnatal age studied (N=8–12 samples per age). To determine whether biosynthesis of the channel mRNA was associated with changes in upstream elements of the ON bipolar transduction cascade, we also determined the expression of the Grm6 gene coding for the mGluR6 receptor. At P21, the Grm6 levels in Pde6brd1 retinas were 7.38 ± 0.93 –fold elevated with respect to wild type C57BL/6 retinas (0.90 ± 0.37; Fig. 1F). This finding suggests that the transduction cascade postsynaptic to photoreceptors responds to loss of input with augmented transcription.
We next examined TRPM1 localization in the Pde6brd1 retina. In P21–P90 wild type retinas, the TRPM1 antibody labeled cell bodies and dendritic processes bipolar neurons (Fig. 2A–C). TRPM1-ir signals in the OPL/INL colocalized with the ON bipolar cell marker protein kinase C (PKCα; data not shown, see Koike et al., 2010). Discrete labeled puncta in the OPL were postsynaptic to presynaptic marker SV2 (Fig. 2B & D), partially overlapping with cone pedicles labeled with peanut agglutinin lectin (PNA; Fig. 2C).
Figure 2.
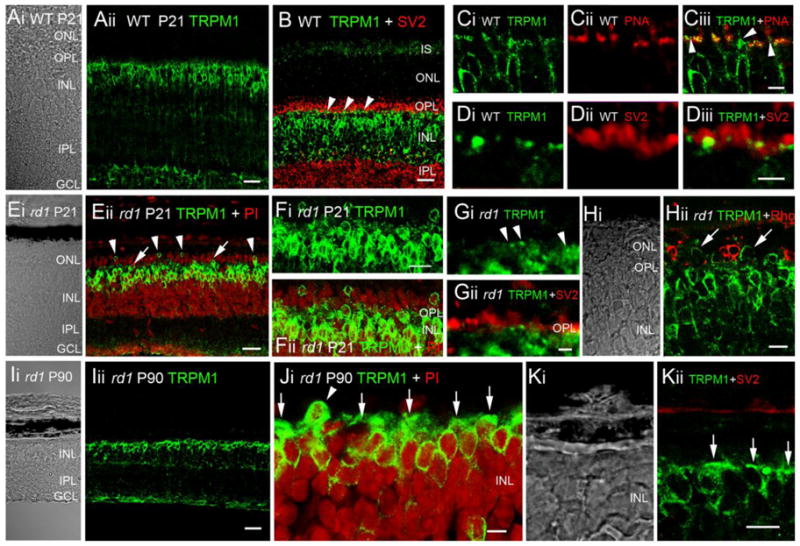
TRPM1 localization in C57BL/6 wild type and Pde6brd1 retina. (A–D) P21 WT retinas. (A) Expression is WT retinas is confined to cells with bipolar morphology. Scale bar = 20 μm. (B) Double labeling with SV2. TRPM1-ir puncta (arrowheads) are proximal to SV2-ir photoreceptor terminals. Scale bar = 20 μm. (C) TRPM1-ir puncta colocalized with PNA; PNA-negative puncta (arrowheads in Ciii) are presumably localized to rod bipolar cells. Scale bar = 5 μm. (D) Double labeling with SV2; scale bar = 2 μm. (E–H) P21 Pde6brd1 retinas. (E–F) Double labeling for TRPM1 and the perikarya marker propidium iodide (PI). The ONL and OPL are shrunk, reflecting loss of ONL perikarya. TRPM1-ir perikarya have migrated (arrowheads) or appear in the process of migration (arrows) into the remaining ONL. Scale bar = 20 μm in E, 10 μm in F. (G) Double labeling with SV2. TRPM1-ir puncta are still observed in P21 rd1 OPL. Scale bar = 2 μm. (H) Double labeling with rhodopsin (Rho). Displaced TRPM1-ir cells (arrows) are not rods. Scale bar = 10 μm. (I–K) P90 Pde6brd1 retinas. (I) ONL and OPL have disappeared. The TRPM1 antibody strongly labels the cell bodies of remaining bipolar neurons. Scale bar = 20 μm. (J & K) Some of the few perikarya displaced into space previously occupied by the ONL are TRPM1-ir (arrowhead in J). TRPM1 signals label cell bodies and horizontal bar-like processes in the distal INL (arrows). Scale bar = 5 μm.
In Pde6brd1 retinas at P21 a marked loss of ONL nuclei and a shrinking of the OPL was observed, reflecting the degeneration and death of rod photoreceptors (Carter-Dawson et al., 1978; Barabas et al., 2010). Despite the loss of photoreceptor input, TRPM1-ir of the distal rd1 INL was prominent, displaying several rows of strongly labeled bipolar perikarya. Surprisingly, a number of TRPM1-immunopositive perikarya were displaced into the degenerated ONL (Fig. E, F & H). The displaced cells were typically associated with empty spaces in the INL directly underneath, suggesting they actively migrated from the INL in search of input. Consistent with this hypothesis, several TRPM1-labeled perikarya observed in mid-OPL (arrows in Fig. Eii) were presumably en route to the ONL. The displaced cells were immunonegative for the rod marker rhodopsin (Fig. 2Hii), suggesting that TRPM1-labeled perikarya in the ONL represented a subset of ON bipolar neurons. At P90, the Pde6brd1 ONL and OPL degenerated with scattered perikarya occasionally observed above the INL (Fig. 2J, arrowhead). Bipolar perikarya in P90 rd1 retinas continued to be strongly labeled by the TRPM1 antibody (Fig. 2I–K). While P21 rd1 retinas continued to express TRPM1-ir puncta within the OPL (Fig. 2F–G), punctate signals were severely attenuated and/or absent from adult rd1 retinas (Fig. 2I) due to the loss of ON bipolar dendritic processes (Strettoi and Pignatelli, 2000). Instead, TRPM1-IR signals assumed a knob- or bar-like appearance (arrow, Figs 2J & K) on the distal side of perikarya themselves. This data shows that continued production of Trpm1 transcripts in nearly photoreceptorless Pde6brd1 retinas is associated with robust expression of TRPM1 protein.
To determine whether Trpm1 transcription was affected by loss of retinal ganglion cells, we examined Trpm1 mRNA content in the DBA/2J strain. Due to recessive mutations in Gpnmb (GpnmbR150X) and Tyrp1 (Tyrp1b) genes that code for a glycoprotein with an unknown function and a membrane-bound melanosomal protein associated with melanin synthesis, respectively, DBA/2J mice develop a retinal phenotype that resembles human pigmentary glaucoma (Chang et al., 1999). Accordingly, the strain is characterized by increased intraocular pressure in animals older than 6 months and by progressive degeneration of RGC axons and perikarya in animals older than 9 months (John et al. 1986; Libby et al., 2005). In our DBA/2J cohort, an age-dependent decrease in the mRNA content for the RGC marker Vglut2 (vesicular glutamate transporter isoform 2) was observed (Fig. 2A) (N= at least 12 eyes per age) together with an increase in intraocular pressure from 13.0 ± 0.55 to 19.2 ± 1.2 mm Hg at 12 months (N=15 animals; Huang et al., 2009). Because the DBA/2J strain exhibits numerous physiological and genetic differences from the C57BL/6 mice, we compared Trpm1 and Grm6 expression in DBA/2J retinas to retinas from DBA-Gpnmb mice that are homozygous for the wild-type allele of Gpnmb on a DBA/2J genetic background and do not develop optic neuropathy (Anderson et al., 2006; Howell et al., 2007). As illustrated in Fig. 2, no significant changes were detected for the Trpm1 and Grm6 mRNA content in DBA/2J compared to DBA-Gpnmb retinas (Fig. 2B & C4; N=4 retinas per age/strain). This result suggests that regulation of Trpm1 in bipolar cells is independent of feedback from postsynaptic retinal ganglion neurons.
Discussion
Our data demonstrates that ON bipolar cells in the rd1 mouse model maintain the GRM6-TRPM1 receptor-channel signaling complex despite almost complete loss of rod photoreceptors and anatomical reorganization of the outer retina. Neither Trpm1 transcription nor posttranscriptional processing was compromised in Pde6brd1 retinas, indicating preservation of the canonical ON transduction complex.
Our results extend previous localization studies of retinal Trpm1 to ON bipolar neurons in wild type mouse retinas (Kim et al., 2008; Koike et al., 2010) by following Trpm1 expression over postnatal development in wild type and degenerating retinas. The report by Kim et al. (1998) who found that Trpm1 mRNA reaches a peak at ~P6 and declined to a low baseline level thereafter, is difficult to reconcile with the proposed function of TRPM1 as the main ON bipolar transduction channel. Indeed, although most bipolar cells are generated in the early postnatal period (Young, 1985) we found that expression of Trpm1 within the first postnatal week was relatively low. The abundance of Trpm1 mRNA was increased by the time of eye opening between P10 and P14, reached adult levels by ~P21 and remained elevated thereafter. The observed increase in transcript levels is coincident with the morphogenesis of photoreceptor synaptic terminals (completed between P7 and P12; Olney, 1968), appearance of GRM6 in bipolar terminals (Nomura et al., 1994), increased density of synaptic arrays of bipolar cells in the IPL (Fisher, 1979; Olney 1968) and the shift from cholinergic to glutamatergic waves in the inner retina (Blankenship et al., 2009). Moreover, our data is consistent with measured excitatory signals within the developing mouse retina (Tian and Copenhagen, 2001) and with increased rod bipolar response amplitudes measured from eye opening to the adulthood (Puthuserry et al., 2009). We conclude that Trpm1 expression matches the observed anatomical and physiological information regarding ON bipolar function in the mouse retina.
A novel finding was the observation of displaced TRPM1-immunoreactive perikarya in the ONL of degenerating P21 retinas. These bipolar nuclei appear to have migrated from the INL following the loss of excitatory input. Although adult Pde6brd1 retinas are devoid of bipolar dendrites (Strettoi and Pignatelli, 2000), several elements that form the signal transduction complex that had been confined to discrete postsynaptic puncta on dendritic arbors of wild type ON bipolar cells continue to be expressed, and trafficked to the outer retina, in rd1 animals. At P21, 1–2 rows of remaining ONL nuclei and associated synaptic terminals of mostly cones appear to be sufficient to maintain at least a fraction of postsynaptic TRPM1 puncta within the OPL. During advanced stages of degeneration in adult rd1 retinas when ON bipolar dendrites are lost, such discrete puncta were replaced by bar- and knob-like TRPM1-immunopositive structures located at the distal side of ON bipolar perikarya (Fig. 2J & K). The altered distribution of TRPM1 is similar to previously observed translocation of the mGluR6/GRM6 receptor (Strettoi and Pignatelli, 2000), suggesting continued association of the receptor-channel complex at the level of the cell body.
Recent molecular and functional studies in the mouse retina have unambiguously localized Trpm1 channels to ON bipolar cells (Kim et al., 2008; Morgans et al., 2009; Koike et al., 2010). We extend these observations by showing that neither loss of rods, RGCs and/or retraction of dendritic tips of ON bipolar cells have a negative impact on Trpm1 expression in Pde6brd1 animals. From the relatively small (~twofold) difference between Trpm1 transcript levels observed in adult C57BL6 and Pde6brd1 retinas we cannot draw a firm conclusion about absolute changes in rd1 Trpm1 expression or TRPM1 protein levels. However, our semi-quantitative approach does suggest that there is little decrease in the overall abundance of Trpm1 transcripts in adult rd1 retinas. This conclusion was supported by immunohistochemical analysis which showed that adult rd1 retinas with severely degenerated ONL express robust TRPM1 immunoreactivity in the distal retina. We therefore hypothesize that the ON bipolar transduction mechanism translocates to dendritic shafts and/or ON bipolar cell bodies so as to preserve cells’ ability to respond to glutamatergic stimulation (e.g., Nomura et al., 1994). An upregulation and redistribution of the GRM6 protein to ON bipolar somata have been previously associated with the progressive loss of rods in the Royal College of Surgeons (RCS) rat retina (Nomura et al., 1994; Armata et al., 2006) whereas a decrease in mGluR6 immunoreactivity was reported for Pde6brd10 retinas (Gargini et al., 2007).
Our data together with reports from the Strettoi laboratory (Strettoi and Pignatelli, 2000; Strettoi et al., 2003) suggests that both the receptor and the transduction channel are targeted appropriately despite extensive remodeling of the rd1 outer retina. If the transduction complex is functional, ON bipolar cells should hyperpolarize following exposure to glutamate. However, Varela et al. (2003) found that acutely dissociated rod bipolar cells are completely insensitive to glutamate stimulation. Uncoupling of the TRPM1 transduction channel from the receptor could result in continuous activation of the channel, leading to depolarization and increased excitatory hyperdrive at ON bipolar output. Such increased excitation, associated with rhythmic bursting, was reported in recent physiological studies (Stasheff, 2008; Margolis et al., 2008). In contrast, other elements of the transduction complex (such as nyctalopin) could independently close the channel and decouple it from Grm6, as might be indicated by results from Marc et al. (2007) who found little AGB+ permeation in intact (ON and OFF) rd1 bipolar cells. Either way, our data opens new and important questions regarding retinal signaling in RP. If the main elements of the ON bipolar transduction complex in the Pde6brd1 retina are in place yet the mechanism remains uncoupled, modulation of other elements that form the transduction cascade, such as the heterotrimeric Gα/βγ subunits or RGS7/11 proteins, might reconstitute glutamate-evoked currents and rescue the downstream phenotype.
These results have implications for developing new strategies for restoring vision in degenerating retinas. Recent breakthrough studies employing genetic manipulations have succeeded in restoring a measure of light-sensitivity to deafferented retinas (Bi et al., 2006; Lagali et al., 2008). These approaches were designed to bypass the GRM6 pathway by relying on incorporation of bacterial channelrhodopsin-2 (ChR2), which directly translates photon capture into cation influx and depolarization, into membranes of bipolar or ganglion neurons. Because ChR2 expression in these studies was not confined to particular cell types, it effectively committed expressor cells to sustained low sensitivity ON firing (Bi et al., 2006). Because light responses in ChR2-transfected OFF bipolar neurons were compromised, OFF spiking in RGCs was lost (Lagali et al., 2008). Parenthetically, our observation that ~50% of Cav1.4 mRNA is still transcribed in the mostly rodless P90 Pde6brd1 retina suggests that these channels, the large majority of which are likely to be localized to bipolar cells (Berntson et al., 2003) could significantly contribute to the excitability of bipolar terminals by boosting the excitation in inner retinal circuits of deafferented retinas. In any case, our findings suggest that outer retinal circuits in deafferented retinas may maintain their ability to transcribe the major transduction channel in rod bipolar neurons and hence remain amenable to more physiological therapeutic interventions.
Research Highlights.
The Trpm1 gene is upregulated during postnatal development of the mouse retina, reaching adult levels at P21.
Loss of photoreceptors does not result in decreased expression of postsynaptic mGluR6 and Trpm1 transcripts.
No changes in Trpm1 and Grm6 expression are seen in the DBA/2J mouse glaucoma model
TRPM1-immunopositive ON bipolar cells migrate into the outer nuclear layer of the degenerating rd1 retina
Remodeling of the deafferented retina promotes plasticity and reorganization of the postsynaptic transduction complex in ON bipolar cells
Acknowledgments
The work was supported by the National Institutes of Health (EY13870; P01 EY014800), The Foundation Fighting Blindness, Moran TIGER award and by an unrestricted grant from Research to Prevent Blindness to Moran Eye Institute at the University of Utah.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson MG, Libby RT, Mao M, Cosma IM, Wilson LA, Smith RS, John SW. Genetic context determines susceptibility to intraocular pressure elevation in a mouse pigmentary glaucoma. BMC Biol. 2006 Jul 7;4:20. doi: 10.1186/1741-7007-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armata IA, Giompres P, Smith A, Stasi K, Kouvelas ED, Mitsacos A. Genetically induced retinal degeneration leads to changes in metabotropic glutamate receptor expression. Neurosci Lett. 2006;393:12–17. doi: 10.1016/j.neulet.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Bellone RR, Brooks SA, Sandmeyer L, Murphy BA, Forsyth G, Archer S, Bailey E, Grahn B. Differential gene expression of TRPM1, the potential cause of congenital stationary night blindness and coat spotting patterns in the Appaloosa horse (Equus caballus) Genetics. 2008;179:1861–1870. doi: 10.1534/genetics.108.088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson A, Taylor WR, Morgans CW. Molecular identity, synaptic localization, and physiology of calcium channels in retinal bipolar cells. J Neurosci Res. 2003;71:146–151. doi: 10.1002/jnr.10459. [DOI] [PubMed] [Google Scholar]
- Bi A, Cui J, Ma YP, Olshevskaya E, Pu M, Dizhoor AM, Pan ZH. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron. 2006;50:23–33. doi: 10.1016/j.neuron.2006.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Ford KJ, Johnson J, Seal RP, Edwards RH, Copenhagen DR, Feller MB. Synaptic and extrasynaptic factors governing glutamatergic retinal waves. Neuron. 2009;62:230–241. doi: 10.1016/j.neuron.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM, Sidman RL. Differential effect of the rd mutation on rods and cones in the mouse retina. Invest Ophthalmol Vis Sci. 1978;17:489–498. [PubMed] [Google Scholar]
- Chang B, Smith RS, Hawes NL, Anderson MG, Zabaleta A, Savinova O, Roderick TH, Heckenlively JR, Davisson MT, John SW. Interacting loci cause severe iris atrophy and glaucoma in DBA/2J mice. Nat Genet. 1999;21:405–409. doi: 10.1038/7741. [DOI] [PubMed] [Google Scholar]
- Chen FS, Shim H, Morhardt D, Dallman R, Krahn E, McWhinney L, Rao A, Gold SJ, Chen CK. Functional redundancy of R7 RGS proteins in ON-bipolar cell dendrites. Invest Ophthalmol Vis Sci. 2010;51:686–693. doi: 10.1167/iovs.09-4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Jiang M, Wang TL, Lyubarsky A, Savchenko A, Bar-Yehuda T, Sterling P, Birnbaumer L, Vardi N. Light response of retinal ON bipolar cells requires a specific splice variant of Galpha(o) J Neurosci. 2002;22:4878–4884. doi: 10.1523/JNEUROSCI.22-12-04878.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber DB, Flannery JG, Bowes-Rickman C. The rd mouse story: seventy years of research on an animal model of inherited retinal degeneration. Prog Retin Eye Res. 1994;13:13–64. [Google Scholar]
- Fisher LJ. Development of synaptic arrays in the inner plexiform layer of neonatal mouse retina. J Comp Neurol. 1979;187:359–372. doi: 10.1002/cne.901870207. [DOI] [PubMed] [Google Scholar]
- Gargini C, Terzibasi E, Mazzoni F, Strettoi E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. J Comp Neurol. 2007;500:222–238. doi: 10.1002/cne.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Libby RT, Marchant JK, Wilson LA, Cosma IM, Smith RS, Anderson MG, John SW. Absence of glaucoma in DBA/2J mice homozygous for wild-type versions of Gpnmb and Tyrp1. BMC Genet. 2007;8:45. doi: 10.1186/1471-2156-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Zou J, Howell GJ, John SMW, Krizaj D. Plasticity of calcium signaling pathways in the DBA/2J mouse model. IOVS. 2009 Abstr 2778a. [Google Scholar]
- Jones BW, Watt CB, Frederick JM, Baehr W, Chen CK, Levine EM, Milam AH, Lavail MM, Marc RE. Retinal remodeling triggered by photoreceptor degenerations. J Comp Neurol. 2003;464:1–16. doi: 10.1002/cne.10703. [DOI] [PubMed] [Google Scholar]
- Kim DS, Ross SE, Trimarchi JM, Aach J, Greenberg ME, Cepko CL. Identification of molecular markers of bipolar cells in the murine retina. J Comp Neurol. 2008;507:1795–1810. doi: 10.1002/cne.21639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike C, Obara T, Uriu Y, Numata T, Sanuki R, Miyata K, Koyasu T, Ueno S, Funabiki K, Tani A, Ueda H, Kondo M, Mori Y, Tachibana M, Furukawa T. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci U S A. 2010;107:332–337. doi: 10.1073/pnas.0912730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagali PS, Balya D, Awatramani GB, Münch TA, Kim DS, Busskamp V, Cepko CL, Roska B. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat Neurosci. 2008;11:667–7. doi: 10.1038/nn.2117. [DOI] [PubMed] [Google Scholar]
- Libby RT, Anderson MG, Pang IH, Robinson ZH, Savinova OV, Cosma IM, Snow A, Wilson LA, Smith RS, Clark AF, John SW. Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neurodegeneration. Vis Neurosci. 2005;22:637–648. doi: 10.1017/S0952523805225130. [DOI] [PubMed] [Google Scholar]
- Lolley RN. The rd gene defect triggers programmed rod cell death. Invest Ophthalmol Vis Sci. 1974;35:4182–4191. [PubMed] [Google Scholar]
- Marc RE, Jones BW, Anderson JR, Kinard K, Marshak DW, Wilson JH, Wensel T, Lucas RJ. Neural reprogramming in retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48:3364–3371. doi: 10.1167/iovs.07-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Zhang J, Jeffrey BG, Nelson SM, Burke NS, Duvoisin RM, Brown RL. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci U S A. 2009;106:19174–8. doi: 10.1073/pnas.0908711106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Sanuki R, Yasuma TR, Onishi A, Nishiguchi KM, Koike C, Kadowaki M, Kondo M, Miyake Y, Furukawa T. TRPM1 mutations are associated with the complete form of congenital stationary night blindness. Mol Vis. 2010;16:425–437. [PMC free article] [PubMed] [Google Scholar]
- Nomura A, Shigemoto R, Nakamura Y, Okamoto N, Mizuno N, Nakanishi S. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell. 1994;77:361–369. doi: 10.1016/0092-8674(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Oancea E, Vriens J, Brauchi S, Jun J, Splawski I, Clapham D. TRPM1 forms ions channels associated with melanin content in melanocytes. Science STKE. 2009;2:1–13. doi: 10.1126/scisignal.2000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW. An electron microscopic study of synapse formation, receptor outer segment development, and other aspects of developing mouse retina. Invest Ophthalmol. 1968;7:250–268. [PubMed] [Google Scholar]
- Patel S, Docampo R. In with the TRP channels: intyracellular functions for TRPM1 and TRPM2. Science STKE. 2009;2:1–3. doi: 10.1126/scisignal.295pe69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punzo C, Cepko C. Cellular responses to photoreceptor death in the rd1 mouse model of retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48:849–857. doi: 10.1167/iovs.05-1555. [DOI] [PubMed] [Google Scholar]
- Puthussery T, Gayet-Primo J, Pandey S, Duvoisin RM, Taylor WR. Differential loss and preservation of glutamate receptor function in bipolar cells in the rd10 mouse model of retinitis pigmentosa. Eur J Neurosci. 2009;29:1533–1542. doi: 10.1111/j.1460-9568.2009.06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Dallman R, Henderson S, Chen CK. Gbeta5 is required for normal light responses and morphology of retinal ON-bipolar cells. J Neurosci. 2007;27:14199–204. doi: 10.1523/JNEUROSCI.4934-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Heimel JA, Kamermans M, Peachey NS, Gregg RG, Nawy S. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J Neurosci. 2009;29:6088–6093. doi: 10.1523/JNEUROSCI.0132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasheff SF. Emergence of sustained spontaneous hyperactivity and temporary preservation of OFF responses in ganglion cells of the retinal degeneration (rd1) mouse. J Neurophysiol. 2008;99:1408–1422. doi: 10.1152/jn.00144.2007. [DOI] [PubMed] [Google Scholar]
- Strettoi E, Pignatelli V. Modifications of retinal neurons in a mouse model of retinitis pigmentosa. Proc Natl Acad Sci USA. 2000;97:11020–11025. doi: 10.1073/pnas.190291097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Pignatelli V, Rossi C, Porciatti V, Falsini B. Remodeling of second-order neurons in the retina of rd/rd mutant mice. Vision Res. 2003;43:867–877. doi: 10.1016/s0042-6989(02)00594-1. [DOI] [PubMed] [Google Scholar]
- Tian N, Copenhagen DR. Visual deprivation alters development of synaptic function in inner retina after eye opening. Neuron. 2001;32:439–449. doi: 10.1016/s0896-6273(01)00470-6. [DOI] [PubMed] [Google Scholar]
- van Genderen MM, Bijveld MM, Claassen YB, Florijn RJ, Pearring JN, Meire FM, McCall MA, Riemslag FC, Gregg RG, Bergen AA, Kamermans M. Mutations in TRPM1 are a common cause of complete congenital stationary night blindness. Am J Hum Genet. 2009;85:730–736. doi: 10.1016/j.ajhg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela C, Igartua I, De la Rosa EJ, De la Villa P. Functional modifications in rod bipolar cells in a mouse model of retinitis pigmentosa. Vision Res. 2003;43:879–85. doi: 10.1016/s0042-6989(02)00493-5. [DOI] [PubMed] [Google Scholar]
- Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- Zhang J, Jeffrey BG, Morgans CW, Burke NS, Haley TL, Duvoisin RM, Brown RL. RGS7 and -11 complexes accelerate the ON-bipolar cell light response. Invest Ophthalmol Vis Sci. 2010;51:1121–1129. doi: 10.1167/iovs.09-4163. [DOI] [PMC free article] [PubMed] [Google Scholar]