Abstract
Survivors of childhood acute lymphoblastic leukemia (ALL) may face an increased risk of metabolic and cardiovascular late effects. In order to determine the prevalence of and risk factors for adverse cardiometabolic traits in a contemporary cohort of pediatric ALL survivors, we recruited 48 off-therapy patients in remission treated with conventional chemotherapy and 26 treated with total body irradiation (TBI) based hematopoietic cell transplantation (HCT) in this cross-sectional pilot study. At a median age of 15 (range 8–21 years), HCT survivors were significantly more likely than non-HCT survivors to manifest multiple cardiometabolic traits including central adiposity, hypertension, insulin resistance, and dyslipidemia. Overall, 23.1% of HCT survivors met criteria for metabolic syndrome (≥3 traits) compared with 4.2% of non-HCT survivors (p=0.02). HCT survivors also had increased C-reactive protein and leptin levels and decreased adiponectin, suggestive of underlying inflammation and increased visceral fat. In multivariate analyses, history of HCT remained associated with ≥2 (OR 5.13, 95% CI 1.54, 17.15) as well as ≥3 (OR 16.72, 95% CI 1.66, 168.80) traits. Other risk factors included any cranial radiation exposure and family history of cardiometabolic disease. In summary, pediatric ALL survivors exposed to TBI-based HCT as well as any cranial radiation may manifest cardiometabolic traits at an early age and should be screened accordingly.
Keywords: acute lymphoblastic leukemia, hematopoietic cell transplantation, metabolic syndrome, radiotherapy, survivor
INTRODUCTION
Cure from childhood acute lymphoblastic leukemia (ALL) now exceeds 85%, resulting in a growing cohort of long-term survivors who potentially face adverse long-term health sequelae as a result of their cancer therapy (1). There is evidence that ALL survivors treated with conventional therapy alone or with hematopoietic cell transplantation (HCT) are at increased risk of developing multiple related cardiovascular/metabolic risk factors, inluding obesity, hypertension, dyslipidemia, and insulin resistance (2–6). Together, these components make up the metabolic syndrome, which is associated with a significantly increased risk of both atherosclerotic cardiovascular disease as well as diabetes mellitus (7–9). Among ALL survivors, risk may be increased secondary to growth hormone deficiency occurring after cranial radiotherapy and total body irradiation (TBI), which has been associated with obesity and dyslipidemia (3;10). As chronic inflammation may have an important role in mediating obesity, insulin resistance, and related cardiovascular diseases (11), chronic graft versus host disease (GVHD) post-transplant also may increase risk among affected survivors (12;13). Other exposures, such as high-dose glucocorticoids (both as part of primary leukemia treatment and GVHD treatment) and more widespread use of immunosuppressive medications such as calcineurin inhibitors used to prevent or treat GVHD, also have been associated with obesity, hypertension, and dyslipidemia (14;15).
Since current ALL therapy is characterized by a reduction in use of cranial radiotherapy and an increased use of more intensive chemotherapy, including more potent glucocorticoids, we conducted this prospective cross-sectional pilot study to determine the prevalence of and risk factors for cardiometabolic traits in pediatric ALL survivors treated since 1990 with conventional chemotherapy and those treated with HCT. We hypothesized that childhood HCT survivors would be at increased risk of these traits compared with ALL survivors treated without HCT, and that this risk would be further modified by history of GVHD and cranial radiotherapy exposure. In exploratory analysis, we also measured selected cytokines in an attempt to determine if levels of cytokines associated with inflammation, adiposity, and endothelial dysfunction would be altered among survivors with multiple cardiometabolic traits.
PATIENTS AND METHODS
Patients
Eligible subjects for this prospective cross-sectional study were diagnosed with ALL at age <22 years, treated at either Seattle Children’s Hospital, Fred Hutchinson Cancer Research Center, or Vanderbilt Children’s Hospital from 1990–2008, and currently age 8–21 years. Two patient cohorts were recruited, one consisting of individuals in first complete remission after treatment with conventional chemotherapy, and the other consisting of individuals treated with HCT, currently in remission, and off any immunosuppression for GVHD. All subjects had to be at least one year off-therapy or from date of HCT. Subjects were recruited in Seattle, Washington, from July 2007 to June 2009, and in Nashville, Tennessee, from April 2009 to June 2009. Among 41 HCT and 83 non-HCT patients approached for this study, 63.4% and 66.3% respectively were enrolled. Seven enrolled non-HCT patients subsequently were excluded (Downs syndrome, n=3; incomplete data, n=4). Final data analysis included 26 HCT and 48 non-HCT survivors. The study protocol was approved by the Institutional Review Boards at all participating centers, and all participants/guardians provided written informed consent prior to participation.
Exposure and outcome measurements
Medical records were abstracted for prior chemotherapy and radiotherapy doses including those associated with HCT, history of extensive or moderate/severe chronic GVHD, and any clinician reported growth hormone deficiency. Medical histories were updated for any patient not seen within the past year at one of the participating centers. Participants and their parents also completed questionnaires on physical activity (16), diet/food frequencies (17), and family history of cardiovascular disease (coronary heart disease, stroke, hypertension, dyslipidemia) and/or diabetes (18). Positive family history was defined by having an affected first degree relative with the relevant disease.
We measured height, weight, waist and hip circumferences, and calculated body mass indices (BMI) and waist-hip ratios. Resting blood pressures were measured twice and a third measurement was obtained if either prior systolic or diastolic pressures were >10 mmHg apart and the most extreme measurement was excluded. Pediatric normative data were used to determine BMI z-scores (19), waist (20) and blood pressure percentiles (21).
At the same research visit when possible, blood, following an 8-hour overnight fast, was obtained for a lipid profile (total cholesterol, high-density lipoprotein [HDL], and triglyceride), glucose, insulin, and selected cytokines (leptin, adiponectin, high sensitivity C-reactive protein [CRP], interleukin-6 [IL-6], tumor necrosis factor alpha [TNF-alpha], E-selectin, and soluble intercellular and vascular cell adhesion molecules [sICAM, sVCAM]). Lipid profiles were collected and processed at participating institutional hospital laboratories. Glucose was measured using an automated hexokinase method (Roche Diagnostics, Indianapolis, IN) while insulin was measured using an automated immuno-enzymometeric assay (Tosoh Bioscience Inc., San Francisco, CA). Cytokines were collected and processed under a standardized protocol (22) and then stored at −80°C before being batch analyzed using commercially available fluorokine multianalyte profiling kits (R&D systems, Minneapolis, MN) on a Luminex 200 analyzer (Luminex Corporation, Austin, TX). As a measure of insulin resistance, we calculated the homeostasis model assessment (HOMA) from fasting glucose and insulin values (23), based on the formula: glucose (mmol/L)×insulin (mU/L) / 22.5.
Cardiometabolic traits were defined a priori via current adult International Diabetes Foundation Consensus criteria (9) for those age ≥18 years and pediatric adapted values for those age <18 years (Table 1). In sensitivity analysis, we applied criteria based on the older but widely used National Cholesterol Education Program Adult Treatment Panel III (ATP III) guidelines (7;8) with fasting glucose ≥100 mg/dL defined as abnormal. For this study, we tabulated the number of abnormal components present in each individual and categorized individuals as having the metabolic syndrome if any 3 or more of the 5 criteria were present.
Table 1.
Cardiometabolic trait definitions.1
| Consensus criteria | NCEP-ATPIII criteria | |||
|---|---|---|---|---|
| Trait | Adult (Ref 9) | Pediatric adaptation |
Adult (Ref 7) | Pediatric (Ref 8) |
| Obesity | BMI ≥30 kg/m2 or waist circumference males ≥94 cm; females ≥80 cm |
BMI ≥95th percentile for age and sex or waist circumference ≥90th percentile for age, sex, and ethnicity |
Waist circumference males >102 cm; females >88 cm |
Waist circumference ≥90th percentile for age and sex |
| High blood Pressure |
≥130/85 mmHg | ≥90th percentile for age, sex, and height or ≥120/80 mmHg (adult pre- hypertension threshold) |
≥130/85 mmHg | ≥90th percentile for age, sex, and height |
| Insulin Resistance |
Fasting glucose ≥100 mg/dL (5.6 mmol) |
Same | Fasting glucose ≥110 mg/dL (6.1 mmol/L)2 |
Same2 |
| High triglyceride levels |
≥150 mg/dL (1.7 mmol/L) |
≥110 mg/dL (1.24 mmol/L) |
≥150 mg/dL (1.7 mmol/L) |
≥110 mg/dL (1.24 mmol/L) |
| Low HDL cholesterol levels |
Males <40 mg/dL (1.03 mmol/L); females <50 mg/dL (1.29 mmol/L) |
≤40 mg/dL (1.0 mmol/L) |
Males <40 mg/dL (1.03 mmol/L); females <50 mg/dL (1.29 mmol/L) |
≤40 mg/dL (1.0 mmol/L) |
HDL: high density lipoprotein; NCEP-ATPIII: National Cholesterol Education Program – Adult Treatment Panel III
Any individual currently taking drugs used for hypertension, diabetes, and dyslipidemia was classified as fulfilling the criterion associated with blood pressure, insulin resistance, and high triglyceride / low HDL levels, respectively.
Redefined as ≥100 mg/dL in this study.
Statistical analyses
Continuous parameters with skewed distributions were transformed when possible. Differences in continuous parameters were compared using the t-test (or Wilcoxon rank sum test if distribution not normal), and differences in proportions assessed by Fisher’s exact test. All tests were two-sided. Multivariate linear regression models that included current age, sex, and participating institution (Seattle vs. Nashville) were used to assess differences in physical activity and diet (calories, fat intake) between patient cohorts. Linear regression models that also included BMI z-scores and presence of multiple cardiometabolic traits (≥2 vs. <2) were used to assess differences in cytokine levels between patient cohorts. Logistic regression models that included the above adjustment variables plus race/ethnicity (White vs. non-White) and family history of cardiovascular disease/diabetes also were used to estimate the odds ratios (OR) and 95% confidence intervals (CI) of meeting ≥2 cardiometabolic traits associated with potential risk factors: HCT status, cranial radiotherapy, chronic GVHD, and growth hormone deficiency. All analyses were performed using STATA, version 10 (Stata Corporation, College Station, TX)
RESULTS
Demographic and treatment characteristics
Basic demographic characteristics were similar for the 2 survivor cohorts (Table 2). Compared with responders, non-responders were slightly more likely to be female (55.8%), but were of similar current age (16 years, range 8–21) and median years since ALL diagnosis (9, range 3–19). The proportion of individuals with any family history of cardiovascular disease and/or diabetes was greater among HCT survivors (61.5%) compared with non-HCT survivors (37.5%; p=0.06). Reflecting contemporary treatment, only 10.4% of the non-HCT group received any cranial radiotherapy (all 1800 cGy), in contrast to the HCT group where 38.5% received some form of cranial radiotherapy, either as upfront therapy or as salvage therapy for recurrence (median 1000 cGy, range 600–2400 cGy). All HCT patients were conditioned with myeloablative doses of cyclophosphamide and TBI (median dose 1320 cGy, range 1200–1575). Most HCT recipients received bone marrow as their stem cell source (n=19; 73.1%), with the remainder receiving peripheral blood (n=5) or cord blood (n=2) products. Twenty-one transplants (80.8%) were HLA-matched with 11 of those using matched unrelated donors. No patient received more than one HCT. Thirteen HCT and 1 non-HCT survivor subsequently were reported to have developed growth hormone deficiency. Nine patients were currently receiving growth hormone supplementation.
Table 2.
Demographic and treatment characteristics of acute lymphoblastic leukemia (ALL) survivors stratified by hematopoietic cell transplantation (HCT) status.
| Characteristic | Non-HCT n=48 |
HCT n=26 |
P-value |
|---|---|---|---|
| Site, n (%) | |||
| Seattle, WA | 30 (62.5) | 21 (80.8) | 0.12 |
| Nashville, TN | 18 (37.5) | 5 (19.2) | |
| Female, n (%) | 26 (54.2) | 10 (38.5) | 0.23 |
| Non-white race/ethnicity | 10 (20.8) | 8 (30.8) | 0.40 |
| Median current age (range), yrs | 14 (8–21) | 15 (8–21) | 0.36 |
| Median years (range) since diagnosis | 10 (3–18) | 10.5 (1–15) | 0.83 |
| Median years (range) since HCT | - | 6 (1–13) | - |
| History of relapse, n (%)1 | - | 16 (61.5) | - |
| Radiation exposure, n (%) | |||
| Cranial radiotherapy | 5 (10.4) | 10 (38.5) | 0.007 |
| Total body irradiation | - | 26 (100) | - |
| History of chronic graft vs. host disease, n (%) | - | 16 (61.5) | - |
Eligibility for the non-HCT cohort was restricted to those in first complete remission. Ten HCT patients were transplanted in first complete remission due to very high risk disease per institutional practice.
Anthropometric and standard laboratory measurements
Although HCT survivors were significantly shorter than non-HCT survivors, the distribution of BMI z-scores and the proportion of overweight or obese individuals as defined by BMI were similar (42.3% vs. 39.6%, respectively; Table 3). The proportions defined as obese using BMI vs. waist circumference were similar for the HCT and non-HCT groups. However, HCT survivors had significantly increased waist-hip ratios, even after adjustment for sex and current age (Table 3).
Table 3.
Metabolic parameters of ALL survivors, stratified by HCT status.
| Outcomes | Non-HCT n=48 |
HCT n=26 |
P-value |
|---|---|---|---|
| Height z-score, mean (±SD) | 0.30 ± 0.99 | −0.59 ± 1.19 | <0.01 |
| BMI z-score, mean (±SD) | 0.80 ± 0.92 | 0.54 ± 1.29 | 0.31 |
| BMI ≥25 or ≥85th percentile for age & sex, n (%) | 19 (39.6) | 11 (42.3) | 1.00 |
| BMI ≥30 or ≥95th percentile for age & sex, n (%) | 13 (27.1) | 6 (23.1) | 0.79 |
| Waist ≥90th percentile for age & sex1, n (%) | 13 (27.1) | 7 (26.9) | 1.00 |
| Waist-hip ratio, mean (±SD) | 0.83 (0.07) | 0.90 (0.06) | <0.01 |
| Blood pressure ≥120/80 or ≥90th percentile for age & height, n (%) | 8 (16.7) | 11 (42.3) | 0.03 |
| Glucose, median (range), mg/dL | 82 (43–96) | 82 (63–110) | 0.76 |
| Insulin, median (range), mU/L | 6.5 (1.0, 26.9) | 11.7 (1.0, 39.6) | <0.01 |
| HOMA-IR, median (range) | 1.32 (0.11, 5.51) | 2.64 (0.18, 10.76) | <0.01 |
| Triglycerides, median (range), mg/dL | 63 (16, 177) | 127 (63, 327) | <0.01 |
| HDL, median (range), mg/dL | 54 (33, 108) | 45 (32, 63) | <0.01 |
| Cardiometabolic traits, n (%)2 | |||
| 0 | 24 (50.0) | 4 (15.4) | |
| 1 | 14 (29.2) | 8 (30.8) | Overall |
| 2 | 8 (16.7) | 8 (30.8) | <0.01 |
| ≥3 | 2 (4.2) | 6 (23.1) |
HOMA-IR: homeostasis model assessment of insulin resistance
Or if waist circumference ≥80 cm (females), ≥94 cm (males).
As defined by the Consensus criteria (Table 1).
HCT survivors were more likely to have blood pressures ≥90th percentile for age, sex, and height (or absolute values ≥120/80 mmHg) compared with non-HCT survivors (Table 3). However, the proportions with blood pressures ≥95th percentile or ≥140/90 mmHg were similar (11.5 vs. 8.3%; p=0.69). While median glucose values were similar for the 2 survivor cohorts, HCT survivors had significantly higher fasting insulin levels and measures of insulin resistance as estimated by HOMA (both p<0.01). HCT survivors also had significantly more adverse lipid profiles with higher triglyceride levels and lower HDL levels (both p<0.01).
When the number of survivors meeting cardiometabolic trait criteria was tabulated, compared with non-HCT survivors, a significantly greater proportion of HCT survivors met at least one criterion (84.6% vs. 50.0%) as well as having at least 3 criteria fulfilled (23.1% vs. 4.2%; global p-value <0.01; Table 3). When criteria were re-analyzed using the ATP III guidelines, the burden of traits remained greater among HCT survivors (p=0.04). However, obesity as defined by BMI was more common among non-HCT survivors with ≥2 traits compared with HCT survivors (9 [90%] vs. 5 [35.7%]; p-value=0.01).
Cytokine levels
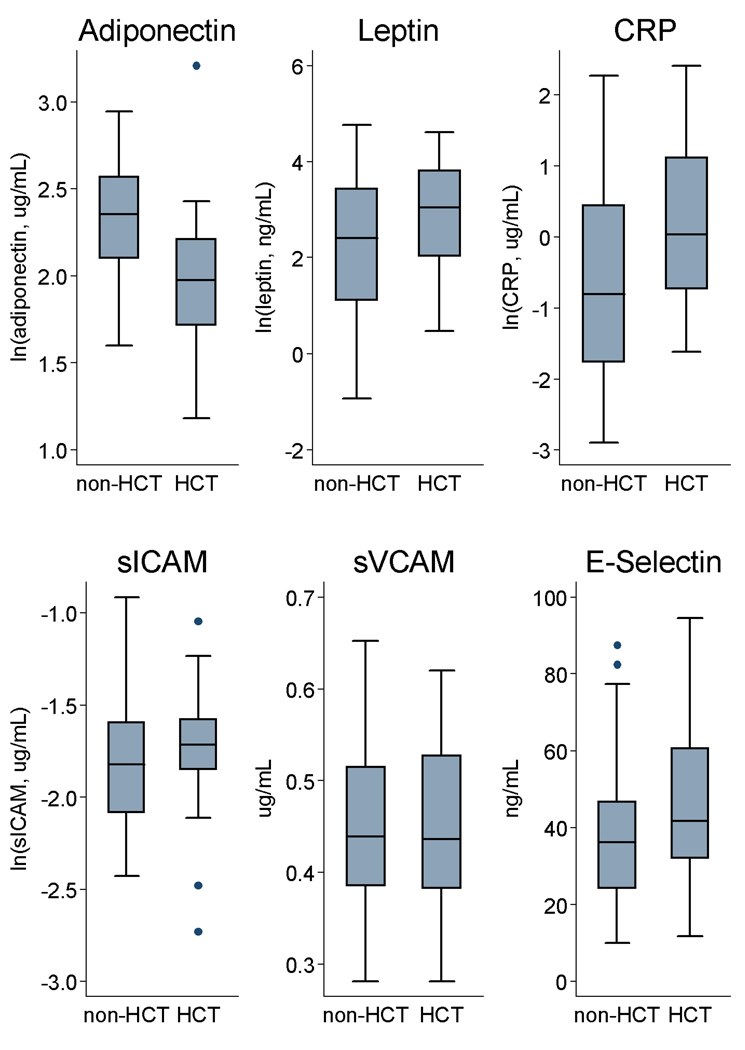
Compared with non-HCT survivors, HCT survivors had significantly decreased adiponectin and increased CRP levels, and borderline increased leptin levels (Figure 1). Markers of endothelial dysfunction (sICAM, vCAM, E-selectin) were similar across the two groups. We also obtained IL-6 and TNF-alpha levels in a subset of individuals (non-HCT, n=23; HCT, n=14), but no significant differences were observed. When HCT and non-HCT survivors were combined and stratified by the presence of <2 vs. ≥2 cardiometabolic traits, levels of leptin, CRP, and E-selectin were significantly increased while adiponectin was significantly decreased among those with ≥2 traits. Among HCT recipients alone, history of HLA-disparity and chronic GVHD were not associated with significant differences in cytokine levels.
Figure 1.
Distribution of selected biomarkers stratified by hematopoietic cell transplantation (HCT) status: adiponectin, leptin, C-reactive protein (CRP), soluble intercellular and vascular cell adhesion molecules (sICAM, sVCAM) and E-selectin. Boxes show median values and interquartile ranges with whiskers denoting upper and lower adjacent values; outside values marked by closed circles. Differences between HCT and non-HCT survivors were significant for adiponectin and CRP (p<0.001 and p=0.02, respectively); distribution of other biomarkers were not significantly different in unadjusted analyses (leptin, p=0.08; sICAM, p=0.17; sVCAM, p=0.96; E-selectin, p=0.19).
Multivariate analyses
In adjusted analyses, history of HCT remained a significant risk factor for having ≥2 cardiometabolic traits (OR 5.13, 95% CI 1.54, 17.15) as well as meeting metabolic syndrome criteria (≥3 traits, OR 16.72, 95% CI 1.66, 168.80). If ATP III criteria were used instead, history of HCT remained significant (≥2 traits, OR 4.16, 95% CI 1.07, 16.10; ≥3 traits, OR 22.99, 95% CI 1.41, 373.65). Compared with those who received no radiotherapy exposure to the brain, survivors treated with cranial radiotherapy/TBI alone and cranial radiotherapy plus TBI both were associated with similar magnitude risks of manifesting ≥2 cardiometabolic traits (ORs ranged 5–6). Positive family history also was significantly associated with ≥2 traits, independent of HCT status (OR 3.65, 95% CI 1.15, 11.57). However, diagnosis age, time interval since diagnosis, sex, and history of chronic GVHD or growth hormone deficiency (even if those currently on supplementation considered separately) were not associated with having ≥2 traits.
Risk estimates were not associated nor modified by the addition of physical activity or caloric intake levels even though HCT survivors had reduced activity scores and borderline decreased caloric intake compared with non-HCT survivors (Table 4). However, the proportion of calories from fats was similar for both groups (data not shown).
Table 4.
Multivariate regression estimates (coefficients [Coeff] with 95% confidence intervals) for selected parameters among ALL survivors, adjusted for sex, current age, race/ethnicity, and institution.
| Parameter | Non-HCT | HCT | P-value |
|---|---|---|---|
| Physical activity1 | Ref | Coeff −1.62 (−2.90, −0.34) | 0.01 |
| Calories1 | Ref | Coeff −0.21 (−0.42, −0.002) | 0.048 |
| Biomarkers2 | |||
| Adiponectin1 | Ref | Coeff −0.32 (−0.52, −0.13) | 0.001 |
| Leptin1 | Ref | Coeff 1.01 (0.55, 1.46) | <0.001 |
| C-reactive protein1 | Ref | Coeff 0.43 (−0.33, 1.19) | 0.26 |
| sICAM1 | Ref | Coeff 0.14 (−0.01 to 0.30) | .07 |
| sVCAM | Ref | Coeff 0.03 (−0.02 to 0.08) | .24 |
| E-selectin | Ref | Coeff 2.54 (−8.11 to 13.19) | .64 |
Ref: referent group; sICAM, sVCAM: soluble intercellular and vascular cell adhesion molecules.
Logarithmically transformed in order to normalize data distribution.
Also adjusted for BMI z-score and presence of ≥2 metabolic syndrome traits.
In linear regression models, HCT status was significantly associated with lower adiponectin levels and higher leptin levels (Table 4). However, HCT status was no longer associated with increased CRP level independent of the presence of ≥2 traits. History of HLA-disparity, chronic GVHD, growth hormone deficiency, and radiation exposure to the brain were not associated with differences in cytokine levels in our adjusted analyses.
DISCUSSION
Various follow-up studies of adult survivors of pediatric ALL have reported increased obesity, insulin resistance, and dyslipidemia (3;5;6). Among studies that have specifically examined transplant survivors, both pediatric and adult HCT recipients appear to be at increased risk of cardiometabolic traits, particularly dyslipidemia (2;4;24–27). HCT survivors also have been reported to have an increased risk of developing diabetes (25;28;29) and CV disease (30–32). However, few studies have directly compared pediatric ALL HCT and non-HCT survivors, particularly survivors treated in the contemporary era when cranial radiotherapy is used less commonly but chemotherapy is more intensive. Our results suggest that young HCT survivors uniformly treated with TBI are at significantly greater risk of cardiometabolic traits and the metabolic syndrome compared with similar-aged non-HCT survivors not exposed to any cranial radiotherapy. Although study power was limited, additional cranial radiotherapy did not appear to markedly increase risk beyond that associated with TBI. Although no standard pediatric definition of metabolic syndrome exists and prevalence estimates can vary depending on the criteria used (33), our findings were consistent across two classification schemes.
The finding that cardiometabolic changes may be occurring soon after treatment in childhood is important as data from the general population suggest that cardiometabolic traits that develop in childhood often persist into adulthood (34;35). However, in contrast to findings from the general population (8) and among our non-HCT ALL survivors, HCT survivors often manifest cardiometabolic traits such as dyslipidemia and insulin resistance without being “obese” as measured by BMI (24;27;29). Instead, more direct measures of central/abdominal adiposity such as waist-hip ratios may be a more useful screening tool in the HCT population. In the general population, central adiposity is correlated with visceral fat, and has been shown to be an independent risk factor for cardiovascular disease and diabetes, even after adjusting for BMI (8;9;36).
As shown in other studies (3;4;6;25;37), we found that TBI and cranial radiotherapy both were strongly associated with subsequent metabolic abnormalities. Both are known risk factors for subsequent growth hormone deficiency. While we did not find history of growth hormone deficiency (regardless of supplementation) to be an independent risk factor for increased cardiometabolic traits, patients were not prospectively tested for this study and it is possible that some patients may have undiagnosed deficiency, particularly post-pubertal patients for whom short stature is less of a concern. Other studies that prospectively tested all participants for growth hormone deficiency have shown that growth hormone deficient individuals were at increased risk (3;38).
In addition to TBI, other factors associated with allogeneic HCT may lead to an increased risk of cardiometabolic complications. Large adult series have found allogeneic HCT recipients, even after adjusting for TBI exposure, are at increased risk of cardiovascular complications compared with autologous HCT (25;30). Some evidence suggests that GVHD following allogeneic HCT can result in chronic low-level inflammation, endothelial dysfunction, and an atherosclerotic phenotype (12;13;39). Although we did not find history of chronic GVHD to be associated with cytokine differences or as an independent risk factor for the development of multiple cardiometabolic traits in our study, this may be due to our limited sample size and our exclusion of patients still actively being treated for GVHD. A larger study with a more detailed analysis of GVHD (e.g. duration of treatment, severity/extent) may yet reveal more subtle effects of GVHD as well as other factors such as stem cell source and HLA-disparity that may influence immune tolerance, inflammation, and the development of cardiometabolic outcomes.
Chronic inflammation appears to be a central pathophysiologic mechanism underlying development of diabetes and atherosclerotic cardiovascular disease in the general population (11). For example, levels of C-reactive protein, E-selectin (mediates leukocyte recruitment and rolling in inflamed tissues), and adipose tissue cytokines such as adiponectin and leptin have been shown to be altered in patients with the metabolic syndrome (36). Adiponectin enhances insulin sensitivity with favorable effects on endothelial function while leptin promotes atherogenesis and is an independent risk factor for cardiovascular disease (40;41). Leptin is produced primarily by subcutaneous adipocytes while adiponectin levels are strongly correlated with visceral fat (40;41). Childhood ALL survivors treated with cranial radiotherapy have been shown to have primarily increased visceral versus subcutaneous fat via computed tomography (42).
Other factors that may contribute to development of cardiometabolic traits include sedentary lifestyle, diet, and family history (both genetic and environmental influences) (43). Studies of adult and pediatric cancer survivors have found that survivors tend to be less physically active versus the general population (44). Dietary studies have generally reported low adherence to recommended dietary guidelines among childhood cancer survivors, suggesting another potential area for intervention (45). Although the proportion of HCT survivors with a positive family history of cardiovascular disease and/or diabetes was greater compared with non-HCT survivors, HCT status remained a significant independent risk factor in our multivariate analyses. Nevertheless, genetic polymorphisms in selected pathways (e.g. adiponectin and leptin receptor genes) may be important in mediating variation in risk in the general population (46) as well as in cancer survivors (47).
In conclusion, we found that cardiometabolic traits, including meeting metabolic syndrome criteria, were common among pediatric ALL survivors treated with TBI-based HCT compared with ALL survivors treated with conventional chemotherapy. Manifestations appeared at an early age and suggest that ALL survivors treated with TBI or cranial radiotherapy should be closely followed and screened for dyslipidemia and diabetes, even if not overweight or obese by BMI standards. Alternative simple measures of central adiposity, such as waist-hip ratios may better identify individuals at potential higher risk. The important role of central adiposity and inflammation in mediating cardiometabolic complications was supported by altered cytokine levels corresponding to a pro-inflammatory state including decreased adiponectin in HCT survivors.
ACKNOWLEDGEMENTS
Supported in part by CTSA grants from the National Center for Research Resources, National Institutes of Health, awarded to the University of Washington (UL1RR025014) and Vanderbilt University (UL1RR024975), as well as a Young Investigator Award from the American Society of Clinical Oncology / Lance Armstrong Foundation (E.J.C.) and a Special Fellowship in Clinical Research from the Leukemia and Lymphoma Society (E.J.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: none
Presented in part at the 50th annual meeting of the American Society of Hematology, San Francisco, CA, December 2008.
REFERENCES
- 1.Margolin JF, Steuber CP, Poplack DG. Acute lymphoblastic leukemia. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. pp. 538–590. [Google Scholar]
- 2.Taskinen M, Saarinen-Pihkala UM, Hovi L, Lipsanen-Nyman M. Impaired glucose tolerance and dyslipidaemia as late effects after bone-marrow transplantation in childhood. Lancet. 2000;356:993–997. doi: 10.1016/S0140-6736(00)02717-3. [DOI] [PubMed] [Google Scholar]
- 3.Gurney JG, Ness KK, Sibley SD, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107:1303–1312. doi: 10.1002/cncr.22120. [DOI] [PubMed] [Google Scholar]
- 4.Steffens M, Beauloye V, Brichard B, et al. Endocrine and metabolic disorders in young adult survivors of childhood acute lymphoblastic leukaemia (ALL) or non-Hodgkin lymphoma (NHL) Clin Endocrinol (Oxf) 2008;69:819–827. doi: 10.1111/j.1365-2265.2008.03283.x. [DOI] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Adams-Huet B, Victor RG, et al. Insulin resistance and risk factors for cardiovascular disease in young adult survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2009;27:3698–3704. doi: 10.1200/JCO.2008.19.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meacham LR, Chow EJ, Ness KK, et al. Cardiovascular risk factors in adult survivors of pediatric cancer--a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2010;19:170–181. doi: 10.1158/1055-9965.EPI-09-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2897. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 8.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 9.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome: a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 10.Talvensaari KK, Lanning M, Tapanainen P, Knip M. Long-term survivors of childhood cancer have an increased risk of manifesting the metabolic syndrome. J Clin Endocrinol Metab. 1996;81:3051–3055. doi: 10.1210/jcem.81.8.8768873. [DOI] [PubMed] [Google Scholar]
- 11.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 12.Tauchmanova L, Matarese G, Carella C, et al. High serum leptin in patients with chronic graft-versus-host disease after hematopoietic stem cell transplantation. Transplantation. 2004;78:1376–1383. doi: 10.1097/01.tp.0000140485.20848.b7. [DOI] [PubMed] [Google Scholar]
- 13.Biedermann BC. Vascular endothelium and graft-versus-host disease. Best Pract Res Clin Haematol. 2008;21:129–138. doi: 10.1016/j.beha.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Chow EJ, Pihoker C, Hunt K, Wilkinson K, Friedman DL. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer. 2007;110:2313–2320. doi: 10.1002/cncr.23050. [DOI] [PubMed] [Google Scholar]
- 15.Chao NJ, Sullivan KM. Pharmacologic prevention of acute graft versus host disease. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas' Hematopoietic Cell Transplantation. 4th ed. West Sussex: Wiley-Blackwell; 2008. pp. 1257–1274. [Google Scholar]
- 16.Godin G, Jobin J, Bouillon J. Assessment of leisure time exercise behavior by self-report: a concurrent validity study. Can J Public Health. 1986;77:359–362. [PubMed] [Google Scholar]
- 17.Neuhouser ML, Rock CL, Eldridge AL, et al. Serum concentrations of retinol, alpha-tocopherol and the carotenoids are influenced by diet, race and obesity in a sample of healthy adolescents. J Nutr. 2001;131:2184–2191. doi: 10.1093/jn/131.8.2184. [DOI] [PubMed] [Google Scholar]
- 18.Higgins M, Province M, Heiss G, et al. NHLBI Family Heart Study: objectives and design. Am J Epidemiol. 1996;143:1219–1228. doi: 10.1093/oxfordjournals.aje.a008709. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Growth charts. [accessed April 6, 2010]; http://www.cdc.gov/growthcharts/computer_programs.htm.
- 20.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 21.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555–576. [PubMed] [Google Scholar]
- 22.Mozaffarian D, Rimm EB, King IB, Lawler RL, McDonald GB, Levy WC. Trans fatty acids and systemic inflammation in heart failure. Am J Clin Nutr. 2004;80:1521–1525. doi: 10.1093/ajcn/80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.Shalitin S, Phillip M, Stein J, Goshen Y, Carmi D, Yaniv I. Endocrine dysfunction and parameters of the metabolic syndrome after bone marrow transplantation during childhood and adolescence. Bone Marrow Transplant. 2006;37:1109–1117. doi: 10.1038/sj.bmt.1705374. [DOI] [PubMed] [Google Scholar]
- 25.Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109:1765–1772. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Annaloro C, Usardi P, Airaghi L, et al. Prevalence of metabolic syndrome in long-term survivors of hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:797–804. doi: 10.1038/sj.bmt.1705972. [DOI] [PubMed] [Google Scholar]
- 27.Majhail NS, Flowers ME, Ness KK, et al. High prevalence of metabolic syndrome after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2009;43:49–54. doi: 10.1038/bmt.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmeister PA, Storer BE, Sanders JE. Diabetes mellitus in long-term survivors of pediatric hematopoietic cell transplantation. J Pediatr Hematol Oncol. 2004;26:81–90. doi: 10.1097/00043426-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Neville KA, Cohn RJ, Steinbeck KS, Johnston K, Walker JL. Hyperinsulinemia, impaired glucose tolerance, and diabetes mellitus in survivors of childhood cancer: prevalence and risk factors. J Clin Endocrinol Metab. 2006;91:4401–4407. doi: 10.1210/jc.2006-0128. [DOI] [PubMed] [Google Scholar]
- 30.Tichelli A, Bucher C, Rovo A, et al. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood. 2007;110:3463–3471. doi: 10.1182/blood-2006-10-054080. [DOI] [PubMed] [Google Scholar]
- 31.Armenian SH, Sun CL, Francisco L, et al. Late congestive heart failure after hematopoietic cell transplantation. J Clin Oncol. 2008;26:5537–5543. doi: 10.1200/JCO.2008.17.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmeister PA, Hingorani SR, Storer BE, Baker KS, Sanders JE. Hypertension in Long-Term Survivors Of Pediatric Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2010;16:515–524. doi: 10.1016/j.bbmt.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ford ES, Li C. Defining the metabolic syndrome in children and adolescents: will the real definition please stand up? J Pediatr. 2008;152:160–164. doi: 10.1016/j.jpeds.2007.07.056. [DOI] [PubMed] [Google Scholar]
- 34.Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 35.Huang TT, Nansel TR, Belsheim AR, Morrison JA. Sensitivity, specificity, and predictive values of pediatric metabolic syndrome components in relation to adult metabolic syndrome: the Princeton LRC follow-up study. J Pediatr. 2008;152:185–190. doi: 10.1016/j.jpeds.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S, Bacha F, Gungor N, Arslanian S. Comparison of different definitions of pediatric metabolic syndrome: relation to abdominal adiposity, insulin resistance, adiponectin, and inflammatory biomarkers. J Pediatr. 2008;152:177–184. doi: 10.1016/j.jpeds.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 37.Jarfelt M, Lannering B, Bosaeus I, Johannsson G, Bjarnason R. Body composition in young adult survivors of childhood acute lymphoblastic leukaemia. Eur J Endocrinol. 2005;153:81–89. doi: 10.1530/eje.1.01931. [DOI] [PubMed] [Google Scholar]
- 38.Taskinen M, Lipsanen-Nyman M, Tiitinen A, Hovi L, Saarinen-Pihkala UM. Insufficient growth hormone secretion is associated with metabolic syndrome after allogeneic stem cell transplantation in childhood. J Pediatr Hematol Oncol. 2007;29:529–534. doi: 10.1097/MPH.0b013e3180f61b67. [DOI] [PubMed] [Google Scholar]
- 39.Tichelli A, Bhatia S, Socie G. Cardiac and cardiovascular consequences after haematopoietic stem cell transplantation. Br J Haematol. 2008;142:11–26. doi: 10.1111/j.1365-2141.2008.07165.x. [DOI] [PubMed] [Google Scholar]
- 40.Hopkins TA, Ouchi N, Shibata R, Walsh K. Adiponectin actions in the cardiovascular system. Cardiovasc Res. 2007;74:11–18. doi: 10.1016/j.cardiores.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antuna-Puente B, Feve B, Fellahi S, Bastard JP. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab. 2008;34:2–11. doi: 10.1016/j.diabet.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Janiszewski PM, Oeffinger KC, Church TS, et al. Abdominal obesity, liver fat, and muscle composition in survivors of childhood acute lymphoblastic leukemia. J Clin Endocrinol Metab. 2007;92:3816–3821. doi: 10.1210/jc.2006-2178. [DOI] [PubMed] [Google Scholar]
- 43.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demark-Wahnefried W, Jones LW. Promoting a healthy lifestyle among cancer survivors. Hematol Oncol Clin North Am. 2008;22:319–342. viii. doi: 10.1016/j.hoc.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robien K, Ness KK, Klesges LM, Baker KS, Gurney JG. Poor adherence to dietary guidelines among adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2008;30:815–822. doi: 10.1097/MPH.0b013e31817e4ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richards JB, Waterworth D, O'Rahilly S, et al. A genome-wide association study reveals variants in ARL15 that influence adiponectin levels. PLoS Genet. 2009;5(12):e1000768. doi: 10.1371/journal.pgen.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ross JA, Oeffinger KC, Davies SM, et al. Genetic variation in the leptin receptor gene and obesity in survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2004;22:3558–3562. doi: 10.1200/JCO.2004.11.152. [DOI] [PubMed] [Google Scholar]