Abstract
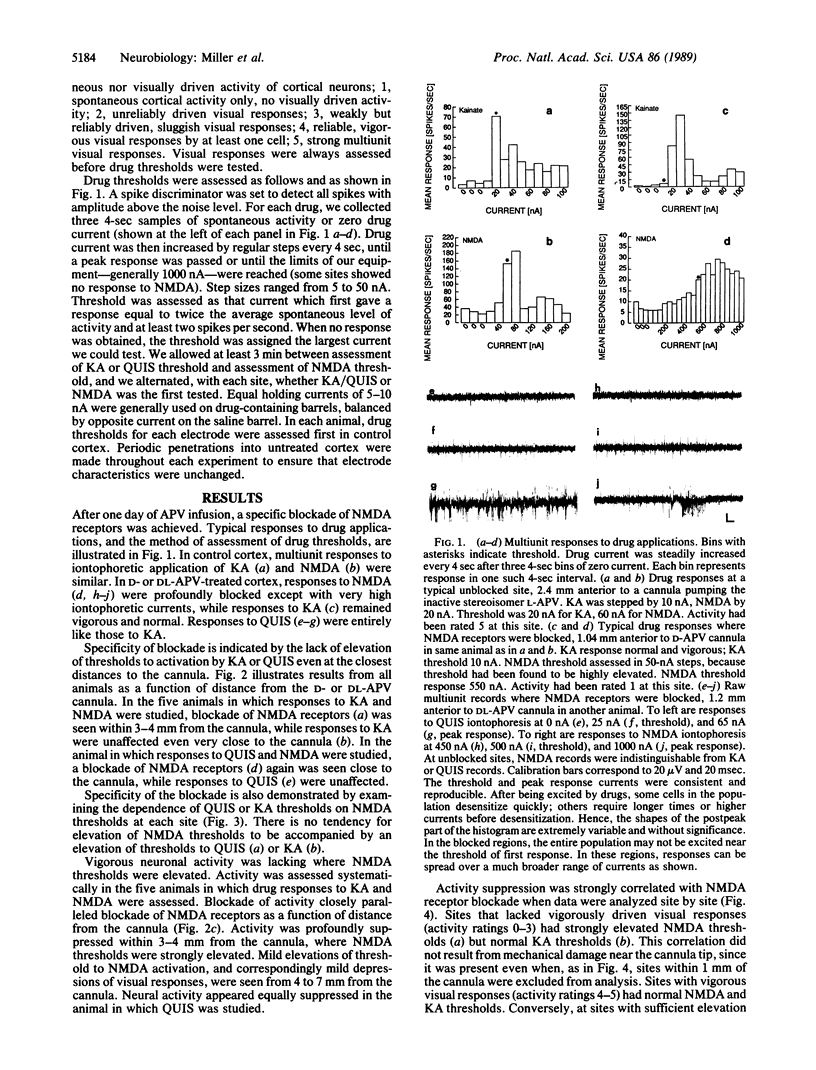
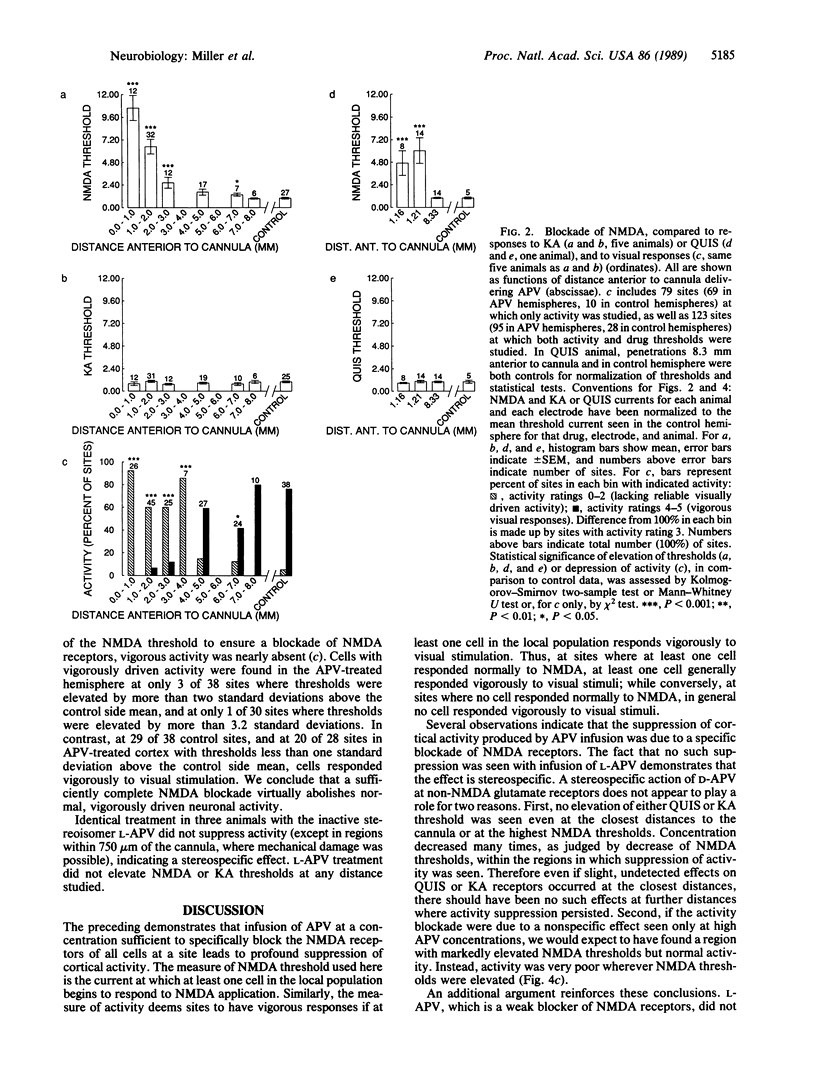
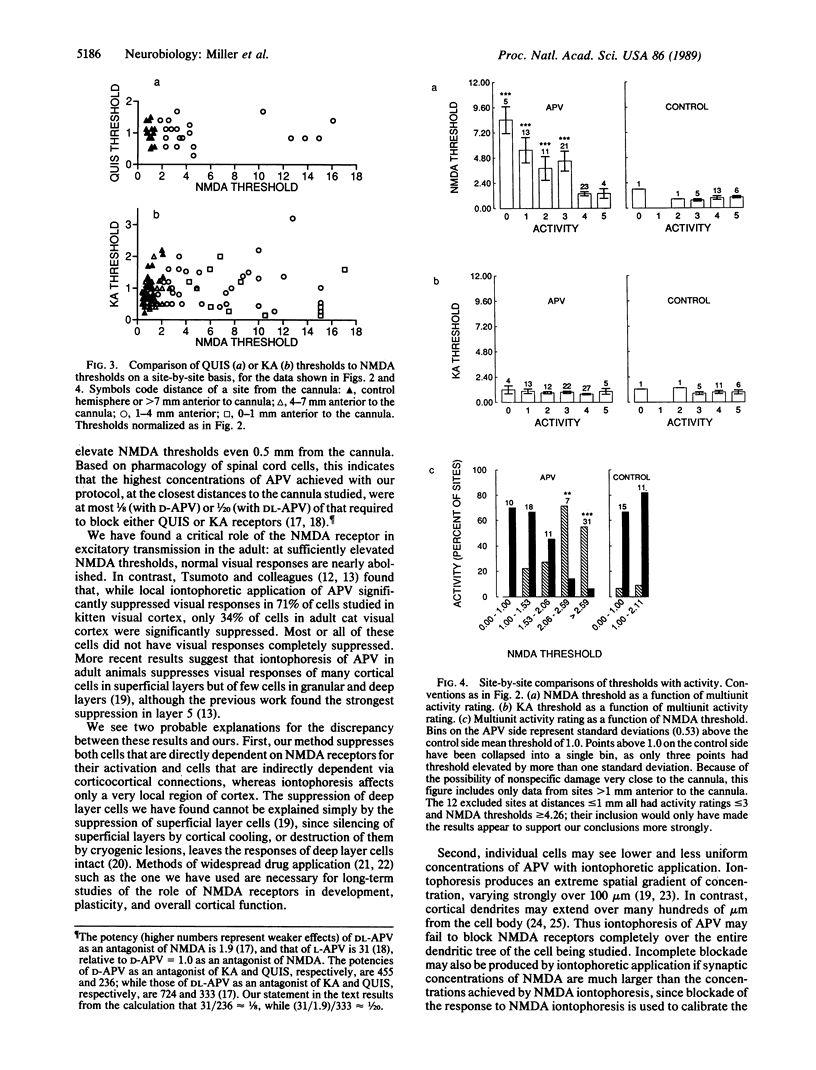
We have investigated the role of the N-methyl-D-aspartate (NMDA) receptor, a subtype of glutamate receptor, in the responses of cells in adult cat visual cortex. After intracortical infusion of the NMDA receptor antagonist DL-2-amino-5-phosphonovalerate (DL-APV) for one day, iontophoretic responses to NMDA, to kainate, and to quisqualate revealed a receptor blockade specific to NMDA receptors and extending several millimeters from the cannula. In this region, neuronal responses to visual stimulation were profoundly suppressed, in a manner strongly correlated with the degree of NMDA receptor blockade. Neither NMDA receptor blockade nor activity suppression was caused by the inactive stereoisomer L-APV. Hence, we conclude that NMDA receptors make a major contribution to normal excitatory transmission in adult visual cortex.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collingridge G. L., Herron C. E., Lester R. A. Frequency-dependent N-methyl-D-aspartate receptor-mediated synaptic transmission in rat hippocampus. J Physiol. 1988 May;399:301–312. doi: 10.1113/jphysiol.1988.sp017081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Herron C. E., Lester R. A. Synaptic activation of N-methyl-D-aspartate receptors in the Schaffer collateral-commissural pathway of rat hippocampus. J Physiol. 1988 May;399:283–300. doi: 10.1113/jphysiol.1988.sp017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Kehl S. J., McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983 Jan;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O., Ito M. Functional synaptic organization of primary visual cortex neurones in the cat. Exp Brain Res. 1968;6(4):324–352. doi: 10.1007/BF00233183. [DOI] [PubMed] [Google Scholar]
- Dale N., Roberts A. Dual-component amino-acid-mediated synaptic potentials: excitatory drive for swimming in Xenopus embryos. J Physiol. 1985 Jun;363:35–59. doi: 10.1113/jphysiol.1985.sp015694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Miller A. J., Sheardown M. J. Amino acid receptor mediated excitatory synaptic transmission in the cat red nucleus. J Physiol. 1986 Jul;376:13–29. doi: 10.1113/jphysiol.1986.sp016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Actions of D and L forms of 2-amino-5-phosphonovalerate and 2-amino-4-phosphonobutyrate in the cat spinal cord. Brain Res. 1982 Mar 11;235(2):378–386. doi: 10.1016/0006-8993(82)91017-4. [DOI] [PubMed] [Google Scholar]
- Evans R. H., Francis A. A., Jones A. W., Smith D. A., Watkins J. C. The effects of a series of omega-phosphonic alpha-carboxylic amino acids on electrically evoked and excitant amino acid-induced responses in isolated spinal cord preparations. Br J Pharmacol. 1982 Jan;75(1):65–75. doi: 10.1111/j.1476-5381.1982.tb08758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L. Slow excitatory postsynaptic currents mediated by N-methyl-D-aspartate receptors on cultured mouse central neurones. J Physiol. 1988 Feb;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K., Armstrong-James M. The role of the anterior intralaminar nuclei and N-methyl D-aspartate receptors in the generation of spontaneous bursts in rat neocortical neurones. Exp Brain Res. 1986;63(3):505–518. doi: 10.1007/BF00237474. [DOI] [PubMed] [Google Scholar]
- Hagihara K., Tsumoto T., Sato H., Hata Y. Actions of excitatory amino acid antagonists on geniculo-cortical transmission in the cat's visual cortex. Exp Brain Res. 1988;69(2):407–416. doi: 10.1007/BF00247586. [DOI] [PubMed] [Google Scholar]
- Herron C. E., Lester R. A., Coan E. J., Collingridge G. L. Frequency-dependent involvement of NMDA receptors in the hippocampus: a novel synaptic mechanism. Nature. 1986 Jul 17;322(6076):265–268. doi: 10.1038/322265a0. [DOI] [PubMed] [Google Scholar]
- Herz A., Zieglgänsberger W., Färber G. Microelectrophoretic studies concerning the spread of glutamic acid and GABA in brain tissue. Exp Brain Res. 1969;9(3):221–235. doi: 10.1007/BF00234456. [DOI] [PubMed] [Google Scholar]
- Hubel D. H. Tungsten Microelectrode for Recording from Single Units. Science. 1957 Mar 22;125(3247):549–550. doi: 10.1126/science.125.3247.549. [DOI] [PubMed] [Google Scholar]
- Huettner J. E., Baughman R. W. The pharmacology of synapses formed by identified corticocollicular neurons in primary cultures of rat visual cortex. J Neurosci. 1988 Jan;8(1):160–175. doi: 10.1523/JNEUROSCI.08-01-00160.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. W., Smith D. A., Watkins J. C. Structure-activity relations of dipeptide antagonists of excitatory amino acids. Neuroscience. 1984 Oct;13(2):573–581. doi: 10.1016/0306-4522(84)90250-1. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Baughman R. W. NMDA- and non-NMDA-receptor components of excitatory synaptic potentials recorded from cells in layer V of rat visual cortex. J Neurosci. 1988 Sep;8(9):3522–3534. doi: 10.1523/JNEUROSCI.08-09-03522.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L. C. Local circuitry of identified projection neurons in cat visual cortex brain slices. J Neurosci. 1987 Apr;7(4):1223–1249. doi: 10.1523/JNEUROSCI.07-04-01223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt A., Bear M. F., Singer W. Blockade of "NMDA" receptors disrupts experience-dependent plasticity of kitten striate cortex. Science. 1987 Oct 16;238(4825):355–358. doi: 10.1126/science.2443978. [DOI] [PubMed] [Google Scholar]
- Lund J. S., Henry G. H., MacQueen C. L., Harvey A. R. Anatomical organization of the primary visual cortex (area 17) of the cat. A comparison with area 17 of the macaque monkey. J Comp Neurol. 1979 Apr 15;184(4):599–618. doi: 10.1002/cne.901840402. [DOI] [PubMed] [Google Scholar]
- Lübbert H., Hoffman B. J., Snutch T. P., van Dyke T., Levine A. J., Hartig P. R., Lester H. A., Davidson N. cDNA cloning of a serotonin 5-HT1C receptor by electrophysiological assays of mRNA-injected Xenopus oocytes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4332–4336. doi: 10.1073/pnas.84.12.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Reiter H. O., Waitzman D. M., Stryker M. P. Cortical activity blockade prevents ocular dominance plasticity in the kitten visual cortex. Exp Brain Res. 1986;65(1):182–188. doi: 10.1007/BF00243841. [DOI] [PubMed] [Google Scholar]
- Salt T. E. Excitatory amino acid receptors and synaptic transmission in the rat ventrobasal thalamus. J Physiol. 1987 Oct;391:499–510. doi: 10.1113/jphysiol.1987.sp016752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt T. E. Mediation of thalamic sensory input by both NMDA receptors and non-NMDA receptors. Nature. 1986 Jul 17;322(6076):263–265. doi: 10.1038/322263a0. [DOI] [PubMed] [Google Scholar]
- Schwark H. D., Malpeli J. G., Weyand T. G., Lee C. Cat area 17. II. Response properties of infragranular layer neurons in the absence of supragranular layer activity. J Neurophysiol. 1986 Oct;56(4):1074–1087. doi: 10.1152/jn.1986.56.4.1074. [DOI] [PubMed] [Google Scholar]
- Stryker M. P., Harris W. A. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci. 1986 Aug;6(8):2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumoto T., Hagihara K., Sato H., Hata Y. NMDA receptors in the visual cortex of young kittens are more effective than those of adult cats. Nature. 1987 Jun 11;327(6122):513–514. doi: 10.1038/327513a0. [DOI] [PubMed] [Google Scholar]
- Wigström H., Gustafsson B., Huang Y. Y. A synaptic potential following single volleys in the hippocampal CA1 region possibly involved in the induction of long-lasting potentiation. Acta Physiol Scand. 1985 Jul;124(3):475–478. doi: 10.1111/j.1748-1716.1985.tb07685.x. [DOI] [PubMed] [Google Scholar]


