Abstract
Though there are multiple routes through which parents can influence their offspring, recent studies of environmentally induced epigenetic variation have highlighted the role of non-genomic pathways. In addition to the experience-dependent modification of DNA methylation that can be achieved via mother-infant interactions, there has been increasing interest in the epigenetic mechanisms through which paternal influences on offspring development can be achieved. Epidemiological and laboratory studies suggest that paternal nutritional and toxicological exposures as well as paternal age and phenotypic variation can lead to variations in offspring and, in some cases, grand-offspring development. These findings suggest a potential epigenetic germline inheritance of paternal effects. However, it may be important to consider the interplay between maternal and paternal influences as well as the experimental dissociation between experience-dependent and germline transmission when exploring the role of epigenetic variation within the germline as a mediator of these effects. In this review, we will explore these issues, with a particular focus on the potential role of paternally-induced maternal investment, highlight the literature illustrating the transgenerational impact of paternal experiences, and discuss the evidence supporting the role of epigenetic mechanisms in maintaining paternal effects both within and across generations.
The study of parental influences, in both epidemiological and laboratory contexts, suggests that there are diverse pathways though which parents can shape their offspring's development. In mammals, the intense prenatal and postnatal investment of mothers in the care of their offspring, and the rarity of bi-parental care, has directed much of the research on parental effects to the role of mother-infant interactions in promoting survival and adaptive development. However, even amongst species in which paternal investment in offspring care is limited, there is evidence for paternal effects. Within the literature, “paternal effects” on development can have a variety of meanings. In addition to describing the influences of male care-giving in species where bi-parental or exclusively paternal care is observed (e.g. marmosets, prairie voles, Peromyscus californicus), this term can also refer to inheritance of genes through the patriline which exhibit parent-of-origin expression patterns (i.e. imprinted genes that are expressed exclusively from the father such as Peg1 & Peg3), or the influence of genes expressed on the Y chromosome that can exert effects on male brain and behavioral development independent of the effects of Sry on hormonally regulated sexual differentiation. More recently, there has been increased interest in exploring the observation that the life-history experiences of males (e.g., nutrition, toxin exposure) can influence the development of both their male and female offspring. These observations, coupled with advances in our understanding of the persistence of environmentally-induced epigenetic modifications, has lead to speculation regarding the germline transmission of environmental experiences and a re-evaluation of the concepts inherent in Lamarckian theories of the inheritance of acquired traits. Though this inheritance system would not be predicted to occur exclusively in the patriline, the information transmitted via sperm during the process of fertilization is thought to be limited to genetic/epigenetic material, whereas the maternal oocyte contributes both genetic/epigenetic factors and a cellular environment which can regulate the activity of those factors, thus making it difficult to separate the unique contribution of maternal epigenetic modifications. Thus, the study of environmentally-induced paternal germline epigenetic effects is currently expanding and may provide an explanation for the transgenerational influence of father's experiences on offspring development. However, we propose that there are important experimental design issues that must be considered when exploring the mechanisms of paternal effects. In particular, it is important to consider the distinction between effects on a germ cell vs. the primordial germ line, the number of generations an effect must persist to be considered a germline transmission, and the possible interplay between maternal and paternal effects that may moderate or mediate the occurrence of paternal effects. In this review, we will highlight the studies in humans and animals that indicate an inheritance of paternal experiences, discuss the theoretical pathways through which these effects may be achieved, and discuss the role of epigenetic mechanisms in mediating paternal influences.
Paternal nutrition: Influences across generations
Large-scale epidemiological studies have established that the diet and nutritional status of fathers and grandfathers can exert transgenerational effects on the phenotypes of sons and grandsons, with particular influences on metabolic functioning. For instance, archival data indicate that food availability during the pre-pubertal slow growth phase (8-12 years of age) of grandfathers is associated with the risk of diabetes and cardiovascular disease as well as mortality in grandsons but not granddaughters (Kaati et al., 2002; Kaati et al., 2007; Pembrey et al., 2006). In rodents, changing the quantity or quality of a male's diet at various developmental time points has also been found to induce phenotypic changes in male offspring. For example, males exposed to prenatal dietary restriction (through reductions in caloric intake of their mother during late gestation), who are then fed ad libitum throughout the rest of their life, sire offspring with reduced birth weights and impaired glucose tolerance compared to fathers who were born to control dams (Jimenez-Chillaron et al., 2009). Restricting the caloric intake of males prior to mating can also lead to altered metabolic functioning of offspring. Males exposed to a single 24 hour period of food deprivation two weeks before they were mated were found to have offspring with reduced serum glucose and altered levels of corticosterone and IGF1 compared to males who did not fast at this time point (Anderson et al., 2006). The nutrient composition of food intake may also have consequences for future generations. For instance, the chewing of betel nuts (which contain nitrosamines) is very popular across South-east Asia and Polynesia, and individuals who do this are known to be at an increased risk of developing metabolic syndrome (Lin et al., 2008). Interestingly, it has been recently demonstrated that the duration and quantity of betel nut intake by males is positively related to the risk of their own offspring developing metabolic syndrome (Chen et al., 2006). Significantly, this finding has been confirmed in a mouse study, with offspring sired by males who were exposed to betel nuts in their diet prior to mating being at an increased risk for developing hyperglycemia (Boucher et al., 1994). Moreover, the inheritance of this phenotype can be transmitted for at least three generations. Increased body length and reduced insulin sensitivity have also been observed amongst mice born to dams fed a high-fat diet from pre-conception to the weaning period (Dunn and Bale, 2009) and offspring and grand-offspring of rat dams fed a low protein diet during gestation have elevated hypertension, despite both these generations being fed control diets (Harrison and Langley-Evans, 2009). In both of these studies, the induced phenotypes were transmissible to the next generation via either the male or female line. Overall, these studies indicate that dietary effects, achieved through both quantity and quality of food intake, can induce effects on male phenotype that may be inherited by subsequent generations.
Exposure to drugs, toxins and endocrine disruptors
Epidemiological studies have demonstrated that the exposure of fathers to various drugs, toxins and other chemicals, such as endocrine disruptors, before mating is associated with altered behavioral development in their children, even after accounting for other potential confounding lifestyle variables. For instance, the early onset of paternal smoking is related to greater body mass index of sons (Pembrey et al., 2006), whereas paternal alcoholism is associated with reduced birth weight in offspring (Little, 1987). Interestingly, children of alcoholic fathers exhibit hyperactivity and reduced cognitive performance, but only if the alcoholic father is also their biological father, demonstrating the potential for these induced effects being preconceptual in nature (Hegedus et al., 1984; Tarter et al., 1984). Laboratory studies of these alcohol-induced effects have indicated that exposure of male mice and rats to alcohol has numerous effects on their offspring, including reduced litter size, reduced birth weight, developmental retardation, increased mortality, compromised immunity as well as behavioral deficits such as impaired discrimination on spatial tasks and altered aggressive, risk-taking and anxiety-like behavior (Abel, 2004; Abel and Tan, 1988; Abel and Bilitzke, 1990; Ledig et al., 1998; Meek et al., 2007; Wozniak et al., 1991). These effects have been established both with males who were exposed to alcohol until the time of mating and also with those males who have had withdrawal periods of various lengths prior to mating. Likewise, cocaine-exposed fathers sire offspring with impairments on tests of visuospatial attention, spatial working memory and spontaneous alternation, and have reduced cerebral volume (Abel et al., 1989; He et al., 2006). Males exposed to various other drugs and toxins such as opiates, cyclophosphamide, ethylene dibromide, and lead, have been found to sire offspring with developmental and behavioral impairments, with these effects in several cases being transmissible via the male line to second and third generations (Hales and Robaire, 2001). The severity of these effects is related to the duration and dosage of drug/toxin exposure as well as the developmental period when paternal exposure occurred (though in most of these studies, males are exposed post-weaning). Though there are species differences and sex-specific consequences of these effects, overall, these studies provide strong evidence that the exposure of males to drugs and other toxins can lead to behavioral changes in offspring, likely via the paternal germline.
Endocrine disruptors, such as the anti-androgenic compound vinclozolin, are another class of pharmacological agents that can induce altered development in the offspring of exposed fathers. However, in contrast to the previous examples, in order for this paternal transmission to occur, males must be exposed within a critical period late in their own embryogenesis during gonadal sex determination (Anway and Skinner, 2008). Hence, rat dams who are exposed to vinclozolin during late-gestation, have offspring who are at an increased risk of tumor formation, kidney disease, immune abnormalities and infertility, phenotypes which are observable for at least four subsequent generations through the male line but are not transmissible through the female line (Anway et al., 2005; Anway et al., 2008; Anway and Skinner, 2008). Moreover, sex-specific changes in anxiety-like behavior are observed in offspring that were separated from the originally exposed dam by as many as four generations through both the male and female lines (Skinner et al., 2008). In addition to these reproductive and behavioral consequences, male offspring within vinclozolin exposed patrilines are distinguished from control offspring on measures of mate preference, with females showing a preference for males from non-exposed lineages (Crews et al., 2007). Thus, the persistence of these paternal effects can have a significant impact on reproductive success.
Developmental consequences of paternal age
Though the detrimental effects of parental age on offspring development have typically focused on the relationship between increased maternal age and offspring's risk of psychopathology, there is also significant evidence for the influence of paternal age. Increasing age of fathers has been found to be related to elevated rates of schizophrenia (Malaspina et al., 2001), autism (Lundstrom et al., 2010; Reichenberg et al., 2006), and early-onset bipolar disorder (Frans et al., 2008) in offspring, as well as with reduced IQ (Malaspina et al., 2005) and social functioning in adolescents (Weiser et al., 2008). Furthermore, very young paternal age (typically under 25) is also associated with negative outcomes such as decreased IQ (Auroux et al., 2009; Auroux et al., 1989; Malaspina et al., 2005), higher rates of autism-spectrum disorder (Lundstrom et al., 2010) and an increased risk of birth defects (Mcintosh et al., 1995), suggesting that there exists an inverted U-shaped relationship between paternal age and offspring health. Significantly, the effects of paternal age reported in these studies are still found after controlling for potential confounding variables such as maternal age, social class, parental education, birth order, and birth weight.
The paternal age effects reported in epidemiological studies have been further supported by laboratory studies in rodents. In one of the first studies of paternal age effects, rats born to very old fathers (>22 months) performed poorly on a conditioned avoidance test compared to rats born to younger males, though no changes in anxiety-like behavior were found (Auroux, 1983). Similarly, mice born to old fathers (>120 weeks) exhibited impairments on a passive-avoidance learning test as well as reduced longevity, lower reproductive success, delayed sensorimotor development and inhibited adult spontaneous activity compared to mice born to younger fathers (Garcia-Palomares et al., 2009a; Garcia-Palomares et al., 2009b). Moreover, male mice born to relatively old fathers (aged 10 months) were also less social in a social-interaction test and less exploratory on a hole-board test compared to males born to younger males (aged 2 months) (Smith et al., 2009). Consistent with the human data, there is also evidence for an inverted U-shaped curve association between paternal age and offspring development in rodents. For example, the locomotor activity of sons and daughters and the learning abilities of sons are significantly reduced in mice born to very young fathers (aged 6 weeks) compared to adult fathers (aged 12-16 weeks) (Auroux et al., 1998).
Inheritance of paternal phenotypic variation
An alternative approach to studying non-genomic paternal inheritance in animal models is to characterize the phenotype of genetically identical males before mating and to then examine whether differences in these traits are inherited by their offspring. In a rodent study using this approach, virgin male mice of the inbred Balb/cJ strain were screened for their anxiety-like and exploratory behavior in the open-field test prior to being mated with Balb/cJ females (Alter et al., 2009). The males were removed from the pregnant female's cage before parturition and thus did not have any postnatal contact with their offspring. Analysis indicated that fathers who were highly exploratory in the open-field had daughters who were also highly exploratory, though this transmission of behavioral phenotype did not occur between fathers and sons. Moreover, father's open-field scores also predicted the hippocampal and brain weight of male and female offspring as well as sons' growth rate. These findings were demonstrated in two separate replicates of the study; once when all males screened for open-field behavior were mated, and again when only males exhibiting extreme open-field behavior (high vs. low exploration) were mated (Alter et al., 2009). This approach illustrates the transmission of naturally occurring individual differences in male behavior in the absence of any postnatal father-offspring interactions.
Pathways linking paternal variation to offspring development
The emerging evidence supporting the occurrence of paternal effects in the absence of male parental care raises intriguing questions regarding the mechanisms driving these effects. Though there has been particular focus on the potential role of germ cell or germline epigenetic variation in mediating paternal effects, there are many experimental design issues that must be overcome to establish the nature of this generational transmission. Here we will discuss two issues of particular theoretical and empirical relevance: I) the interplay between paternal and maternal effects in shaping offspring development, and II) the determination of experience-dependent vs. germline effects.
I. Paternal via maternal: The role of male-induced maternal effects
It is well acknowledged in behavioral ecology and evolutionary biology that individuals make decisions regarding how much parental care to provide to current offspring dependent upon how costly this may be to future fitness (Clutton-Brock, 1991). In particular, females are predicted to dynamically adjust their reproductive investment in response to prevailing environmental factors, with consequences for offspring development (Harris and Uller, 2009; Ratikainen and Kokko, 2009). The phenotypic quality of the male that a female mates with could potentially be one source of environmental variation that is a highly significant predictor for how females should adjust their care (see Figure 1). This relationship between mate quality and maternal care was first outlined by the differential allocation hypothesis (DAH), which stated that females who mate with high quality (typically attractive) males should increase their investment in offspring if the cost of reproducing is high (Burley, 1988; Sheldon, 2000). An alternative or variant to DAH is the compensation hypothesis, whereby females paired with unattractive or non-preferred males would increase investment to counteract the disadvantages that their offspring may inherit from their father (Gowaty et al., 2007; Gowaty, 2008). Reproductive compensation is especially likely in stressful environments where low genetic quality males are at a large disadvantage compared to high genetic quality males, and in mating systems with low reproductive skew, where even low quality males will be able to reproduce (Gowaty et al., 2007). These hypotheses have been tested across a wide variety of taxa with support for both hypotheses being reported (Harris and Uller, 2009; Ratikainen and Kokko, 2009; Sheldon, 2000). For example, when mated with males sporting red leg bands (i.e. artificially made more attractive), female zebra finches laid heavier eggs, had offspring that spent more time begging, and had faster growth rates than offspring of females who were paired randomly with males sporting green leg bands (i.e. artificially made unattractive) (Gilbert et al., 2006). In contrast, another study reported increased egg volumes, elevated yolk carotenoids and testosterone levels when female zebra finches were paired with less attractive males (Bolund et al., 2009). Likewise, young female mallards increased their egg volume when mated with highly attractive males (Cunningham and Russell, 2000), but older females increase their egg volume when mated with less attractive males (Bluhm and Gowaty, 2004). The decision to withhold resources or increase maternal investment may involve an interaction between mate quality and the reproductive life-history of the female, the availability of attractive males within a population, or the degree of reproductive skew (Harris and Uller, 2009). It has also been argued that reproductive compensation is more likely to occur in situations where females are not allowed to make a free choice with whom to mate (Gowaty et al., 2007; Harris and Uller, 2009; Sheldon, 2000).
Fig. 1.
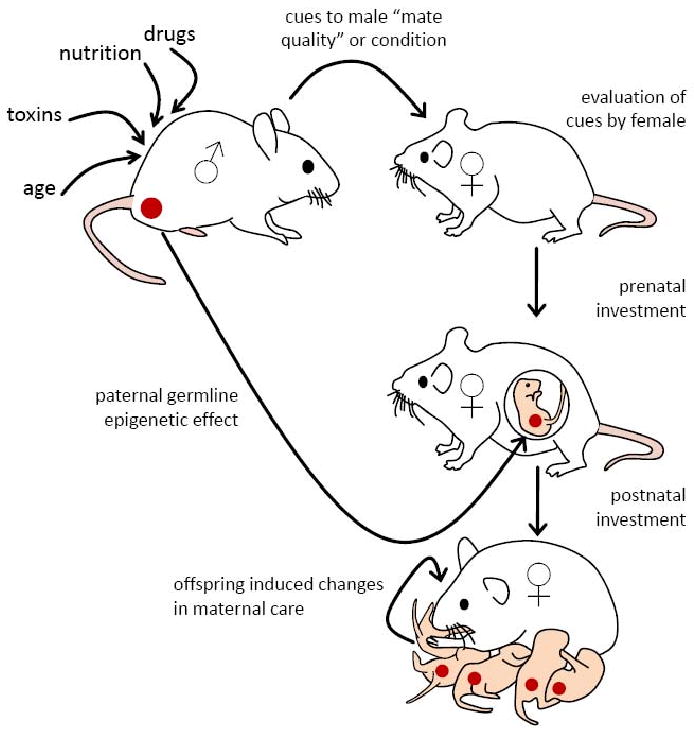
Illustration of the non-genomic pathways through which paternal effects on offspring development can be achieved. Experiences of males (drugs, nutrition, toxin, age), particularly those experienced during early development, may lead to epigenetic alterations in the male germline (red circle) which are then transmitted to offspring with consequences for phenotypic variation. Alternatively, or likely in combination with these direct paternal effects, the experiences of a male prior to mating may lead to changes in mate quality or preference as assessed by the female at the time of mating. This assessment may then lead to differential prenatal and/or postnatal maternal investment in the growth and development of offspring generated from this mating with consequences for offspring phenotypic variation. Maternal investment may also vary as a function of paternally mediated variations in offspring phenotype during both the prenatal and/or postnatal periods. Differential maternal investment as a function of paternal experiences or offspring traits may serve either to enhance the transmission of paternal exposures or compensate for deficits in functioning that are induced by these environmental experiences.
Though studies of male-induced maternal care have been explored predominantly in birds, there are also several examples of variation in maternal investment induced by male quality amongst rodents. In one study, mate preference was assessed in female house mice with subsequent mating of these females with preferred or non-preferred males (Drickamer et al., 2000). When females were mated with a preferred male, they gave birth to larger litters and offspring were found to be socially dominant, better nest-builders, exhibit more freezing behavior in a predator-avoidance test, and had decreased mortality rates compared to the offspring of females who mated with non-preferred males (Drickamer et al., 2000; Drickamer et al., 2003). Significantly, these paternally driven maternal effects were found when females were given a free-choice to show a preference for or against individual males.
It is evident that paternally driven maternal effects are an important consideration when designing experiments that attempt to explore the transmission of paternal effects. As is apparent in the case of exposure to endocrine disruptors, female mate choice can be altered by male environmental exposures, even those exposures occurring in a previous generation, and this selectivity may also apply to variations in male nutrition, toxin/drug exposure, and age, with consequences for prenatal and postnatal maternal investment in offspring (see Figure 1). Male phenotype could also have direct effects on the physiology of females (i.e. increasing hormone levels (Lupo di Prisco et al., 1978)) during the mating period with consequences for offspring development. However, few studies of paternal effects have considered the potential confounding role of maternal investment. Interestingly, in the Alter et al. (2009) study, maternal modulation of the paternal transmission of open-field behavior was examined in two ways. Firstly, the postnatal maternal behavior of dams was recorded and when entered into the regression model was found not to change the association between fathers' and daughters' open-field scores even though frequency of postnatal nursing and licking/grooming were also themselves related to offspring behavior. Secondly, the time that fathers spent cohabiting with females during their gestational period was assessed. It was determined that when the male remained with the dam during gestation for a lengthy period, there was a decreased association between male open-field score and son's open-field performance. This finding is suggestive that in some cases, maternal effects may override paternal germline effects and thus may be an important consideration in predicting the magnitude and direction of paternal effects.
Related to the concept of paternal effects via maternal investment is the consequence of direct paternal genetic/epigenetic effects on offspring quality leading to variations in the level of prenatal and postnatal care the offspring will receive. Offspring are not passive recipients of maternal care but can actively influence their own development. Following implantation, embryonic and maternal physiology become intricately coordinated and the continued growth and development of the embryo/fetus is dependent on the release of growth factors and hormones from the fetoplacental unit which then alter maternal respiration, food intake, glucose metabolism, and prime the maternal brain for the demands of postnatal maternal care (e.g. lactation and nurturing behaviors) (Brunton and Russell, 2008). In rodents, it has been demonstrated that during the postnatal period, offspring traits such as locomotor activity, suckling ability, and ultrasound production enable pups to regulate the levels of maternal care they receive from the dam, leading to altered developmental trajectories (Curley et al., 2010; Wohr et al., 2009). Interestingly, there is evidence that fathers are able to uniquely adjust the characteristics of their offspring leading to altered prenatal and postnatal development. Paternally expressed genes (i.e. paternal gene copy is expressed, maternal gene copy is epigenetically silenced) are highly expressed in the placenta and are critical to normal growth of the fetus (Constancia et al., 2004). In addition, paternally expressed genes such as Peg3 and Gnasxl also regulate postnatal mother-infant interactions and promote the suckling behavior of pups (Curley et al., 2004; Plagge et al., 2004) and there is growing evidence for the susceptibility of these imprinted genes to modification in expression in response to paternal environmental exposures (Dolinoy and Jirtle, 2008). Thus, the inheritance by offspring paternal genetic/epigenetic variation can have both direct effects on offspring and/or indirect effects on offspring via maternal investment, subsequently leading to complex interactions between mothers and offspring with consequences for development.
II. Experience-dependent vs. germline paternal transmission
Another critical issue for the study of paternal effects is regarding how many generations the transmission of a phenotype should be observed before it may be considered to be transmitted via the germline as opposed to being the result of developmental plasticity. For instance, in the case of pregnant dams who are exposed to some perturbation (e.g. high fat diet, endocrine disruptors, ethanol), there are three generations being exposed to the same environmental experience. Within the pregnant F0 female, not only are her fetal F1 offspring developing but so too are the F2 generation primordial germ cells (PGCs) within the F1 fetuses. Thus, to demonstrate that any induced change in phenotype can be transmitted through the germline, the phenotype must also be exhibited by the first non-exposed generation which would be the F3 generation. Skinner (2008) has proposed that if such a phenotypic inheritance can be observed to the F3 generation then this effect can be termed a transgenerational inheritance, whereas if phenotypic changes are only observed in the exposed generations then this should be considered a multigenerational phenotype (Skinner, 2008). For example, F1 male offspring of F0 rat dams who are exposed gestationally to chronic levels of dexamethasone exhibit deficits in metabolic and stress phenotypes as adults, and their own F2 offspring also show reduced birth weight, glucose intolerance and altered hepatic enzyme activity even when they are mated with control unexposed females (Drake et al., 2005). Significantly, however, no differences were seen in the F3 generation suggesting a multigenerational phenotype but not a transgenerational inheritance. Likewise, male F1 offspring of F0 rat dams who are fed a low protein diet during gestation have F2 offspring with raised blood pressure and reduced nephron numbers, but this phenotype is not observed in the F3 generation (Harrison and Langley-Evans, 2009). In the case of F0 males exposed to environmental experiences postnatally (i.e. outside of the window of primordial germ cell development), then the first non-exposed generation would be the F2, though relatively few studies using this methodological approach have thus far examined whether such induced phenotypes are transmitted to the next generation. While such a demonstration of the transgenerational inheritance of phenotypes certainly strengthens the likelihood that these effects are epigenetic, it should also be noted that the absence of an observed transmission to a subsequent generation does not necessarily preclude an initial (F1) germline epigenetic effect (Drake and Liu, 2010). For instance, it is possible that induced phenotypes may wane over successive generations due to either the absence of the initial environmental stimulus or the removal of acquired epigenetic marks (as described in the next section).
Role of epigenetic mechanisms in the transmission of paternal effects
In the absence of male parental care or paternally induced maternal effects, how are the experiences of males able to shape the development of subsequent generations? One possibility is that de novo mutations in the DNA sequence of male sperm may be induced by exposures or increased with age, and that these genetic effects account for the behavioral changes observed in offspring. In the field of developmental toxicology, there is evidence for an association between male exposure to various drugs/toxins and an increased occurrence of mutations, including numerical and structural chromosomal abnormalities, point mutations, copy number variant (CNV) changes, and duplications/deletions of microsatellites (Delbes et al., 2010; Hales and Robaire, 2001). Though these mutational events certainly account for some of the induced phenotypic variation observed in offspring, a key question that arises from these studies is whether mutations are the only germline mechanism responsible for this inheritance, or whether other mechanisms may play a role. A commonly acknowledged average baseline mutational rate frequency in humans of 2.3 × 10-8 per nucleotide per generation appears to be too low to account for all transgenerational phenotypic inheritance (Arnheim and Calabrese, 2009; Nachman and Crowell, 2000), although it should be noted that certain mutational events (e.g. CNVs) can occur at a much higher frequency than baseline and that certain sections of the DNA (hotspots) may be particularly susceptible to de novo mutations (Egan et al., 2007). Interestingly, with specific regard to paternal age, the increase in sporadic cases of genetic disorders is exponential with increasing age of the father despite there being ambiguous evidence for an exponential increase in the mutational rate of sperm with increasing paternal age, suggesting that other mechanisms may be significant (Arnheim and Calabrese, 2009; Risch et al., 1987; Walter et al., 1998). Moreover, a genetic mutation hypothesis cannot explain the inheritance of male phenotypes to offspring in genetically identical animals in the absence of exposures (Alter et al., 2009). Therefore the possibility that non-genomic factors might influence the male germline has been raised, with epigenetic modifications (e.g. DNA methylation, histone modifications and non-coding RNAs) that the mature sperm presents to the oocyte at fertilization, being strong candidates for being the mediators of these effects (Probst et al., 2009). To establish the role of epigenetic factors in paternal transgenerational effects, it must be demonstrated that the environmental experience induces epigenetic modification in the germ cells, and that these effects are transmitted across generations.
In order for environmentally induced or stochastic changes in these epigenetic modifications to be faithfully transmitted to offspring they have to escape two major phases of DNA epigenetic reprogramming when the epigenome (i.e. genome wide methylation patterns) are subjected to extensive demethylation and remethylation. The first wave occurs in the zygote, where the paternal genome is actively demethylated shortly after fertilization and then remethylated just prior to implantation of the blastocyst (Shi and Wu, 2009). The second wave occurs during embryogenesis with DNA demethylation occurring in the PGCs as they migrate down the genital ridge to the early bipotential gonad. Following sex-determination, DNA remethylation occurs in germ cells in a sex-specific fashion (Allegrucci et al., 2005). Significantly, some classes of genes within the germline have the unique capacity to retain their altered methylation states across multiple generations despite these waves of epigenetic reprogramming during development. In particular, retrotransposable elements and imprinted genes appear to be both sensitive to environmental exposures and capable of retaining epigenetic marks (Lane et al., 2003).
Retrotransposons comprise approximately 45% of the human genome and are mostly remnants of ancestral infections that became fixed in the genome. Over time, most of these sequences have become silenced by extensive DNA methylation. For example, Intracisteral-A particle (IAP) elements (a long terminal repeat retrotransposon) that became incorporated into the genome are resistant to post-fertilization demethylation and have the capacity to regulate the transcription of multiple neighboring genes. Further, in mice, variation in IAP methylation status (that may arise stochastically or with diet/toxin exposure) has been shown to result in heritable phenotypic variation. For example, an IAP element inserted into the 5′ region of the AxinFu allele (a gene responsible of embryonic axis formation), when methylated will result in the expression of aberrant gene transcripts and a kinked-tail phenotype (Rakyan et al., 2003). Interestingly, methylation status of the IAP is highly correlated across tissues (germ and somatic) and with the degree of tail kink. Both the phenotype and methylation status can be inherited by offspring through maternal and paternal lineages. Similarly, the insertion of an IAP element into an exon of the agouti gene (Avy) results in a number of phenotypic effects; including a range of coat color pigmentations and the propensity to develop obesity (Morgan et al., 1999). Like in the AxinFu mouse, the epigenetic state of Avy can be modified by environmental factors such as diet (methyl supplementation) and environmental toxicants (bisphenol A) and is inherited by offspring via the maternal line (Cropley et al., 2006; Dolinoy et al., 2007; Morgan et al., 1999). Interestingly, the epigenetic state of Avy and the accompanying phenotype can also be paternally transmitted, but this is dependent upon the genetic strain of the wild-type mother with whom the male is mated (Rakyan et al., 2003). Females of the C57BL/6J strain did not facilitate paternal transmission of the Avy phenotype whereas females of the 129P4 strain did, suggesting that genetic differences in maternal cytoplasmic factors may be able to influence the transmission of paternal epigenetic modifications.
The other class of genes that retain a ‘memory’ of their ancestral epigenetic marks are imprinted genes. These genes are expressed in a parent-of-origin manner which is achieved through either the maternal or paternal allele being silenced via epigenetic modifications (typically DNA methylation) that have avoided being reprogrammed following fertilization (Keverne and Curley, 2008). There are approximately 100 of these genes in both rodents and humans, though a recent study suggests that many more alleles may show parent-of-origin specific DNA methylation profiles (Schalkwyk et al., 2010). Interestingly, it has been established that gene expression and methylation status of imprinted genes in sperm can be modified by paternal alcohol exposure (Ouko et al., 2009). Similar changes have also been observed in the sperm of mice who were conceived through artificial reproductive technologies, and significantly, the altered methylation patterns of particular imprinted genes (Snrpn and H19) were observed in the sperm of F2 males demonstrating that these changes had avoided reprogramming and had been transgenerationally inherited (Stouder et al., 2009).
Other environmental exposures have also been found to result in altered gene expression, DNA methylation, and the activity of enzymes involved in regulating epigenetic modifications in both the soma and germline. For instance, the exposure of gestating rat dams to the endocrine disruptor, vinclozolin, during PGC development is associated with altered levels of sperm DNA methylation and subsequent genome-wide transcriptome changes in multiple tissues (e.g. testis, brain, prostate) of offspring for four generations. These effects presumably arise as a result of differential methylation at imprinted and imprinted-like genes in the male germline (Anway et al., 2005; Stouder and Paoloni-Giacobino, 2010). Indeed, the gene expression of nearly 400 genes (in male offspring) and 1500 genes (in female offspring) was altered in the hippocampus and amygdala up to three generations post-vinclozolin exposure, demonstrating that many genes can retain an imprint or memory of the initial environmental exposure (Skinner et al., 2008). Significantly, affected genes were predominantly those involved in the regulation of axon guidance and long-term potentiation, and could thus alter brain development and behavior. DNA methylation changes can also accumulate with increasing paternal age in multiple tissues including the gametes (Flanagan et al., 2006; Fraga et al., 2005; Oakes et al., 2003). Further, exposure to drugs such as alcohol and cocaine induce changes in gene expression that are correlated with the degree of DNA methylation and chromatin remodeling at multiple gene sequences in both the brain and periphery (Bielawski et al., 2002; Ouko et al., 2009; Pandey et al., 2008). Chronic cocaine and alcohol exposure are also associated with significant decreases in the mRNA levels of enzymes responsible for DNA methylation (DNMTs) in the testes and sperm of adult male rodents (Bielawski et al., 2002; He et al., 2006; Ouko et al., 2009). Finally, exposure of male mice to toxins can induce changes in the epigenome of germ cells. Males subjected to chromium chloride two weeks before mating exhibit hypomethylation of the 45S ribosomal RNA gene in their sperm, and these males sire offspring with elevated body weight and thyroxine levels (Cheng et al., 2004), whereas, male mice exposed to steel plant air (comprised of various pollutants) have hypermethylated DNA in sperm compared to control animals even following removal from the exposure (Yauk et al., 2008).
In addition to the epigenetic marks of DNA, the germ cells transmit various cytoplasmic RNAs (which include mRNAas, siRNAs, piRNAs and miRNAs) that are essential for post-fertilization development. There is now accumulating evidence that the RNAs (particularly non-coding RNAs) of germ cells may carry functional epigenetic information that can be inherited transgenerationally through the germline. One example of this RNA-mediated inheritance comes from studies of paramutation in which the interaction between two alleles of a single locus results in heritable variation. This phenomenon is well-described in the plant literature but was only recently reported to occur in the mouse. One example of paramutation involves the Kit gene that encodes a tyrosine kinase receptor involved in the synthesis of melanin (Rassoulzadegan et al., 2006). Individuals heterozygous for a mutation in the Kit gene have reduced Kit mRNA expression and distinctive white pigmentations on their feet and tails. Interestingly, when either male or female heterozygotes are crossed with wild-type individuals, some of the wild-type offspring (Kit*) have reduced Kit mRNA levels and inherit the paramutation phenotype (white pigmentations) (Rassoulzadegan et al., 2006). These individuals are then able to pass on their altered phenotype to their own offspring. The presence of low levels of Kit mRNAs and various abnormal RNA molecules in the testes and mature sperm led to the hypothesis that the inheritance was likely to be mediated by RNAs. Indeed, injection of Kit mRNA prepared from heterozygotes and microRNAs (miRNAs) against Kit mRNAs into zygotes reproduced the white pigmentations (Rassoulzadegan et al., 2006).
Further support for RNA-mediated mechanisms in driving germline transgenerational inheritance has come from the demonstration of phenotypic inheritance following the injection of various miRNAs into the fertilized eggs of mice. For instance, injection of the cardiac tissue-specific miRNA, miR-1 into fertilized eggs (that are then transplanted into recipient females) results in offspring with anatomical and physiological indicators of cardiac hypertrophy (Wagner et al., 2008). Similarly, injection of miR-124 (critical for brain development) resulted in offspring that showed significant increases in growth rate throughout development (Grandjean et al., 2009). Significantly, both of these manipulations resulted in developmental and adult changes in gene expression of multiple genes known to be targeted by the respective miRNAs and in some cases even altered the chromatin structure at those genes (Grandjean et al., 2009). Further, like the Kit paramutation, miRNAs were expressed at detectable levels in sperm and although these studies did not measure RNA levels in eggs, the phenotypes were transmitted through both the paternal and maternal lineages for up to 3 generations. Taken together, these studies suggest that RNAs expressed in the germ cells (of both males and females) may carry functional epigenetic information that induce persistent transgenerational effects. Relevant to this review, environmental variables such as aging and smoking result in changes in RNA expression in germ cells (Hamatani et al., 2004; Linschooten et al., 2009) although it is still unknown if experience-dependent changes in RNA contribute to germline inheritance in a similar fashion.
The interplay between maternal and paternal influences should also be considered within the context of these “gametic epigenetic effects” (Youngson and Whitelaw, 2008). Maintaining paternal epigenetic modifications may ultimately depend on the presence of factors within the oocyte that can remove or re-establish epigenetic marks, as suggested with the maternal strain specific paternal inheritance of the Avy phenotype (Rakyan et al., 2003). Using interspecies intracytoplasmic sperm injection (ICSI), it has been demonstrated that changes in methylation patterns are both oocyte and sperm dependent. For example, the level of demethylation that is observed in the male pronucelus following fertilization is inversely associated with the degree of condensed chromatin packaging. Studies of the post-fertilization development of interspecific hybrids generated via ICSI suggest that mouse oocytes (compared to sheep and bovine) have a greater capacity to demethylate the male pronuclei (Beaujean et al., 2004). Though individual differences in within species oocyte re-programming mechanisms have not yet been explored, there is evidence suggestive of these effects.
Finally, it is important to note that though there is increasing evidence for the role of epigenetic factors in explaining the effects of paternal experience on offspring phenotype, it remains difficult to disentangle the effects of genetic variation and mutations from epigenetic modifications in perpetuating paternal effects. Mutational events or DNA damage has the potential to induce heritable epigenetic marks (Jablonka and Raz, 2009). Further, depending on where these mutations occur, it is possible to influence the epigenetic structure of specific genes or of the entire genome (particularly if genes coding for epigenetic machinery are affected) (Jablonka and Raz, 2009; Mohn and Schubeler, 2009). Conversely, there is the potential for epigenetic marks (i.e. loose chromatin structure) to increase the probability of mutation, transposition and and/or the recombination of DNA sequences (Jablonka and Lamb, 1995; Jablonka and Raz, 2009). This bidirectional relationship between epigenetics and genetics remains an important caveat in the interpretation of the mechanisms leading to paternal effects and a critical factor to consider in understanding the origins of phenotypic variation.
Conclusions & Future Directions
The study of paternal effects is expanding in novel directions through the incorporation of an epigenetic perspective on the transmission of parental traits and experiences. Though much of the evidence is, at this stage, speculative, there is emerging support for the inducement of paternal germline epigenetic changes in response to environmental exposures that have consequences for subsequent generations of offspring. This relationship between parental experience and offspring development is a rapidly expanding field of study that challenges our notions of the mechanisms of inheritance and has revived interest in Lamarckian views on the role in evolution of the inheritance of acquired traits. Ultimately, the non-genomic mechanisms described in this review may provide a route through which developmental plasticity in one generation can be transmitted across multiple generations. However, with increasing interest in paternal germline inheritance, there must also be increased scrutiny regarding the experimental design of studies which explore this phenomenon. The interplay between maternal and paternal effects is an important consideration, and likely to occur in varying degrees in response to specific environmental exposures, and may require the use of techniques such as ICSI and embryo transfer to dissociate. Likewise, the dissociation between an experience-dependent vs. a germline epigenetic effect in response to the environment may require the extension of analysis to F3 and F4 generations. One final consideration, which relates to both the conceptual and mechanistic pathways that have been discussed, is the compensatory processes that can augment the transmission of paternal effects. Thus, though there may be potential for a paternal effect to be perpetuated transgenerationally, this may only occur under conditions when maternal factors (ecological, behavioral, physiological, and/or molecular) enable this transmission to occur. Exploring these complex relationships between paternal, maternal, and offspring phenotypes and the effect of the environment on this dynamic, represents a challenging yet fascinating approach within the study of behavioral epigenetics.
Acknowledgments
This work was supported by Grant Number DP2OD001674 from the Office of the Director, National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel E. Paternal contribution to fetal alcohol syndrome. Addict Biol. 2004;9:127–33. doi: 10.1080/13556210410001716980. discussion 135-6. [DOI] [PubMed] [Google Scholar]
- Abel EL, Tan SE. Effects of paternal alcohol consumption on pregnancy outcome in rats. Neurotoxicol Teratol. 1988;10:187–92. doi: 10.1016/0892-0362(88)90016-5. [DOI] [PubMed] [Google Scholar]
- Abel EL, Moore C, Waselewsky D, Zajac C, Russell LD. Effects of cocaine hydrochloride on reproductive function and sexual behavior of male rats and on the behavior of their offspring. J Androl. 1989;10:17–27. doi: 10.1002/j.1939-4640.1989.tb00051.x. [DOI] [PubMed] [Google Scholar]
- Abel EL, Bilitzke P. Paternal alcohol exposure: paradoxical effect in mice and rats. Psychopharmacology (Berl) 1990;100:159–64. doi: 10.1007/BF02244399. [DOI] [PubMed] [Google Scholar]
- Allegrucci C, Thurston A, Lucas E, Young L. Epigenetics and the germline. Reproduction. 2005;129:137–49. doi: 10.1530/rep.1.00360. [DOI] [PubMed] [Google Scholar]
- Alter MD, Gilani AI, Champagne FA, Curley JP, Turner JB, Hen R. Paternal Transmission of Complex Phenotypes in Inbred Mice. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LM, Riffle L, Wilson R, Travlos GS, Lubomirski MS, Alvord WG. Preconceptional fasting of fathers alters serum glucose in offspring of mice. Nutrition. 2006;22:327–31. doi: 10.1016/j.nut.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–9. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway MD, Rekow SS, Skinner MK. Comparative anti-androgenic actions of vinclozolin and flutamide on transgenerational adult onset disease and spermatogenesis. Reprod Toxicol. 2008;26:100–6. doi: 10.1016/j.reprotox.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway MD, Skinner MK. Epigenetic programming of the germ line: effects of endocrine disruptors on the development of transgenerational disease. Reprod Biomed Online. 2008;16:23–5. doi: 10.1016/s1472-6483(10)60553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnheim N, Calabrese P. Understanding what determines the frequency and pattern of human germline mutations. Nat Rev Genet. 2009;10:478–88. doi: 10.1038/nrg2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auroux M. Decrease of learning capacity in offspring with increasing paternal age in the rat. Teratology. 1983;27:141–8. doi: 10.1002/tera.1420270202. [DOI] [PubMed] [Google Scholar]
- Auroux M, Nawar NN, Naguib M, Baud M, Lapaquellerie N. Post-pubescent to mature fathers: increase in progeny quality? Hum Reprod. 1998;13:55–9. doi: 10.1093/humrep/13.1.55. [DOI] [PubMed] [Google Scholar]
- Auroux M, Volteau M, Ducot B, Wack T, Letierce A, Meyer L, Mayaux MJ. Progeny's mental aptitudes in man: relationship with parental age at conception and with some environmental factors. C R Biol. 2009;332:603–12. doi: 10.1016/j.crvi.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Auroux MR, Mayaux MJ, Guihard-Moscato ML, Fromantin M, Barthe J, Schwartz D. Paternal age and mental functions of progeny in man. Hum Reprod. 1989;4:794–7. doi: 10.1093/oxfordjournals.humrep.a136988. [DOI] [PubMed] [Google Scholar]
- Beaujean N, Taylor JE, McGarry M, Gardner JO, Wilmut I, Loi P, Ptak G, Galli C, Lazzari G, Bird A, Young LE, Meehan RR. The effect of interspecific oocytes on demethylation of sperm DNA. Proc Natl Acad Sci U S A. 2004;101:7636–40. doi: 10.1073/pnas.0400730101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielawski DM, Zaher FM, Svinarich DM, Abel EL. Paternal alcohol exposure affects sperm cytosine methyltransferase messenger RNA levels. Alcohol Clin Exp Res. 2002;26:347–51. [PubMed] [Google Scholar]
- Bluhm CK, Gowaty PA. Reproductive compensation for offspring viability deficits by female mallards, Anas platyrhynchos. Animal Behaviour. 2004;68:985–992. [Google Scholar]
- Bolund E, Schielzeth H, Forstmeier W. Compensatory investment in zebra finches: females lay larger eggs when paired to sexually unattractive males. Proc Biol Sci. 2009;276:707–15. doi: 10.1098/rspb.2008.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher BJ, Ewen SW, Stowers JM. Betel nut (Areca catechu) consumption and the induction of glucose intolerance in adult CD1 mice and in their F1 and F2 offspring. Diabetologia. 1994;37:49–55. doi: 10.1007/BF00428777. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. The expectant brain: adapting for motherhood. Nat Rev Neurosci. 2008;9:11–25. doi: 10.1038/nrn2280. [DOI] [PubMed] [Google Scholar]
- Burley N. The Differential-Allocation Hypothesis - An Experimental Test. American Naturalist. 1988;132:611–628. [Google Scholar]
- Chen TH, Chiu YH, Boucher BJ. Transgenerational effects of betel-quid chewing on the development of the metabolic syndrome in the Keelung Community-based Integrated Screening. Program Am J Clin Nutr. 2006;83:688–92. doi: 10.1093/ajcn.83.3.688. [DOI] [PubMed] [Google Scholar]
- Cheng RY, Hockman T, Crawford E, Anderson LM, Shiao YH. Epigenetic and gene expression changes related to transgenerational carcinogenesis. Mol Carcinog. 2004;40:1–11. doi: 10.1002/mc.20022. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH. The evolution of parental care. Vol. 1. Princeton University Press; Princeton, New Jersey: 1991. [Google Scholar]
- Constancia M, Kelsey G, Reik W. Resourceful imprinting. Nature. 2004;432:53–7. doi: 10.1038/432053a. [DOI] [PubMed] [Google Scholar]
- Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, Anway MD, Skinner MK. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci U S A. 2007;104:5942–6. doi: 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropley JE, Suter CM, Beckman KB, Martin DI. Germ-line epigenetic modification of the murine A vy allele by nutritional supplementation. Proc Natl Acad Sci U S A. 2006;103:17308–12. doi: 10.1073/pnas.0607090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham EJ, Russell AF. Egg investment is influenced by male attractiveness in the mallard. Nature. 2000;404:74–7. doi: 10.1038/35003565. [DOI] [PubMed] [Google Scholar]
- Curley JP, Barton S, Surani A, Keverne EB. Coadaptation in mother and infant regulated by a paternally expressed imprinted gene. Proc Biol Sci. 2004;271:1303–9. doi: 10.1098/rspb.2004.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, Rock V, Moynihan AM, Bateson P, Keverne EB, Champagne FA. Developmental shifts in the behavioral phenotypes of inbred mice: the role of postnatal and juvenile social experiences. Behav Genet. 2010;40:220–32. doi: 10.1007/s10519-010-9334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbes G, Hales BF, Robaire B. Toxicants and human sperm chromatin integrity. Mol Hum Reprod. 2010;16:14–22. doi: 10.1093/molehr/gap087. [DOI] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104:13056–61. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Jirtle RL. Environmental epigenomics in human health and disease. Environ Mol Mutagen. 2008;49:4–8. doi: 10.1002/em.20366. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R34–8. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Liu L. Intergenerational transmission of programmed effects: public health consequences. Trends Endocrinol Metab. 2010;21:206–13. doi: 10.1016/j.tem.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Drickamer LC, Gowaty PA, Holmes CM. Free female mate choice in house mice affects reproductive success and offspring viability and performance. Animal Behaviour. 2000;59:371–378. doi: 10.1006/anbe.1999.1316. [DOI] [PubMed] [Google Scholar]
- Drickamer LC, Gowaty PA, Wagner DM. Free mutual mate preferences in house mice affect reproductive success and offspring performance. Animal Behaviour. 2003;65:105–114. doi: 10.1006/anbe.1999.1316. [DOI] [PubMed] [Google Scholar]
- Dunn GA, Bale TL. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology. 2009;150:4999–5009. doi: 10.1210/en.2009-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan CM, Sridhar S, Wigler M, Hall IM. Recurrent DNA copy number variation in the laboratory mouse. Nat Genet. 2007;39:1384–9. doi: 10.1038/ng.2007.19. [DOI] [PubMed] [Google Scholar]
- Flanagan JM, Popendikyte V, Pozdniakovaite N, Sobolev M, Assadzadeh A, Schumacher A, Zangeneh M, Lau L, Virtanen C, Wang SC, Petronis A. Intra- and interindividual epigenetic variation in human germ cells. Am J Hum Genet. 2006;79:67–84. doi: 10.1086/504729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suner D, Cigudosa JC, Urioste M, Benitez J, Boix-Chornet M, Sanchez-Aguilera A, Ling C, Carlsson E, Poulsen P, Vaag A, Stephan Z, Spector TD, Wu YZ, Plass C, Esteller M. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102:10604–9. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frans EM, Sandin S, Reichenberg A, Lichtenstein P, Langstrom N, Hultman CM. Advancing paternal age and bipolar disorder. Arch Gen Psychiatry. 2008;65:1034–40. doi: 10.1001/archpsyc.65.9.1034. [DOI] [PubMed] [Google Scholar]
- Garcia-Palomares S, Navarro S, Pertusa JF, Hermenegildo C, Garcia-Perez MA, Rausell F, Cano A, Tarin JJ. Delayed fatherhood in mice decreases reproductive fitness and longevity of offspring. Biol Reprod. 2009a;80:343–9. doi: 10.1095/biolreprod.108.073395. [DOI] [PubMed] [Google Scholar]
- Garcia-Palomares S, Pertusa JF, Minarro J, Garcia-Perez MA, Hermenegildo C, Rausell F, Cano A, Tarin JJ. Long-term effects of delayed fatherhood in mice on postnatal development and behavioral traits of offspring. Biol Reprod. 2009b;80:337–42. doi: 10.1095/biolreprod.108.072066. [DOI] [PubMed] [Google Scholar]
- Gilbert L, Williamson KA, Hazon N, Graves JA. Maternal effects due to male attractiveness affect offspring development in the zebra finch. Proc Biol Sci. 2006;273:1765–71. doi: 10.1098/rspb.2006.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowaty PA, Anderson WW, Bluhm CK, Drickamer LC, Kim YK, Moore AJ. The hypothesis of reproductive compensation and its assumptions about mate preferences and offspring viability. Proc Natl Acad Sci U S A. 2007;104:15023–7. doi: 10.1073/pnas.0706622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowaty PA. Reproductive compensation. J Evol Biol. 2008;21:1189–200. doi: 10.1111/j.1420-9101.2008.01559.x. [DOI] [PubMed] [Google Scholar]
- Grandjean V, Gounon P, Wagner N, Martin L, Wagner KD, Bernex F, Cuzin F, Rassoulzadegan M. The miR-124-Sox9 paramutation: RNA-mediated epigenetic control of embryonic and adult growth. Development. 2009;136:3647–55. doi: 10.1242/dev.041061. [DOI] [PubMed] [Google Scholar]
- Hales BF, Robaire B. Paternal exposure to drugs and environmental chemicals: effects on progeny outcome. J Androl. 2001;22:927–36. doi: 10.1002/j.1939-4640.2001.tb03431.x. [DOI] [PubMed] [Google Scholar]
- Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, Dudekula DB, VanBuren V, Ko MS. Age-associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet. 2004;13:2263–78. doi: 10.1093/hmg/ddh241. [DOI] [PubMed] [Google Scholar]
- Harris WE, Uller T. Reproductive investment when mate quality varies: differential allocation versus reproductive compensation. Philos Trans R Soc Lond B Biol Sci. 2009;364:1039–48. doi: 10.1098/rstb.2008.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M, Langley-Evans SC. Intergenerational programming of impaired nephrogenesis and hypertension in rats following maternal protein restriction during pregnancy. Br J Nutr. 2009;101:1020–30. doi: 10.1017/S0007114508057607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Lidow IA, Lidow MS. Consequences of paternal cocaine exposure in mice. Neurotoxicol Teratol. 2006;28:198–209. doi: 10.1016/j.ntt.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Hegedus AM, Alterman AI, Tarter RE. Learning achievement in sons of alcoholics. Alcohol Clin Exp Res. 1984;8:330–3. doi: 10.1111/j.1530-0277.1984.tb05522.x. [DOI] [PubMed] [Google Scholar]
- Jablonka E, Lamb MJ. Epigenetic Inheritance and Evolution: the Lamarckian Dimension. Vol. Oxford University Press; 1995. [Google Scholar]
- Jablonka E, Raz G. Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. Q Rev Biol. 2009;84:131–76. doi: 10.1086/598822. [DOI] [PubMed] [Google Scholar]
- Jimenez-Chillaron JC, Isganaitis E, Charalambous M, Gesta S, Pentinat-Pelegrin T, Faucette RR, Otis JP, Chow A, Diaz R, Ferguson-Smith A, Patti ME. Intergenerational transmission of glucose intolerance and obesity by in utero undernutrition in mice. Diabetes. 2009;58:460–8. doi: 10.2337/db08-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaati G, Bygren LO, Edvinsson S. Cardiovascular and diabetes mortality determined by nutrition during parents' and grandparents' slow growth period. Eur J Hum Genet. 2002;10:682–8. doi: 10.1038/sj.ejhg.5200859. [DOI] [PubMed] [Google Scholar]
- Kaati G, Bygren LO, Pembrey M, Sjostrom M. Transgenerational response to nutrition, early life circumstances and longevity. Eur J Hum Genet. 2007;15:784–90. doi: 10.1038/sj.ejhg.5201832. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Curley JP. Epigenetics, brain evolution and behaviour. Front Neuroendocrinol. 2008;29:398–412. doi: 10.1016/j.yfrne.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Lane N, Dean W, Erhardt S, Hajkova P, Surani A, Walter J, Reik W. Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis. 2003;35:88–93. doi: 10.1002/gene.10168. [DOI] [PubMed] [Google Scholar]
- Ledig M, Misslin R, Vogel E, Holownia A, Copin JC, Tholey G. Paternal alcohol exposure: developmental and behavioral effects on the offspring of rats. Neuropharmacology. 1998;37:57–66. doi: 10.1016/s0028-3908(97)00185-8. [DOI] [PubMed] [Google Scholar]
- Lin WY, Chiu TY, Lee LT, Lin CC, Huang CY, Huang KC. Betel nut chewing is associated with increased risk of cardiovascular disease and all-cause mortality in Taiwanese men. Am J Clin Nutr. 2008;87:1204–11. doi: 10.1093/ajcn/87.5.1204. [DOI] [PubMed] [Google Scholar]
- Linschooten JO, Van Schooten FJ, Baumgartner A, Cemeli E, Van Delft J, Anderson D, Godschalk RW. Use of spermatozoal mRNA profiles to study gene-environment interactions in human germ cells. Mutat Res. 2009;667:70–6. doi: 10.1016/j.mrfmmm.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Little RE. Mother's and father's birthweight as predictors of infant birthweight. Paediatr Perinat Epidemiol. 1987;1:19–31. doi: 10.1111/j.1365-3016.1987.tb00084.x. [DOI] [PubMed] [Google Scholar]
- Lundstrom S, Haworth CM, Carlstrom E, Gillberg C, Mill J, Rastam M, Hultman CM, Ronald A, Anckarsater H, Plomin R, Lichtenstein P, Reichenberg A. Trajectories leading to autism spectrum disorders are affected by paternal age: findings from two nationally representative twin studies. J Child Psychol Psychiatry. 2010 doi: 10.1111/j.1469-7610.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- Lupo di Prisco C, Lucarini N, Dessi-Fulgheri F. Testosterone aromatization in rat brain is modulated by social environment. Physiol Behav. 1978;20:345–8. doi: 10.1016/0031-9384(78)90230-5. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Harlap S, Fennig S, Heiman D, Nahon D, Feldman D, Susser ES. Advancing paternal age and the risk of schizophrenia. Arch Gen Psychiatry. 2001;58:361–7. doi: 10.1001/archpsyc.58.4.361. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Reichenberg A, Weiser M, Fennig S, Davidson M, Harlap S, Wolitzky R, Rabinowitz J, Susser E, Knobler HY. Paternal age and intelligence: implications for age-related genomic changes in male germ cells. Psychiatr Genet. 2005;15:117–25. doi: 10.1097/00041444-200506000-00008. [DOI] [PubMed] [Google Scholar]
- Mcintosh GC, Olshan AF, Baird PA. Paternal age and the risk of birth defects in offspring. Epidemiology. 1995;6:282–8. doi: 10.1097/00001648-199505000-00016. [DOI] [PubMed] [Google Scholar]
- Meek LR, Myren K, Sturm J, Burau D. Acute paternal alcohol use affects offspring development and adult behavior. Physiol Behav. 2007;91:154–60. doi: 10.1016/j.physbeh.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Mohn F, Schubeler D. Genetics and epigenetics: stability and plasticity during cellular differentiation. Trends Genet. 2009;25:129–36. doi: 10.1016/j.tig.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Morgan HD, Sutherland HG, Martin DI, Whitelaw E. Epigenetic inheritance at the agouti locus in the mouse. Nat Genet. 1999;23:314–8. doi: 10.1038/15490. [DOI] [PubMed] [Google Scholar]
- Nachman MW, Crowell SL. Estimate of the mutation rate per nucleotide in humans. Genetics. 2000;156:297–304. doi: 10.1093/genetics/156.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes CC, Smiraglia DJ, Plass C, Trasler JM, Robaire B. Aging results in hypermethylation of ribosomal DNA in sperm and liver of male rats. Proc Natl Acad Sci U S A. 2003;100:1775–80. doi: 10.1073/pnas.0437971100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouko LA, Shantikumar K, Knezovich J, Haycock P, Schnugh DJ, Ramsay M. Effect of alcohol consumption on CpG methylation in the differentially methylated regions of H19 and IG-DMR in male gametes: implications for fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2009;33:1615–27. doi: 10.1111/j.1530-0277.2009.00993.x. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008;28:3729–37. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjostrom M, Golding J. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet. 2006;14:159–66. doi: 10.1038/sj.ejhg.5201538. [DOI] [PubMed] [Google Scholar]
- Plagge A, Gordon E, Dean W, Boiani R, Cinti S, Peters J, Kelsey G. The imprinted signaling protein XL alpha s is required for postnatal adaptation to feeding. Nat Genet. 2004;36:818–26. doi: 10.1038/ng1397. [DOI] [PubMed] [Google Scholar]
- Probst AV, Dunleavy E, Almouzni G. Epigenetic inheritance during the cell cycle. Nat Rev Mol Cell Biol. 2009;10:192–206. doi: 10.1038/nrm2640. [DOI] [PubMed] [Google Scholar]
- Rakyan VK, Chong S, Champ ME, Cuthbert PC, Morgan HD, Luu KV, Whitelaw E. Transgenerational inheritance of epigenetic states at the murine Axin(Fu) allele occurs after maternal and paternal transmission. Proc Natl Acad Sci U S A. 2003;100:2538–43. doi: 10.1073/pnas.0436776100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M, Grandjean V, Gounon P, Vincent S, Gillot I, Cuzin F. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature. 2006;441:469–74. doi: 10.1038/nature04674. [DOI] [PubMed] [Google Scholar]
- Ratikainen II, Kokko H. Differential allocation and compensation: who deserves the silver spoon? Behav Ecol. 2009 arp168. [Google Scholar]
- Reichenberg A, Gross R, Weiser M, Bresnahan M, Silverman J, Harlap S, Rabinowitz J, Shulman C, Malaspina D, Lubin G, Knobler HY, Davidson M, Susser E. Advancing paternal age and autism. Arch Gen Psychiatry. 2006;63:1026–32. doi: 10.1001/archpsyc.63.9.1026. [DOI] [PubMed] [Google Scholar]
- Risch N, Reich EW, Wishnick MM, McCarthy JG. Spontaneous mutation and parental age in humans. Am J Hum Genet. 1987;41:218–48. [PMC free article] [PubMed] [Google Scholar]
- Schalkwyk LC, Meaburn EL, Smith R, Dempster EL, Jeffries AR, Davies MN, Plomin R, Mill J. Allelic skewing of DNA methylation is widespread across the genome. Am J Hum Genet. 2010;86:196–212. doi: 10.1016/j.ajhg.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon BC. Differential allocation: tests, mechanisms and implications. Trends Ecol Evol. 2000;15:397–402. doi: 10.1016/s0169-5347(00)01953-4. [DOI] [PubMed] [Google Scholar]
- Shi L, Wu J. Epigenetic regulation in mammalian preimplantation embryo development. Reprod Biol Endocrinol. 2009;7:59. doi: 10.1186/1477-7827-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK. What is an epigenetic transgenerational phenotype? F3 or F2. Reprod Toxicol. 2008;25:2–6. doi: 10.1016/j.reprotox.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS One. 2008;3:e3745. doi: 10.1371/journal.pone.0003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RG, Kember RL, Mill J, Fernandes C, Schalkwyk LC, Buxbaum JD, Reichenberg A. Advancing paternal age is associated with deficits in social and exploratory behaviors in the offspring: a mouse model. PLoS One. 2009;4:e8456. doi: 10.1371/journal.pone.0008456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouder C, Deutsch S, Paoloni-Giacobino A. Superovulation in mice alters the methylation pattern of imprinted genes in the sperm of the offspring. Reprod Toxicol. 2009;28:536–41. doi: 10.1016/j.reprotox.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Stouder C, Paoloni-Giacobino A. Transgenerational effects of the endocrine disruptor vinclozolin on the methylation pattern of imprinted genes in the mouse sperm. Reproduction. 2010;139:373–9. doi: 10.1530/REP-09-0340. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Hegedus AM, Goldstein G, Shelly C, Alterman AI. Adolescent sons of alcoholics: neuropsychological and personality characteristics. Alcohol Clin Exp Res. 1984;8:216–22. doi: 10.1111/j.1530-0277.1984.tb05842.x. [DOI] [PubMed] [Google Scholar]
- Wagner KD, Wagner N, Ghanbarian H, Grandjean V, Gounon P, Cuzin F, Rassoulzadegan M. RNA induction and inheritance of epigenetic cardiac hypertrophy in the mouse. Dev Cell. 2008;14:962–9. doi: 10.1016/j.devcel.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Walter CA, Intano GW, McCarrey JR, McMahan CA, Walter RB. Mutation frequency declines during spermatogenesis in young mice but increases in old mice. Proc Natl Acad Sci U S A. 1998;95:10015–9. doi: 10.1073/pnas.95.17.10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M, Reichenberg A, Werbeloff N, Kleinhaus K, Lubin G, Shmushkevitch M, Caspi A, Malaspina D, Davidson M. Advanced parental age at birth is associated with poorer social functioning in adolescent males: shedding light on a core symptom of schizophrenia and autism. Schizophr Bull. 2008;34:1042–6. doi: 10.1093/schbul/sbn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohr M, Oddi D, D'Amato FR. Effect of altricial pup ultrasonic vocalization on maternal behavior. Handbook of Behavioral Neuroscience. 2009;19:159–166. [Google Scholar]
- Wozniak DF, Cicero TJ, Kettinger L, 3rd, Meyer ER. Paternal alcohol consumption in the rat impairs spatial learning performance in male offspring. Psychopharmacology (Berl) 1991;105:289–302. doi: 10.1007/BF02244324. [DOI] [PubMed] [Google Scholar]
- Yauk C, Polyzos A, Rowan-Carroll A, Somers CM, Godschalk RW, Van Schooten FJ, Berndt ML, Pogribny IP, Koturbash I, Williams A, Douglas GR, Kovalchuk O. Germ-line mutations, DNA damage, and global hypermethylation in mice exposed to particulate air pollution in an urban/industrial location. Proc Natl Acad Sci U S A. 2008;105:605–10. doi: 10.1073/pnas.0705896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngson NA, Whitelaw E. Transgenerational epigenetic effects. Annu Rev Genomics Hum Genet. 2008;9:233–57. doi: 10.1146/annurev.genom.9.081307.164445. [DOI] [PubMed] [Google Scholar]


