Abstract
Background
Garcinia buchananii bark extract is a traditional African remedy for diarrhea, dysentery, abdominal discomfort and pain. We investigated the mechanisms and efficacy of this extract using the guinea pig distal colon model of gastrointestinal motility.
Methods
Stem bark was collected from G. buchananii trees in their natural habitat of Karagwe, Tanzania. Bark was sun dried and ground into fine powder, which was suspended in Krebs to obtain an aqueous extract. Isolated guinea pig distal colon was used to determine the effect of the G. buchananii bark extract on fecal pellet propulsion. Intracellular recording was used to evaluate the extract action on evoked fast excitatory post-synaptic potentials (fEPSPs) in S- neurons of the myenteric plexus.
Key Results
G. buchananii bark extract inhibited pellet propulsion in a concentration-dependent manner, with an optimal concentration of ~10 mg powder ml−1. Interestingly, washout of the extract resulted in an increase in pellet propulsion to a level above basal activity. The extract reversibly reduced the amplitude of evoked fEPSPs in myenteric neurons. The extract’s inhibitory action on propulsive motility and fEPSPs was not affected by the opioid receptor antagonist, naloxone, or the alpha- 2 adrenoceptor antagonist, yohimbine. The extract inhibited pellet motility in the presence of gamma-aminobutyric acid (GABA), GABAA and GABAB receptor antagonists picrotoxin and phaclofen, respectively. However, phaclofen and picrotoxin inhibited recovery rebound of motility during washout.
Conclusions & Inferences
G. buchananii extract has the potential to provide an effective, non-opiate anti-diarrheal drug. Further studies are required to characterize bioactive components and elucidate the mechanisms of action, efficacy and safety.
Keywords: Garcinia, intestinal motility, myenteric, diarrhea, herbal medicine, botanical extract and gamma-aminobutyric acid (GABA)
INTRODUCTION
In developing nations diarrheal diseases are a significant cause of debilitation, weakened immunity, susceptibility to infection, stunted growth, morbidities and mortalities of high-risk groups (children, elderly, and HIV/AIDS patients1-5. Each year approximately 2 billion cases of diarrheal diseases occur, resulting in approximately 1.5 million deaths of children under the age of five 1, 4, 5. Diarrheal disease is a major complication in HIV/AIDS patients. In sub-Saharan Africa, ~ 1.5 million HIV/AIDS patients die directly or indirectly due to diarrhea each year 2, 3. In the developed countries, diarrhea illnesses result in a significant number of mortalities, hospitalizations, and outpatient clinic visits, especially in children under 5 years 6-8, irritable bowel syndrome patients 9, 10, and the elderly 11. These diseases are a significant financial burden to families, businesses, and governments worldwide 4-8.
Despite complex etiology and pathobiology, diarrheal disease is fundamentally caused by agents that induce and increase propulsive motility, hypersecretion, variable degrees of inflammation and pain, as well as altered immunity of the bowel 4, 5, 7, 9, 10. Many strategies have been used for the treatment of diarrheal diseases. These include anti-motility agents such as opiates and somatostatin analogs, absorbents, anti-secretory medicines (ekephalinase inhibitors), vaccinations, antibiotics, and oral rehydration therapy 3-5, 12-15. Unfortunately, financial constraints limit synthetic drug availability in developing nations. Today, only 39 per cent of children with diarrhea in developing countries receive the low-osmolarity fluid replacement kit with zinc, a recommended treatment, to prevent dehydration and reduce mortality 5. Consequently, there is need for development of new interventions and approaches to treat diarrhea, with efforts being directed toward the manufacture of effective, affordable, and accessible formulations that target both symptoms and underlying causes of these diseases.
Herbal extracts have been used for several millennia to treat diarrheal diseases, and it is estimated that up to 80% of the developing countries is currently dependent on such options. This suggests that population in some herbal remedies have the potential to fill the aforementioned niche 2, 14, 16, 17. Nevertheless, such remedies continue to be regarded with skepticism due to a lack of objective data regarding their safety, mechanisms of action, and efficacy 16. Widely used anti-diarrheal folk remedies include extracts from blackberry roots and bark, Croton lechleri, Galla chinensis, blueberry leaves and fruit, chamomile leaves, apples, green bananas, wood creosote 2, 14, 16, 17 and Garcinia plant bark and fruit2, 17. Extracts from stem and root bark of G. buchananii are currently used to treat diarrhea 2, but the mechanism of action, dose-responsiveness, and bioactive ingredients are unknown.
The aim of this study was to determine the effects of an aqueous G. buchananii stem bark extract on peristalsis and neurotransmission activity of the guinea pig distal colon.
MATERIALS AND METHODS
Animals and solutions
Male adult guinea pigs (Charles River, Montreal, Canada and Elm Hill Breeding Labs, Chelmsford, Massachusetts, USA) weighing 250-350g were housed in metal cages with soft bedding. Animals had access to food and water ad libitum and were maintained at 23-24°C on a 12:12 h light-dark cycle. Animals were anesthetized with isoflurane and exsanguinated. The entire distal colon was removed, stored in Krebs solution (mM: NaCl, 121; KCl, 5.9; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 25; NaH2PO4, 1.2; glucose, 8; aerated with 95% O2/5% CO2; all from Sigma-Aldrich, St. Louis, MO, USA) and used for subsequent motility or electrophysiology experimentation. The Institutional Animal Care and Use Committees of both the University of Idaho and the University of Vermont approved all animal procedures.
Preparation of aqueous G. buchananii extract
Bark samples were collected from stems of G. buchananii trees (family name Clusiaceae; vernacular name Omusharazi; see Kisangau et al., 2) in their natural habitat in Nyakasimbi village (GPS: Latitudes: −1.852247, Longitudes 31.024017; ~ 1600 m), Karagwe, Kagera, Tanzania. The bark specimens used are similar to G. buchananii bark deposited at the Department of Botany herbarium, University of Dar es alaam (voucher specimen # DK 063/06, see Kisangau and others 3). Bark was collected in October 2006 and September 2009, trimmed into small pieces, sun dried for one week and ground into powder using a wooden mortar and pestle. The powder was sieved using 1-mm mesh with criss-cross patterning. The powder was transported using airtight bags, and was stored at 4°C while protected from light. Freshly diluted extract solutions were used for each experiment. Appropriate amounts of the powder were weighed, mixed with 100 ml Krebs, and stirred for 30 min. at room temperature. The mixtures were filtered using analytical filter paper (Schleicher and Schuell Blue Ribbbon filter paper 589/3; 0.2 μm retention) and the filtrate (G. buchananii bark extract) was used as indicated in the procedures.
Gastrointestinal Motility Assays
Segments (~ 10 cm long) of distal colon were pinned on either end in a Sylgard-lined 50 ml organ bath, continuously perfused with oxygenated Krebs solution (rate: 10 ml min−1) and maintained at temperatures between 36-37°C. Tissues were initially allowed to equilibrate for 30 minutes in re-circulating Krebs solution. Colonic motility was studied using the Gastronintestinal Motility Monitoring system (GIMM; Med-Associates Inc., Saint Albans, Vermont, USA), which included a digital video camera (Catamount Research and Development Inc, St Albans, VT) to film fecal pellet propulsion. Trials were separated by five-minute recovery periods between successive pellet runs. The GIMM system software calculated velocities by tracking pellets as they traversed colon segments. After initial equilibration, five pellet propulsion trials in vehicle solution were obtained to determine the basal velocity for each tissue preparation. To reduce variations between runs and experiments, the values obtained were used to generate a normalized basal velocity by dividing the values of the 4th – 5th runs by the value of the 1-2nd runs. G. buchananii stem bark extract and test compounds dissolved in 100 ml of Krebs solution were superfused either into the organ bath or into the lumen of the colon using polyethylene tubing (PE 205; outside diameter 9.5 mm). The effects of extract and test compounds were evaluated by obtaining velocities of four trials taken at 5 minute intervals (5-20 min.). Normalized velocity data for these treatments were obtained by dividing the test velocities acquired by the average velocity of the five pellet propulsion runs performed in vehicle solution (runs 1-5, above). Normalized data became ratios without units, and were presented as a percent velocity of basal activity for each tissue. Velocities of pellet propulsion were also studied during washout of tissue with Krebs solution (after extract or test compound application) for 20 min.
Intracellular Recording
To study fast excitatory post-synaptic potentials, longitudinal muscle-myenteric plexus preparations of guinea pig colon were pinned and stretched in a 2.5 ml Sylgard lined recording chamber. The tissues were maintained at 36-37°C by continuous perfusion with re-circulating oxygenated Krebs solution (10 ml min−1). Nifedipine (5 μM) and atropine (200 nM) were added to Krebs solution to limit muscle contractions. Myenteric ganglia were visualized at x200 using Hoffman modulation contrast optics on an inverted microscope (Nikon Diaphot, Melvilee, NY, USA). Individual neurons were randomly impaled using glass microelectrodes, which were filled to the shoulder with 1.0 M KCl and topped off with 2.0 M KCl, generating a range of 50-150 MΩ input resistance. Membrane potential was measured with an Axoclamp-2A amplifier (Axon Instruments, Union City, CA, USA) and the electrical signals were acquired and analyzed using PowerLab Chart version 5.01 (ADInstruments, Castle Hills, Australia). Input resistance and resting membrane potential were determined for neurons before and after exposure to G. buchananii extract. Using monopolar extracellular electrodes made from Teflon-insulated platinum wire, synaptic input to myenteric neurons was elicited by direct single pulse stimuli (0.5 ms duration) applied to interganglionic fiber tracts. S-type neurons were identified based on the existence of fast excitatory postsynaptic potentials (fEPSPs) and the lack of a shoulder on the re-polarizing phase of the action potential 18-20. Amplitudes of the maximum fEPSPs were acquired while injecting hyperpolarizing currents to maintain membrane potential (approximately −90 MV) and avoid action potentials. The amplitude was measured by taking the difference in voltage between the peak of each fEPSP and the membrane holding potential. Only neurons with an input resistance > 50 MΩ were considered to be healthy and selected for study.
Data Analysis
Statistical analysis of data was done using Student’s t-test, one-way ANOVA, and the Newman-Keul’s multiple comparison post-test using GraphPad Prism 4 software (GraphPad Software Inc., San Diego, CA, USA). Data were expressed as the mean ± SEM for n values representing the number of colon segments from different animals used in each experiment. Statistical differences were considered significant at a P value <0.05.
Drugs and other materials
phaclofen, picrotoxin, yohimbine hydrochloride, GABA (γ-aminobutyric acid), nifedipine, atropine, and naloxone hydrochloride were purchased from Sigma Aldrich Chemical (St. Louis, MO).
RESULTS
Intraluminal versus serosal application of G. buchananii bark extract and rapid reversal of induced inhibition
The preliminary findings of this study were reported in an abstract at Neurogastroenterology and Motility 2009 Joint International Meeting 21.
Control Velocity
Average basal velocity of pellet propulsion was 2.4 +/− 0.6 mm sec−1 (n = 28). The normalized basal pellet velocity computed by dividing the values of pellet velocities of the 4th – 5th runs by the value of the 1-2nd runs, and the ratios expressed as 100% was: ~ 99.6%, +/− 2.0%; n = 18.
Effect of G. buchananii extract on basal pellet motility
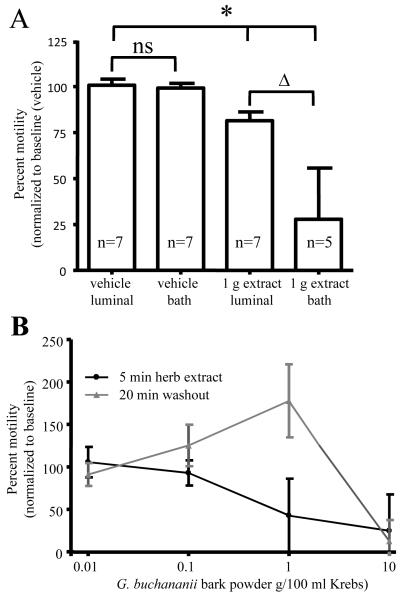
Aqueous extract from G. buchananii bark powder did not alter the pH of Krebs (vehicle solutions). When added to the bathing solution, the extract rapidly inhibited pellet propulsion in a concentration-dependent manner (Figs. 1A-B). The onset of a response occurred within 1 minute of exposure. The extract caused a less extensive reduction in pellet propulsion when administered to the colon intraluminally (1 g bark powder/100 ml extract intraluminal: 80.9% +/− 6.3%, n= 7 vs. 1 g bark powder /100 ml extract bath: 27. 8% +/− 27.8%; n= 5; 10 min.; P< 0.001; Fig. 1A). The extract-induced inhibition of propulsive motility was rapidly reversed by washout, which increased pellet velocity by 30-100% above basal activity with exception of an extracted prepared using 10 g bark powder/100 ml Krebs (Fig. 1B).
Figure 1.
Intraluminal and bath applications of G. buchananii bark extract inhibit pellet propulsion in isolated segments of guinea pig distal colon. A. Bar graph showing the effects of intraluminal vs. bath delivery of the extract (1 g bark powder/100 ml Krebs solution; 5 minute application) on propulsive motility. No difference was detected between intraluminal and bath applications of the vehicle with regard to pellet velocity (bracket with ns). Intraluminal and bath extract applications reduced pellet velocity (bracket with asterisks). 1 g powder in 100 ml Krebs had larger inhibitory effects when applied in the bath vs. an intraluminal delivery (bracket with triangle). B. The concentration-dependent effects of the extract and the effects of washout on pellet propulsion. Lower concentrations (0.01- 0.1 g bark powder/100 ml Krebs) did not alter motility. Higher concentrations (1 - 10 g/100 ml Krebs) inhibited propulsion in a concentration-dependent fashion. At concentrations of 1 g bark powder/100 ml Krebs and below, pellet propulsion was rapidly restored to normal values and an increased rate of propulsive motility (30-100% above baseline) was observed following washout for 10-20 min. Normal pellet propulsion was not restored by 20 min. of washout after treatment with G. buchananii bark powder at a concentration of 10 g/100 ml Krebs.
Garcinia buchananii extract inhibits enteric neurotransmission
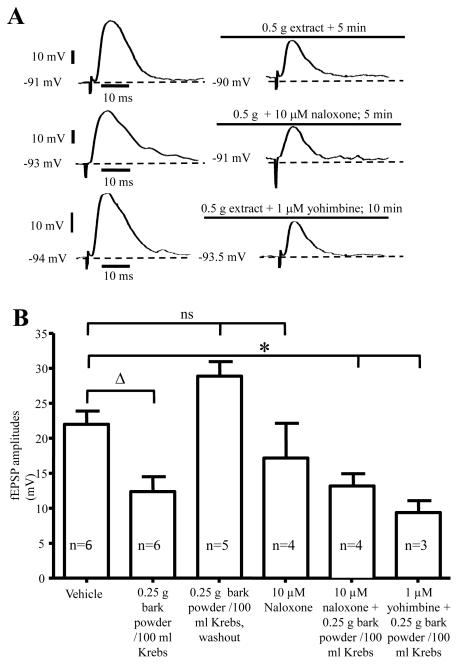
Intracellular recording was used to study the effect of G. buchananii extract on 24 S- neurons in the myenteric ganglia of guinea pig distal colon using longitudinal smooth muscle- myenteric plexus preparations (Figs. 2A-B). S- type neurons exhibit fEPSPs after application of single electrical stimuli to interganglionic nerve fiber tracts 18-20, 22. We found that compared with the motility assay, lower concentrations of G. buchananii extract (0.25-0.5 g bark powder/100 ml Krebs) caused a considerable decrease of fEPSPs amplitudes. Application of G. buchananii extract (0.25 g bark powder/ 100 ml Krebs) did not alter the resting membrane potential (RMP; vehicle: −52.08 ± 0.8, n=6 vs. −50.78 ± 0.6 n=4, P= 0.33) or input resistance (vehicle: 111.2 ± 14.3, n=6 vs.: 97.7 ± 11.5, n=3; P= 0.92) of S- neurons. Application of the extract in the presence of naloxone (n=4) and yohimbine (n=3) did not alter the membrane potential of S- type myenteric neurons. Changes in neuronal excitability, in terms of rheobase or presence of anodal break action potentials, were not observed. When evoked synaptic potentials were evaluated, the extract (0.25-0.5 g bark powder/ 100 ml Krebs) reduced fEPSPs amplitudes of S-neurons after 2-5 minutes of application (vehicle: 21.9 ± 1.9 MV, n=6 vs. 0.25 g bark powder/100 ml Krebs: 12.3 ± 2.1 MV, n=6; P=0.007; 5 minute interval; Figs. 2A-B). The effect of the extract on fEPSP amplitudes was readily reversed by washout (0.25 g bark powder/100 ml Krebs: 13.4 ± 1.2 MV vs. 10 min washout: 28.8 ± 2.08 MV; n=5; P=0.007; Fig. 2B). The amplitude of fEPSPs in S-neurons rebounded to slightly above basal level following washout (vehicle: 21.9 ± 1.9 MV, n=13 vs. 10 min. washout: 28.8 ± 2.08 MV, n=5; P=0.06).
Figure 2.
Demonstration that evoked fEPSPs activity was inhibited by application of G. buchananii extract (0.25 - 0. 5 g bark powder/100 ml Krebs), and these actions were not mediated by opioid or alpha-2 adrenoceptor receptors. A. Representative traces of fEPSPs, demonstrating that G. buchananii extract inhibited fEPSPs in S neurons in the myenteric ganglia of guinea pig distal colon. These actions were not affected by the opioid receptor antagonist, naloxone (10 μM), or by the alpha-2 adrenoceptor antagonist, yohimbine (1 μM). B. Summary data showing that G. buchananii bark extract (0.25 g bark powder/100 ml Krebs) reduced fEPSPs amplitudes in the myenteric neurons after 5 min (bracket with triangle). The inhibitory actions of G. buchananii extract on fEPSPs persisted in the presence of the opioid receptor antagonist, naloxone (10 μM) and the alpha-2 adrenoceptor antagonist, yohimbine (1 μM) (bracket with asterisk). The fEPSPs amplitudes were restored to normal values by washout for 5-10 minutes, and were not affected by naloxone (ns bracket).
The effects G. buchananii extract are not altered by opioid or alpha-2 adrenergic receptor antagonists
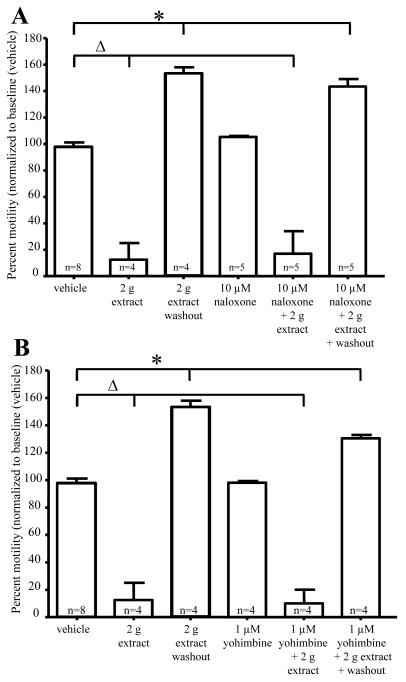
Previous studies have demonstrated that opioid and alpha-2 adrenergic receptor agonists result in inhibition of enteric ganglia fEPSPs 23, 24. To determine whether G. buchananii extract acts via activation of these receptors, the actions of the extract was tested in the presence of receptor antagonists. The μ-opiod receptor antagonist naloxone (10 μM) and alpha-2 (2a, 2b, 2c)-adrenergic receptor antagonist yohimbine (1 μM) had no effect on fEPSPs. Application of the extract in the presence of naloxone (vehicle: 21.9 ± 1.9 MV, n=6 vs. 0.25 g bark powder/100 ml Krebs + 10 μM naloxone: 13.1 ± 1.7 MV; n=4; P=0.03) and yohimbine (vehicle: 21.9 ± 1.9 MV, n=6 vs. 0.25 g bark powder/100 ml Krebs + 1 μM yohimbine: 9.3 ± 1.7 MV, n=3; P=0.009) did not alter the inhibitory actions of the extract on fEPSPs (Fig. 2B). In motility studies, G. buchananii extract 2 g bark powder/100 ml Krebs rapidly reduced pellet velocity (vehicle: 99.7% +/− 3.7%, n= 8 vs. 2 g bark powder/100 ml Krebs extract: 12.5% +/− 12.0%, n= 4; P< 0.001; 10 min; Figs. 3 A-B). Like in fEPSP studies, motility assays showed no change in the ability of the G. buchananii bark extract to reduce pellet propulsion while in the presence of naloxone (10 μM) or yohimbine (1 μM) (vehicle: 99.7% +/− 3.7%, n= 8 vs. 2 g bark powder/100 ml Krebs extract + 10 μM naloxone: 20.4% +/− 20.4%; n = 5; P< 0.001; vehicle: 99.7% +/− 3.7%, n= 8 vs. 2 g bark powder/100 ml Krebs extract + 1 μM yohimbine: 10.0% +/− 10.0%; n = 6; 10 min interval; P< 0.001; Figs. 3 A-B).
Figure 3.
Inhibitory actions of G. buchananii extract (2 g bark powder/100 ml Krebs) on pellet motility do not involve opioid or α-2 adrenergic receptor activation. A-B: Summary data showing that neither naloxone (10 μM; A, bracket with triangle) nor yohimbine (1μM; B, bracket with triangle) altered the ability of G. buchananii extract to reduce propulsive motility. The rebound prokinetic effect of G. buchananii extract detected after washout also persisted in the presence of naloxone and yohimbine (3 A-B, brackets with asterisks).
Garcinia buchananii bark extract inhibits GABA receptors
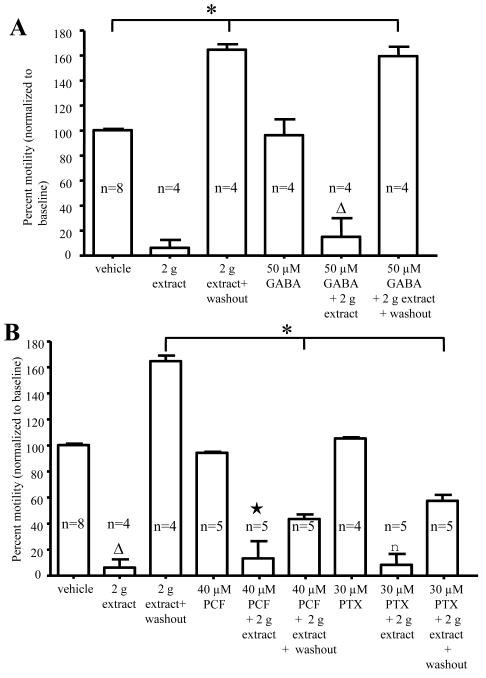
Flavonoids, which have been found in the bark of Garcinia plants 17, 25, inhibit neurotransmission in the brain by acting via gamma-aminobutyric acid (GABA) receptors 26, 27. Colonic motility assays were used to examine whether G. buchananii extract interacts with GABA receptors. G. buchananii extract (2 g bark powder/100 ml Krebs) inhibited pellet propulsion in the presence of GABA agonist GABA (50 μM; Fig. 4A), the GABAA-antagonist picrotoxin (PTX, 30 μM) and the GABAB-antagonist phaclofen (PCF, 40 μM), respectively (Fig. 4B). While GABA showed no influence on recovery of pellet propulsion from the inhibitory effects of the extract, both phaclofen and picrotoxin caused a slower rate of recovery, such that normal propulsive activity was not achieved after 20 minutes of Krebs washout (2 g extract washout: 157.0% +/− 6.0%, n= 4 vs. 2 g extract + PCF washout: 43.5% +/− 21.4%; n = 5; P< 0.001 and 2 g extract + PTX washout: 58.5% +/− 8.1%; n = 5; P< 0.001; Figs. 4 A-B).
Figure 4.
G. buchananii extract contains bioactive components that act as GABA receptor ligands. A-B: Summary data showing that GABA (50 μM, A, triangle), the GABAB receptor antagonist, phaclofen (PCF, 40 μM; B, star), and the GABAA receptor antagonist picrotoxin (PTX, 30 μM; B, square), did not alter the effects of G. buchananii extract (2 g bark powder/100 ml Krebs, delivered by bath application) on propulsive motility after 5-10 minutes. Recovery of propulsive motility by post-treatment washout was similar to vehicle results for GABA treatment (increasing propulsive motility by up to 70% above baseline at 15-20 minute washout intervals; (A, bracket with asterisk; P<0.05). A slower recovery was observed during washout after treatment using the extract in combination with picrotoxin (PTX, 30 μM) or phaclofen (PCF, 40 μM). In these cases, motility was still significantly lower than that of vehicle after 20 minutes of washout (bracket with asterisk in B; P<0.05).
DISCUSSION
The purpose of this study was to determine whether G. buchananii extract, a herbal remedy for diarrheal diseases in sub-Saharan Africa, inhibits colonic motility, and to begin to identify the target tissues and the cellular mechanisms underlying these effects. We found that G. buchananii extract contains readily soluble bioactive components that rapidly inhibit propulsive motility in guinea pig distal colon segments. This inhibitory activity occurs via activation of yet to be identified targets in the mucosa and myenteric ganglia. The extract’s action involves an inhibition of synaptic transmission presumably by either reducing pre-synaptic neurotransmitter release, or by a blockade of post-synaptic excitatory responses in the myenteric ganglia. The motility and synaptic inhibitory actions were not mediated by the activation of pre-synaptic opioid 24, 28 or α –adrenergic 10, 23, 29 receptors, which are the targets of most anti-motilic drugs commonly used to treat diarrheal diseases 3-5, 10, 29.
G. buchananii bark extract likely contains an assortment of bioactive components that have receptor targets in the myenteric plexus. The findings of this study demonstrate that the components of the G. buchananii bark extract inhibit motility from both the mucosal and serosal surfaces of the guinea pig colon. Although an anti-motility response with a higher magnitude was obtained by applying the extract on the serosal surface, the onset of responses in both cases did not differ, occurring as early as 1 minute after exposure to the extract. The variation between the two routes suggests that when G. buchananii bark extract was applied to the bathing solution, bioactive components activated a larger subset of myenteric neurons, since this bath application exposed the serosal surface to the extract. Consequently the myenteric plexus was rapidly exposed to a larger extract volume. The findings also suggest a reduced rate of target site access following oral administration. These ideas are supported by the findings that lower concentrations of the bark extract are required to inhibit fEPSPs in longitudinal smooth muscle-myenteric preparations vs. higher concentrations need for bath and intraluminal application in motility studies using intact colon segments. In either case, the responses appear to be mainly a result of inhibition of neurotransmission in the myenteric plexus.
Interestingly, at concentrations of less than 10 g G. buchananii bark powder per100 ml Krebs, the inhibition of propulsion was reversed after 10-20 minute washout. Upon washout, the velocities rebounded beyond the basal level by up to seventy percent. The reasons for these findings are not entirely clear. However, this suggests that G. buchananii extract may contain pro-motility components. This idea is supported by observations that G. buchananii bark extract can be separated into fractions of anti- motilic and pro-kinetic activities using thin layer chromatography (Boakye, unpublished findings). Several other possibilities for these observations exist. Differences in the activation rates of cellular processes needed for inhibition/stimulation of propulsion and tissue metabolism of prokinetic versus anti-motilic components are proposed explanations. The findings support the concept that mild overdose following consumption of large amounts of G. buchananii extract can readily be reversed.
One major action of G. buchananii bark extract reported here is inhibition of interneuronal transmission in the myenteric ganglia. Fast excitatory synaptic transmission in the ENS plays a critical role of regulating intestinal motility 18, 20, 30. Basal resting membrane potentials, input resistance, and fEPSP amplitudes observed in this study correspond with previous findings in the myenteric ganglia of guinea pig distal colon18, 19. On the basis of the findings reported here, G. buchananii bark extract contains bioactive components that either inhibit pre-synaptic neurotransmitter release from myenteric nerve terminals, or interfere with post-synaptic responses. Furthermore, this study has demonstrated that inhibition was not mediated by activation of pre-synaptic opioid or α -2 adrenergic receptors, which are the targets of some anti-diarrheal drugs 3, 4, 10, 23, 24, 29. In the guinea pig distal colon, myenteric neurons exhibit mixed fEPSPs with a larger proportion being regulated by nicotinic acetylcholine receptors 18. It has been proposed that plant polyphenols can selectively block nicotinic acetylcholine receptors 31, showing another potential mechanism for G. buchananii bark extract to inhibit fEPSPs. Pre-synaptic inhibition of neurotransmission in the ENS can involve activation of α2-adrenoceptors, opioid receptors, muscarinic M2 receptors, Adenosine A1, and 5-HT1A receptors 32, all of which couple to G-proteins and eventually modulate neurotransmitter release. The effect of the extract on cholinergic neurons and synaptic mechanisms of enteric neurotransmission is the next goal of our research.
The bioactive components in G. buchananii bark extract appear to affect enteric neurotransmission at least in part via GABA receptors. Recent studies using botanical extracts suggest that bioflavanoids inhibit neurotransmission in the brain 26, 27. These botanical metabolites either inhibit 26, 27 or activate 33 GABA receptors. There is also evidence suggesting that flavanoids and xanthones constitute the major components of some Garcinia bark extracts 17, 25. GABA is a neurotransmitter found in interneurons, which mediates activation of cholinergic excitatory neurons of the guinea pig distal colon 32, 34, 35. In the present study, GABA did not affect G. buchananii bark extract’s activity. However, GABAA and GABAB receptor antagonists suppressed the rebound of propulsive activity during washout, suggesting that if rebound is due to activity of pro-kinetic components, they act as ligands of GABAA and GABAB receptors. It is likely that these pro-kinetic components cannot readily be washed out. In light of these findings, further studies are needed to distinguish how the components of the extract affect GABA neurotransmission in the ENS.
Within our model, it is likely that bioactive components of G. buchananii bark extract are acting via the colonic mucosa to inhibit peristaltic activity, and the specific mucosal target receptors are not known. The anti-motility effects are elicited rapidly. Dissolving the bark powder into Krebs solution seems to effectively incorporate the bioactive components of G. buchananii bark into vehicle solution. This indicates that the traditional way of consuming G. buchananii bark by chewing the bark itself or mixing the bark powder with a beverage, (Fidelis Kanyamisibo, personal communication) is an efficient indigenous way to deliver the extract for therapeutic purposes. Botanical extracts and their derivatives are decomposed and extensively metabolized or modified in the GI tract 36. It is unclear whether the components that inhibit colonic motility in this study would reach the colon following oral administration, or whether it is the systemically delivered metabolic derivatives that act as anti-diarrheal remedies. There is need to study this bioavailability and how G. buchananii bark extract along with its metabolic derivatives affect mucosal serotonin signaling (including activation of nerve endings) and other signaling mechanisms that are key to GI motility 9, 37.
Taken together, these findings indicate that G. buchananii bark extract is capable of acting via mucosal targets and/or myenteric S-type neurons to inhibit propulsive motility in guinea pig distal colon. Inhibition of synaptic transmission is likely to reduce bowel pain and discomfort, often associated as symptoms of diarrhea 10. Garcinia plant extracts contain components with anti-inflammatory, anti-protozoa, anti-bacterial, anti-viral, and anti-oxidant activity 17, 25, 38. These characteristics and our findings suggest the bark of G. buchananii is promising to become an effective anti-diarrheal botanical remedy 21. It needs to be determined whether the active compounds include flavonoids, xanthones, glycosides or alkaloids (which are present in these plants 17, 25), or a synergistic activity of any combination of these. The efficacy and safety of G. bucananii cannot be adequately measured until these questions are answered. This is especially necessary because our data suggest that at a higher dose, G. buchananii bark extract may shut down propulsive motility in guinea pig distal colon.
In conclusion, our findings suggest that G. buchananii bark extract contains readily soluble bioactive compounds that act via mucosal targets or directly on the ENS to reduce propulsive motility through inhibition of S- neuron synaptic activity. G. buchananii bark extract has the potential to be developed as an affordable and effective anti-diarrheal drug for high-risk groups.
ACKNOWLEDGMENTS
The University of Idaho Start-up to Dr. Onesmo B. Balemba and NIH grant DK62267 to Dr. Gary M. Mawe supported this study. The authors are grateful to Ms. Ailene MacPherson and Ms Hayato Norimine for their help in motility assays.
Footnotes
DISCLOSURES
The authors declare have no competing interests
REFERENCE
- 1.Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet. 2005;365(9465):1147–52. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 2.Kisangau DP, Lyaruu HV, Hosea KM, Joseph CC. Use of traditional medicines in the management of HIV/AIDS opportunistic infections in Tanzania: a case in the Bukoba rural district. J Ethnobiol Ethnomed. 2007;3:29. doi: 10.1186/1746-4269-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nwachukwu CE, Okebe JU. Antimotility agents for chronic diarrhoea in people with HIV/AIDS. Cochrane Database Syst Rev. 2008;(4) doi: 10.1002/14651858.CD005644.pub2. CD005644. [DOI] [PubMed] [Google Scholar]
- 4.Thapar N, Sanderson IR. Diarrhoea in children: an interface between developing and developed countries. Lancet. 2004;363(9409):641–53. doi: 10.1016/S0140-6736(04)15599-2. [DOI] [PubMed] [Google Scholar]
- 5.UNICEF/WHO Diarrhoea: why children are still dying and what can be done WHO: New report 2009. 2009:1–68. [Google Scholar]
- 6.Gravel D, Miller M, Simor A, et al. Health care-associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: a Canadian Nosocomial Infection Surveillance Program Study. Clin Infect Dis. 2009;48(5):568–76. doi: 10.1086/596703. [DOI] [PubMed] [Google Scholar]
- 7.Vernacchio L, Vezina RM, Mitchell AA, Lesko SM, Plaut AG, Acheson DW. Characteristics of persistent diarrhea in a community-based cohort of young US children. J Pediatr Gastroenterol Nutr. 2006;43(1):52–8. doi: 10.1097/01.mpg.0000228094.74207.39. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman CM, Bresee JS, Parashar UD, Riggs TL, Holman RC, Glass RI. Cost of diarrhea-associated hospitalizations and outpatient visits in an insured population of young children in the United States. Pediatr Infect Dis J. 2001;20(1):14–9. doi: 10.1097/00006454-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol. 2005;39(5 Suppl 3):S184–93. doi: 10.1097/01.mcg.0000156403.37240.30. [DOI] [PubMed] [Google Scholar]
- 10.Wood JD. Effects of bacteria on the enteric nervous system: implications for the irritable bowel syndrome. J Clin Gastroenterol. 2007;41(5 Suppl 1):S7–19. doi: 10.1097/MCG.0b013e31802f1331. [DOI] [PubMed] [Google Scholar]
- 11.Lew JF, Glass RI, Gangarosa RE, Cohen IP, Bern C, Moe CL. Diarrheal deaths in the United States, 1979 through 1987. A special problem for the elderly. JAMA. 1991;265(24):3280–4. [PubMed] [Google Scholar]
- 12.Atia AN, Buchman AL. Oral rehydration solutions in non-cholera diarrhea: a review. Am J Gastroenterol. 2009;104(10):2596–604. doi: 10.1038/ajg.2009.329. quiz 605. [DOI] [PubMed] [Google Scholar]
- 13.Farthing M. Antisecretory drugs for diarrheal disease. Dig Dis. 2006;24(1-2):47–58. doi: 10.1159/000090308. [DOI] [PubMed] [Google Scholar]
- 14.Greenwood-Van Meerveld B, Tyler KR, Venkova K, Kuge T. Comparison of the antidiarrheal effects of wood creosote and loperamide in the rat jejunum and colon in vitro. Biol Pharm Bull. 2000;23(8):952–6. doi: 10.1248/bpb.23.952. [DOI] [PubMed] [Google Scholar]
- 15.Scrimgeour AG, Lukaski HC. Zinc and diarrheal disease: current status and future perspectives. Curr Opin Clin Nutr Metab Care. 2008;11(6):711–7. doi: 10.1097/MCO.0b013e3283109092. [DOI] [PubMed] [Google Scholar]
- 16.Palombo EA. Phytochemicals from traditional medicinal plants used in the treatment of diarrhoea: modes of action and effects on intestinal function. Phytother Res. 2006;20(9):717–24. doi: 10.1002/ptr.1907. [DOI] [PubMed] [Google Scholar]
- 17.Pedraza-Chaverri J, Cardenas-Rodriguez N, Orozco-Ibarra M, Perez-Rojas JM. Medicinal properties of mangosteen (Garcinia mangostana) Food Chem Toxicol. 2008;46(10):3227–39. doi: 10.1016/j.fct.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Krauter EM, Linden DR, Sharkey KA, Mawe GM. Synaptic plasticity in myenteric neurons of the guinea-pig distal colon: presynaptic mechanisms of inflammation-induced synaptic facilitation. J Physiol. 2007;581(Pt 2):787–800. doi: 10.1113/jphysiol.2007.128082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linden DR, Sharkey KA, Mawe GM. Enhanced excitability of myenteric AH neurones in the inflamed guinea-pig distal colon. J Physiol. 2003;547(Pt 2):589–601. doi: 10.1113/jphysiol.2002.035147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wade PR, Wood JD. Synaptic behavior of myenteric neurons in guinea pig distal colon. Am J Physiol. 1988;255(2 Pt 1):G184–90. doi: 10.1152/ajpgi.1988.255.2.G184. [DOI] [PubMed] [Google Scholar]
- 21.Balemba OB, Strong DS, Mawe GM. Garcinia buchananii bark extracts inhibit propulsive motility and fast synaptic potentials in the guinea pig distal colon. Neurogastroenterol Motil. 2009;21(Suppl 1):31–2. doi: 10.1111/j.1365-2982.2010.01583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertrand P, Thomas EA. Multiple levels of sensory integration in the intrinsic sensory neurons of the enteric nervous system. Clin Exp Pharmacol Physiol. 2004;31(11):745–55. doi: 10.1111/j.1440-1681.2004.04092.x. [DOI] [PubMed] [Google Scholar]
- 23.Wood JD. Neurotransmission at the interface of sympathetic and enteric divisions of the autonomic nervous system. Chin J Physiol. 1999;42(4):201–10. [PubMed] [Google Scholar]
- 24.Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil. 2004;16(Suppl 2):17–28. doi: 10.1111/j.1743-3150.2004.00554.x. [DOI] [PubMed] [Google Scholar]
- 25.Mbwambo ZH, Kapingu MC, Moshi MJ, et al. Antiparasitic activity of some xanthones and biflavonoids from the root bark of Garcinia livingstonei. J Nat Prod. 2006;69(3):369–72. doi: 10.1021/np050406v. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez SP, Wasowski C, Loscalzo LM, et al. Central nervous system depressant action of flavonoid glycosides. Eur J Pharmacol. 2006;539(3):168–76. doi: 10.1016/j.ejphar.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Spencer JP. The interactions of flavonoids within neuronal signalling pathways. Genes Nutr. 2007;2(3):257–73. doi: 10.1007/s12263-007-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood MJ, Hyman NH, Mawe GM. The Effects of Daikenchuto (DKT) on Propulsive Motility in the Colon. J Surg Res. 2009 doi: 10.1016/j.jss.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Callahan MJ. Irritable bowel syndrome neuropharmacology. A review of approved and investigational compounds. J Clin Gastroenterol. 2002;35(1 Suppl):S58–67. doi: 10.1097/00004836-200207001-00011. [DOI] [PubMed] [Google Scholar]
- 30.Ren J, Zhou X, Galligan JJ. 5-HT4 receptor activation facilitates recovery from synaptic rundown and increases transmitter release from single varicosities of myenteric neurons. Am J Physiol Gastrointest Liver Physiol. 2008;294(6):G1376–83. doi: 10.1152/ajpgi.00078.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pithayanukul P, Ruenraroengsak P, Bavovada R, Pakmanee N, Suttisri R, Saenoon S. Inhibition of Naja kaouthia venom activities by plant polyphenols. J Ethnopharmacol. 2005;97(3):527–33. doi: 10.1016/j.jep.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 32.Galligan JJ. Ligand-gated ion channels in the enteric nervous system. Neurogastroenterol Motil. 2002;14(6):611–23. doi: 10.1046/j.1365-2982.2002.00363.x. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez SP, Mewett KN, Hanrahan JR, Chebib M, Johnston GA. Flavan-3-ol derivatives are positive modulators of GABA(A) receptors with higher efficacy for the alpha(2) subtype and anxiolytic action in mice. Neuropharmacology. 2008;55(5):900–7. doi: 10.1016/j.neuropharm.2008.06.069. [DOI] [PubMed] [Google Scholar]
- 34.Grider JR. Regulation of excitatory neural input to longitudinal intestinal muscle by myenteric interneurons. Am J Physiol. 1998;275(5 Pt 1):G973–8. doi: 10.1152/ajpgi.1998.275.5.G973. [DOI] [PubMed] [Google Scholar]
- 35.Krantis A. GABA in the Mammalian Enteric Nervous System. News Physiol Sci. 2000;15:284–90. doi: 10.1152/physiologyonline.2000.15.6.284. [DOI] [PubMed] [Google Scholar]
- 36.Spencer JP. Metabolism of tea flavonoids in the gastrointestinal tract. J Nutr. 2003;133(10):3255S–61S. doi: 10.1093/jn/133.10.3255S. [DOI] [PubMed] [Google Scholar]
- 37.Costedio MM, Hyman N, Mawe GM. Serotonin and its role in colonic function and in gastrointestinal disorders. Dis Colon Rectum. 2007;50(3):376–88. doi: 10.1007/s10350-006-0763-3. [DOI] [PubMed] [Google Scholar]
- 38.dos Reis SB, de Oliveira CC, Acedo SC, et al. Attenuation of colitis injury in rats using Garcinia cambogia extract. Phytother Res. 2009;23(3):324–9. doi: 10.1002/ptr.2626. [DOI] [PubMed] [Google Scholar]