Abstract
Objectives
The Prostate Cancer Prevention Trial (PCPT) prostate cancer risk calculator was developed to aid physicians in counseling men for consideration of prostate biopsy based on prostate-specific antigen (PSA) and other clinical risk factors. This study investigated the role of body mass index (BMI) in this assessment.
Methods
BMI category was defined as < 25 (under/normal weight), 25.0-29.9 (overweight), 30.0-34.9 (obese [OB] I), 35.0-39.9 (OB II), and ≥ 40 (OB III). BMI-adjusted PSA for a man was determined by multiplying his PSA to the ratio of the geometric mean of PSA for BMI<25 to the geometric mean of PSA for his BMI category. Operating characteristics of PSA and BMI-adjusted PSA were compared to PCPT risks using area underneath the receiver operating characteristic curve (AUC). Statistical tests of differences between AUCs for different diagnostic tests were performed via nonparametric U-statistic method.
Results
BMI-adjusted PSA equaled to unadjusted PSA multiplying 1.09, 1.20, 1.50, 1.71 for men in overweight, OBI, OBII and OBIII categories, respectively. The area underneath the operating characteristic curve (AUC) for BMI-adjusted PSA values (0.84) did not differ from PSA; that of the PCPT calculator with BMI-adjusted PSA (0.87) did not differ from the calculator with PSA. Of 2816 men with a PSA less than or equal to 2.5 ng/mL who did not undergo biopsy, 126 (4.5%) would have a BMI-adjusted PSA exceeding 2.5 ng/mL.
Conclusions
Due to lower levels of PSA, overweight and obese men may suffer diminished cancer detection opportunities when undergoing PSA-based screening.
Keywords: BMI, PSA, BMI-adjusted PSA, PCPT risk calculator, prostate cancer
Introduction
One in six men will be diagnosed with prostate cancer (PCa) during their lifetime and one in thirty-five will die from the disease. While prostate-specific antigen (PSA) is commonly used in determining who may be at risk for PCa and should undergo a prostate biopsy, use of PSA alone can expose patients to unnecessary biopsy, but sometimes fail to timely perform biopsy resulting in delayed diagnosis.1,2 Traditionally, the PSA value has been treated as dichotomous with a value above a specific threshold (PSA>4.0 ng/mL or >2.5 ng/mL) leading to a prostate biopsy recommendation. More recently, the continuum of PCa risk with PSA level, even below 4.0 ng/mL, and the contribution of other risk factors, including digital rectal examination (DRE), family history of PCa, a prior biopsy, race and age to risk of disease have led to development of personalized risk assessment tools such as the Prostate Cancer Prevention Trial (PCPT) prostate cancer risk calculator.2,3 In addition, many researchers have reported that increased body mass index (BMI) is associated with decreased PSA.3-10 In this study we investigated the role of BMI-adjusted PSA in PCa screening.
Materials and Methods
The San Antonio center for Biomarkers Of Risk of prostate cancer (SABOR) is a National Cancer Institute, Early Detection Research Network-sponsored Clinical Validation Center. SABOR has enrolled community-dwelling men in a biomarker validation cohort. From this cohort, we identified 3697 men (3432 non-cancers and 265 cancers) who were enrolled through May 2009 for this analysis. In these subjects, age, PSA, body mass index (BMI), DRE, race, first-degree family history of PCa, and history of a negative prostate biopsy were collected at or within 2.5 years before the date of diagnosis for participants with PCa, and at or within 2.5 years before the date of the last visit for those without.
Descriptive statistics were used to summarize PSA and BMI characteristics and all clinical risk factors. Associations between PSA and each of the risk factors were assessed using Kruskal-Wallis or Mann-Whitney test for continuous PSA and Kendall's tau test for categorical PSA (<1, 1-2.49, 2.5-3.99, 4-9.99, ≥10). Median BMIs among the races were compared using the Kruskal-Wallis rank sum test. Linear regression was used to assess the relationship between PSA and BMI categories (< 25 under/normal weight, 25.0-29.9 overweight, 30.0-34.9 obese [OB] I, 35.0-39.9 OBII, ≥ 40 OBIII) while controlling for the risk factors: age, PSA, DRE, family history of PCa, history of a negative biopsy, and race. Logistic regression with backward stepwise model selection was used to model the association of BMI and other risk factors to PCa on biopsy.
BMI-adjusted PSA values were calculated by computing the geometric mean of PSA values for each BMI category in participants without PCa, and then taking ratios of the geometric mean PSA for underweight/normal men relative to the geometric mean PSA of each overweight and obese category. The BMI-adjusted PSA for a man who is underweight/normal is simply his PSA value; for a man in the other classes, his BMI-adjusted PSA is his PSA times the ratio of the geometric mean of PSA for underweight/normals to the geometric mean of PSA for his class.
Evaluation of the performance of the PSA, BMI-adjusted PSA, PCPT Risk Calculator and updated PCPT Risk Calculator with BMI-adjusted PSA was performed by assessment of the operating characteristics: area underneath the receiver operating characteristic curve (AUC), sensitivity and specificity. AUCs of PSA, BMI-adjusted PSA, PCPT risk and PCPT risks based on BMI-adjusted PSA were calculated as the Wilcoxon statistic, and statistical tests of differences between AUCs for the different diagnostic tests were performed via the nonparametric U-statistic method.11 All statistical tests were performed at the α=0.05 (two-sided) level of statistical significance and all statistical analyses in the R statistical package (Version 2.9.0, Copyright 2009, R Foundation for Statistical Computing).
Results
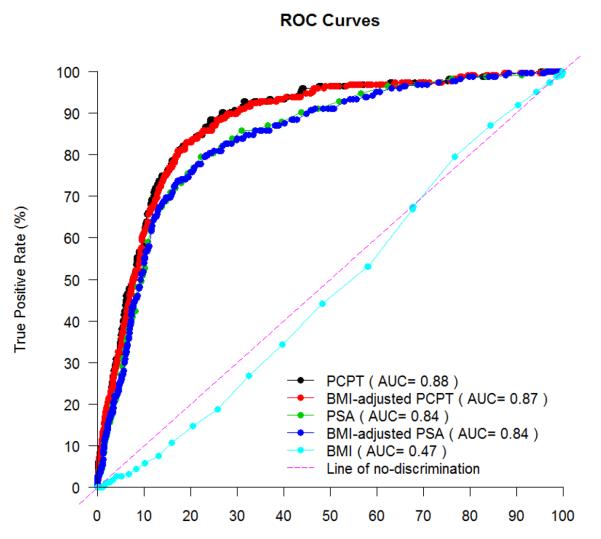
Characteristics of participants are summarized in Table 1. There was no significant difference between cancers and non-cancers with regard to mean BMI (p=0.20) but some evidence of a shift in the distribution across categories of BMI (p=0.06 in Table 1 and p=0.03 after combining the OBII and OBIII categories). Among cancers, BMI category did not correlate with high-grade (Gleason score≥7) PCa (p=0.75 and p=0.74 for before and after the OBII/OBIII combination, respectively). The area under the ROC (Receiver Operating Characteristic) curve using continuous BMI was 0.474 (Figure 1; 95% CI: 0.438-0.510; p=0.92).
Table 1.
Characteristics of Patients in SABOR.
| Cancer cases | Non-cancers | All subjects | |||||
|---|---|---|---|---|---|---|---|
| Variables | N | Statistics* | N | Statistics* | N | Statistics* | P value† |
| Age (yrs) | 265 | 64.8(44.4,88.8) | 3432 | 60.4(22.8,91.2) | 3697 | 60.8(22.8,91.2) | <0.001 |
| 20-39.9 | 0 | 0.0% | 141 | 4.1% | 141 | 3.8% | <0.001 |
| 40-49.9 | 11 | 4.2% | 529 | 15.4% | 540 | 14.6% | |
| 50-59.9 | 72 | 27.2% | 1006 | 29.3% | 1078 | 29.2% | |
| 60-69.9 | 110 | 41.5% | 1060 | 30.9% | 1170 | 31.7% | |
| ≥70 | 72 | 27.2% | 696 | 20.3% | 768 | 20.8% | |
|
| |||||||
| Height (in.) | 249 | 69.5(57,78) | 3349 | 70(55,79) | 3598 | 70(55,79) | 0.93 |
|
| |||||||
| Weight (lbs.) | 249 | 191(103,354) | 3349 | 195(108,430) | 3598 | 195(103,430) | 0.25 |
|
| |||||||
| BMI (kg/m2)‡ | 249 | 28 (16,45) | 3349 | 28 (15,67) | 3598 | 28 (15,67) | 0.20 |
| <24.9 | 32 | 12.1% | 521 | 15.2% | 553 | 15.0% | 0.06 |
| 25.0-29.9 | 131 | 49.4% | 1501 | 43.7% | 1632 | 44.1% | |
| 30.0-34.9 | 66 | 24.9% | 890 | 25.9% | 956 | 25.9% | |
| 35.0- 39.9 | 13 | 4.9% | 303 | 8.8% | 316 | 8.6% | |
| ≥40.0 | 7 | 2.6% | 134 | 3.9% | 141 | 3.8% | |
| Missing | 16 | 6.0% | 83 | 2.4% | 99 | 2.7% | |
|
| |||||||
| PSA (ng/mL) | 263 | 3.6(0.3,93.8) | 3418 | 1(0.1,29.2) | 3681 | 1.1(0.1,93.8) | <0.001 |
| <1 | 16 | 6.0% | 1652 | 48.1% | 1668 | 45.1% | <0.001 |
| 1-2.5 | 52 | 19.6% | 1186 | 34.6% | 1238 | 33.5% | |
| 2.5-4 | 89 | 33.6% | 332 | 9.7% | 421 | 11.4% | |
| 4-10 | 92 | 34.7% | 217 | 6.3% | 309 | 8.4% | |
| ≥10 | 14 | 5.3% | 31 | 0.9% | 45 | 1.2% | |
| Missing | 2 | 0.8% | 14 | 0.4% | 16 | 0.4% | |
|
| |||||||
| Race/ethnicity | |||||||
| Hispanic | 73 | 27.6% | 1258 | 36.7% | 1331 | 36.0% | 0.02 |
| White/non-Hispanic | 150 | 56.6% | 1688 | 49.2% | 1838 | 49.7% | |
| Black/non- Hispanic | 41 | 15.5% | 450 | 13.1% | 491 | 13.3% | |
| Other | 1 | 0.4% | 36 | 1.1% | 37 | 1.0% | |
|
| |||||||
| Prior negative biopsy | |||||||
| Never | 208 | 78.5% | 2801 | 81.6% | 3009 | 81.4% | 0.21 |
| At least one | 57 | 21.5% | 631 | 18.4% | 688 | 18.6% | |
|
| |||||||
| DRE | |||||||
| Normal | 169 | 63.8% | 3153 | 91.9% | 3322 | 89.9% | <0.001 |
| Abnormal | 67 | 25.3% | 148 | 4.3% | 215 | 5.8% | |
| Missing | 29 | 10.9% | 131 | 3.8% | 160 | 4.3% | |
|
| |||||||
| Family history | |||||||
| No | 202 | 76.2% | 2803 | 81.7% | 3005 | 81.3% | 0.03 |
| Yes | 63 | 23.8% | 629 | 18.3% | 692 | 18.7% | |
|
| |||||||
| Gleason score | |||||||
| 5 | 7 | 2.6% | NA | ||||
| 6 | 162 | 61.1% | |||||
| 7 | 62 | 23.4% | |||||
| 8 | 11 | 4.2% | |||||
| 9 | 8 | 3.0% | |||||
| Missing | 15 | 5.7% | |||||
BMI: body mass index; PSA: prostate-specific antigen; DRE: digital rectal examination
Statistics are median (range) and count (%) for continuous and categorical variables, respectively.
P values for test of differences between Prostate Cancer and non-cancers using Mann-Whitney U test and Chi-Square test for continuous and categorical variables, respectively.
BMI category was defined as < 25 (under/normal weight), 25.0-29.9 (overweight), 30.0-34.9 (obese [OB] I), 35.0-39.9 (OB II), and ≥ 40 (OB III).
Figure 1.
ROC curves
In logistic modeling of PCa risk, there was no significant association between BMI and PCa after adjusting for other risk factors, including age, PSA, DRE, first degree family history of PCa, history of a previous negative biopsy, and race (p=0.35 for continuous BMI; p=0.29 and p=0.18 for categorical BMI before and after the OBII/OBIII combination, respectively).
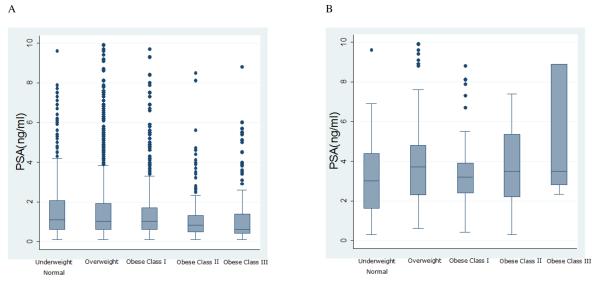
For men without PCa, serum PSA level was significantly influenced by age, race, BMI, prior negative biopsy, and DRE, and mean PSA decreased significantly with increasing BMI (p<0.001) after adjustment for other covariates (Figures 2A and 3A). Among cancers, mean PSA increased non-significantly with increasing BMI (p=0.42; Figures 2B and 3B; p=0.48 after after adjusting for high-grade versus low-grade PCa (p=0.49). The regression of log PSA on BMI category in non-cancers indicated statistically significant reduction with increasing BMI category, with geometric mean PSA of 1.2, 1.1, 1.0, 0.8, 0.7 ng/mL for the under and normal weight, overweight, OBI, OBII and OBIII categories, respectively (F=14.42, df=4, p<0.001). This led to adjustment factors (ratios of under and normal weight relative to each class) equal to: 1.09, 1.20, 1.50, 1.71 for the overweight, OBI, OBII and OBIII categories, respectively. In other words, the unadjusted PSA for men in these weight classes should be multiplied by these factors. After OBII and OBIII were combined, the nearly identical associations between PSA and BMI were observed as described above, and the adjustment factor became 1.5 for the OBII/OBIII category.
Figure 2.
Distribution of PSA according to BMI category. Boxes range from the 25th to the 75th quantiles of the distribution with horizontal lines within the boxes denoting the median; whiskers extend from the edge of the box out to 1.5 times the interquartile range, and individual points beyond the whiskers are denoted by asterisks. 45 PSA's beyond 10 are not shown to improve readability.
Panel A: n=3432 non-cancers
Panel B: n=265 prostate cancer cases
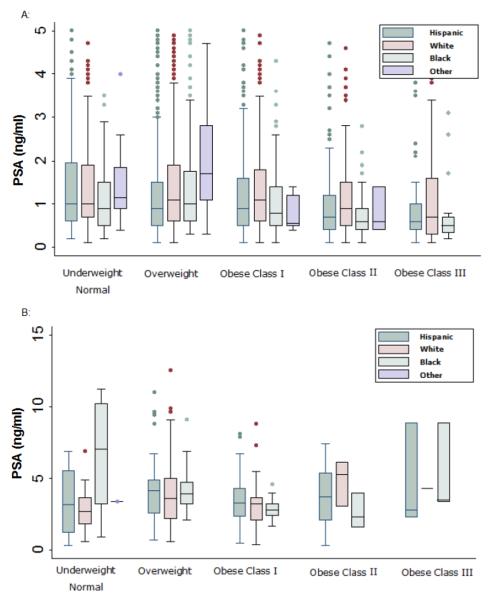
Figure 3.
Distributions of PSA by BMI and racial categories
Panel A: n=3432 non-cancers. 153 PSA's beyond 5 are not shown to improve readability.
Panel B: n=265 cases. 9 PSA's beyond 15 are not shown to improve readability.
The AUC for BMI-adjusted PSA values was 0.84, identical to the AUC for unadjusted PSA. The AUC of the PCPT calculator was 0.88, which was slightly better than the AUC of the PCPT calculator with BMI-adjusted PSA of 0.87 (Figure 1). For those men with a BMI≥35 (OBII/OBIII), the AUCs were 0.88 (95%CI: 0.79 to 0.98), 0.88 (95%CI: 0.78 to 0.98), 0.91(95%CI: 0.82 to 0.99) and 0.90 (95%CI: 0.81 to 0.99) for PSA, BMI-adjusted PSA, the PCPT calculator and the BMI-adjusted PCPT calculator, respectively, which were higher than, but did not differ significantly (p=0.33) from, those men with a BMI<35 (the AUCs were 0.83 [95%CI: 0.81 to 0.86], 0.83 [95%CI: 0.81 to 0.86], 0.87 [95%CI: 0.85 to 0.90] and 0.87 [95%CI: 0.85 to 0.90] for PSA, BMI-adjusted PSA, PCPT calculator and BMI-adjusted PCPT calculator, respectively).
Many prostate biopsy recommendations suggest referral for prostate biopsy if PSA exceeds 2.5 ng/mL or for an abnormal DRE. To post-hoc assess the implication of using BMI-adjusted PSA, the proportion of men with PSA less than or equal to 2.5 ng/mL who would have been considered for prostate biopsy if BMI-adjusted PSA values were used was computed. Of 2816 men with a PSA less than or equal to 2.5 ng/mL who did not receive a biopsy, 126 (4.5%) would have a BMI-adjusted PSA exceeding 2.5 ng/mL. When stratified by race these percentages were 3.9%, 5.1% and 4.1% for Hispanic, white non-Hispanic and black non-Hispanics, respectively, and did not differ significantly (p=0.31).
Discussion
Associations between increased BMI and decreased PSA have previously been reported by many researchers.3-10 Two explanations have been advanced to explain the lower levels of PSA among obese men: a hemodilution effect due to greater blood volume or suppression of PSA production due to lower testosterone levels and higher estrogen levels among obese men.12-16 Beyond PCa detection, outcomes after treatment for PCa have been found by several investigators to be worse in obese men.17-23 Among cancers our study found no association between BMI and high versus low-grade PCa on biopsy. One possible explanation for this observation could be delayed detection among obese men due to artifactual suppression of PSA.
The PCPT prostate cancer risk calculator was developed based on 5519 men from the placebo group of the PCPT of whom all had prostate biopsy, regardless of PSA and DRE findings. The calculator includes risk factors shown to have independent predictive value for risk of PCa and includes race/ethnicity, age, PSA, family history of PCa, DRE, and results of prior prostate biopsy).3 The original cohort used to develop the PCPT calculator was limited to men in the control arm of the PCPT trial, who (based on trial eligibility) had no previous history of PCa, were age 55 years or older and had a normal DRE and PSA<3.0 ng/mL at the time of study enrollment. The URL for the pcpt risk calculator is http://deb.uthscsa.edu/URORiskCalc/Pages/uroriskcalc.jsp.
Based on our previous observations of the relationship between obesity and PSA as well as the observations of others that obesity is a significant risk factor for inferior treatment outcomes for PCa, we felt that an adjustment for obesity might enhance the performance of the PCPT Prostate Cancer Risk Calculator by accounting for the significantly-lower levels of PSA among obese men. A limitation of this approach is that the PCPT population used to build the calculator is a healthier screening population, with required PSA<3.0 ng/mL and normal DRE for entry to the study, compared to population in this study. In our large, prospectively followed community-dwelling cohort we did confirm the stepwise reduction in PSA levels among obese men but were unable to demonstrate a significant improvement in the performance of the PCPT Risk Calculator with the addition of BMI in this population. One explanation for our observation of no significant change in the AUC of BMI-modified risk is that this metric may be too stringent as the effect may apply only to a small fraction of the population; in these men, the impact may be significant. For example, only 457 of 3598 men studied (12.7%) had a BMI>35. However, it was among these men that the adjustment factor for PSA would be 1.5 – 1.7 fold greater, potentially significantly affecting their risk assessment. For these men with a BMI≥35, the AUCs of all PSA and risk calculator measures were higher than for men with BMI<35, but not statistically significantly so. As noted by many researchers, the AUC is an insensitive measure and an additional risk factor must have a very strong association with the disease outcome than we ordinarily see in etiologic research to perform well as a prognostic test for the individual patient.24-27 Given the substantial impact of PSA on risk of cancer, risk of high grade cancer, and decision to pursue prostate biopsy, PSA adjustment in obese men may thus be a consideration.
We have confirmed the strong inverse association between PSA and BMI among controls. While among cancers we observed that mean PSA increased non-significantly with increasing BMI which was consistent with some previous reports on men undergoing radical prostatectomy (RP).13 Although others found that total PSA increased with an increasing BMI in men undergoing RP by a single surgeon.28 For the general population, incorporation of BMI into a risk assessment tool such as the PCPT Prostate Cancer Risk Calculator is unnecessary. However, consideration should be given for men with a BMI>35 to include a BMI adjustment, multiplying the PSA by 1.5 for a BMI of 35-40 and by 1.71 for a BMI>40. Such an action would lead to lower PSA threshold values in these most obese men and may prompt improved detection for patients that may be at a greater risk of treatment failure in part due to delayed diagnosis caused by lower PSA values.
Conclusions
We observed that increased BMI is not a risk factor for PCa among our SABOR participants. Adjustment for diminished levels of PSA in the general population does not increase the accuracy of PSA-based risk assessment. However, due to lower levels of PSA, overweight and obese men may suffer diminished cancer detection opportunities when undergoing PSA screening and a BMI-based PSA adjustment may be considered.
Acknowledgments
Funding was provided by the Early Detection Research Network, National Cancer Institute, National Institutes of Health Grant [U01-CA86402] and the San Antonio Cancer Institute [P30-CA54174].
Abbreviations
- AUC
Area Under the ROC Curve
- BMI
Body mass index
- DRE
Digital Rectal Exam
- EDRN
Early Detection Research Network
- PCPT
Prostate Cancer Prevention Trial
- PSA
Prostate Specific Antigen
- ROC
Receiver-Operator Characteristics
- SABOR
San Antonio center of Biomarkers Of Risk for prostate cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schroder FH, van den Bergh RC, Wolters T, et al. Eleven year outcome of patients with prostate cancers diagnosed during screening after initial negative sextant biopsies. Eur Urol. 2010;57:256–266. doi: 10.1016/j.eururo.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 2.Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 3.Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98:529–534. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]
- 4.Ahn JO, Ku JH. Relationship between serum prostate-specific antigen levels and body mass index in healthy younger men. Urology. 2006;68:570–574. doi: 10.1016/j.urology.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Baillargeon J, Pollock BH, Kristal AR, et al. The association of body mass index and prostate-specific antigen in a population-based study. Cancer. 2005;103:1092–1095. doi: 10.1002/cncr.20856. [DOI] [PubMed] [Google Scholar]
- 6.Culp S, Porter M. The effect of obesity and lower serum prostate-specific antigen levels on prostate-cancer screening results in American men. BJU Int. 2009;104:1457–1461. doi: 10.1111/j.1464-410X.2009.08646.x. [DOI] [PubMed] [Google Scholar]
- 7.Han JH, Chang IH, Ahn SH, et al. Association between serum prostate-specific antigen level, liver function tests and lipid profile in healthy men. BJU Int. 2008;102:1097–1101. doi: 10.1111/j.1464-410X.2008.07774.x. [DOI] [PubMed] [Google Scholar]
- 8.Han JH, Choi NY, Bang SH, et al. Relationship between serum prostate-specific antigen levels and components of metabolic syndrome in healthy men. Urology. 2008;72:749–754. doi: 10.1016/j.urology.2008.01.084. [DOI] [PubMed] [Google Scholar]
- 9.Kim YJ, Han BK, Hong SK, et al. Body mass index influences prostate-specific antigen in men younger than 60 years of age. Int J Urol. 2007;14:1009–1012. doi: 10.1111/j.1442-2042.2007.01879.x. [DOI] [PubMed] [Google Scholar]
- 10.Kristal AR, Chi C, Tangen CM, et al. Associations of demographic and lifestyle characteristics with prostate-specific antigen (PSA) concentration and rate of PSA increase. Cancer. 2005;106:320–328. doi: 10.1002/cncr.21603. [DOI] [PubMed] [Google Scholar]
- 11.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 12.Rodriguez C, Patel AV, Calle EE, et al. Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer Epidemiol Biomarkers Prev. 2001;10:345–353. [PubMed] [Google Scholar]
- 13.Freedland SJ, Platz EA, Presti JC, Jr, et al. Obesity, serum prostate specific antigen and prostate size: implications for prostate cancer detection. J Urol. 2006;175:500–504. doi: 10.1016/S0022-5347(05)00162-X. [DOI] [PubMed] [Google Scholar]
- 14.Banez LL, Hamilton RJ, Partin AW, et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA. 2007;298:2275–2280. doi: 10.1001/jama.298.19.2275. [DOI] [PubMed] [Google Scholar]
- 15.Rundle A, Neugut AI. Obesity and screening PSA levels among men undergoing an annual physical exam. Prostate. 2008;68:373–380. doi: 10.1002/pros.20704. [DOI] [PubMed] [Google Scholar]
- 16.Grubb RL, 3rd, Black A, Izmirlian G, Hickey TP, et al. Serum prostate-specific antigen hemodilution among obese men undergoing screening in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol Biomarkers Prev. 2009;18:748–751. doi: 10.1158/1055-9965.EPI-08-0938. [DOI] [PubMed] [Google Scholar]
- 17.Andersson SO, Wolk A, Bergstrom R, et al. Body size and prostate cancer: a 20-year follow-up study among 135,006 Swedish construction workers. J Natl Cancer Inst. 1997;89:385–389. doi: 10.1093/jnci/89.5.385. [DOI] [PubMed] [Google Scholar]
- 18.Aziz NM, Hartman T, Barrett M, et al. Weight and prostate cancer in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) trial. Proceedings of ASCO. 2000;19:647a. (abstract 2550) [Google Scholar]
- 19.Schuurman AG, Goldbohm RA, Dorant E, et al. Anthropometry in relation to prostate cancer risk in The Netherlands Cohort Study. Am J Epidemiol. 2000;151:541–549. doi: 10.1093/oxfordjournals.aje.a010241. [DOI] [PubMed] [Google Scholar]
- 20.Amling CL, Riffenburgh RH, Sun L, et al. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol. 2004;22:439–445. doi: 10.1200/JCO.2004.03.132. [DOI] [PubMed] [Google Scholar]
- 21.Freedland SJ, Aronson WJ, Kane CJ, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22:446–453. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 22.Freedland SJ, Terris MK, Presti JC, Jr, et al. Obesity and biochemical outcome following radical prostatectomy for organ confined disease with negative surgical margins. J Urol. 2004;172:520–524. doi: 10.1097/01.ju.0000135302.58378.ae. [DOI] [PubMed] [Google Scholar]
- 23.Bassetta WW, Cooperberga MR, Sadetskya N, et al. Impact of obesity on prostate cancer recurrence after radical prostatectomy: Data from CaPSURE. Urology. 2005;66:1060–1065. doi: 10.1016/j.urology.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 24.Wald NJ, Hackshaw AK, Frost CD. When can a risk factor be used as a worthwhile screening test? BMJ. 1999;319:1562–1565. doi: 10.1136/bmj.319.7224.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pepe MS, Janes H, Longton G, et al. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159:882–890. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- 26.Ware JH. The limitations of risk factors as prognostic tools. N Engl J Med. 2006;355:2615–2617. doi: 10.1056/NEJMp068249. [DOI] [PubMed] [Google Scholar]
- 27.Ware JH, Cai T. Comments on ‘Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond’ by M. J. Pencina et al., Statistics in Medicine (DOI: 10.1002/sim.2929) Stat Med. 2008;27:185–187. doi: 10.1002/sim.2985. [DOI] [PubMed] [Google Scholar]
- 28.Loeb S, Yu X, Nadler RB, et al. Does body mass index affect preoperative prostate specific antigen velocity or pathological outcome after radical prostatectomy? J Urol. 2007;177:102–106. doi: 10.1016/j.juro.2006.08.097. [DOI] [PubMed] [Google Scholar]