Abstract
Effects of estradiol benzoate (EB), ERα-selective agonist, propyl pyrazole triol (PPT) and ERβ-selective agonists, diarylpropionitrile (DPN) and Compound 19 (C-19) on memory were investigated in OVX rats using object recognition (OR) and placement (OP) memory tasks. Treatments were acute (behavior 4 h later) or sub chronic (daily injections for 2 days with behavior 48 h later). Objects were explored in sample trials (T1), and discrimination between sample (old) and new object/location in recognition trials (T2) was examined after 2–4 h inter-trial delays. Subjects treated sub chronically with EB, DPN, and C-19, but not PPT, discriminated between old and new objects and objects in old and new locations, suggesting that, at these doses and duration of treatments, estrogenic interactions with ERβ contributes to enhancements in recognition memory. Acute injections of DPN, but not PPT, immediately after T1, also enhanced discrimination for both tasks (C19 was not investigated). Effects of EB, DPN and PPT on anxiety and locomotion, measured on elevated plus maze and open field, did not appear to account for the mnemonic enhancements. Monoamines and metabolites were measured following DPN treatment in subjects that did not receive behavioral testing. DPN was associated with alterations in monoamines in several brain areas: indexed by the metabolite, 3-methoxy-4-hydroxyphenylglycol (MHPG), or the MHPG/norepinephrine (NE) ratio, NE activity was increased by 60–130% in the prefrontal cortex (PFC) and ventral hippocampus, and NE activity was decreased by 40–80% in the v. diagonal bands and CA1. Levels of the dopamine (DA) metabolite, homovanillic acid (HVA), increased 100% in the PFC and decreased by 50% in the dentate gyrus following DPN treatment. The metabolite of serotonin, 5-hydroxyindole acetic acid (5-HIAA), was increased in the PFC and CA3, by approximately 20%. No monoaminergic changes were noted in striatum or medial septum. Results suggest that ERβ mediates sub chronic and acute effects of estrogens on recognition memory and that memory enhancements by DPN may occur, in part, through alterations in monoaminergic containing systems primarily in PFC and hippocampus.
Keywords: Estradiol, memory, estrogen receptor alpha agonists, estrogen receptor beta agonists, monoamines
Introduction
That estradiol modulates neural functions like mood, affect, anxiety, fear and vulnerability to addictive drugs in addition to its well documented role in reproduction is now well accepted (Watson, Alyea, Cunningham, & Jeng, 2010). Moreover, estradiol also exerts influence over higher order cognitive function, predominantly enhancing learning and memory (Luine, 2008). However, regulation of cognition by estrogens and other hormones is complex with efficacy dependent on dose, duration of treatment and nature of the cognitive demand (Luine, 2008; Frick, 2009). For example, high levels of estradiol have been shown to inhibit performance of some memory tasks and may even impair performance once subjects have learned how to solve the task (Dohanich, 2002), and recent studies indicate that estrogens may influence strategies used for solving memory tasks which may lead to poorer performance in females as compared to males (Korol, 2004; Davis, Jacobson, Aliakbari & Mizumori, 2005). However, a large body of evidence in both young and aged rodent subjects indicates that estradiol promotes memory (Daniel, 2006; Luine, 2008; Frick, 2009). For example, enhanced memory has been shown when estrogens are given by chronic (i.e. days to weeks, Davis et a;. 2005; Gibbs, Gabor, Cox, & Johnson, 2004; Luine, Richards, Wu, & Beck, 1998), sub chronic (i.e. a few days, Daniel & Dohanich, 2001; Frye & Rhodes, 2002; Leuner, Mendolia-Loffredo, & Shors, 2004; Sandstrom & Williams, 2004), or acute (i.e. min to hrs, Luine, Jacome, & MacLusky, 2003; Packard & Teather, 1997; Rhodes & Frye, 2006; Inagaki, Gautreaux and Luine, 2010) treatment regimens to ovariectomized (OVX) rats in a variety of tasks, such as the delayed matching-to-position (DMP) T-maze, Morris water maze, eight-arm radial maze, object recognition, trace eyeblink conditioning and inhibitory avoidance tasks.
Effects of estrogens are mediated through receptors (ERs), and for almost fifty years, it was thought that this receptor was a single ligand-dependent transcription factor acting in the nucleus to enhance nuclear transcription (genomic effects). A second estrogen receptor was identified approximately 15 years ago (Kuiper, Enmark, Pelto-Huikko, Nilsson, & Gustafsson, 1996), and receptors are currently designated as ERα and ERβ. In addition, more recent results support nongenomic steroid actions initiated at the level of the cell membrane through ERs spanning the plasma membrane that mediate effects on pathways linked to G-protein and tyrosine kinase pathways (Roepke, Ronneklei & Kelly, In Press). ERs may also act directly at nuclear sites (CREB and AP1) to regulate transcription (Watson et al, 2010). Thus, estrogen effects are no longer limited to those that are delayed in onset and long lasting (chronic, genomic), but may also be rapid and short-lived (acute, membrane dependent).
In relation to memory, evidence suggests that ERβ, rather than ERα, contributes. Experiments in mice with knockouts of ERα (ERKO) or ERβ (BERKO) indicate that ERβ mediates learning and memory function by estradiol (Liu, Day, Muñiz, & Bitran, 2008; Rissman, Heck, Leonard, Shupnik, & Gustafsson, 2002; Walf, Koonce, & Frye, 2008). These results are supported by other experiments where estradiol and ER-specific agonists were given to OVX rodents. For example, estradiol and the ERβ agonist, WAY-200070, but not the ERα agonist, PPT (Harris, H., Katzenellenbogen, J. and Katzenellenbogen, B., 2002), given for two days, enhanced spatial memory on the radial arm maze (Liu et al, 2008). A similar pattern was found on the Morris water maze where estradiol and DPN, another ERβ agonist (Minutolo, F., Macchia, M., Katzenellenbogen, B. and Katzenellenbogen, J., 2009), but not PPT, given acutely, enhanced performance in OVX rats (Rhodes and Frye, 2006). However, other results do not support the view that estradiol effects on memory are mediated solely by ERβ. Using the spatial memory task, object placement, and acute treatments, Frye, Duffy, & Walf (2007) reported that estradiol and PPT, but not DPN, enhanced memory, and in the non-spatial memory test, object recognition, acute estradiol, PPT and DPN enhanced performance (Walf, Rhodes, & Frye, 2006). Finally, a recent study gave chronic treatments to OVX rats and tested acquisition and memory in a delayed matching-to-position (DMT) T-maze task (Hammond, Mauk, Ninaci, Nelson, & Gibbs, 2009). Estradiol, PPT and DPN all enhanced acquisition/learning as compared to OVX females; however, no treatment altered memory in tests with long inter-trial delays following the acquisition trials. Thus, it is currently unclear which ERs contribute to cognitive function or whether the two receptors may differentially regulate learning as compared to memory function or regulate different types of memory. Further confounding cognitive analyses is the observation that estradiol and ER agonists can influence overall activity and anxiety which might indirectly alter mnemonic function (Bowman, Ferguson, & Luine, 2002; Díaz-Véliz, Alarcón, Espinoza, Dussaubat, & Mora, 1997; Lund, Rovis, Chung, & Handa, 2005; Tomihara, Soga, Nomura, Korach, Gustafsson, Pfaff, & Ogawa, 2009).
In this study, we examined estrogenic influences on cognitive function using the non-spatial memory task, objection recognition (OR), and the spatial memory task, object placement (OP). These tasks are advantageous since they provide an assessment of working memory with little learning component (Ennaceur, A., Neave, N. and Aggleton, J., 1997). Effects of estradiol were first evaluated, and then the ERα agonist, PPT, and the ERβ agonists DPN and compound C-19 (Wilkening, R., Ratcliffe, R., Tynebor, E., Wildonger, K., Fried, A., Hammond, M., Mosley, R., Fitzgerald, P., et al, 2006) were tested. Both sub chronic (days) and acute (hr) treatments were assessed in an attempt to determine which ER receptors may be responsible for enhancements in memory. In addition, the effects of estradiol and some of the ER ligands were evaluated in the open field and elevated plus maze to assess possible non-mnemonic contributions to performance in memory tasks. Finally, effects of DPN on monoamine and metabolite levels in specific brain areas were measured to determine whether agonist-dependent changes in monoaminergic function are present in areas contributing to cognitive function because monoamines have been previously shown to contribute to cognition (Brozoski, Brown, Rosvold, & Goldman, 1979; Ramos and Arnsten, 2006).
Materials and Methods
Subjects
Two-month old, female Sprague Dawley rats were OVX by the vendor (Harlan Sprague Dawley, Inc., Indianapolis, IN) and delivered to Hunter College where they were double-housed in plastic cages and kept on a 12/12h light/dark cycle (lights on at 07:00am) with access to food and water ad libitum. A diet very low in phytoestrogens (Chow 2016, 16% protein rodent diet, Harlan Teklad Global Diets, Madison, WI) was provided because OVX rats on regular rat chow show enhanced spatial memory and increased dendritic spine density in hippocampal and prefrontal cortex (PFC) pyramidal neurons (Luine, Attalla, Mohan, Costa, & Frankfurt, 2006). All experiments conformed to the guidelines of the NIH Guide for Care and Use of Animals and were approved by the Institutional Animal Care and Use Committee of Hunter College.
Sub chronic hormonal treatment
Cohorts of 16–18 OVX rats each were used in the behavioral testing (EB, PPT and DPN, C19), and an additional, separate cohort of 16 OVX rats was used for monoamine measurements. After acclimation (discussed below), half of the subjects (n=8–9/group) received a single daily sc injection of either EB (50 μg/kg), PPT (3 and 5 mg/kg), DPN (3 mg/kg), or C-19 (3 and 5 mg/kg), or vehicle (sesame oil or propylene glycol; 200–300 μl) for two days and were tested two days after the last injection. EB, rather than estradiol, was utilized because it provides more sustained and longer elevations in circulating estradiol (Scharfman, H., Hintz, T., Gomez, J., Stormes, K., Barouk, S., Malthankar-Phatak, D., McLoskey, D., Luine, V. and MacLusky, N., 2007). We have previously shown that this EB dose increases CA1 dendritic spine synapse density (MacLusky, Luine, Hajszan, & Leranth, 2005). PPT and DPN, at the doses given or at similar doses, exert estrogenic effects. For example, PPT and E2, but not DPN, increase uterine weight (Harris et al., 2002; Le Saux & Di Paolo, 2005; Lubbers, Alves, Zafian, Gautreaux, Gordon, Correa, Lorrain, Hong, Luine, Rohrer, & Hickey, 2006; Lund et al., 2005), whereas DPN and E2, but not PPT, increase specific binding to dopamine transporters (DAT) in the rat striatum (Le Saux & Di Paolo, 2006). The doses of C-19 were chosen based on the compound’s affinity and specificity for ERβ (Opas, Scafonas, Nanterment, Wilkening, Birzin, Wilkinson, Colwell, Schaeffer, Towler, Rodan, & Schmidt, 2009; Wilkening, et al, 2006) since information on its effects in the CNS are not available. Two additional cohorts of subjects received a single daily sc injection of EB, drugs or vehicle for two days and were tested on the EPM 24 h after the second injection day and then tested for OR or OP on the following day to assess drug effects on anxiety and to confirm positive treatment effects on memory. Another cohort received acute DPN and was tested on the EPM 4 h later.
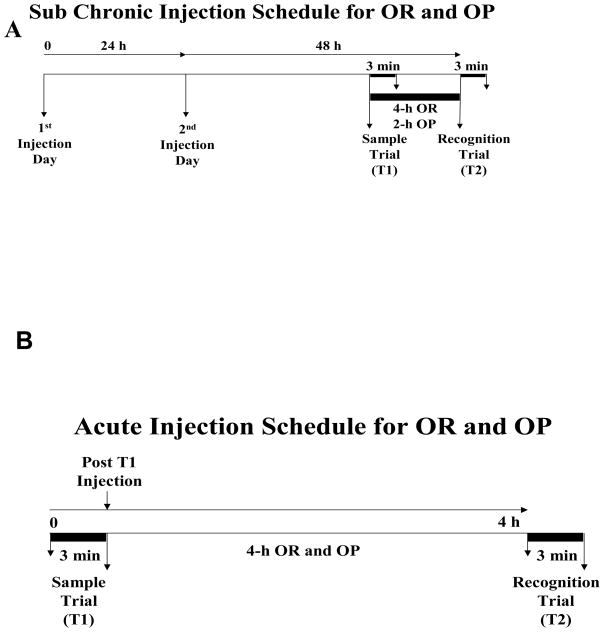
PPT and DPN were purchased from Tocris Bioscience (Ellisville, MO). EB was purchased from SIGMA (St. Louis, MO), and C-19 was a gift from Merck Research Laboratories. EB and PPT were dissolved in ethanol and then diluted in sesame oil. DPN and C-19 were dissolved in propylene glycol because they are not soluble in ethanol/sesame oil. Control subjects received the appropriate vehicle, and hormones/drugs, dissolved in either sesame oil or propylene glycol. The sub chronic injection schedule for both OR and OP is depicted in Fig. 1A. One week after subjects completed behavioral trials, some were given vehicle, EB or agonists in the sub chronic treatment regimen and were sacrificed at the time that behavior would have been tested, and uteri were removed and trimmed of fat and weighed.
Figure 1. Sub chronic and acute injection schedules.
(A) In the sub chronic treatment paradigm, subjects received a single daily sc injection for two days and were tested on OR and OP 48 h after the second injection. The inter trial delays for OR and OP were 4 h and 2 h, respectively. (B) In the acute treatment paradigm, subjects received a single sc injection immediately after the sample trial (post T1). The inter trial delay was 4 h.
Acute hormonal treatment
A cohort of 18 OVX rats was used for the two behavioral tests: OR and OP, each separated by 6–8 days. Subjects received a single sc injection of PPT (1 mg/kg), DPN (1 mg/kg), or vehicle immediately after (post) the sample trial (described in the next section). Lower doses than used for sub chronic treatment were chosen since membrane receptors have higher affinities for hormones (Watson, et al, 2010). Acute EB was not tested in the present study since estrogens have been previously shown to rapidly enhance recognition memory in this paradigm (Luine et al., 2003; Inagaki et al, 2010). The acute injection schedule for both OR and OP is shown in Fig. 1B.
Behavioral tasks
Object recognition
OR and OP tests were adapted from Ennaceur et al. (1997) and have been recently described in detail by this laboratory (Bowman, Micik, Gautreaux, Fernandez, and Luine, 2009). Tests consist of two trials: a sample trial (T1) and a recognition/retention trial (T2). Acclimation and habituation began approximately two weeks after ovariectomy and one week after arrival. During acclimation, trials were separated by 1 min, 10 min, 1 h, and 2 h inter-trial delay intervals for OR, and 10 min, 40 min, and 1 h for OP in a 3 × 3 open field chamber (See Beck & Luine, 2002; Luine et al., 2003 for details). In the OR memory task, subjects in T1 were placed in the center of the chamber with two identical objects (e.g., soda cans, plastic water bottles, various containers) placed equidistant from two corners of the chamber. Animals were allowed 3 min to explore the objects in the chamber. Exploration was defined as sniffing, whisking or looking at the objects within a 2-cm range. Time spent exploring the objects was recorded. After the inter-trial delay of 4 hr, subjects were returned to the chamber, and one of the old, familiar objects from T1 was replaced with a new (novel) object, and subjects were allowed 3 min to explore T2. Time spent exploring the old and novel object was recorded by an observer blind to treatments, and data are expressed as the exploration ratio (time with new object/time with old object + time with new object). A ratio of 0.5 indicates chance performance, i.e. the subject spent the same amount of time exploring each object and thus did not discriminate between old and new objects. Data from a few trials where subjects did not explore objects in T1 or T2 were omitted from the analyses (four out of approximately 192 trials). In the acute experiment, T1 was immediately followed by hormone or drug injection, and T2 was performed 4 h later. Old and new objects were counterbalanced for all groups, and chamber floor and objects were cleaned with disinfectant spray at the culmination of each trial.
Object placement
Procedures for OP were similar to OR, but in OP one of the identical objects was moved to a new location in T2, and the objects were more intricate in shape (e.g., figurines, funnels, candle holders). The inter-trial delay was 2 hr for OP and 4 hr for OR because OVX female rats are unable to discriminate, respectively, at these delays (Beck & Luine, 2002; Luine, 2008). Counterbalancing, cleaning and data calculation was the same as in OR.
Open field
Activity on the open field was assessed as previously described (Bowman, MacLusky, Sarmiento, Frankfurt, Gordon, & Luine, 2004). Briefly, subjects were individually placed in a box (14 × 14 × 20 cm) with a sliding door attached to a 3 × 5 chamber. Latency to enter the chamber was recorded. Subjects were allowed to explore the field for 6 min, divided into two 3-min segments. Measures included outer visits (crossings on the periphery of the chamber), inner visits (crossings on the center of the chamber), rearing (standing up on hind limbs), grooming, and defecation.
Elevated plus maze
This maze assesses anxiety-related behaviors (Lund et al., 2005; Pellow & File, 1986) and consisted of four arms: two alternate, open arms (50 × 10 cm each) and two alternate, closed arms (50 × 10 × 40 cm). The maze was 50 cm above the floor, and subjects were individually placed in the center of the four arms (10 × 10 cm) and allowed to explore for 5 min. Arm entry was considered complete and therefore counted when all four of the animals’ limbs were in the arm. The number of entries and time spent in open and closed arms were recorded.
Monoamine and metabolite measurement following DPN sub chronic treatment
Monoamines and metabolites were measured by HPLC with electrochemical detection according to the method originally documented by Renner and Luine (1986) and recently updated by this lab (Bowman, Micik, Gautreaux, Fernandez, & Luine, 2009). OVX rats, treated with vehicle or 3 mg/kg of DPN using the sub chronic regimen, were sacrificed 48 h after the last injection, at the time when recognition memory would be tested. The brains were rapidly removed, frozen and stored at −70°C. Brains were serially cut into 300-micron thick sections and mounted on microscope slides. A 500-μm diameter steel cannula sample tissue in the target areas with the aid of a dissecting microscope and a freezing stage maintained at approximately −11°C. Tissue samples were placed in 1.5ml Eppendorf tubes. The brain areas sampled and number of punches (P) obtained from each area were as follows: medial prefrontal cortex (PFC; 4 P); CA1 (10–12 P), CA3 (10–12 P), and dentate gyrus (8 P) of the hippocampus; ventral hippocampus (10–12 P), vertical diagonal bands (VDB; 6 P); striatum (4 P); medial septum (6 P).
Monoamines and metabolites were measured by dissolving the punches in 60μl of sodium acetate buffer pH 5.0), and obtaining the released neurotransmitter through a process of freezing and thawing, which disrupts cellular structures and releases cellular components including monoamines. α-Methyl-dopamine was added as an internal standard, and samples were centrifuged at 12000 rpm for 12 minutes. The supernatant was removed and the pellet was re-suspended in 200 μl (PFC) or 100 μl of 2.0N NaOH (other brain areas) for protein analysis using Bio-Rad reagent (Bio-Rad Laboratories, Hercules, CA, USA).
High-performance liquid chromatography (HPLC) with electrochemical analysis was used to quantify neurotransmitter levels. The 40 μl supernatant was used in the detection of monoamines and metabolites, including dopamine (DA) and its metabolites, 3–4-dihydroxyphenylalanine (DOPAC) and homovanillic acid (HVA); norepinephrine (NE) and its metabolite 3-methoxy-4-hydroxyphenylglycol (MHPG); and serotonin (5-HT) and its metabolite 5-hydroxyindole acetic acid (5-HIAA). The supernatant was injected into a Waters Associates chromatographic system (Waters 2690), consisting of an alliance module containing an automated refrigerated, injector pump, Symmetry C18 5μm 4.6 × 150mm reverse-phase column (Novapak three micron), and an ESA coulochem III detector (screening electrode at +.50V and detecting electrode at +50V potential). The mobile phase contained 3% acetonitrile and peak sharpness was increased by the addition of 100% methanol (99.5% mobile: 0.5% methanol).
Millennium software (Waters Associates) was used to run the chromatography system, in which concentrations of neurotransmitters and metabolites were calculated by reference to standards and the internal standard using peak integration. Monoamine and metabolite concentrations are expressed as pg/μg protein and turnover ratios (metabolite/monoamine) were calculated as an index of activity.
Statistical analyses
Data were analyzed using NCSS (NCSS Statistical Software, Kaysville, UT). T-tests were used to analyze for group differences in exploration time during T1 of recognition tests. Non-parametric Mann-Whitney U tests were used to test group differences in ratios in T2 (time with new/old+new for OR and OP) because ratios are not normally distributed, and therefore, parametric statistics (t-tests) cannot be applied. Open field activity was analyzed by two-way, repeated measures ANOVA (group × time), and post hoc tests (Fisher LSD test) were applied where appropriate. Elevated plus maze data were analyzed by either student’s t-test or one-way ANOVA, depending on the number of groups. Monoamine levels were analyzed by Student t-tests.
Results
Effect of Sub chronic EB treatment on OR and OP
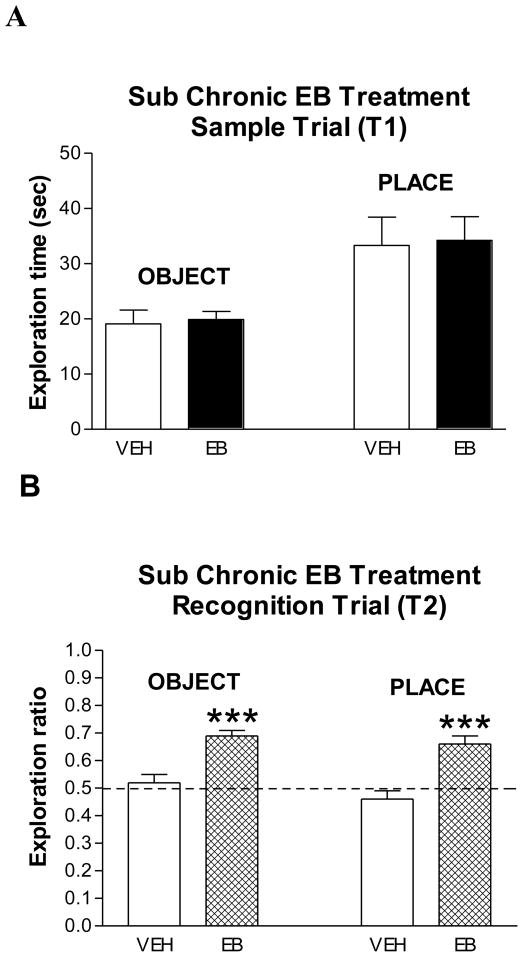
In the OR (non-spatial memory) task, both EB- and vehicle-treated subjects spent the same amount of time (approximately 20 sec) exploring objects in T1 (Fig. 2A). In T2, however, the exploration ratio (time with new object/time with new + time with old object) was significantly higher for EB- than for vehicle-treated subjects (Mann-Whitney u-test where Z = 3.32, p < 0.0004) (Fig. 2B). EB-treated rats had an exploration ratio of approximately 0.70 whereas vehicle-treated rats had a chance ratio, 0.53. In addition, EB-treated subjects explored the new object significantly more than the old object whereas the vehicle-treated group did not (data not shown, p < 0.001). Similar exploration times between the control and EB-treated group in T1 indicate that enhanced recognition by the EB-treated group did not result from greater object exploration.
Figure 2. Effects of sub chronic EB treatment on object and place recognition.
(A) In the sample trial (T1), exploration times for the object and place tests are shown in vehicle- and EB-treated subjects (n=9/group). No significant differences. (B) In the recognition trial (T2), entries are ratios (new/old + new) of time spent exploring each object and objects in each location for vehicle- and EB-treated subjects. Dotted line at 0.5 indicates spending the same amount of time exploring new and old objects or locations. *** p < 0.001, see results for statistical details.
In the OP (spatial memory) task, both EB- and vehicle-treated subjects spent equal amounts of time (approximately 34 sec) exploring objects in T1 (Fig. 2A). As in OR, the exploration ratio for EB-treated subjects in T2 (approximately 0.7), was significantly higher than that for the vehicle-treated group, approximately 0.45 (Z = 3.45, p < 0.0002) (Fig. 2B). In addition, EB-treated subjects spent significantly more time exploring the object in the new than the old location whereas the vehicle treated group did not (data not shown, p < 01). Thus, treatment of OVX subjects with EB enhances both object and place recognition memory.
Effect of sub chronic treatment with ER agonists on OR and OP
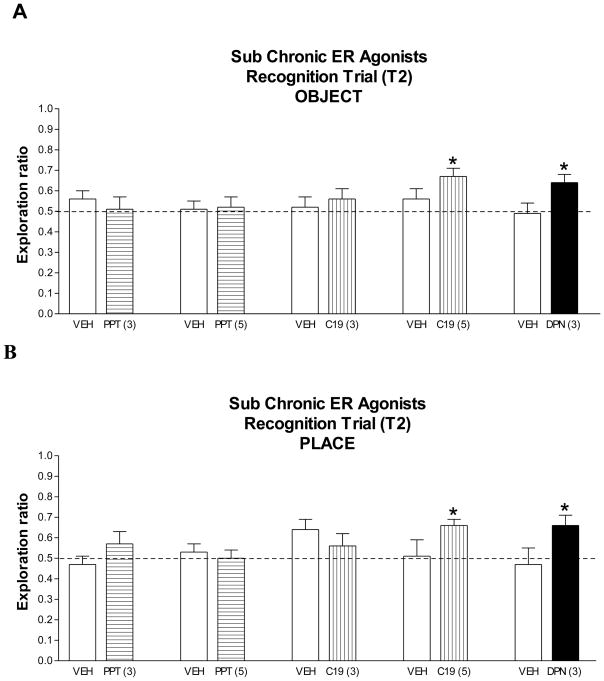
No significant differences between agonist- (PPT, DPN and C-19) and vehicle-treated subjects were found in T1 object exploration time for OR and OP tests (data not shown). In the recognition trial of the OR task, the exploration ratio for PPT and C-19, approximately 0.50, was not significantly different from the respective vehicle group when a 3-mg/kg dose was given (Fig. 3A). However, when the dose was increased to 5 mg/kg, the exploration ratio was significantly higher for C-19- than for vehicle-treated subjects (Z = 1.74, p < 0.04), whereas in the PPT-treated group, increasing the dose did not influence performance (Fig. 3A). The exploration ratio was also significantly higher for DPN- than for vehicle-treated subjects (Z = 1.97, p < 0.02) (Fig. 3A). In addition, rats treated with either of the ERβ agonists, DPN (3 mg/kg) or C-19 (5 mg/kg), spent significantly more time with the new than with the old object (data not shown, p < 0.01). Thus, ERβ-specific agonists, DPN and C-19, but not the ERα-specific agonist, PPT, enhanced object recognition performance.
Figure 3. Effects of sub chronic treatment with ER agonists on OR and OP.
Entries are T2 ratios (new/old + new) of time spent exploring objects in OR (A) and objects in each location in OP (B) for vehicle-, PPT- (3 and 5 mg/kg), C-19- (3 and 5 mg/kg) and DPN- (3 mg/kg) treated subjects. Entries are means ± SEM for 8–13 subjects/group. Treatments were tested in separate experiments with control and treated subjects but are shown together in the graph. * p < 0.05, see results for statistical details.
A similar pattern of results for the agonists was found in the OP task. The exploration ratios for PPT and C-19, when given at a dose of 3 mg/kg, were not significantly different from their vehicle group (Fig. 3B). When the doses were increased to 5 mg/kg, the exploration ratio was significantly higher for C-19 than for vehicle-treated subjects (Z = 1.67, p < 0.05), but not for PPT-treated subjects vs control subjects (Fig. 3B). The exploration ratio was significantly higher for DPN- (3 mg/kg) than for vehicle-treated groups (Z = 2.0, p < 0.02) (Fig. 3B). Also, DPN- (3 mg/kg) and C-19- (5 mg/kg) treated subjects spent significantly more time with the object in the new than the old location (p < 0.05 and p < 0.01, respectively; data not shown). Thus, treatment with the ERβ-specific agonists, DPN and C-19, but not the ERα-specific agonist, PPT, enhanced place recognition.
Effects of EB and ER agonists on uterine weight
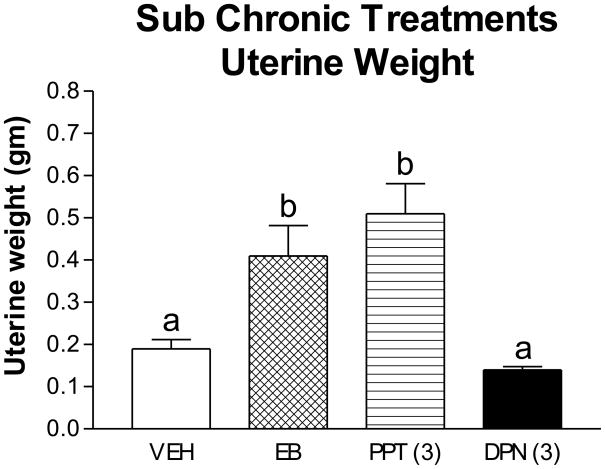
Effects of EB, PPT and DPN on uterine weight are shown in Figure 4. C-19 was not assessed because its effect on uterine weight in this paradigm has been previously shown (Lubbers, L., Alves, S., Zafian, P., Gautreaux, C., Gordon, M., Correa, L. et al, 2006). One-way ANOVA showed a significant treatment effect, F3,37 = 14.3, p < 0.00002. Newman-Keuls post hoc testing revealed that uterine weight was significantly greater for EB and PPT compared to the vehicle-treated group (p < 0.01 and 0.0001, respectively) and to the DPN-treated group (p < 0.001, p < 0.001, respectively). Uterine weights of DPN and vehicle-treated subjects did not differ from each other. Thus, these data indicate that, at the given doses, PPT and EB, but not DPN activated ERα in the uterus.
Figure 4. Effect of sub chronic treatment with EB and ER agonists on uterine weight.
Entries are means ± SEM of uterine wet weight (gm) for vehicle- (n=20), EB- (50 μg/kg; n=8), PPT- (3 mg/kg; n=4) and DPN- (3 mg/kg; n=9) treated subjects. Bars with different superscripts are different from one another by at least p < 0.01, see results for statistical details.
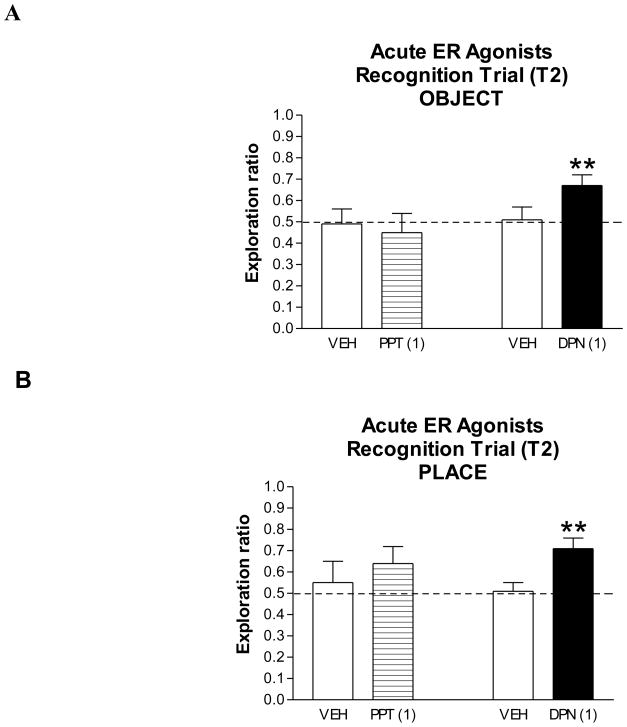
Acute effects of ER agonists on OR and OP
We next investigated effects on memory when hormones or agonists were given and recognition memory tested 4 h later. EB was not tested as it has been previously shown to enhance OR and OP in this paradigm (Luine et al, 2003; Inagaki et al, 2010) and only one agonist of each receptor class was assessed, PPT and DPN. Exploration ratios of PPT- and vehicle-treated subjects were not significantly different from each other in the recognition trial (Fig. 5A). On the other hand, the exploration ratio was significantly higher for DPN- than for the vehicle-treated group (Z = 2.17, p < 0.01; Fig. 5A). As with sub chronic treatment, DPN-treated subjects spent significantly more time with the new than with the old object (p < 0.01), whereas the vehicle-treated group did not (data not shown). In the OP task, exploration ratios of PPT- and vehicle-treated groups did not significantly differ from each other, but were significantly higher for DPN- than vehicle-treated subjects (Z = 2.70, p < 0.004; Fig. 5B). DPN-treated subjects also spent significantly more time exploring the object in the new than in the old location in OP (p < 0.001); in contrast, time spent with the object in the new and old locations was not different for vehicle-treated subjects (data not shown). Thus, acute treatment with the ERβ-specific agonist, DPN, but not the ERα-specific agonist, PPT, enhances both object and place recognition.
Figure 5. Effects of acute PPT and DPN treatments on OR and OP.
Entries are T2 ratios (new/old + new) of time spent exploring each object in OR (A) and objects in each location in OP (B) for vehicle-, PPT- (1 mg/kg) and DPN- (1 mg/kg) treated subjects. Treatments were tested in separate experiments with control and treated subjects (n = 8–9) but are shown together in the graph. ** p < 0.01, see results for statistical details.
Effects of EB and ER agonists on activity in the open field
The effects of treatment with EB (Experiment 1, Table 1) and one ERα, PPT, and one ERβ, DPN, agonist (Experiment 2, Table 1) on the open field were examined. No treatment differences in entry latency were found. No significant treatment effects in overall locomotor activity were found in outer or inner visits in either experiment, but a significant effect of time was found in both experiments (F1,16 = 41.43; P < 0.00001 and F1,15 = 9.16, P < 0.008, respectively); subjects, treated or control, were less active during the second 3-min period on the field. The decrease in outer visits over time is indicative of habituation to the field by the subjects. In the second experiment, there was also a significant treatment by time interaction (F2,15 = 6.06, P < 0.01) in outer visits, and the DPN-treated group had significantly more outer visits than the PPT- and vehicle-treated subjects in time 1 (P < 0.01). Rearing, grooming and defecation did not show any group or time effects (data not shown).
Table 1.
Effects of sub chronic EB, PPT and DPN treatments on the open field.
| Group | Outer visits |
Inner visits |
Entry Latency | ||
|---|---|---|---|---|---|
| Time 1 | Time 2 | Time 1 | Time 2 | ||
| Vehicle | 40.0 ± 6.4 | 24.1 ± 4.8++ | 1.3 ± 0.8 | 2.9 ± 1.4 | 12.2 ± 1.4 |
| EB | 56.9 ± 7.2 | 28.3 ± 3.5+++ | 3.7 ± 1.1 | 1.8 ± 0.8 | 10.7 ± 1.8 |
| Vehicle | 34.8 ± 9.6 | 38.7 ± 10.0 | 0.5 ± 0.5 | 2.8 ± 1.3 | 14.0 ± 3.1 |
| PPT | 36.8 ± 9.3 | 27.0 ± 9.6 | 0.7 ± 0.2 | 0.5 ± 0.3 | 15.6 ± 5.0 |
| DPN | 57.7 ± 6.9** | 29.8 ± 5.8+++ | 1.5 ± 0.7 | 2.0 ± 0.9 | 12.7 ± 2.8 |
Locomotor activity on the open field is shown and analyzed individually for each of the two cohorts. Entries are means ± SEM. Time 1 and Time 2 refer to the 1st and 2nd 3 min on the open field, respectively. See text for statistical details.
(P < 0.01) indicates group difference within time 1.
(P < 0.001),
(P < 0.01) indicates within group difference between time 1 and 2.
Effects of EB and ER agonists on the elevated plus maze
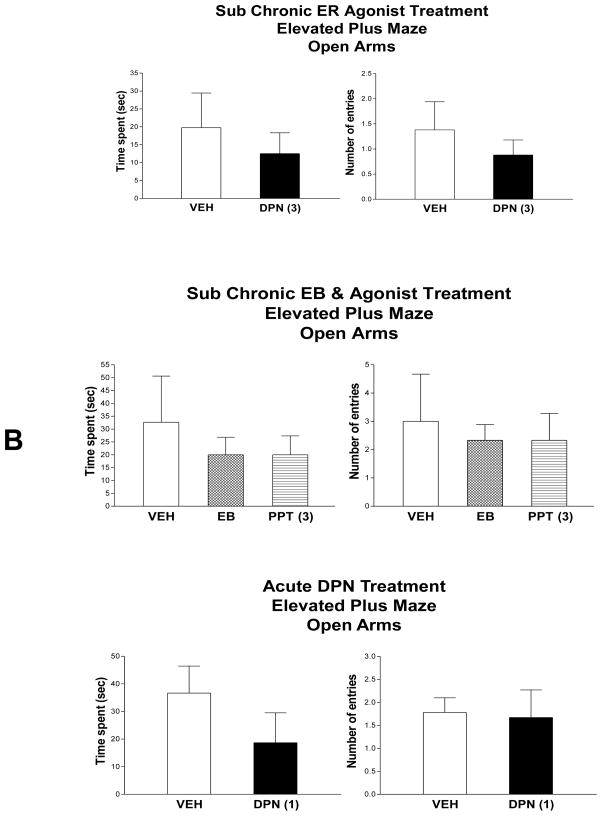
Following sub chronic treatment to OVX rats, DPN treatment did not alter time spent in the open arms (t = 1.01, p < 0.84) or the number of entries into the open arms (t = 0.45, p < 0.66) of the elevated plus maze (Fig 6A). Likewise, shown in Figure 6B, treatment with EB or PPT did not alter time spent in the open arms (F2,15 = .38, p < 0.69) or the number of entries into the open arms (F2,15 = 0.11, p < 0.89). Acute DPN treatment did not alter performance of OVX rats on the elevated plus maze (Fig. 6C); no changes were noted in time spent in the open arms (t =1.23, p < 0.) or in number of entries into the open arms (t = 0.16, p < 0.). Thus, neither EB nor the agonists DPN or PPT altered behavior on the elevated plus maze.
Figure 6. Effects of EB and ER agonists on the elevated plus maze.
Entries are means ± SEM of time spent and number of entries into the open arms following sub chronic treatment with (A) vehicle and DPN (3 mg/kg) (n=8/group) and (B) vehicle, EB (50 μg/kg) and PPT (3 mg/kg) (n=6/group). (C) Testing was conducted acutely, 4 h following DPN at 1mg/kg, with n=9/group. No significant differences. See results for statistical details.
Effects of DPN on monoamines and metabolites
Sub chronic treatment with DPN was associated with substantial changes in monoamines and metabolites in areas important for learning and memory (Table 2). In the PFC, levels of DA significantly increased by 35% (p < 0.05), and its metabolite, HVA, and the HVA/DA ratios significantly increased by approximately 100% (p < 0.001 and p < 0.05, respectively) following DPN treatment, and the norepinephrine metabolite, MHPG, was also significantly higher, 75%, (p < 0.05). In CA1 of the hippocampus, NE levels significantly increased 35% (p < 0.05) while the MHPG/NE ratio significantly decreased by 50% (p < 0.05). In contrast, the ventral hippocampus had significantly increased MHPG and MHPG/NE ratios, 60 and 130%, respectively (p < 0.01, p < 0.05) following DPN treatment. Dentate gyrus HVA levels significantly declined 50% with treatment (p < 0.01). In the VDB, MHPG and the MHPG/NE ratio significantly declined following treatment with DPN, 80 and 70%, respectively (p < 0.000001 and p < 0.01) and HVA significantly decreased by 43% (p < 0.05). The only changes noted in the 5-HT system were in the PFC and CA3 where the metabolite of 5-HT, 5-HIAA, showed modest increases, approximately 20%, in both areas (p < 0.05, p < 0.05) following DPN treatment. No changes in monoamines or metabolites were noted in medial septum or striatum following DPN treatment.
Table 2.
Effects of sub chronic DPN treatment on monoamine levels in the brain
| Group | DA | HVA | HVA/DA | NE | MHPG | MHPG/NE | 5HT | 5HIAA | 5HIAA/5HT |
|---|---|---|---|---|---|---|---|---|---|
| CA1 | |||||||||
| Control | 1.12±0.25 | 0.40±0.07 | 0.39±0.07 | 1.18±0.16 | 5.37±0.84 | 5.68±1.33 | 0.96±0.09 | 2.37±0.27 | 2.53±0.28 |
| DPN | 0.93±0.28 | 0.37±0.07 | 0.57±0.07 | 1.60±0.12* | 3.48±0.37 | 2.37±0.34* | 1.13±0.07 | 2.51±0.22 | 2.22±0.14 |
| CA3 | |||||||||
| Control | 0.46±0.08 | 0.28±0.06 | 0.40±0.08 | 2.42±0.27 | 5.31±0.65 | 2.30±0.29 | 1.66±0.18 | 3.78±0.29 | 2.59±0.27 |
| DPN | 0.68±0.11 | 0.28±0.03 | 0.46±0.06 | 2.73±0.23 | 7.25±0.78 | 2.74±0.27 | 1.49±0.09 | 4.60±0.25* | 3.16±0.24 |
| Dentate Gyrus | |||||||||
| Control | 0.37±0.09 | 0.32±0.04 | 0.76±0.19 | 3.69±0.32 | 1.25±0.16 | 0.35±0.04 | 0.85±0.06 | 2.81±0.18 | 3.45±0.37 |
| DPN | 0.42±0.14 | 0.16±0.03** | 0.57±0.11 | 4.18±0.34 | 1.35±0.13 | 0.33±0.03 | 1.05±0.09 | 3.40±0.39 | 3.24±0.28 |
| Ventral Hippocampus | |||||||||
| Control | 3.08±0.37 | 0.36±0.07 | 0.08±0.02 | 5.13±0.54 | 2.07±0.16 | 0.43±0.05 | 4.34±0.47 | 6.00±0.48 | 1.44±0.10 |
| DPN | 7.56±2.66 | 0.38±0.04 | 0.06±0.01 | 4.66±0.84 | 3.37±0.32** | 0.99±0.22* | 4.66±0.52 | 5.19±0.34 | 1.18±0.09 |
| Prefrontal Cortex | |||||||||
| Control | 0.64±0.04 | 5.28±0.70 | 7.44±0.61 | 2.03±0.11 | 3.14±0.44 | 1.60±0.24 | 1.82±0.13 | 2.12±0.15 | 1.2±0.10 |
| DPN | 0.87±0.07* | 10.66±1.0*** | 12.13±1.63* | 2.40±0.21 | 5.51±0.74* | 2.52±0.48 | 2.42±0.29 | 2.68±0.19* | 1.24±0.17 |
| VDB | |||||||||
| Control | 5.15±0.94 | 0.30±0.04 | 0.06±0.01 | 6.80±0.81 | 18.18±1.32 | 2.92±0.49 | 2.27±0.24 | 2.92±0.15 | 1.41±0.17 |
| DPN | 3.81±0.52 | 0.17±0.02* | 0.05±0.003 | 6.30±0.73 | 4.68±0.76****** | 0.85±0.22** | 2.30±0.23 | 3.05±0.13 | 1.43±0.16 |
| Medial Septum | |||||||||
| Control | 4.01±0.69 | 0.64±0.08 | 0.17±0.03 | 5.32±0.44 | 20.06±2.54 | 3.78±0.39 | 3.58±0.41 | 3.75±0.28 | 1.11±0.09 |
| DPN | 4.24±0.44 | 0.72±0.09 | 0.20±0.03 | 5.72±0.31 | 19.90±1.68 | 4.01±0.56 | 3.53±0.25 | 3.86±0.13 | 1.12±0.06 |
| Striatum | |||||||||
| Control | 61.93±4.34 | 0.58±0.06 | 0.009±0.001 | 0.27±0.05 | 6.52±0.82 | 31.98±5.08 | 1.68±0.20 | 1.95±0.14 | 1.22±0.09 |
| DPN | 75.92±6.22 | 0.57±0.06 | 0.008±0.001 | 0.26±0.02 | 5.68±0.71 | 23.01±3.27 | 1.80±0.11 | 1.99±0.11 | 1.12±0.06 |
Data were analyzed by two-sample t-tests to test for group differences in each individual monoamine, metabolite and turnover ratio. Entries are means ± SEM for 7–9/group. Significant differences are indicated by
(P<0.05),
(P<0.01),
(P<0.001), and
(P<0.000001).
No significant differences in monoamines, metabolites or turnover ratios were found between groups in medial septum and striatum.
Discussion
Consistent with previous results from a number of labs (Luine et al., 2003; Walf et al., 2006; Scharfman et al., 2007; Inagaki et al, 2010), EB administration to OVX rats improved working memory for two recognition memory tasks: OR, which is non-spatial and OP, which is a spatial memory task dependent on the hippocampus. The current studies, in which OVX rats were given EB (50 ug/kg) for two days and tested 48 h after the last injection, are the first to show that both types of recognition memory are enhanced at this dose and duration of estradiol treatment in this species. Previously, we showed that estrogen treatments to OVX rats immediately following the viewing of objects (T1) improve OR and OP memory 4 h later (Luine et al., 2003; Inagaki et al, 2010), and Frye and colleagues found similar effects when estradiol was given immediately after T1 and memory tested 24 h later (Frye et al., 2007; Walf et al., 2006). Acute or sub chronic estrogen treatments also enhance memory in these tasks in mice (Fernandez, Lewis, Pechenino, Harburger, Orr, Gresack, Schafe, & Frick, 2008; Li, Brake, Romeo, Dunlop, Gordon, Buzescu, Magarinos, Allen, Greengard, Luine, & McEwen, 2004; Walf et al., 2008). The presumably delayed onset of EB action (behavior was enhanced 48 h after the last treatment) suggests that, in this sub chronic treatment paradigm, mediation of cognitive effects occurs through genomic mechanisms via classical ERα and ERβ (however, possible rapid estrogen interactions with other receptors cannot be discounted, see further discussion below). Both ERs are present in the brain and thus could mediate cognitive changes. ERα is highly expressed in areas of the hypothalamus-POA responsible for reproduction and in the uterus and breast (Hewitt and Korach, 2003) as well as in brain areas involved in learning, memory, emotionality and attention such as the hippocampus and amygdala (Milner, McEwen, Hayashi, Li, Reagan & Alves, 2001). ERβ is present in those same areas as well as many areas of the neocortex, but is notably absent in some hypothalamic areas and peripheral estrogen target organs (Shughrue, Lane and Merchenthaler, l997; Mitra, Hoskin, Yudkovitz, Pear, Wilkinson, et al, 2003). In addition, ERβ is higher than ERα in the hippocampus and frontal cortex, areas important for memory (Mitra et al, 2003).
To determine which estrogen receptor(s) might be responsible for EB’s enhancing effects in these memory tasks, we tested selective agonists for ERα (PPT; Harris et al., 2002) and ERβ (C-19; Wilkening et al., 2006; DPN; Minutolo et al, 2009) under the same conditions as EB. The two ERβ agonists enhanced object and place memory, but the ERα agonist did not. These results are consistent with Rissman et al. (2002) who showed that estrogen-treated BERKO mice did not learn the Morris water maze task and with Liu et al. (2008) who showed that estrogen treatment to ERKO, but not BERKO, mice, improved hippocampus-dependent Y-maze performance. Also, Walf et al. (2008) reported that estradiol or DPN administration to wild type, but not BERKO mice, enhanced both OR and OP performance.
Although the current experiments did not verify that the ER selective agonists occupied the ERs in the brain, studies indicate that PPT and DPN can penetrate the blood brain barrier and alter ER-dependent pathways (Harris et al., 2002; Lund et al., 2005). C-19 has been shown to affect thermoregulation and alter levels of monoamines in the brain providing indirect evidence for activity within the CNS (Lubbers et al., 2006; Opas et al., 2009). Furthermore, we assessed uterine wet weight, which provides additional validation of agonist specificity and effectiveness because the uterus contains only ERα (Harris et al., 2002). DPN, at a dose, which enhanced recognition memory, did not increase uterine weight. While not tested here, C-19, given daily for 4 consecutive days, does not increase uterine weight (Lubbers et al., 2006). The lack of uterine effects shows that, at these doses, the ERβ-specific agonists did not activate ERα, at least in the uterus, which is known to be more sensitive to estrogens than the brain. On the other hand, PPT, given for two days at 3 mg/kg, led to a doubling of uterine wet weight but no effect on the memory tasks. PPT, given at 3 mg/kg, is known to activate ERα and increase progesterone receptors in hypothalamic nuclei that specifically contain ERα (Harris et al., 2002) and 2.5 mg/kg activates female sexual behavior (Mazzucco et al., 2008). Thus, the doses of PPT used here (3 and 5 mg/kg for 2 days) should have been sufficient to activate classical ERα-dependent responses. The PPT dose was not increased further because of the potential for loss of specificity and activation of both α and β receptors (Harris et al., 2002). Thus, our results with ER selective agonists suggest that estrogen action on recognition memory, at least at the current doses and duration of treatment, are mediated primarily or exclusively through ERβ.
In contrast to these current results, another recent study implicated both ERs in mediating estrogen’s effects in a memory task. Hammond et al. (2009) found that estradiol, PPT and DPN all enhanced acquisition of a spatial task, the DMP T-maze task. In this case, treatment was chronic, not sub chronic, as acquisition was tested beginning 10 days after initiation of treatments. Thus, it is possible that the longer duration of treatment led to loss of receptor selectivity of the agonists. In addition, the two receptors have been shown to influence the expression of each other (Rissman, 2008), so it is possible that the chronic treatments led to changes in receptor expression, which resulted in ERα activation of memory (see Rissman, 2008, for further discussion). Another possible explanation for the difference in efficacy between these and Hammond’s results is that different ERs might contribute to acquisition as compared to memory. This idea has not received any direct testing; however, estrogen has been shown to enhance performance during the acquisition phase of spatial tasks, but when subjects have acquired how to solve the task, estrogens often no longer enhance performance (Dohanich, 2002; Luine, 2008; Frick, 2009). OR and OP are primarily memory tasks (Ennaceur et al, 1997), and thus effects of estradiol or agonists on learning would not contribute to task performance in the current experiments.
The effects of PPT and DPN (C-19 was not evaluated) on memory were also tested in an acute treatment paradigm since estradiol has been shown to rapidly enhance memory consolidation in four memory tasks – water maze (Rhodes & Frey, 2006; Sandstrom & Williams, 2004), inhibitory avoidance (Frye & Rhodes, 2002), OR and OP (Luine et al., 2003; Scharfman et al., 2007; Walf et al., 2008; Inagaki, 2010). Estradiol rapidly alters some central signaling pathways (Bryant et al., 2005; Roepke et al, in press) suggesting a basis for the rapid initiation of some behavioral effects. For recognition memory tasks, immediate, but not delayed, sc injections of a variety of estrogens after the sample trial enhance discrimination 4 h (Luine et al., 2003) or 24 h later in OVX rats (Frye et al., 2007) and mice (Fernandez et al., 2008). Interestingly, 17α-estradiol has a greater potency than 17β-estradiol in acute enhancements of memory (Luine et al, 2003; Ingaki et al, 2010). This difference in potency is consistent with effects on membrane signaling by estrogens (Roepke et al, in press) and suggest differences between nuclear and membrane receptors; however, little information on the identity of central membrane receptors is available (Watson et al, 2010). In the water maze, estrogens given sc or directly into the hippocampus immediately following, but not 2 h after, the training trials enhanced the ability of OVX or castrated rats to locate the hidden platform (Packard & Teather, 1997). Likewise, estradiol administration to OVX rats immediately, but not 1, 2 or 3 h post training, increases crossover latencies for inhibitory avoidance (Rhodes & Frye, 2006). Thus, the temporal relationship between estrogen application and performance enhancement is consistent with augmentation of mnemonic processes. The current results show that post T1 injections of DPN, but not PPT, rapidly enhance memory in both OR and OP. Similar memory enhancing effects by DPN, but not PPT, were obtained by Rhodes and Frye (2006) in a post training treatment paradigm in the water maze task. However, Frye’s group has recently reported that when the agonists are given post T1 and memory tested 24 h later, both PPT and DPN enhance OP (Frye et al., 2007). Furthermore, PPT, but not DPN, enhances OR (Rhodes & Frye, 2006). Differences in agonist doses do not appear to account for the differences in effects (1 vs. 0.9 mg/kg), but the differences in inter-trial delays (4 h vs. 24 h) may be important. The additional 20 h may allow for receptor interactions or other unknown processes to occur. It has also been noted by Rissman (2008) that cognitive responses to estrogens and agonists might be different in knockout vs. mice bearing both ERs because of the lack of receptor interactions in knockout subjects. In addition, Mitra et al. (2003) reported some differences in the distribution of ERβ in rats vs. mice. Collectively, these data indicate that both rapid and sub chronic effects of estradiol on memory function are mediated through ERs, however, the precise roles these receptors play in enhancing memory function require further study.
Both estrogens and DPN have been shown to exert anxiolytic effects under certain treatment conditions, and estrogen often increases activity (Koss, Gehlert, & Shekhar, 2004; Martínez-Mota, Estrada-Camarena, López-Rubalcava, Contreras, & Fernández-Guasti, 2000; Morgan & Pfaff, 2002; Tomihara et al., 2009). Thus, we assessed locomotion and anxiety with our treatment regimen since increased activity and decreased anxiety could contribute to better performance in memory tasks. As indicated by entries into the open arms of the elevated plus maze and number of inner visits or entry latencies on the open field, neither EB, DPN nor PPT exerted anxiolytic effects with the current treatments. It should however be noted that anxiety testing was done 24 h post treatment while memory testing was done 48 h post treatment. On the open field, there were no treatment effects on activity by EB or agonists, but all subjects showed a marked decrease in visits from time 1 to time 2, which is an indication of habituation to the open field. DPN-treated rats, on the other hand, made more outer visits than vehicle- or PPT-treated subjects during time1 but did show habituation over time. Thus, DPN, but not PPT or EB, enhanced locomotion and exploration on the open field, and this enhanced exploration could, in turn, contribute to DPN’s enhancements in recognition memory. In contrast to the open field, object exploration during T1 for both OR and OP tests, did not differ between DPN- and vehicle-treated subjects. Taken together, these data suggest that DPN may enhance exploration of space, but not objects. The lack of DPN effects on the elevated plus maze contrasts with those of Lund et al. (2005) who found that a single daily sc dose of 1 mg/kg of DPN, given to OVX rats for four consecutive days with testing on the elevated plus maze one half hour after the last injection, decreased anxiety. Consistent with current data, those investigators also found that PPT did not affect anxiety. Lack of effects on anxiety and activity in the present study, as compared to previous studies are most likely related to higher doses and/or longer duration of treatments since others have also reported no effects by E2 and/or ERβ agonists (Andrade, Nakamuta, Avanzi, & Graeff, 2005; Koss et al., 2004; Martinez-Mota et al., 2000; Morgan & Pfaff, 2002). Our own previously reported observation on the positive effects of estradiol on locomotion supports the idea that longer treatments may result in a different outcome (Bowman et al., 2002). Thus, current and previous data suggest that the sub chronic estrogenic treatments used here affected memory and not psychological factors that can enhance performance in memory tasks.
We examined the effects of DPN at the sub chronic dose treatment which enhanced recognition memory on levels of catechol- and indole-amines and metabolites in areas of brain that contribute to memory. We did not examine estradiol since previous studies link this hormone to monoaminergic alterations (see below). Previous studies show that indices of other neurotransmitters, such as AMPA and NMDA receptor subunits in the hippocampus (Liu et al., 2008; Waters, Mitterling, Spencer, Mazid, McEwen, & Milner, 2009), and striatal dopamine receptors and transporters are increased by DPN but not PPT, but prepoenkephalin gene expression is increased by both DPN and PPT (Morisette, Le Saux, D’Astous, Jourdain, Al Sweidi, Morin, Estrada-Camarena, Mendez, Garcia-Segura, & Di Paolo, 2008); however, little information appears available on DPN effects on monoamine levels. In the current study, effects of DPN were most prominent on noradrenergic terminals in the dorsal (CA1) and ventral hippocampus and PFC where activity, as assessed by MHPG levels or MHPG/NE ratios, changed by 60–130%. NE activity increased following DPN treatment in the PFC and ventral hippocampus, whereas it decreased in CA1 and also in the VDB. Furthermore, DA activity increased by 60% (HVA/DA ratio) in the PFC, and HVA levels decreased 50% in the dentate gyrus, following DPN. PFC dopaminergic activity has long been linked to cognitive function (Brozoski et al., 1979), and recent studies have shown that multiple, general cognitive constructs known to be regulated by PFC dopaminergic terminals are influenced by circulating gonadal hormones (Kritzer, Brewer, Montalmant, Davenport, & Robinson, 2007; Luine, 2007). In addition, estradiol treatments increase DA activity and axon densities in the PFC (Kritzer, 2000). Thus, the 35% increase in DA levels and the 60% increase in DA activity in the PFC, following DPN, may contribute to its improvement in recognition memory in OVX rats. The PFC also receives NE neuronal projections, which contribute to cognitive function (Arnsten & Li, 2005). DPN-dependent increases in the NE metabolite, MHPG, in this area are consistent with enhancement of OR and OP. Chronic estrogen treatment also increases NE activity in the PFC (Luine et al., 1998). In addition, a small increase of 25% in 5HIAA levels, the metabolite of 5-HT, was noted in PFC. In a previous study, we observed that chronic estradiol treatment also increased PFC 5-HT activity (Luine, Richards, Wu & Beck, 1998). In a separate study that compared the effects of 4 days of treatment with PPT or C-19, both agonists appear to have similar effects on NE in frontal cortex, and there was a trend toward higher MHPG levels. In addition, PPT caused a significant increase in the DA metabolite, DOPAC, in that area (Lubbers et al., 2006). Thus, while there is some overlap between estradiol and both agonists in inducing changes in norepinephrine in the frontal cortex, dopaminergic and serotonergic changes following either estradiol or DPN, but not PPT, may be more important for enhancing memory.
In the hippocampus, NE activity decreased in CA1 (50% decrease in MHPG/NE) while it increased in the ventral hippocampus (130% increase in MHPG/NE), and CA3 5HIAA showed a small 20% increase, following DPN treatment. The role of the hippocampus in spatial memory, including object placement, is well documented, and the object recognition task also relies on the hippocampus, but to a lesser extent than object placement (Broadbent, Squire, & Clark, 2004). Little information is available on estrogen-dependent changes in monoamines in the ventral hippocampus, but this region contains both ERs with ERβ being especially abundant in rodents (Mitra et al., 2003; Shughrue et al., 1997). Changes in dorsal hippocampal monoamines by estrogen (Luine et al., 1998) and stress (Bowman, Micik, Gautreaux, Fernandez and Luine, 2009) have been linked to spatial memory. For example, better performance in radial arm maze and OP was associated with increased 5-HT (Luine, Spencer, & McEwen, 1993) and NE in CA3 hippocampus (Bowman et al., 2004; see also Bowman, Beck, & Luine, 2003 for review). Finally, we have recently reported that EB, given in the same acute paradigm as here, alters monoamines (Inagaki et al, 2010). Subjects underwent a T1 trial for recognition memory, immediately received 20ug/kg of estradiol and were sacrificed 30 min later, during the time of memory consolidation. Activity of 5-HT, NE and DA were increased in PFC and NE activity was decreased in the hippocampus. Thus, acute estradiol also alters monoamine activity, but the pattern of change is not identical after acute EB vs sub chronic administration of DPN.
In conclusion, the current results suggest that ERβ, but not ERα, contributes to estradiol-dependent enhancements in recognition memory. Moreover, changes in monoaminergic activity in the PFC and hippocampus following an ERβ selective agonist, DPN, may contribute to mnemonic enhancements; however, further investigations of learning and memory tasks as well as neurochemical consequences of ER activation are necessary to substantiate these conclusions. The importance of the current research is that ERβ agonists might provide important alternative treatments to estradiol for cognitive loss following oophorectomy or menopause without the deleterious effects in the uterus, breast and circulatory system caused from the activation of ERα by estradiol or currently utilized estrogens like Premarin (Sherwin, 2007).
Acknowledgments
Grant Support: R25-GM-60665 (MBRS/RISE), S06-GM-60654 (SCORE), and G12-RR-03037 (RCMI) grants from NIH and by CUNY Research grants for doctoral students. This research is part of the doctoral thesis of L. Jacome.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrade T, Nakamuta J, Avanzi V, Graeff F. Anxiolytic effect of estradiol in the median raphe nucleus mediated by 5-HT1a receptors. Behavioural Brain Research. 2005;163:18–25. doi: 10.1016/j.bbr.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Arnsten A, Li B. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biological Psychiatry. 2005;57 (11):1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Beck K, Luine V. Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiology and Behavior. 2002;75:661–673. doi: 10.1016/s0031-9384(02)00670-4. [DOI] [PubMed] [Google Scholar]
- Bowman R, Ferguson D, Luine V. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:401–10. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Bowman R, Beck K, Luine V. Chronic stress effects on memory: Sex differences in performance and monoamines. Hormones and Behavior. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Bowman R, MacLusky N, Sarmiento Y, Frankfurt M, Gordon M, Luine V. Sexually dimorphic effects of prenatal stress on cognition, hormonal responses, and central neurotransmitters. Endocrinology. 2004;145:3778–3787. doi: 10.1210/en.2003-1759. [DOI] [PubMed] [Google Scholar]
- Bowman R, Micik R, Gautreaux C, Fernandez L, Luine V. Sex-dependent changes in anxiety, memory, and monoamines following one week of stress. Physiology and Behavior. 2009;97:21–29. doi: 10.1016/j.physbeh.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Broadbent N, Squire L, Clark R. Spatial memory, recognition memory, and the hippocampus. Proceeding of the National Academy of Sciences USA. 2004;101 (40):14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski T, Brown R, Rosvold H, Goldman P. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkeys. Science. 1979;205:929–31. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Bryant D, Bosch M, Rønnekleiv O, Dorsa D. 17-β estradiol rapidly enhances extracellular signal-regulated kinase 2 phosphorylation in the rat brain. Neuroscience. 2005;133:343–352. doi: 10.1016/j.neuroscience.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Daniel JM. Effects of estrogen on cognition: what have we learned from basic research? J Neuroendocrinol. 2006;18:787–95. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- Daniel J, Dohanich G. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. The Journal of Neuroscience. 2001;21 (17):6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D, Jacobson T, Aliakbari S, Mizumori S. Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiology of Learning and Memory. 2005;84:132–137. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Díaz-Véliz G, Alarcón T, Espinoza C, Dussaubat N, Mora S. Ketanserin and anxiety levels: influence of gender, estrous cycle, ovariectomy and ovarian hormones in female rats. Pharmacology Biochemistry and Behavior. 1997;58 (3):637–642. doi: 10.1016/s0091-3057(97)90004-6. [DOI] [PubMed] [Google Scholar]
- Dohanich GP. Gonadal steroids, learning and memory. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RI, editors. Hormones, Brain and Behavior. San Diego: Academic Press; 2002. pp. 265–327. [Google Scholar]
- Ennaceur A, Neave N, Aggleton J. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Experimental Brain Research. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Fernandez S, Lewis M, Pechenino A, Harburger L, Orr P, Gresack J, Schafe G, Frick K. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. Journal of Neuroscience. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick K. Estrogens and age-related memory decline in rodents: What have we learned and where do we go from here? Hormones and Behavior. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye C, Rhodes M. Enhancing effects of estrogen on inhibitory avoidance performance may be in part independent of intracellular estrogen receptors in the hippocampus. Brain Research. 2002;956:285–293. doi: 10.1016/s0006-8993(02)03559-x. [DOI] [PubMed] [Google Scholar]
- Frye C, Duffy C, Walf A. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiology of Learning and Memory. 2007;88:208–16. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R, Gabor R, Cox T, Johnson D. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29:741–748. doi: 10.1016/S0306-4530(03)00118-5. [DOI] [PubMed] [Google Scholar]
- Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs R. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Hormones and Behavior. 2009;56:309–314. doi: 10.1016/j.yhbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H, Katzenellenbogen J, Katzenellenbogen B. Characterization of the biological roles of the estrogen receptors, ERα and ERβ, in estrogen target tissues in vivo through the use of an ERα-selective ligand. Endocrinology. 2002;143 (11):4172–4177. doi: 10.1210/en.2002-220403. [DOI] [PubMed] [Google Scholar]
- Hewitt S, Korach K. Oestrogen receptor knockout mice: roles for oestrogen receptors α and β in reproductive tissues. Reproduction. 2003;125:143–149. doi: 10.1530/rep.0.1250143. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine V. Acute Estrogen Treatment Facilitates Recognition Memory Consolidation and Alters Monoamine levels in Memory-related Brain Areas. Hormones & Behavior. 2010;58:415–426. doi: 10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in blancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82:309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Koss W, Gehlert D, Shekhar A. Different effects of subchronic doses of 17-β estradiol in two ethologically based models of anxiety utilizing female rats. Hormones and Behavior. 2004;46:158–164. doi: 10.1016/j.yhbeh.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Kritzer M. Effects of acute and chronic gonadectomy on the catecholamine innervation of the cerebral cortex in adult male rats: insensitivity of axons immunoreactive for dopamine-beta-hydroxylase to gonadal steroids, and differential sensitivity of axons immunoreactive for tyrosine hydroxylase to ovarian and testicular hormones. The Journal of Comparative Neurology. 2000;427:617–33. [PubMed] [Google Scholar]
- Kritzer M, Brewer A, Montalmant F, Davenport M, Robinson J. Effects of gonadectomy on performance in operant tasks measuring prefrontal cortical function in adult male rats. Hormones and Behavior. 2007;51:183–195. doi: 10.1016/j.yhbeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Kuiper G, Enmark J, Pelto-Huikko M, Nilsson S, Gustafsson J. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proceeding of the National Academy of Sciences USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Shors T. High levels of estrogen enhance associative memory formation in ovariectomized females. Psychoneuroendocrinology. 2004;29:883–890. doi: 10.1016/j.psyneuen.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux M, Di Paolo T. Chronic estrogenic drug treatment increases preproenkephalin mRNA levels in the rat striatum and nucleus accumbens. Psychoneuroendocrinology. 2005;30:251–260. doi: 10.1016/j.psyneuen.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Le Saux M, Di Paolo T. Influence of oestrogenic compounds on monoamine transporters in rat striatum. Journal of Neuroendocrinology. 2006;18:25–32. doi: 10.1111/j.1365-2826.2005.01380.x. [DOI] [PubMed] [Google Scholar]
- Li C, Brake W, Romeo R, Dunlop J, Gordon M, Buzescu R, Magarinos A, Allen P, Greengard P, Luine V, McEwen B. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proceeding of the National Academy of Sciences USA. 2004;101 (7):2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Day M, Muñiz L, Bitran D. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nature Neuroscience. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Lubbers L, Alves S, Zafian P, Gautreaux C, Gordon M, Correa L, Lorrain D, Hong K, Luine V, Rohrer S, Hickey G. Subtype-selective estrogen receptor (ER) agonists affect monoamine levels in discrete regions of the rat brain. Society for Neuroscience Abstract. 2006:111. [Google Scholar]
- Luine V, Spencer R, McEwen B. Effects of chronic corticosterone ingestion on spatial memory performance and hippocampal serotonergic function. Brain Research. 1993;616:65–70. doi: 10.1016/0006-8993(93)90193-q. [DOI] [PubMed] [Google Scholar]
- Luine V, Richards S, Wu V, Beck K. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Hormones and Behavior. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Luine V, Jacome L, MacLusky N. Rapid enhancements of visual and place memory by estrogens in rats. Endocrinology. 2003;144 (7):2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luine V, Attalla S, Mohan G, Costa A, Frankfurt M. Dietary phytoestrogens enhance spatial memory and spine density in the hippocampus and prefrontal cortex of ovariectomized rats. Brain Research. 2006;1126:183–187. doi: 10.1016/j.brainres.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Luine V. The prefrontal cortex, gonadal hormones and memory. Hormones and Behavior. 2007;51:181–182. doi: 10.1016/j.yhbeh.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Luine V. Sex steroids and cognitive function. Journal of Neuroendocrinology. 2008;20:866–72. doi: 10.1111/j.1365-2826.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- Lund T, Rovis T, Chung W, Handa R. Novel actions of estrogen receptor-β on anxiety-related behaviors. Endocrinology. 2005;146 (2):797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- MacLusky N, Luine V, Hajszan T, Leranth C. The 17α and 17β isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146 (1):287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- Martínez-Mota L, Estrada-Camarena E, López-Rubalcava C, Contreras C, Fernández-Guasti A. Interaction of desipramine with steroid hormones on experimental anxiety. Psychoneuroendocrinology. 2000;25:109–120. doi: 10.1016/s0306-4530(99)00042-6. [DOI] [PubMed] [Google Scholar]
- Mazzucco C, Lieblich E, Bingham B, Williamson M, Viau V, Galea L. Both estrogen receptor α and estrogen receptor β agonists enhance cell proliferation in the dentate gyrus of adult female rats. Neuroscience. 2006;141:1793–1800. doi: 10.1016/j.neuroscience.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Mazzucco D, Walker HA, Pawluski J, Lieblich S, Galea L. ERalpha, but not ERbeta, mediates the expression of sexual behavior in the female rat. Behavioural Brain Research. 2008;19:111–7. doi: 10.1016/j.bbr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Milner T, McEwen B, Hayashi S, Li C, Reagan L, Alves S. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. Journal of Comparative Neurology. 2001;429:355–71. [PubMed] [Google Scholar]
- Minutolo F, Macchia M, Katzenellenbogen B, Katzenellenbogen J. Estrogen receptor beta ligands: Recent advances and biomedical applications. Medicinal Research Reviews. 2009 Dec 4; doi: 10.1002/med.20186. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Mitra S, Hoskin E, Yudkovitz J, Pear L, Wilkinson H, Hayashi S, Pfaff D, Ogawa S, Rohrer S, Schaeffer J, McEwen B, Alves S. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–67. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Morgan M, Pfaff D. Estrogen’s effects on activity, anxiety, and fear in two mouse strains. Behavioural Brain Research. 2002;132:85–93. doi: 10.1016/s0166-4328(01)00398-9. [DOI] [PubMed] [Google Scholar]
- Morisette M, Le Saux M, D’Astous M, Jourdain S, Al Sweidi S, Morin N, Estrada-Camarena E, Mendez P, Garcia-Segura L, Di Paolo T. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. The Journal of Steroid Biochemistry and Molecular Biology. 2008;108:327–38. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Opas E, Scafonas A, Nanterment P, Wilkening R, Birzin E, Wilkinson H, Colwell L, Schaeffer J, Towler D, Rodan G, Schmidt A. Control of rat tail skin temperature regulation by estrogen receptor-beta selective ligand. Maturitas. 2009;64:46–51. doi: 10.1016/j.maturitas.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Packard M, Teather L. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiology of Learning and Memory. 1997;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- Pellow S, File S. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacology Biochemistry and Behavior. 1986;24 (3):525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Ramos B, Arnsten A. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacology and Therapeutics. 2006;113 (3):523–36. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner K, Luine V. Analysis of temporal and dose-dependent effects of estrogen on monoamines in brain nuclei. Brain Research. 1986;366:64–71. doi: 10.1016/0006-8993(86)91281-3. [DOI] [PubMed] [Google Scholar]
- Renner K, Luine V. Analysis of temporal and dose-dependent effects of estrogen on monoamines in brain nuclei. Brain Research. 1986;366:64–71. doi: 10.1016/0006-8993(86)91281-3. [DOI] [PubMed] [Google Scholar]
- Rhodes M, Frye C. ERβ-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiology of Learning and Memory. 2006;85:183–191. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rissman E, Heck A, Leonard J, Shupnik M, Gustafsson J. Disruption of estrogen receptor β gene impairs spatial learning in female mice. Proceeding of the National Academy of Sciences USA. 2002;99 (6):3996–4001. doi: 10.1073/pnas.012032699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman E. Roles of oestrogen receptors α and β in behavioural neuroendocrinology: beyond Yin/Yang. Journal of Neuroendocrinology. 2008;20:873–879. doi: 10.1111/j.1365-2826.2008.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Ronnekleiv OK, Kelly MJ. Physiological consequences of membrane-initiated estrogen signaling in the brain. Frontiers in bioscience. doi: 10.2741/3805. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom N, Williams C. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Hormones and Behavior. 2004;45:128–135. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Hintz T, Gomez J, Stormes K, Barouk S, Malthankar-Phatak D, McLoskey D, Luine V, MacLusky N. Changes in hippocampal function of ovariectomized rats after sequential low doses of estradiol to simulate the preovulatory estrogen surge. European Journal of Neuroscience. 2007;26:2595–2612. doi: 10.1111/j.1460-9568.2007.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. The clinical relevance of the relationship between estrogen and cognition in women. J Steroid Biochem Mol Biol. 2007;106:151–156. doi: 10.1016/j.jsbmb.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Shughrue P, Lane M, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. The Journal of Comparative Neurology. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Tomihara K, Soga T, Nomura M, Korach K, Gustafsson J, Pfaff D, Ogawa S. Effect of ER-beta gene disruption on estrogenic regulation of anxiety in female mice. Physiology and Behavior. 2009;16:300–6. doi: 10.1016/j.physbeh.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf A, Rhodes M, Frye C. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiology of Learning and Memory. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf A, Koonce C, Frye C. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiology of Learning and Memory. 2008;89:513–21. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters E, Mitterling K, Spencer J, Mazid S, McEwen B, Milner T. Estrogen receptor alpha and beta specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain Research. 2009;1290:1–11. doi: 10.1016/j.brainres.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CS, Alyea RA, Cunningham KA, Jeng Y. Estrogens of multiple classes and their role in mental health disease mechanisms. Internat’l J Women’s Health. 2010;2:153–66. doi: 10.2147/ijwh.s6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkening R, Ratcliffe R, Tynebor E, Wildonger K, Fried A, Hammond M, Mosley R, Fitzgerald P, Sharma N, McKeever B, Nilsson S, Carlquist M, Thorsell A, Locco L, Katz R, Frisch K, Birzin E, Wilkinson H, Mitra S, Cai S, Hayes E, Schaeffer J, Rohrer S. The discovery of tetrahydrofluorenones as a new class of estrogen receptor β-subtype selective ligands. Bioorganic and Medicinal Chemistry Letters. 2006;16:3489–94. doi: 10.1016/j.bmcl.2006.03.098. [DOI] [PubMed] [Google Scholar]