Abstract
Pigeons were trained in a new procedure to test for visual binding errors between the dimensions of color and shape. In Experiment 1, pigeons learned to discriminate a target compound from 15 non-target compounds (constructed from four colors and shapes) by choosing one of two hoppers in a two-hopper choice task. The similarity of the target to non-target stimuli influenced choice responding. In Experiment 2, pigeons learned to detect a target compound when presented with a non-target compound within the same trial under conditions of simultaneity and sequentiality. Non-target trials were arranged to allow for the testing of binding errors (i.e., false identifications of the target on certain non-target trials). Transient evidence for binding errors in two of the birds occurred at the start of two-item training, but decreased with training. The experiments represent an important step toward developing a framework for the evaluation of visual feature binding in nonhumans.
Over the last several decades, experiments examining the binding of visual information of multidimensional stimuli have been extensively performed in human perception (e.g., Brockmole & Franconeri, 2009; Crick, 1984; Luck & Vecera, 2002; Robertson, 2003; Saiki & Miyatsuji, 2007; Treisman, 1996). A common finding is that object features are often correctly identified, but occasionally incorrectly bound, resulting in binding errors. Binding errors are also referred to as illusory conjunctions, a term first coined by Treisman and Schmidt (1982). Treisman and Schmidt found that with brief (i.e., around 120 ms) presentations of colored letters, humans would occasionally report illusory conjunctions of the color and form of the letters. For example, presented with a green O and a red T, a subject would sometimes report having seen a green T. Since Treisman and Schmidt’s landmark experiments, research on illusory conjunctions has progressed considerably, exploring how location (e.g., Ashby, Prinzmetal, Ivry & Maddox, 1996), time (e.g., McLean, Broadbent, & Broadbent, 1982), gestalt grouping mechanisms (Prinzmetal, 1981), and memory mechanisms (e.g., Prinzmetal, 1995), each play a role in producing illusory conjunctions.
One issue in regard to space and time is whether location or time holds a higher priority in the binding process. Both factors are critical to binding. For instance, as the spatial distance between two simultaneously presented stimuli increases, binding errors are reduced (e.g., Ashby, et al., 1996; Cohen & Ivry, 1989; Ivry & Prinzmetal, 1991; Keele, Cohen, Ivry, Liotti, & Yee, 1988; McLean, et al., 1982). As for time, as the time between two sequentially presented stimuli increases, binding errors are reduced (Keele et al., 1988; McLean et al., 1982). If location is more important than time to the binding of visual features, then rapidly presenting two stimuli in the same location should increase the probability of binding errors relative to the case where two stimuli are presented at the same time in two different but proximal locations. Using variants of the rapid serial visual presentation (RSVP) detection task, several studies have found that location is more important than time in the binding of visual information (Keele et al., 1988; McLean et al., 1982).
In vision, an object’s visual features such as its color, shape, and orientation are unified in an organism’s environment. In mammalian brains, however, visual features are analyzed in different areas of the brain (e.g., Lamme & Roelfesma, 2000; Zeki, 1993). For example, there are cells that are selectively tuned in area V4 to color, striate cortex to orientation, the medial temporal area to motion, and the inferotemporal cortex to complex form. However, after this distributed processing the end result is the perception of a bound, three-dimensional representation of the object. Hence, the object’s visual features are somehow recombined. How this reintegration process occurs is known as the binding problem. The binding problem continues to be one of cognitive science’s more vexing riddles. The behavioral research devoted to the binding problem has been conducted mostly with humans. However, this integration process is likely a general process that would be stable and common across a wide variety of vertebrate species (Papini, 2002). Nonhuman animals that rely heavily on vision for their everyday survival would be an ideal choice for developing a framework to address the comparative mechanisms underlying feature binding.
Pigeons serve as excellent subjects for comparative studies because their non-mammalian visual system has evolved to rapidly analyze visual information from the environment much like our own (e.g., Cook, 2000, 2001). In regard to the binding problem, prior physiological and psychological findings have suggested that color and shape features may be separately processed and reintegrated by their visual system, as well. The avian brain has two different ascending visual pathways, the thalamofugal and tectofugal, which relay information to the visual Wulst and entopallium respectively. The thalamofugal and tectofugal pathways are considered to be homologous to the mammalian geniculostriate and extrageniculate pathways (Husband & Shimizu, 2001). Like humans, there are also specialized areas of the avian visual pathways, which analyze specific visual information (for a review see Wylie, Gutierrez-Ibanez, Pakan, & Iwaniuk, 2009). For instance, retinal ganglion cells are responsive to directional movement and horizontal edge detection (Maturana & Frenk, 1963) and optic tectum cells are responsive to color, brightness, and line orientation (Engelage & Bischof, 1993; Li, Xiao, & Wang, 2007). Thus, the avian brain has specialized areas for the analysis of color and shape information. In regard to the discriminative response, different feature dimensions (e.g., color, shape, orientation, size) are processed separably and independently (e.g. Brown, Cook, Lamb, & Riley, 1984; Cook, 1992; Cook, Cavoto, & Cavoto, 1996; Cook, Riley, & Brown, 1992; Riley & Brown, 1991). If two dimensions are processed separably and independently, then the integration of these dimensions can occur if both dimensions are relevant to a task. For example, using a texture discrimination task, Cook et al. 1996, found that pigeons (like humans) find feature searches easier than conjunctive searches. Feature searches require subjects to find a target (or region as in texture segregation) that differs from distractors in one dimension (e.g., blue U amongst orange Us). Conjunctive searches require subjects to find a target that combines feature dimensions shared with distractors (e.g., blue U amongst blue As and orange Us). Conjunctive searches are typically more difficult because they require binding across separable and independent dimensions. Like visual search and texture discriminations, compound stimulus discriminations requiring feature binding to complete successfully. In fact, George and Pearce (2003) demonstrated such feature binding between the visual dimensions of color and orientation in pigeons.
George & Peace (2003) trained pigeons to discriminate orientation-color pairs on the basis of specific configurations (bindings) of color and orientation in particular spatial locations. Importantly, attending to a single dimension (i.e., color or orientation) did not provide sufficient information to solve the discrimination. After learning the discrimination, the stimuli were rotated 90° and retested. If pigeons had learned feature-bound representations, the rotated stimuli should elicit the reverse behavior of the trained contingencies. If the pigeons had learned visual patterns (templates), they may rotate the image back to its training orientation (i.e., a -90° rotation) to solve the discrimination, with no change in behavior. The results suggested that the pigeons were relying on feature-bound representations rather than a template-like pattern. Although the procedure of George & Pearce does not allow for the direct assessment of binding errors, their results suggest that binding errors may occur in the perception of conjunction stimuli. Because compound stimuli may be processed in a feature-based manner, misperceptions of the features in a non-target display may activate the “target” compound and evoke a target-present response.
The goal of the following experiments was to develop procedures to examine visual binding in pigeons by manipulating spatial and temporal factors, within a trial, of multiple presentations of color-shape compound stimuli. We were interested in whether binding errors occur in pigeons when presented with specific compound objects within a two-object array in different locations (simultaneously or sequentially) or the same location (sequentially). Before we began exploring variables related to binding, we needed to determine whether or not pigeons could discriminate a specific compound stimulus.
Experiment 1
The purpose of this experiment was to establish a conditional compound discrimination requiring the processing of two dimensions (cf. Blough, 1969; Chatlosh & Wasserman, 1993; George & Pearce, 2003; Heinemann & Chase, 1970). The visual dimensions selected were color and shape because both physiological and behavioral evidence suggests that birds process these dimensions independently. It was critical to establish that features along both dimensions were accurately discriminated by the pigeons in order to explore subsequently the possibility of binding errors between the two dimensions in Experiment 2. If the pigeons only discriminated along one dimension, and failed to discriminate along the other, it would be impossible to detect any binding errors.
In the conditional discrimination one compound was designated the target and the remaining compound stimuli were designated non-targets. A two-hopper choice task was used, which required the pigeon to select either the left or right hopper conditional on the compound stimulus presented on a given trial. For example, for the target compound the left hopper was the correct choice and for the non-target compounds the right hopper was the correct choice. Once this choice discrimination was learned, we could measure the presence of binding errors by using different combinations of the non-target compounds in Experiment 2.
Method
Animals
Four experimentally-naive male White Carneaux pigeons served as subjects: Chief, Dr J, Larry, and Reggie. Pigeons were maintained at 80% of their free-feeding weights via mixed grain during experimental sessions with supplemental feeding as needed. They had free access to grit and vitamin-enriched water in their individual home cages. A 12:12 hr light/dark cycle was kept throughout the study.
Apparatus
A flat-black Plexiglass chamber (38-cm wide × 36-cm deep × 39.3-cm high) contained the pigeon. A 28 × 21-cm viewing window was centered in the middle of the front panel. A 28-V houselight was located in the center of the ceiling and was illuminated at all times, except during the timeout that followed incorrect responses. Two identical hoppers (Coulbourn #E14-10, Allentown, PA) were located at the front of the left and right walls of the operant chamber, each 3.5 cm from the front viewing panel and flush with the floor. Infrared LEDs were mounted 1.5 cm within each hopper and detected the entrance of a pigeon’s head. A color monitor (COMPAQ 151FS, Houston, TX) was located immediately behind the front panel and was visible through the viewing window. Mounted in this window, 2 cm in front of the color monitor was a touchscreen (functional resolution of 324 × 206 pecking locations; Elographics AccuTouch Model E274-SFC; Oak Ridge, TN) that detected pecks directed to the monitor screen. A .02 mm sheet of acetate placed in front of the touchscreen in the viewing window protected it from scratching caused by the pigeons’ beaks.
All experimental events were controlled and recorded by computer. Stimulus generation and event programming were executed using QuickBasic (Microsoft, Redmond, WA) and an attached graphics library (GX Graphics, Houston, TX). Computer-controlled relays (Metrabyte, Taunton, MA) governed the operation of the hoppers and houselight. A color video card controlled the monitor in the SVGA graphics mode (800 × 600 pixels).
Stimuli
Sixteen multidimensional stimuli were used and were pairwise combinations of 4 colors (green SVGA #2, cyan #3, red #4, yellow #7) and 4 shapes (U, T, square, and triangle). An additional shape, O, was also used during acquisition. All stimuli were presented on a black background. Large and small stimulus sizes were used during the initial training of the compounds. The large stimuli were 3 cm × 3 cm. The small stimuli were 1.8 cm × 1.8 cm. The large stimuli were eventually dropped from testing (Dr J & Larry, session 71; Chief, 35; Reggie, 29), because no size difference was found for any pigeon (mean choice accuracy large: 80.5%; small: 81%).
Procedure
Initial training
Pigeons were first trained to eat from both hoppers, and then auto and handshaped (if necessary) to peck a ready signal presented in the middle of the viewing window. Food reinforcement occurred upon pigeon head entry into one of the two randomly lit hoppers. Upon the pigeon’s head entry, the food hopper was raised for 2-s. Autoshaping began with a white 2.5-cm circular ready signal. The ready signal was permanently changed a few sessions into the autoshaping procedure to a larger 13 × 6 cm (width × height) white ready signal with a diagonal hatched texture. The ready signal was located 9.5 cm from the bottom and 7.5 cm from both sides of the viewing window. This change was implemented to allow the ready signal to cover the entire response area used for stimulus presentations. Once responding to the ready signal was established, a target or non-target compound stimulus was immediately presented after the ready signal for 5 s or until pecked once. Compound stimuli could appear in one of three positions in a 1 × 3 array of locations: left, center, or right. Each position was located 12.5 cm above the bottom of the viewing window. The left position was 9 cm from the left side and 19 cm from right side of the viewing window. The center position was 14 cm from both sides of the viewing window. The right position was 19 cm from the left side and 9 cm from the right side of the viewing window. All stimuli were centered in the three positions. Following stimulus presentation only the correct choice hopper was illuminated. For two pigeons, the left hopper was the correct choice on target trials and the right hopper was the correct choice on non-target trials. This assignment was reversed for the other two pigeons. Each pigeon had a unique target stimulus to identify: Chief = cyan T, Dr J = green triangle, Larry = red U, Reggie = yellow square. Target stimuli were selected with the constraint that each of the colors and shapes be used once. A 30-s intertrial interval (ITI) followed reinforcement. These 120-trial sessions, occurring over the course of several days, consisted of 60 target (30 large / 30 small) and 60 non-target trials randomly intermixed. Each of the fifteen non-target stimuli appeared 4 times (2 large / 2 small). Target and non-target trials appeared equally often at each of the three stimulus locations in the array.
Discrimination training
Discrimination training began when both the correct and incorrect hoppers were simultaneously lit following stimulus presentation, thus requiring a discriminative choice to be made between the two hoppers. Each trial began with a peck to the ready signal, followed by a peck to a compound stimulus. Both hopper lights were then illuminated and the stimulus was displayed until a choice occurred. If the correct hopper was selected, a pigeon was allowed 1.6-1.8-s (depending on a pigeon’s weight) access to mixed grain. If the incorrect hopper was chosen, the pigeon experienced a 15-s timeout. A 30-s ITI followed either consequence. Within a few sessions, a variable ratio (VR) schedule to the compound stimulus was implemented, requiring 1-5 pecks to be directed at the stimulus before the test hoppers were illuminated. Sessions consisted of 120 randomly intermixed trials: 60 target (30 large / 30 small) and 60 non-target trials. Each non-target compound stimulus was tested 4 times (2 large / 2 small). Target and non-target trials were tested equally often at each of the three stimulus locations in the array.
To demonstrate the pigeons were discriminating both dimensions of color and shape, two criteria had to be met for three consecutive sessions. Percentage of target hopper choice was used to judge these criteria. The first criterion required that the pigeons be accurately discriminating the target compound. Therefore, the hit rate on target trials had to be ≥ 90%. The second criterion required that the pigeons be accurately discriminating both dimensions of the compounds. Thus, any stimulus that shared a feature (e.g., red T, cyan U) in common with the target (e.g., red U) had to be discriminated with a false alarm rate of ≤ 33%. Together, these criteria assured that choice responding was both significantly different from chance (50%) and that the pigeons were discriminating both dimensions.
During discrimination training several adjustments were made to increase the pigeons’ choice accuracy to meet these criteria. These adjustments were instigated mainly to counter poor discrimination along one of the two dimensions. The adjustments consisted of intermittent use of a correction procedure, feature-discrimination training, and replacing one of the shapes (O for the square). For correction procedure sessions each trial in a session was repeated until a correct choice occurred. Feature-discrimination training consisted of sessions in which non-target stimuli consisted only of compounds that shared a target color or shape. For example, if Dr J was mainly discriminating the compounds by the color dimension, he experienced sessions that consisted of only green triangles, green Us, green Ts, and green squares. Hence, such feature-discrimination training required a pigeon to discriminate the dimension producing less stimulus control to succeed at the task. Finally, because Dr J and Reggie were unable to discriminate between the square and triangle shape features, the square was replaced with the O shape. With these adjustments, the pigeons eventually learned the task as configured and met our criteria.
Results and Discussion
All pigeons learned to discriminate between the target and the non-target compounds. Figure 1 displays the percentage of target hopper choice responses for each pigeon across five-session blocks. On target trials (filled circles) a perfect score is 100% (hit rate). For non-target trials (open symbols) a perfect score is 0% (false alarm rate). The 16 training stimuli were divided into four stimulus groupings: target, target-color, target-shape, and different-target features. Target trials consisted of trials containing the target stimulus, for instance, red Us if that was the pigeon’s target (red U is continued throughout this article as the target example). Target-color trials consisted of non-target trials in which the target’s color but not the target’s shape was presented (e.g., red Ts, squares, and triangles). Target-shape trials consisted of non-target trials in which the target’s shape but not the target’s color was presented (e.g., yellow, green, and cyan Us). Different-target features trials consisted of non-target trials in which the target’s color and shape were not presented (e.g., cyan triangles, cyan Ts, cyan square, green triangles, green Ts, green squares, yellow triangles, yellow Ts, and yellow squares). The correction procedure and feature-discrimination training sessions have been omitted from the plots. The similarity of the non-target stimuli to the target stimuli influenced choice responding, as both target-shape and target-color trials had significantly higher false alarms than different-target features trials. Thus, if a non-target stimulus differed in both dimensions from the target stimulus the pigeons were more likely to correctly select the non-target hopper than if a non-target stimulus differed in only one dimension from the target.
Figure 1.
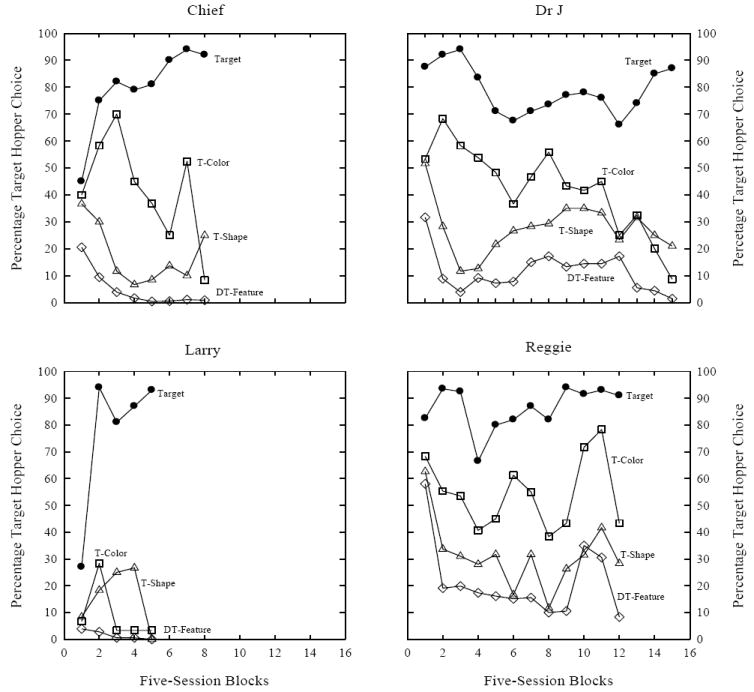
Mean percentage target hopper choice for each pigeon across 120-trial five-session blocks from Experiment 1. The 16 training stimuli were dived into four stimulus groupings: target, target-color (T-Color), target-shape (T-Shape), and different-target features (DT-Feature).
The results of Figure 1 also show that over training three pigeons (Chief, Dr J, & Reggie) responded to target-color trials as more similar to the target than target-shape trials, as indicated by the higher percentage of false alarms on target-color trials (46.3%) than on target-shape trials (25.6%). A paired t-test confirmed this comparison using the data for these three pigeons, t(2) = 7.0, p < .05. This result suggests that for these three pigeons the color of the target object controlled responding more than its shape. For the remaining pigeon (Larry), the color and shape of the target object equally controlled responding.
By the end of training each pigeon met or exceeded the criteria set for the three trial types. Recall that the criterion measure for target trials is a hit rate ≥ 90% and for target-color and target-shape trials a false alarm rate ≤ 33%. The pigeon’s hit rate on target trials (92.5%) was well above chance (50%, one-sample t-test, t(3) = 53.2, p < .01), and their false alarms on non-target trials (target-color: 11.8%, target-shape: 18.8, different-target features: 2.1%) were well below chance, ts(3) > 5, p < .02. Additionally, individual d’ values ranged from 1.89 to 5.75 indicating excellent discrimination.
The results of Experiment 1 show that each pigeon succeeded in discriminating the compound stimuli using information from both the color and shape dimensions. The similarity of the non-target stimuli to the target stimulus influenced choice responding results in systematic patterns of errors. The evidence for this was more false alarms occurred when the non-target shared either its color or shape with the target than if it did not. Over most of the experiment, the majority of the pigeons seemed to rely more on color information to discriminate the target compound from the non-target compounds than shape. Because the relative discriminability of the color and shape features was a priori impossible to control, it is perhaps not surprising that stimulus control was greater for one dimension. With adjustments and training, however, by the end of Experiment 1 the pigeons clearly solved the current conditional compound stimulus discrimination by discriminating and using both the dimensions of color and shape. This positioned us in Experiment 2 to begin to explore the possibility of binding errors in the processing of these compounds.
Experiment 2
In Experiment 2, the pigeons were trained to detect their target compound stimulus using both simultaneous and sequential presentations where two compound stimuli were presented on each trial. We explored the nature of binding errors by pairing certain non-target stimuli with other non-target stimuli or with target stimuli. In this way, we determined the components of the stimulus compound that controlled responding. In this altered procedure for the pigeons, if the target appeared as one of the two compound stimuli (items) during a trial, then the target hopper was the correct choice. This was considered a target trial. If both items were non-targets, then the non-target hopper was the correct choice. This was considered a non-target trial. Target and non-target trials were equally likely in a session. In order to determine the presence of binding errors, five trial types (see Figure 2) were used: target, target-color, target-shape, target-color-shape, and different-target-color-shape. As above, target trials consisted of the target item and a different-target-features item; Target-color trials consisted of a shared target-color item and a different-target-features item; Target-shape trials consisted of a shared target-shape item and a different-target-features item; Target-color-shape trials consisted of a shared target-color item and a shared target-shape item; and different-target-color-shape trials consisted of two different-target-features item.
Figure 2.
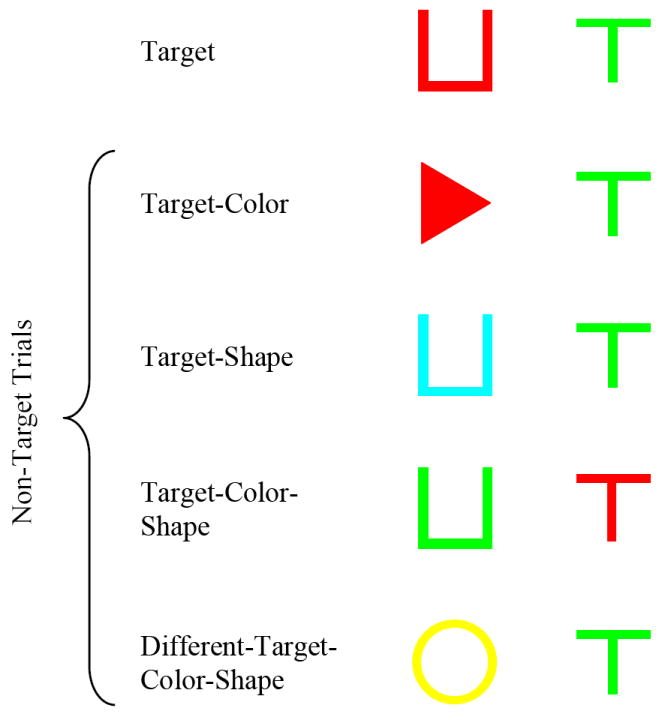
The five trial types used in the three two-item conditions of Experiment 2 and the target item for these examples is Red U. Binding errors were determine by comparing false alarms on target-color-shape trials with those on target-color and target-shape trials.
The five trial types yield specific information in determining binding errors. Hit rate on target trials provided a measure of how accurate the pigeons are at discriminating the target compound stimulus in the presence of a different-target-feature item. Much as in Experiment 1, false alarms on target-color trials measured the pigeons’ errors to the target’s color but not shape. False alarms on target-shape trials measured the pigeons’ errors to the target’s shape but not color. New to Experiment 2 were the target-color-shape trials. False alarms on these target-color-shape trials measured the pigeons’ errors to the target’s color and shape (perhaps incorrectly combined). We predicted that more false alarms should occur on target-color-shape trials relative to either target-color or target-shape trials. There were two reasons for this prediction. First there are two features of the target present on target-color-shape trials instead of only one feature on the target-color or target-shape trials. Beyond this factor, however, was the secondary possibility of binding errors do to the incorrect combination of these two features. If binding errors occurred, according to Treisman & Schmidt (1982), then the false alarm rate to these target-color-shape trials should be greater than the additive and independent effect of target-color and target-shape trials combined.
The effect of space and time on the binding of visual information was also explored in Experiment 2. To measure performance for spatial and temporal factors, the pigeons learned to discriminate a target compound paired with a non-target compound within sequential and simultaneous presentations of the two compounds. To determine whether binding errors were more likely to occur under manipulations of time or location, a comparison between the binding errors on simultaneous and sequential trials can be made. To make this comparison there were three two-item conditions: Sequential same location, sequential alternate locations, and simultaneous. The sequential same location condition consisted of two items presented in the same location one right after the other. The sequential alternate locations condition consisted of two items presented in different locations one right after the other. The simultaneous condition consisted of two items presented at the same time in two different locations. These conditions were analogous to those used to explore spatiotemporal factors with human subjects (Keele et al., 1988; McLean et al., 1982). Therefore, a comparative analysis between humans and pigeons could potentially be done. Unique to our experiments was that we could assess binding errors at different points in time across training. Hence, we could test whether binding errors in visual working memory might change over time as the task was learned.
Several questions were of main interest during this two-item testing. First, could the pigeons learn to discriminate two different compounds during the same trial under simultaneous and sequential conditions? Second, would binding errors occur under these manipulations of spatiotemporal factors? Third, would binding errors be different for time versus space (i.e., sequential versus simultaneous)? Fourth, would the number of binding errors change over two-item training? This last issue has received little attention (Colzato, Raffone, & Hommel, 2006). Colzato et al. suggested that if binding and learning are strongly dependent on each other then binding errors would decrease over time. For instance, in the present experiment how the pigeons represent two-item trials in long term memory may enter visual working memory and influence feature binding in visual working memory. If binding and learning are weakly dependent on each other, then binding errors should not change over time.
Method
Animals and apparatus
The pigeons and apparatus were the same as in Experiment 1.
Two-item training
Three conditions were implemented to assess the role of spatial and temporal factors in the perception of two compound items in a trial. The sequential alternate locations (SAL) and sequential same location (SSL) conditions were two-item sequences (or lists). The first and second items of a sequence were presented in the first and second positions of the sequence respectively. A 0-s interstimulus interval separated the two items. In the SAL condition the two items were presented in two different locations. In the SSL condition the two items were presented in the same location. In the simultaneous (SIM) condition the two items were presented at the same time in different locations. Using the three locations from Experiment 1, the SIM and SAL conditions were arranged so that two contiguous locations were used on each trial. The spatial distance between the edges of the two items in these trials was 2.3 cm. An equal number of pecks (VR 2-5) were required to each item on a sequential trial. For instance, if three pecks (randomly determined) were required then on a sequential trial three pecks would be emitted to the first item and then three to the second item. For SIM trials, pecks were distributed in any manner to the two simultaneously presented stimuli until the VR was completed to each separate item.
There were five trial types used in order to determine the presence or absence of binding errors (see Figure 2). For target trials, a pigeon’s unique target item (e.g., red U) was presented with a different-target-features item (e.g., green T). For target-color trials, a target-color item (e.g., red T; bolded item features indicate a shared value with the target) was presented with a different-target-features item (e.g., cyan triangle). For target-shape trials, a target-shape item (e.g., cyan U) was presented with a different-target-features item (e.g., yellow O). For target-color-shape trials, a target-color item (e.g., red triangle) was presented with a target-shape item (e.g., green U). For different-target-color-shape trials, a different-target-features item (e.g., cyan T) was presented with a different-target-features item (e.g., green O).
Sessions consisted of 126 randomly intermixed trials (63 target, 63 non-target trials). Each session consisted of 32 trials of the three two-item conditions: SAL, SIM, and SSL. Of the five trial types, the three two-item conditions contained 16 target, 4 target-color, 4 target-shape, 4 target-color-shape, and 4 different-target-color-shape trials. These trials were further divided so that equal frequencies of the target, target-color, and target-shape items occurred in the first and second position during the sequential conditions or left and right during the simultaneous condition. Non-target items were randomly selected with the restriction that they were only used during the appropriate trial type. For instance, if red U was the target then red T, square, and triangle occurred equally often on target-color trials. There were also 30 one-item trials, identical to those tested in Experiment 1. One-item trials consisted of 15 target trials and 15 non-target trials with each of the 15 non-target items occurring once. All conditions and trial types were randomly presented throughout a session. All other aspects of the experiment were the same as in Experiment 1, except the stimuli were immediately turned off (0-s delay) following hopper illumination. The total number of sessions conducted for each pigeon was 70 for Chief and Larry, 34 for Dr J, and 28 for Reggie.
Results
Two-item discrimination
All pigeons learned to discriminate two-item trials both sequentially and simultaneously. Figure 3 depicts the first thirty days of acquisition for the three two-item conditions (filled symbols) and the one-item condition (open circles) across five-session blocks. These four conditions were further divided into target (solid lines) and non-target (dashed lines) trials. Pigeons learned to discriminate target from non-target two-item trials by detecting their target item, as performance improved on target, but not non-target two-item trials. The following analyses confirmed this interpretation.
Figure 3.
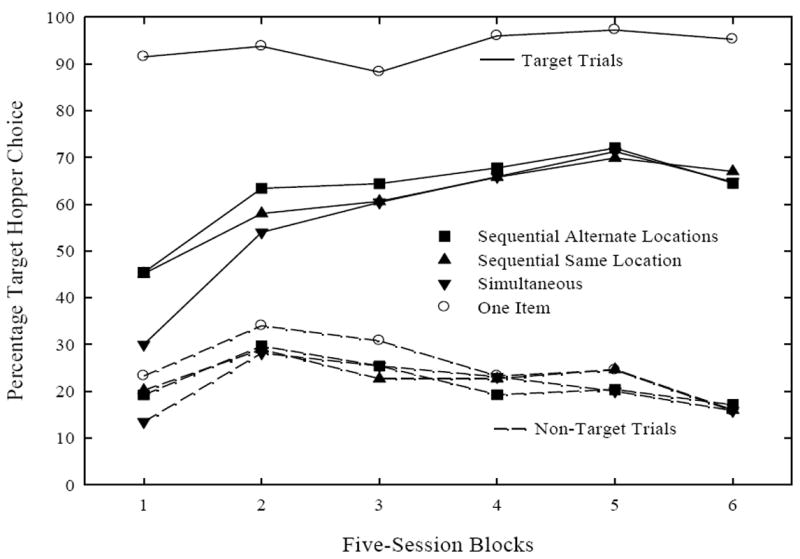
Mean percentage target hopper choice for the three two-item conditions (filled symbols) and the one-item condition (open circles) divided into target (solid lines) and non-target trials (dashed lines).
A three-way repeated measures analysis of variance (ANOVA) of Trial Type (target, non-target) × Two-Item Condition (SAL, SIM, SSL) × Five-Session Blocks (1, 2, 3, 4, 5, 6) was conducted on percentage target hopper choice. Significant Trial Type × Five-Session Blocks interaction, F(5, 15) = 14.49, p < .00005, Trial Type main effect, F(1, 3) = 29.4, p < .02, and Five-Session Blocks main effect, F(5, 15) = 3.06, p < .05, were found. These effects were primarily due to the improved performance on target trials and were further confirmed by the following analyses. For target trials, a two-way repeated measures ANOVA of Two-Item Condition (SAL, SIM, SSL) × Five-Session Blocks conducted on percentage target hopper choice found a significant interaction, F(10, 30) = 2.84, p < .05. This interaction was due to poorer performance on SIM trials during the first block in relation to SSL and SAL, but by the end of acquisition there was no difference between simultaneous and sequential trials. By the last block there were no differences between the two-item trial types. Additionally, separate one-way repeated measures ANOVA for each two-item condition yielded main effects, Fs(5, 15) > 3.8 p < .03, which indicated that pigeons learned to identify their target stimulus in each of the two-item conditions. For non-target two-item trials, an identical two-way repeated measures ANOVA found that both factors and the interaction did not approach significance. Therefore, there was no difference between the two-item non-target conditions, and overall discrimination remained stable over training.
To address whether any changes occurred over training on two-item non-target trials for target-color, target-shape, target-color-shape, or different-target-color-shape trial types separate one-way repeated measures ANOVAs of Five-Session Blocks were conducted. These examined each of the four two-item non-target trial types averaged over simultaneous and sequential presentations. Discrimination on non-target trials remained stable, except on target-color-shape trials, which significantly improved over training, as there was a 13.3% decrease in false alarms from block 1 to 6, F(5, 15) = 4.19, p < .05, (28.9%, 38.8%, 30.6%, 27.2%, 22.3%, 15.6%; respectively for Five-Session Blocks 1-6).
Recency effect
Prior procedures using sequential presentation of stimuli have typically found a pronounced recency effect for the last item displayed in a sequence (e.g., Alsop & Honig, 1991; MacDonald, 1993; Roberts & Grant, 1976; Santiago & Wright, 1984). In the present experiment, a similar recency effect was expected for the sequential presentation conditions. If so, it is critical to establish that the pigeons were also discriminating the compound stimuli in the first position of sequentially presented stimuli. If they were not coding and using information from the first position, then binding errors could not occur in the sequential conditions.
A recency effect would be represented by a higher accuracy on target trials when the target stimulus appeared in the second position as opposed to the first position in the sequence. Figure 4 shows percentage target hopper choice for sequentially presented target trials across two-item training by first and second position. For comparison, target trials on one-item and simultaneous trials are also shown. The ordinal position of the target clearly influenced choice responding. A two-way repeated measures ANOVA of Position (first, second) × Five-Session Blocks on percentage target hopper choice resulted in a significant main effect of Position, F(1, 3) = 565.11, p < .0005, indicating a recency effect (first position: 41.1%, second position 82.9%). Performance improved across training, as supported by a Five-Session Blocks main effect, F(5, 15) = 4.72, p < .01, but the recency effect remained constant, as there was no interaction between the factors.
Figure 4.
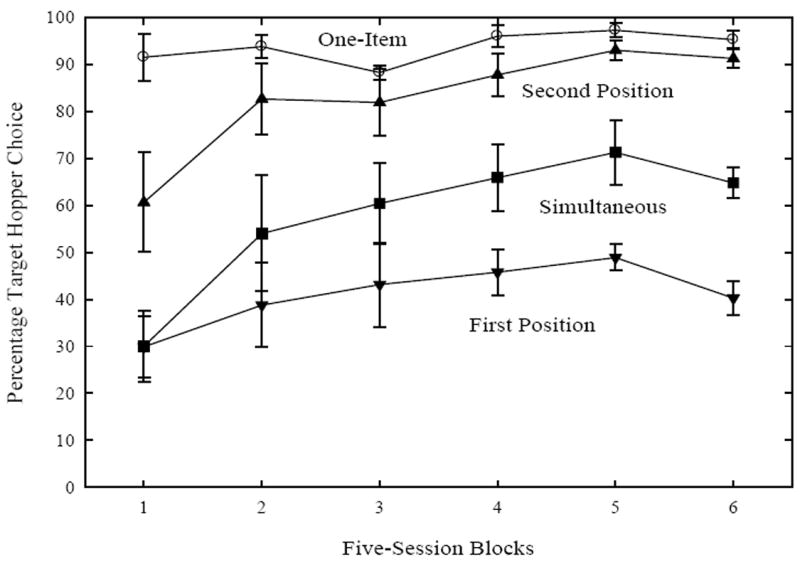
Mean percentage target hopper choice for target trials only, divided into target sequential trials by first and second position, simultaneous, and one-item trials. Error bars are standard errors of the means.
The strongest evidence that the pigeons were using first position information comes from comparing performance on the first position target trials to different-target-color-shape trials for two-item sequential conditions. Because both of these trial types have a different-target-features item in the second position, if accuracy for first position target trials is above the false-alarm rate for different-target-color-shape trials, discrimination of the first item in the sequence must have occurred. A two-way repeated measures ANOVA of Trial Type (first position target, different-target-color shape) × Condition (SAL, SSL) on the last five-session block for percentage target hopper choice resulted in only a significant main effect of Trial Type, F(1, 3) = 18.2, p < .03. Overall, the pigeons were discriminating the item in the first position. However, individual d’ values indicated that three pigeons were discriminating the first position quite well (range 1.07 to 5.12) , although Reggie was not very good (SAL: .11, SSL: .39)
Simultaneity vs. sequentiality
We were also concerned that simultaneous presentations may have been treated as sequential discriminations by the pigeons. Such similarity could have occurred because the pigeons were required to peck at each item before a hopper choice happened. That is, on simultaneous trials a pigeon had to peck at one item and then another, potentially making the trial a sequential presentation. If so, then a recency effect might occur for the last item pecked on simultaneous trials. However, because of the peck contingency on simultaneous trials, the pigeons did not always peck at each item the same number of times. This unequal peck distribution on simultaneous trials could have influenced memory for the item and, hence, choice responding on target trials (cf. MacDonald, 1993). The following proportional analysis of pecks to the target showed this outcome not to be true.
The simultaneous target trials for the last ten sessions were broken down into nine bins based on the percentage of pecks to the target item: >85%, 75-85%, 65-75%, 55-65%, 45-55%, 35-45%, 25-35%, 15-25%, and <15%. The means for each of these bins for percentage target hopper choice were 63.6%, 76.6%, 73.6%, 69.9%, 77.7%, 73%, 71.6%, 74.4%, and 85.7% respectively. All paired t-tests between each of these bins failed to reach significance. Thus, the percentage of pecks to the target item in relation to the non-target item was irrelevant in determining a choice response.
To best compare simultaneous to sequential trials the 45-55% bin was used. This bin was selected because it represents those simultaneous trials in which, on the average, equal pecks occurred to both items, which always occurred on sequential trials. These data was further divided by whether the target was pecked last. There was no statistically-significant difference between trials in which the target was pecked last (77.9%) or not (76.1%). Hence, no recency effect was found, suggesting that simultaneous trials may not have been analyzed sequentially.
Returning to Figure 4, further evidence for the difference between simultaneous and sequential target trials is shown over the five-session blocks of two-item training. In this figure, all data from the simultaneous target trials are used and recall that the sequential trials are broken down into first or second position as determined by the target’s placement. Simultaneous trials (57.7%) are discriminated poorer than second position sequential trials (82.9%). A two-way repeated measures ANOVA of Trial Type (simultaneous, second position) × Five-Session Blocks confirmed this difference, F(1, 3) = 29.21, p < .05. A Five-Session Blocks effect was also found to be significant F(5, 15) = 7.92, p < .001, and the interaction was not. Thus, the pigeons improved with these two discriminations at the same rate. A similar ANOVA comparing simultaneous to first position sequential trials (41.1%) also yielded a marginally significant main effect of Trial Type, F(1, 3) = 9.7, p = .053, Five-Session Blocks, F(5, 15) = 4.27, p < .05, and an interaction F(5, 15) = 3.95, p < .05. Hence, simultaneous target trials were discriminated better than first position sequential trials and performance improved faster with the simultaneous condition.
Binding errors
Binding error analyses were conducted on the first and the last five sessions of two-item training. This separation was done because the prior analyses indicated that performance on target-color-shape trials improved over training. Table 1 shows each pigeon’s false alarm rate averaged over the first five sessions of two-item discrimination training for the target-color-shape, target-color, and target-shape trial types for each of the two-item conditions. Binding errors were calculated based on the formula from Treisman and Schmidt (1982). If binding errors occurred, according to Treisman and Schmidt (1982), then the false alarm rate (i.e., target hopper choices) should be greater during target-color-shape trials than the additive effect of target-color and target-shape trials. Their reasoning was that false alarms on target-color and target-shape trials would give independent measures of correctly identifying one of the target features. Target-color-shape trials would also be a measure of correctly identifying target features, except the number of features in common with the target is doubled. Therefore, to show evidence of binding errors, false alarms on target-color-shape trials should exceed the additive effect of the target-color and target-shape trials. Binding errors derived from false alarms were calculated using the following formula:
| (1) |
Table 1.
Percentage of Binding Errors from the First Five Sessions in Experiment 2 as Derived by Subtracting the Sum of Target-Color and Target-Shape Trials from Target-Color-Shape Trials
| Two-Item Condition | Target Color-Shape | Target-Color | Target-Shape | Binding Errors |
|---|---|---|---|---|
| Seq. Alt. Loc. | ||||
| Chief | 40 | 10 | 25 | 5 |
| Dr J | 10 | 10 | 5 | -5 |
| Larry | 28 | 5 | 15 | 8 |
| Reggie | 45 | 45 | 15 | -15 |
| Seq. Same Loc. | ||||
| Chief | 25 | 20 | 15 | -10 |
| Dr J | 25 | 10 | 5 | 10 |
| Larry | 35 | 5 | 0 | 30 |
| Reggie | 45 | 35 | 45 | -35 |
| Simultaneous | ||||
| Chief | 35 | 25 | 25 | -15 |
| Dr J | 5 | 0 | 0 | 5 |
| Larry | 10 | 0 | 10 | 0 |
| Reggie | 20 | 25 | 15 | -15 |
There was some evidence of binding errors for the SAL (Chief and Larry), SSL (Dr J and Larry), and SIM (Dr J) conditions. For Dr J and Larry, it appears location was more important than time for feature binding, as binding errors were elevated on SSL in comparison with SIM trials. However, the variation in the data across the animals and conditions makes the evidence for the existence of binding errors less compelling than one would desire. If such errors did exist at the beginning of training, they had disappeared by the end of training. Table 2 shows each pigeon’s false alarm rate averaged over the last five sessions of two-item discrimination training for the target-color-shape, target-color, and target-shape trial types for each of the two-item conditions. There was no evidence of binding errors for any pigeon in any condition. A two-way repeated measures ANOVA of Trial Type (SAL, SSL, SIM) × Block (1, 2) on binding errors showed some support for this decrease as the main effect of block approached significance, F(1, 3) = 8.5, p = .06. Isolating this data to the individual pigeons that showed positive binding errors in the first 5 sessions (M = 11.6) to their performance on the last five sessions (M = -22) did show a significant decrease in binding errors, paired t-test t(4) = 6.1, p < .005. In summary, for those pigeons in which binding errors occurred the number of binding errors decreased over two-item training.
Table 2.
Percentage of Binding Errors from the Last Five Sessions in Experiment 2 as Derived by Subtracting the Sum of Target-Color and Target-Shape Trials from Target-Color-Shape Trials
| Two-Item Condition | Target Color-Shape | Target-Color | Target-Shape | Binding Errors |
|---|---|---|---|---|
| Seq. Alt. Loc. | ||||
| Chief | 5 | 15 | 20 | -30 |
| Dr J | 10 | 10 | 10 | -10 |
| Larry | 0 | 0 | 5 | -5 |
| Reggie | 30 | 21.6 | 16.6 | -8.2 |
| Seq. Same Loc. | ||||
| Chief | 10 | 10 | 5 | -5 |
| Dr J | 10 | 10 | 30 | -30 |
| Larry | 0 | 0 | 5 | -5 |
| Reggie | 26.6 | 26.6 | 26.6 | -26.6 |
| Simultaneous | ||||
| Chief | 0 | 50 | 10 | -60 |
| Dr J | 0 | 30 | 10 | -40 |
| Larry | 20 | 15 | 15 | -10 |
| Reggie | 26.6 | 15 | 43.4 | -31.8 |
Discussion
Discriminative performance was investigated on compound stimuli presented in two-item simultaneous and sequential conditions. Comparisons between specific trial types allowed for the assessment of whether or not binding errors influenced choice responding. Two main findings were established: 1) all pigeons were able to learn to discriminate target from non-target trials whether they occurred under conditions of simultaneity or sequentiality, and 2) some limited evidence of binding errors was initially found at the beginning of training, but this evidence disappeared with training.
How did the pigeons learn to discriminate target trials from non-target trials for two-item presentations? Of note, all pigeons continued to perform their one-item discrimination throughout two-item training indicating that the established stimulus control from Experiment 1 was maintained. Such stimulus control likely allowed the pigeons to readily transfer to two-item non-target trials, as overall performance on non-target trials remained constant over training. The pigeons initially had difficulty responding “target” on target two-item trials because of the interfering stimulus control of the non-target item on such trials. The pigeons did gradually improve on two-item target trials indicating that the development of processes that reduced this interference (e.g., longer visual search, greater impulse control, better suppression of non-target responses). Nevertheless, target two-item performance never reached the level of target one-item performance, even when the target was in the second serial position indicating that this interference was never completely eliminated. There was also a potential processing difference among sequential and simultaneous presentation trials. The difference between these trials may have possibly occurred because the two items on simultaneous trials may have become “unified” over training. That is, the pigeons began to represent the two items as a single configuration instead of two separate items. If so, the pigeons would have to learn the novel two-item pairings whereas, for sequential trials, the two items continued to be represented as separate items. To learn the two-item target discriminations the pigeons would have to inhibit their choice response to a non-target item on such trials. The recency effect found only on sequential trials is evidence consistent with the items being represented separately. The effect is not uncommon as many researchers have found strong recency effects with sequential stimulus presentation (e.g., Alsop & Honig, 1991; MacDonald, 1993; Roberts & Grant, 1976; Weisman, Wasserman, Dodd, & Larew, 1980; Wright, Santiago, Sands, Kendrick & Cook, 1985). Because of the changes in responding based on the serial position of the target item, recency effects (and primacy effects) are considered evidence that sequential presentation of stimuli does not produce unification of the individual items (Wright, 1998). In the current experiment such recency effects could be explained by interference processes within visual working memory. That is, processing of the first position item can be retroactively interfered with by the second position item which has a strong influence when a short delay (< 1 s) occurs between the presentation of the second item and choice (as in Experiment 2). Regardless of the underlying process, it was clearly difficult for the pigeons to learn to respond to the weaker available target in the first position than to the more strongly available non-target in the second position. It is interesting to note that performance on target simultaneous trials was in between performance on the first and second position sequential trials (see Figure 4). For the simultaneous target trials each item has an equal strength (and interfering influence) in visual working memory. Hence, from the separate item perspective the final ordering of the data in Figure 4 makes perfect sense. Of course, this does not rule out unification of items on simultaneous trials but it does seem less likely.
In regard to binding errors, we expected, regardless of whether or not the Treisman and Schmidt (1982) binding error criterion was satisfied, that more false alarms should have occurred on target-color-shape trials relative to either target-color or target-shape trials. The hypothesis was based on there being two features of the target present on target-color-shape trials instead of only one feature on the target-color or target-shape trials. That is, the more features shared with the target (i.e., an increase in similarity) on non-target trials, the more likely a false alarm will occur. The results from Experiment 1 supported this hypothesis because more false alarms occurred on target-color and target-shape trials than different-target-feature trials. Performance at the start of two-item training during the first five-session block of acquisition also supported this expectation too, as false alarms were significantly higher on target-color-shape trials in comparison to the mean of target-color and target-shape two-item trials, as confirmed by planned comparisons, F (1, 3) = 19.2 p = .022. This finding was strong for three of the pigeons (Chief, Dr J, & Larry; see Table 1). This suggests at the very least that two shared features of targets were being recognized by the pigeons across the different items and contributing to responding.
Regarding the more intriguing question of whether binding errors occurred, the answer is mixed. We did find some evidence that perhaps two of the birds exhibiting greater than expected errors on the target-color-shape trials as determined by the Treisman and Schmidt criterion at the very beginning of testing and on sequential trials. There was very little if any evidence with simultaneously presented items. Responding that a target was present for the simultaneous condition was initially difficult for the pigeons and may have mitigated the possibility of binding errors for the simultaneous condition because they were no longer under stimulus control by the target features in this condition. These selective hints of binding errors are mitigated however by the fact that when combined across all birds, there was no evidence for binding errors above that expected for the conjoint presentation of two shared target features.
What is clear, however, is that the processing of the target-color-shape trial type capable of generating such binding errors changed across training. Acquiring the two-item discrimination resulted in the disappearance of any evidence of binding errors at the end of training (Table 2). During the last five-session block, false alarm rates were equivalent on target-color-shape trials in comparison to target-color and target-shape two-item trials. These findings indicate that the pigeons learned something about this type of stimulus presentation over two-item training that altered the recognition of these shared target features when they occurred in different items.
General Discussion
In these experiments, a novel experimental procedure for pigeons involving sequential and simultaneous target detection was developed to assess whether binding errors occurred in the visual processing of the presentation of multiple compound stimuli by pigeons. In Experiment 1, pigeons learned a conditional discrimination that required one choice response in the presence of a target compound and a different choice response in the presence of a non-target compound. This conditional discrimination required the processing of both color and shape information. The shared dimensionality of the non-target stimuli in relation to the target stimulus clearly influenced choice responding indicating that both dimensions did come to control choice. In Experiment 2, pigeons learned a two-item discrimination under simultaneous and sequential presentation conditions. A recency effect was found for the sequential conditions indicating interference in visual working memory. Such interfering stimulus control is the ideal situation to produce binding errors. Two-item pairings were arranged to allow for the examination of binding errors in time (simultaneous) and location (sequential). At the start of two-item training transient evidence for binding errors in some birds was observed. By the end of training, however, the effect disappeared because the processing of shared target features in different items had changed.
What exactly did the pigeons learn during two-item training that may have affected binding? We offer two possibilities. First, if the pigeons had learned to configure target-color-shape item pairings over two-item training this would have affected feature binding in visual working memory. Such configural learning of item combinations may have acted as conjunction detectors (Colzato et al., 2006). These conjunctions detectors can facilitate processing of the appropriate feature binding by fine-tuning attention to the appropriate features in different visual dimensions. Such tuning can reduce the occurrence of binding errors.
Second, if the pigeons learned position information pertaining to a target-color and target-shape item in the first position of a sequence, then any possibility of binding errors would be reduced. Pigeons could have learned such position information. This strategy was possible because when presented with a target-color or target-shape item in the first position, selecting the non-target hopper always resulted in food reinforcement because the target-color and target-shape items never occurred with the target item on target trials. Thus, the pigeons could have reached a decision based on the first item they encountered and essentially ignored the second item. However, if our pigeons did reach a decision after processing the first item in a sequence then one would expect a primacy and not recency effect on target trials which did not occur. Nevertheless, two experiments have shown that pigeons can respond to a sequence’s identity before its completion, if given the opportunity (Terrace, 1986; Weisman, Gibson, & Rochford, 1984). For example, Terrace trained pigeons in a conditional discrimination to peck a “target” key after presentation of a target sequence (e.g., red - green - blue) and peck a “non-target” key after presentation of a non-target sequence (e.g., green - orange - red). If the non-target sequence differed from the target sequence in the first position, the pigeons would begin pecking the non-target key before the sequence ended. Similarly, Weisman et al. trained pigeons to discriminate between positive (e.g., red - yellow) and negative sequences (e.g., red - red, yellow - yellow, yellow - red) of houselights in an advance-key procedure. Pecking the advance-key allowed pigeons to terminate a sequence and begin a new trial. Pigeons were able to accurately use the advance-key to terminate negative sequences that were recognizable during presentation of the first houselight color (e.g., yellow in the example given). Therefore, when possible, pigeons can reach a decision concerning the sequence’s identity before the end of a trial. Thus, in the present experiment, the pigeons could have detected whether or not a sequence was a target or non-target trial for all items in the first position except for different-target-feature items. To correct this problem the target should be allowed to occur with any non-target item. This solution might produce a difference between target-color-shape, target-color, and target-shape trials. Of note, we later allowed the target to occur with any non-target item. This change had no effect on binding errors at that time. However, prior learning in the task may have blocked the learning of any new strategies.
The decrease in binding errors across training also suggests that binding is not confined completely within visual working memory. At least one component of the process may involve long term memory, ensuring that well-trained, stable object discriminations (e.g., grape juice is purple, despite being stored in a white container) are performed with near perfect accuracies (Treisman & Schmidt, 1982). The binding errors observed in this experiment are perhaps qualitatively different from the errors observed in experiments involving rapid non-contingent stimulus presentation (i.e., memory vs. perceptually based binding errors). Although many experiments on binding have used rapid visual presentation to increase the frequency of errors, there is evidence that binding errors can occur at longer exposure durations and without explicitly diverting attention (Prinzmetal, Henderson, & Ivry, 1995). Prinzmetal et al. (1995) found evidence for binding errors in a colored-letter identification task with view times as long as 1.5 seconds. Thus another possibility to consider is that our pigeons got to observe the stimuli for times much longer than most human studies. These longer viewing times may have helped the pigeons to correctly conjoin the features of each object. Thus, the requirements of our procedure likely made it far more difficult to detect perceptually-related binding errors. A future direction of our research should be to explore the influence of viewing times < 1 second of the stimuli presented in our task.
Another important consideration is “when” the binding errors occurred during the perception of the stimuli. The notion that the binding process is composed of functionally distinct sub-processes is not new (e.g., Treisman, 1996) and provides a way of categorizing results related to the binding problem. Using Treisman’s categories, the current experiments were designed to understand property binding, the combination of information from specialized neural regions; and location binding, the combining of differentially coded “what” and “where” information. The use of extended presentation times and the VR requirement make it unlikely that the errors were occurring at the property binding stage, unless we assume that these errors arise under ample viewing conditions, which is unlikely (Treisman & Schmidt, 1982). It is more likely that the errors were arising as the birds attempted to bind the location and object information. This reasoning is supported by the increased number of binding errors for at least one bird (Larry, also the highest performer in the task) under the sequential condition only. As mentioned above, over time the birds may have learned to deal with these errors by adopting a strategy to consider only a portion of the available stimuli on a given trial. Transfer tests involving non-trained conjunction stimuli in untrained location configurations could more clearly show the prevalence of binding errors in pigeon visual perception.
The accomplishment of the sequential and simultaneous target detection task with compound stimuli was the first of its kind with pigeons. While this target detection task was specifically designed to explore binding processes in a conditional discrimination, it could be used further in the analysis of several other interesting domains in animal learning and cognition such as the exploration of perceiving and remembering, structure and capacity of visual working memory, configural processing of sequences, and the elemental/configural debate. The task can offer new perspectives on these domains because comparisons within and between sequential and simultaneous discriminations can be made with the same group of animals.
Moreover, these experiments begin to ask and study questions in avian perception that have either not been explored or have received little previous attention. Do pigeons combine visual information? If they do, is the binding process fallible? How might spatial and temporal factors influence binding if errors do occur? How does learning influence feature binding? Answering these questions will allow us to better assess whether binding is a general process common among vertebrate species. The present experiments were the first designed to investigate the psychological processes underlying binding errors in any nonhuman visual system. They represent an important step toward developing a framework for the evaluation of feature binding in nonhumans. They add to the growing literature in animal cognition on the mechanisms underlying the pigeon visual system and the comparative psychology of mammalian and non-mammalian visual systems.
Acknowledgments
Preparation of this article was supported by National Institute of Health grant MH61798 and National Science Foundation Grant #0718804. The research was part of a Masters thesis in Psychology submitted to Tufts University by Jeffrey S. Katz. The committee for this thesis, Emily Bushnell, Robert G. Cook, and Daniel C. Dennett provided helpful comments on earlier drafts of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alsop B, Honig WK. Sequential stimuli and relative numerosity discriminations in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:386–395. [Google Scholar]
- Ashby FG, Prinzmetal W, Ivry R, Maddox WT. A formal theory of feature binding in object perception. Psychological Review. 1996;103:165–192. doi: 10.1037/0033-295x.103.1.165. [DOI] [PubMed] [Google Scholar]
- Blough DS. Attention shifts in a maintained discrimination. Science. 1969;166:125–126. doi: 10.1126/science.166.3901.125. [DOI] [PubMed] [Google Scholar]
- Brockmole JR, Franconeri SL. Binding: A special issue of the journal Visual Cognition. New York: Psychology Press; 2009. [Google Scholar]
- Brown MF. Dissociation of stimulus compounds by pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1987;13:80–91. [Google Scholar]
- Brown MF, Cook RG, Lamb MR, Riley DA. The relation between response and attentional shifts in pigeon compound matching-to-sample performance. Animal Learning & Behavior. 1984;12:41–49. [Google Scholar]
- Chatlosh DL, Wasserman EA. Multidimensional stimulus control in pigeons: Selective attention and other issues. In: Zentall TR, editor. Animal cognition: A tribute to Donald A Riley. Hillsdale, NJ: Erlbaum; 1993. pp. 271–292. [Google Scholar]
- Cohen A, Ivry RB. Illusory conjunctions inside and outside the focus of attention. Journal of Experimental Psychology: Human Perception & Performance. 1989;15:650–663. doi: 10.1037//0096-1523.15.4.650. [DOI] [PubMed] [Google Scholar]
- Colzato LS, Raffone A, Hommel B. What do we learn from binding features? Evidence for multilevel feature integration. Journal of Experimental Psychology: Human Perception & Performance. 2006;32:705–716. doi: 10.1037/0096-1523.32.3.705. [DOI] [PubMed] [Google Scholar]
- Cook RG. Dimensional organization and texture discrimination in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1992;21:354–363. doi: 10.1037//0097-7403.23.4.401. [DOI] [PubMed] [Google Scholar]
- Cook RG. The comparative psychology of avian visual cognition. Current Directions in Psychological Science. 2000;9:83–89. [Google Scholar]
- Cook RG. Avian visual cognition. 2000 Retrieved May 1, 2010, from http://www.pigeon.psy.tufts.edu/avc/
- Cook RG, Cavoto KK, Cavoto BR. Same-different texture discrimination and concept learning by pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1995;21:253–260. [Google Scholar]
- Cook RG, Cavoto KK, Cavoto BR. Mechanisms of multidimensional grouping, fusion, and search in avian texture discrimination. Animal Learning & Behavior. 1996;24:150–167. [Google Scholar]
- Cook RG, Cavoto BR, Katz JS, Cavoto KK. Pigeon perception and discrimination of rapidly changing texture stimuli. Journal of Experimental Psychology: Animal Behavior Processes. 1997;23:390–400. doi: 10.1037//0097-7403.23.4.390. [DOI] [PubMed] [Google Scholar]
- Cook RG, Katz JS, Cavoto BR. Pigeon same-different concept learning with multiple stimulus classes. Journal of Experimental Psychology: Animal Behavior Processes. 1997;23:417–433. doi: 10.1037//0097-7403.23.4.417. [DOI] [PubMed] [Google Scholar]
- Cook RG, Riley DA, Brown MF. Spatial and configural factors in compound stimulus processing by pigeons. Animal Learning & Behavior. 1992;20:41–55. [Google Scholar]
- Cook RG, Wixted JT. Same-different discrimination in pigeons: Testing competing models of discrimination and stimulus integration. Journal of Experimental Psychology: Animal Behavior Processes. 1997;23:401–416. doi: 10.1037//0097-7403.23.4.401. [DOI] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: The searchlight hypothesis. Proceedings of the National Academy of Sciences. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelage J, Bischof HJ. The organization of the tectofugal pathway in birds: A comparative review. In: Zeigler HP, Bischof HJ, editors. Vision, brain, and behavior in birds. Cambridge, MA: MIT Press; 1993. pp. 137–158. [Google Scholar]
- Gathercole SE, Broadbent DE. Combining attributes in specified and categorized target search: Further evidence for strategic differences. Memory & Cognition. 1984;12:329–337. doi: 10.3758/bf03198292. [DOI] [PubMed] [Google Scholar]
- George DN, Pearce JM. Discrimination of structure: II. Feature binding. Journal of Experimental Psychology: Animal Behavior Processes. 2003;29:107–117. doi: 10.1037/0097-7403.29.2.107. [DOI] [PubMed] [Google Scholar]
- Heinemann EG, Chase S. Conditional stimulus control. Journal of Experimental Psychology. 1970;84:187–197. doi: 10.1037/h0032083. [DOI] [PubMed] [Google Scholar]
- Husband S, Shimizu T. Evolution of the avian visual system. Cook RG, editor. Avian visual cognition. 2001 Retrieved from http://www.pigeon.psy.tufts.edu/avc/
- Ivry RB, Prinzmetal W. Effect of feature similarity on illusory conjunctions. Perception & Psychophysics. 1991;49:105–116. doi: 10.3758/bf03205032. [DOI] [PubMed] [Google Scholar]
- Keele SW, Cohen A, Ivry RB, Liotti M, Yee P. Tests of a temporal theory of attentional binding. Journal of Experimental Psychology: Human Perception & Performance. 1988;14:444–452. doi: 10.1037//0096-1523.14.3.444. [DOI] [PubMed] [Google Scholar]
- Lamme VAF, Roelfsema PR. The distinct modes of vision offered by feedforward and recurrent processing. Trends in Neuroscience. 2000;23:571–579. doi: 10.1016/s0166-2236(00)01657-x. [DOI] [PubMed] [Google Scholar]
- Li DP, Xiao Q, Wang SR. Feedforward construction of the receptive field and orientation selectivity of visual neurons in the pigeon. Cerebral Cortex. 2007;17:885–893. doi: 10.1093/cercor/bhk043. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Vecera SP. Attention. In: Pashler H, Yantis S, editors. Stevens’ handbook of experimental psychology: vol. 1. Sensation and perception. 3. New York: John Wiley & Sons; 2002. pp. 235–286. [Google Scholar]
- MacDonald SE. Delayed matching-to-successive-samples in pigeons: Short-term memory for item and order information. Animal Learning and Behavior. 1993;21:59–67. [Google Scholar]
- Mackintosh NJ. Overshadowing and stimulus intensity. Animal Learning and Behavior. 1976;4:186–192. doi: 10.3758/bf03214033. [DOI] [PubMed] [Google Scholar]
- Maturana HR, Frenk S. Directional movement and horizontal edge detectors in the pigeon retina. Science. 1963;142:977–979. doi: 10.1126/science.142.3594.977. [DOI] [PubMed] [Google Scholar]
- McLean JP, Broadbent DE, Broadbent MH. Combining attributes in rapid serial visual presentation tasks. Quarterly Journal of Experimental Psychology. 1982;35A:171–186. doi: 10.1080/14640748308402123. [DOI] [PubMed] [Google Scholar]
- Papini MR. Pattern and process in the evolution of learning. Psychological Review. 2002;109:86–201. doi: 10.1037/0033-295x.109.1.186. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditional reflexes. London: Oxford University Press; 1927. [Google Scholar]
- Pearce JM. Similarity and discrimination: A selective review and a connectionist model. Psychological Review. 1994;101:587–607. doi: 10.1037/0033-295x.101.4.587. [DOI] [PubMed] [Google Scholar]
- Prinzmetal W. Principles of feature integration in visual perception. Perception & Psychophysics. 1981;56:551–564. doi: 10.3758/bf03206147. [DOI] [PubMed] [Google Scholar]
- Prinzmetal W. Visual feature integration in a world of objects. Current Directions in Psychological Science. 1995;4:90–94. [Google Scholar]
- Prinzmetal W, Henderson D, Ivry R. Loosening the constraints on illusory conjunctions: Assessing the roles of exposure duration and attention. Journal of Experimental Psychology: Human Perception & Performance. 1995;6:1362–1375. doi: 10.1037//0096-1523.21.6.1362. [DOI] [PubMed] [Google Scholar]
- Riley DA, Brown MF. Representation of multidimensional stimuli in pigeons. In: Lockhead GR, Pomerantz James R, editors. The perception of structure. Washington, DC: American Psychological Association; 1991. [Google Scholar]
- Roberts WA, Grant DS. Studies in short-term memory in the pigeon using the delayed matching-to-sample procedure. In: Medin DL, Roberts WA, Davis RT, editors. Processes of animal memory. Hillsdale, NJ: Erlbaum; 1976. [Google Scholar]
- Robertson LC. Binding, spatial attention and perceptual awareness. Nature Reviews Neuroscience. 2003;4:93–102. doi: 10.1038/nrn1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki J, Miyatsuji H. Feature binding in visual working memory evaluated by type identification paradigm. Cognition. 2007;102:49–83. doi: 10.1016/j.cognition.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Santiago HC, Wright AA. Pigeon memory: Same/different concept learning, serial probe recognition acquisition, and probe delay effects on the serial-position function. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10:498–512. [PubMed] [Google Scholar]
- Terrace HS. Positive transfer from sequence production to sequence discrimination in a nonverbal organism. Journal of Experimental Psychology: Animal Behavior Processes. 1986;12:215–234. [Google Scholar]
- Treisman AM. The binding problem. Current Opinion in Neurobiology. 1996;6:171–178. doi: 10.1016/s0959-4388(96)80070-5. [DOI] [PubMed] [Google Scholar]
- Treisman AM, Schmidt H. Illusory conjunctions in the perception of objects. Cognitive Psychology. 1982;14:107–141. doi: 10.1016/0010-0285(82)90006-8. [DOI] [PubMed] [Google Scholar]
- Weisman RG, Gibson M, Rochford J. Testing models of delayed sequence discrimination in pigeons: The advance key procedure. Canadian Journal of Psychology. 1984;38:256–268. [Google Scholar]
- Weisman RG, Wasserman EA, Dodd PWD, Larew MB. On-line choice and the representation of serially structured stimuli. Journal of Experimental Psychology: Animal Behavior Processes. 1980;6:312–325. doi: 10.1037//0097-7403.17.1.55. [DOI] [PubMed] [Google Scholar]
- Wylie DRW, Guitierrez-Ibanez C, Pakan JMP, Iwaniuk AN. The optic tectum of birds: Mapping our way to understanding visual processing. Canadian Journal of Experimental Psychology. 2009;63:328–338. doi: 10.1037/a0016826. [DOI] [PubMed] [Google Scholar]
- Wright AA. Auditory and visual serial position functions obey different laws. Psychonomic Bulletin and Review. 1998;5:564–584. [Google Scholar]
- Wright AA, Santiago HC, Sands SF, Kendrick DF, Cook RG. Memory processing of serial lists by pigeons, monkeys, and people. Science. 1985;229:287–289. doi: 10.1126/science.9304205. [DOI] [PubMed] [Google Scholar]
- Zeki S. A vision of the brain. Cambridge: Blackwell Scientific Publications; 1993. [Google Scholar]


