Abstract
When environmental or sensory conditions change suddenly, the brain must be capable of learning different behavioral modes to produce accurate movements under multiple circumstances. A form of this dual-state adaptation known as “context-specific adaptation” has been widely investigated using the saccade gain adaptation paradigm in humans. In this study, we asked whether or not context-specific adaptation of saccade gain exists in monkeys and if so to explore its properties. Here, vertical eye position was used as a context cue for adaptation of horizontal saccade gain. We asked for a gain increase in one context and gain decrease in another context, and then determined if a change in the context would invoke switching between the adapted states. After training, our monkeys developed context-specific adaptation: in most cases gain-decrease adaptation could be induced, but there was little or no gain-increase adaptation. This context-specific adaptation developed gradually and switching of gains was evident on the first saccades with each change in context. Along with these results, the retention of an adaptation aftereffect overnight indicates that contextual-specific adaptation in monkeys is not a strategy, but involves a true adaptive process of reorganization in the brain. We suggest that context-specific adaptation in monkeys could be an important tool to provide insights into the mechanisms of saccade adaptation that occurs during the more natural circumstances of daily life.
Keywords: Saccade, Adaptation, Context, Motor learning, Monkey
INTRODUCTION
Motor learning as it is usually studied in the laboratory occurs in a constrained, artificial environment in which the stimulus and desired response are rigidly controlled. Indeed, such experiments allow one to hone in on fundamental, relatively low-level mechanisms that underlie adaptive motor control. On the other hand, the learning that occurs during the more natural circumstances of daily life must be optimized for a complex sensory (and motor) environment in which the particular ‘contextual’ circumstances in which the learning takes place dictate the necessary type of response. In other words, because environmental or sensory conditions can change suddenly, the brain must be capable of learning different behavioral modes to produce accurate movements under multiple circumstances. For example, pointing movements can be adapted in a dual-state manner in the presence and absence of a prism-induced perturbation (Mcgonigle & Flook, 1978; Welch, Bridgeman, Anand, & Browman, 1993). This form of dual-state adaptation is termed “context-specific adaptation”, and can be defined as the ability to learn and store simultaneously two different adapted responses of a motor task, and to invoke the appropriate adapted states with the associate context cue (e.g., Shelhamer & Clendaniel, 2002a; Shelhamer, Aboukhalil, & Clendaniel, 2005). Once learned such contextual cues can be extremely powerful; witness the inappropriate postural response and near fall that occurs when one takes a first step upon a broken escalator (Bronstein, Bunday, & Reynolds, 2009; Reynolds & Bronstein, 2003).
Saccades are an ideal model to study motor adaptation because it is relatively easy both to artificially manipulate the stimuli that drive saccades, and to measure the consequent change in the motor response (Hopp & Fuchs, 2004; Tian, Ethier, Shadmehr, Fujita, & Zee, 2009). Adaptation of saccade gain in both humans and monkeys has been studied extensively with a conventional double-step paradigm (McLaughlin, 1967). Adaptation is usually more rapid and robust for gain decrease than increase and is slower in monkeys than in humans (Deubel, Wolf, & Hauske, 1986; Straube, Fuchs, Usher, & Robinson, 1997). Much evidence indicates that the dorsal vermis (lobules VI–VII), the underlying posterior fastigial nuclei and perhaps the adjacent cerebellar hemispheres are crucial areas for saccadic adaptation (Alahyane et al., 2008; Barash et al., 1999; Catz, Dicke, & Thier, 2008; Choi, Kim, Cho, & Kim, 2008; Optican & Robinson, 1980; Robinson, Fuchs, & Noto, 2002; Soetedjo, Kojima, & Fuchs, 2008; Takagi, Zee, & Tamargo, 1998).
In humans, it has been well established that saccades can be made to have two different gains in response to the same target displacement: which gain can depend on various contextual cues: e.g., target distance (Chaturvedi & Van Gisbergen, 1997), horizontal and vertical eye position in the orbit (Alahyane & Pelisson, 2004; Shelhamer & Clendaniel, 2002a; Shelhamer et al., 2005), head orientation (Shelhamer & Clendaniel, 2002a,b), and the visual properties of the target (Herman, Harwood, & Wallman, 2009). However, little is known about the neural mechanisms responsible for context-dependent adaptation of saccade gain, and especially its anatomical substrate.
While much has been learned about saccade gain adaptation using experimental studies in monkeys, and especially its neurophysiological underpinnings, context-specific saccade adaptation of the type described above has not been studied in monkeys. Monkeys have been shown to develop different gains depending upon saccade amplitude (Watanabe, Noto, & Fuchs, 2000) and the degree of disconjugate saccade adaptation that occurs to wearing a prism in front of one eye can be made to depend upon orbital position (Oohira & Zee, 1992). While such adaptive capabilities can be important, for example, in adaptation to a paresis of a single eye muscle, it is not the ‘higher-level’ type of adaptation that we propose to study here. Furthermore, context-specific adaptation in monkeys could be an important tool to provide insights into how saccade adaptation occurs in the more natural behaviors within a complex sensory environment. Thus, we asked here whether or not context-specific adaptation of saccade gain exists in monkeys and if so to explore some of its properties. In this study we use the conventional saccade gain adaptation paradigm, in which horizontal saccades of equal amplitude are alternately subjected to different adaptive demands (gain-increase and gain-decrease) in two different vertical eye positions (context) during the same adaptation session.
METHODS
General experimental procedures
Experiments were conducted on three male rhesus monkeys (M1, M2 and M3) weighing from 4 to 6 kg. All experimental and surgical procedures, including anesthesia and post-operative analgesia, were performed according to a protocol that was approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University and following the NIH Guidelines. The animals were first trained to come out of their cages and sit comfortably in a primate chair. For head immobilization during experiments, a plastic head plate was attached to the skull with dental acrylic. In single procedures for each eye, dual scleral coils made of stainless steel wire were implanted binocularly in M1 and in the left eye in M2, as has been previously described (Tian, Zee, & Walker, 2007). Dual resonant “leadless” coils, small multiple-turn loop of fine wire connected to a capacitor (Roberts, Shelhamer, & Wong, 2008), were implanted in the right eye in M2 and M3. Animals were trained to fixate and follow a small visual target for a liquid reward.
Both M1 and M2 underwent a left trochlear nerve section and M2 had an ipsilateral trigeminal nerve (V1 and V2) section as well as part of a different study. The lesion produced a vertical misalignment of the eyes: the paretic eye was higher than the normal eye, and this misalignment was greatest in down gaze when the paretic eye was adducted. Experiments reported here were conducted in M1 about 14 months after the superior oblique palsy surgery and in M2 both before and 5 months after the surgery. For the adaptation data collected after the animal had the trochlear nerve section, the normal eye was always the viewing eye (the paretic eye was patched) during the recording session and we show the data from the normal eye. The vertical misalignment was stable in both monkeys (6.7° ± 0.3° in M1, 5.7° ± 0.3° in M2). For M2, there was no difference in the performance of gain adaptation of horizontal saccades before and after trochlear nerve section. Accordingly we are confident that the vertical strabismus produced by the trochlear nerve section did not alter performance on the horizontal saccade adaptation paradigm. Thus all the results in a given adaptation paradigm are pooled.
Eye movement measurements
During each experimental session, the animals were seated in a primate chair with head fixed. Eye movements were measured with the magnetic field search coil method in M1 and in the left eye of M2. The coil frame consisting of three orthogonal magnetic fields was attached to the top of the primate chair, such that the head was centered within it. Signals from each coil were demodulated by frequency detectors, sampled at 1000 Hz, and stored on computer for later analysis. For the right eye of M2 and M3, eye movements were measured with a “leadless” search coil system (Roberts et al., 2008), which uses a resonant coil and no connecting lead wire. A transmitter emits a stream of pulses to the eye coil, and a receiver then detects the resonant oscillations emitted from the eye coil. The relative intensity of the signal as received by sets of orthogonal receiver coils determined the orientation of the eye coil. The leadless coil system has a noise level of 0.05° peak to peak, and provides a sample rate up to 1000 Hz.
The coil signal was calibrated by requiring the animal to fix successively on targets at 5° intervals over a range of ± 20° horizontal and vertical. Calibration data were obtained with one eye viewing and the other occluded. Visual targets were back-projected using a mirror-controlled laser beam onto a translucent screen (72° × 72°) located 1 m in front of the animal. The room was dimly illuminated.
Experimental paradigms
A standard double-step paradigm was used to induce an increase or a decrease in saccade amplitude (McLaughlin, 1967). As monkeys made saccades in response to 15° horizontal target movements, we detected each saccade as the eye crossed a virtual 2° window placed around the starting position. The occurrence of a saccade triggered a brief target step either 5° away from (gain-increase) or 5° closer to (gain-decrease), the starting position of the saccade. This created a visual error at the end of the movement as if the saccade had been too small or too large, requiring the animal to make a corrective saccade to fixate the target. This procedure, when repeated over several hundred times, gradually increased or decreased the saccade amplitude. We adapted saccades to both leftward and rightward target movements. Adapting saccades in one direction does not influence saccades in the opposite direction (Deubel et al., 1986; Miller, Anstis, & Templeton, 1981; Straube et al., 1997), so we treated saccades in the two directions independently.
Contextual adaptation
In this paradigm, adaptation to backward and forward target steps were elicited concurrently and associated with different vertical eye positions. For the gain-increase trials, an initial target was presented at left 10° (−10°). After the monkey fixated the target for 500–1,000 ms, the target jumped to +5°. During the saccade, this target was displaced to +10° and maintained for 500 ms. This location then became the fixation point for the next trial and the same sequence was presented in the opposite direction. For the gain-decrease trials, the initial fixation target was presented at −5°. The first target step was to +10°, and the second target step was to +5°. This location then became the fixation point for the next trial in the opposite direction. Gain-increase adaptation was carried out with the eyes directed up 10° above straight-ahead gaze, and gain-decrease adaptation with the eyes directed down 10°. These two context/adaptation conditions were alternately presented ~30 times each, with 30 adaptation trials in each block (~1800 trials total). The intertrial time was 750 ms. Between each change in context/adaptation condition, the animal received 20 s of a rest to avoid fatigue. This protocol was also repeated with the conditions reversed. A total of 23 experiments were performed with the three monkeys.
Control adaptation
To determine the effectiveness of context-specific adaptation, a control adaptation paradigm was performed in M3. A single type of adaptation (gain-increase or gain-decrease) was elicited with the eyes either looking up 10° or looking down 10°. This experiment was designed to look for generalization of adaptation in one context to the second context.
Test sessions
Test trials in each context state (vertical position) were performed before (pretest, 15 trials in each direction) and after (posttest, 20 trials in each direction, tested twice) adaptation. These were similar to the adaptation trials, except that there was no second target jump: the target did not reappear after the first target jump. There was thus no visual feedback as to the accuracy of the saccade, providing an open-loop measure of saccade gain. The fixation target for the next trial appeared 1000 ms later.
Data analysis
Data were analyzed offline with custom software developed in MATLAB™ (The Mathworks, Natick, MA). An interactive program was used to mark automatically the onset and the end of saccades using velocity criteria. The onset of the saccade was defined when the eye velocity first exceeded 30°/sec and the end of the saccade when the eye velocity first dropped below 30°/sec. The correctness of these markings was verified by the experimenter. Targeting saccades elicited by an initial target step were selected for analysis. Trials were rejected if the latency of saccade was less than 60 ms, or if the trial was contaminated by blinks. These criteria excluded approximately 5% of all saccades during contextual adaptation in M1, 7% in M3 and 11% in M2, who was a less motivated worker. The average latency over all the adaptation experiments was 179 ± 23 ms in M1, 236 ± 29 ms in M2 and 244 ± 41 ms in M3. When we applied a latency criterion of 100 ms to assess the extent of predictive saccades, the number of excluded trials increased by 4% in M1 and there was no difference in M2 and M3.
The horizontal amplitude of saccade was calculated as the difference between initial and final eye positions. The percent amplitude change of the saccade between the posttest and the pretest sessions was calculated as follows: [(posttest mean amplitude − pretest mean amplitude)/pretest mean amplitude] × 100. To assess the time course of contextual adaptation, the first saccade upon each change in context was identified. This saccade gives an indication of whether or not gain switching indeed occurs immediately upon a change in context cue, before any adaptation trials are performed. A linear regression line was fit separately to the gain-increase and gain-decrease trials. The rate of adaptation was estimated by the slope of regression line. We presented the results of both rightward and leftward saccades in normal monkeys (M2 before the surgery and M3), but only leftward saccades in M1 and M2 after the surgery, since there were more adaptation trials available for analysis in the leftward direction than the rightward.
RESULTS
General features
In general, we found some degree of context-specific adaptation when in one context the target steps backward during saccades and in the other context the target steps forward. Figure 1 shows an example of the time course of adaptation from monkey 1 (M1), who was trained with gain-decrease adaptation with eyes down and gain-increase adaptation with eyes up. The amplitude of the leftward saccade for gain-increase and gain-decrease trials is plotted as a function of the saccade number. The saccades have an initial amplitude of approximately 15°, the size of the first target displacement. Little adaptation is evident during the first set of gain-increase trials and the subsequent set of gain-decrease trials. As the session progresses (the two contexts alternate), gain-decrease adaptation becomes more clear, meanwhile gain-increase adaptation still shows a decline but at a much slower rate. At the end of this process, the saccades in the gain-decrease trials have become significantly smaller; the saccades in the gain-increase trials have also become smaller but by much less than the gain-decrease trials. Note as was typical for all monkeys that the saccade amplitude within each individual context setting in the adaptation process was variable but, on average, it changed monotonically over the entire adaptation process in each context as shown by the exponential fit.
Figure 1.
An example of the time course of context-specific adaptation from M1. The amplitude of the leftward saccade for gain-increase and gain-decrease trials is plotted as a function of saccade numbers. × indicates the saccade amplitudes for gain-increase trials with eye up 10°, and ○ for gain-decrease trials with eyes down 10°. Solid curves are exponential fits. Test trials before and after adaptation are set off by dotted vertical lines.
Aftereffect of adaptation
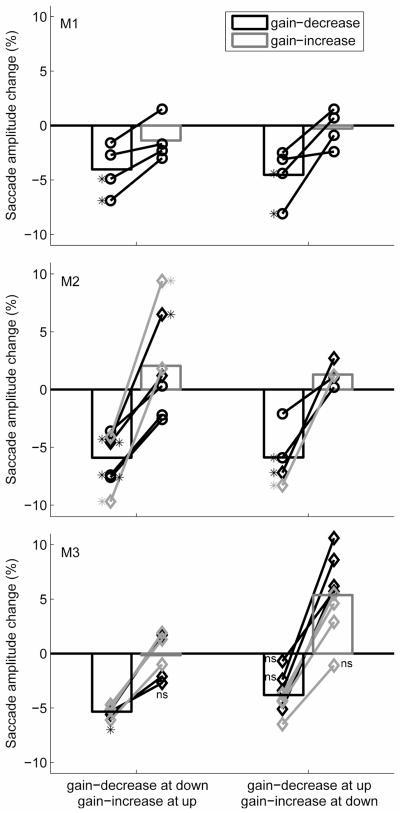
The aftereffect of adaptation was measured by comparing, before and after training, the amplitude of saccades directed towards targets that had been extinguished once the saccade began. Figure 2 summarizes the changes of saccade amplitude in all 23 experiments performed with the three monkeys. The aftereffect of training was variable across experiments as seen in Figure 2 (each line represents the results from different days). On average, saccade amplitude was reduced by 4.3% ± 2.3% (means ± SD) in M1, 5.9% ± 2.3% in M2 and 4.5% ± 1.5% in M3 after gain-decrease adaptation, which is consistent with the expected effect in the appropriate context (gaze directed down as well as up). However, the adaptation was less effective in the gain-increase context (gaze directed up as well as down except for gaze down in M3), as the saccade amplitude slightly decreased by 0.8% ± 1.8% in M1, increased by 1.8% ± 3.5% in M2 and 3.0% ± 4.0% in M3. Nevertheless, in all animals, the changes generated in the gain-increase and gain-decrease conditions were significantly different (Wilcoxon signed rank test; p < 0.01), which suggests that context specificity can occur in saccadic gain adaptation in monkeys when the training involves conflicting saccade amplitude changes.
Figure 2.
Changes of saccade amplitude between the posttest and the pretest trials in all 23 context-specific adaptation experiments. Each black line represents the results of leftward saccades for each individual day and grey line represents the results of rightward saccades. The superimposed bars indicate the mean values. ◇ indicates the results if the monkeys were intact. * beside the data points indicates the change was significantly different in the panel of M1 and M2. For M3, * and ns below the data points indicate the change was significant and nonsignificant for all the points above; otherwise all the changes were significant except indicated as ns.
To find out whether the context-specific adaptation was as effective as a single adaptation and to assess the generalization of adaptation in one context to the second context, we also tested gain decrease or gain increase at up or down positions as controls in M3. Figure 3 summarizes the changes of saccade amplitude in all 8 control experiments. On average, saccade amplitude was reduced by 9.7% ± 3.0% at up position and 11.7% ± 3.8% at down after gain-decrease adaptation; average gain transfer to the second position was 48% and 74%, respectively. The amplitude increased by 4.0% ± 0.7% at up position and 10.3% ± 5.1% at down after gain-increase adaptation; average gain transfer to the second position was 162% and 46%, respectively. Both the gain-increase and gain-decrease adaptations were more effective in control conditions than in context-specific conditions, which indicates that gain-increase and gain-decrease adaptation interacted when they were performed simultaneously in a single session. Secondly, the gain-increase adaptation was more robust when gaze was directed down than up, which was consistent with what we found during contextual adaptation (Fig. 2, right side of M3 panel).
Figure 3.
Changes of saccade amplitude between the posttest and the pretest trials in all 8 control adaptation experiments in M3. Each black line represents the results of leftward saccades for each individual day and grey line represents the results of rightward saccades. The superimposed bars indicate the mean values. All the changes were significant except indicated as ns.
Time course of adaptation
To quantify the time course of adaptation, we plotted the amplitude of the first saccade upon each change in context state as a function of the set number (Figure 4, from the same data as shown in Fig 1). This indicates whether or not the amplitudes of the saccades change immediately upon a change in context state. Regression lines were fit separately to the gain-increase trials and to the gain-decrease trials. Figure 4 shows the evidence of switching between the increased-gain state and the decreased-gain state immediately upon each change in context. As adaptation progresses, this context specificity increases in size as seen by the divergence of the regression lines.
Figure 4.
The first adaptation trial upon each change in context state during the course of adaptation in M1. Consecutive set numbers are along the abscissa. Lines were fit separately to the gain-increase and gain-decrease trials using a least squares algorithm, to quantify the time course of adaptation.
Figure 5 summarize the slopes of the regression lines (as illustrated in Fig. 4) in all 23 contextual adaptations. Similar to the aftereffect of adaptation (Fig 2), the rate of adaptation varied from animal to animal and from day to day for a particular animal. On average, the gain-decrease adaptation was more rapid than the gain-increase adaptation except for gaze up in M3. The differences between the slopes of the gain-increase trials and the gain-decrease trials were significant in M2 and M3 (Wilcoxon signed rank test; p < 0.01), but missed being significant in M1 (p = 0.078).
Figure 5.
Summary of the slopes of the regression lines illustrated in Figure 4 for all 23 context-specific adaptation experiments. Each black line represents the results of leftward saccades for each individual day and grey line represents the results of rightward saccades. The superimposed bars indicate the mean values. ◇ indicates the results if the monkeys were intact. * beside the data points indicates the slope was significantly different from zero.
The data above demonstrated that the adapted state switched immediately upon the change in context state, suggesting a mechanism for contextual switching that did not require the reappearance of an error signal to invoke the context-specific response, i.e., a feedforward control mechanism. To assess this further, we examined the difference in amplitudes between the first two saccades upon each change in context state. If retinal error from the first saccade is used to determine that a change in context state has occurred, then the second saccade after experiencing an error should show increased context-specificity (larger regression slopes) relative to the first. The slopes of regression lines fit to the first saccade and the second saccade for both gain-increase and gain-decrease trials are shown in Figure 6. It is clear that there is no significant difference in the time course of adaptation as assessed with first saccades or second saccades upon each context switch for both rightward and leftward saccades (Wilcoxon signed rank test; p > 0.1). Again, this result supports a feedforward mechanism for control of context switching.
Figure 6.
The slopes of regression lines fit to the first saccade and the second saccade upon each context switch for both gain-increase and gain-decrease trials in all 23 context-specific adaptation experiments. Black line represents the results of leftward saccades and grey line represents the results of rightward saccades. It is clear that the second saccade does not systematically exhibit greater adaptation than the first for both rightward and leftward saccades.
Long-term context-specific adaptation
In an attempt to test whether or not the long-term training over days can improve the contextual learning, monkey 2 was subject to the same context-specific adaptation session (only rightward saccades were adapted) for three days. The animal underwent normal visual experience after each day’s test. Figure 7 shows the amplitude of the adapted saccades during the three day contextual adaptation. On all three days, M2 exhibited a typical pattern of context-specific learning as shown in Fig 1: gain-decrease adaptation is evident, but gain-increase adaptation remains marginal.
Figure 7.
Saccade amplitude changes during the three-day context-specific adaptation in M2. × indicates the saccade amplitudes for gain-increase trials with eye up 10°, and ○ for gain-decrease trials with eyes down 10°. Test trials before and after adaptation are set off by dotted vertical lines for each day. Arrows indicate the retention of adaptation at the beginning of day 2 and day 3.
To compare saccade amplitude between different days, we plot in Figure 8 saccade amplitude as a function of the test sessions (from before to after adaptation). For the saccades in the gain-increase context, the amplitude changed little over three days (Kruskal-Wallis test; p = 0.12). For the saccades in the gain-decrease context, the mean amplitude of test trials decreased significantly from 14.9° ± 0.7° to 13.0° ± 0.6° on day 1 (Wilcoxon rank sum test; p< 0.01). At the start of day 2, saccade amplitude recovered to 13.9° ± 0.4°, but was still significantly smaller than day 1 pretest amplitude (p< 0.01). By the end of day 2, saccade amplitude decreased to 13.2° ± 0.5°. At the start of day 3, saccade amplitude changed very little overnight (p = 0.13). By the end of day 3, it decreased to 11.9° ± 0.5° and the difference between the two adapted states was larger than that on day 1. Most striking is the retention of the context-specific adaptation at the beginning of day 2 and day 3 as indicated by the arrows in Fig 7. This indicates the two different gain states produced by the contextual paradigm reflect a true neuronal plasticity. From this we conclude that eye position information is used as a contextual signal both during the adaptation and retention periods, allowing the saccadic system to switch state between high or low gains.
Figure 8.
Mean saccade amplitude plotted as a function of the test sessions of the three- day context-specific adaptation in M2. × indicates the saccade amplitudes of test trials in the gain-increase context (with eye up 10°), and ○ in the gain-decrease context (with eyes down 10°). Error bars are SD.
DISCUSSION
Here we show in monkeys that saccades in response to the same-sized target displacement can be simultaneously adapted to exhibit different gains which depend on vertical eye position (context). As has been shown in humans, this context-specific adaptation develops gradually and switching of gains is evident on the first saccades with each change in context. Furthermore, our results demonstrate that contextual adaptation involving conflicting error signals was less robust than the non-contextual adaptation, probably due to the interference between context/adaptation states.
Context-specific adaptation can occur in monkeys
A previous study has reported that saccades can be made to have two different gains depending on their amplitude in monkeys (Watanabe et al., 2000). Here we asked if monkeys can perform a more complicated task, in which we ask them to invoke a more complicated, presumably higher-level adaptive response that is tailored to a specific context. “Context” here refers to a condition that is not usually thought of as influencing the performance of a motor task (Shelhamer & Clendaniel, 2002a; Shelhamer et al., 2005). Thus, the adaptation may be low-level, whereas the contextual switching is higher-level. We used vertical eye position as a context for adaptation of horizontal saccades in response to the same target displacement. We asked if the monkeys could learn a gain increase in one context and a gain decrease in another context, and then determined if a change in the context would invoke immediate switching between the two different gain states. In the absence of context-specificity, one might expect the gain-increase and gain-decrease adaptation to cancel each other and produce no adaptive changes at worst or only one of the two adaptations at best. Our results indicate this is not the case: in most experiments gain-decrease adaptation could be induced, but there was little or no gain-increase adaptation. Nevertheless, there was a significant difference between the two simultaneous modifications of saccade amplitude according to vertical eye position. As pointed out in prior studies in human beings (Shelhamer & Clendaniel, 2002a,b; Shelhamer et al., 2005), this is nevertheless a demonstration of context-specific adaptation, since each change in context invokes clear switching of gain states, even if one of the states does not exhibit significant adaptation. One might ask why the brain needs a mechanism to make horizontal eye movements dependent on vertical eye position. Takagi et al. (2000) suggested a mechanism based on context-specific adaptation of pursuit and similar considerations apply to saccades. Obliquely directed movements, which occur often naturally, demand different horizontal innervations depending on the vertical position of the eye because of the change in pulling directions of the horizontal muscles with vertical eye position. Accordingly vertical eye position could be an effective contextual cue for the appropriate horizontal innervations needed to make an accurate saccade.
One can ask if the vertical strabismus produced by the trochlear nerve section (part of a different study) affected the contextual adaptation in our monkeys, since two of the three contextual adaptation experiments performed before M2 had the nerve section appeared to have a greater aftereffect than those after, and the adaptation in M1 was even weaker. Taking the results together in all three monkeys, however, we think it unlikely that the strabismus had a major effect on the contextual adaptation in the two operated monkeys. The variability of adaptation itself, from animal to animal and from day to day for a specific animal makes it difficult to make a firm conclusion about the strabismus. Furthermore, the amount of aftereffect after conventional adaptation was similar before and after the nerve section in M2 (data not shown). Accordingly we are confident that the vertical strabismus did not alter the performance of the horizontal saccade adaptation per se and, especially considering that the monkey was always tested with only the intact eye viewing.
Comparison to studies with humans
In humans, two previous studies have investigated the context specificity of saccade adaptation using vertical eye position as a context (Alahyane & Pelisson, 2004; Shelhamer & Clendaniel, 2002a). Our findings in monkeys are most similar to those of Shelhamer and Clendaniel (2002a), who found there was considerable undesired transfer from gain-decrease adaptation to gain-increase adaptation, with the result that there was little gain-increase adaptation. However, Alahyane and Pelisson (2004) reported that significant adaptation can be induced not only in the gain-decrease context but also in the gain-increase context. One possible reason for this difference, as has been suggested (Alahyane & Pelisson, 2004), is that the two vertical eye positions where the conflicting adaptations were tested were farther away from each other in their study (up 12.5° and down 25°) than in ours and Shelhamer and Clendaniel’s experiments (up 10° and down 10°). The closer the two contexts are, the less effective it might be to prevent transfer of adaptation from gain-decrease condition to the gain-increase condition. A similar spatial extent effect is seen, for example, in disconjugate saccade adaptation in monkeys in response to wearing a laterally-displacing hemifield prism in front of one eye (Oohira & Zee, 1992).
The efficacy of different context cues (sensory and motor) has been assessed in prior studies (Shelhamer & Clendaniel, 2002a,b). Eye position in the orbit is an example of a motor context cue: the motor commands sent to the muscles to generate a particular movement are different in the two context states. Head roll-tilt is an example of a sensory cue as head tilt is sensed by the otoliths and proprioception but saccades are always same in the head. It has been demonstrated that the motor context (change in saccade direction) is relatively stronger than the sensory context (head roll-tilt) (Shelhamer & Clendaniel, 2002b), and horizontal eye position was more effective than vertical eye position for demonstrating context-specific adaptation of horizontal saccades (Shelhamer & Clendaniel, 2002a). Taken together, these results suggest that a context cue is more effective when it is immediately relevant to the movement being adapted since naturally occurring adaptation is more commonly a motor repair than a sensory remapping mechanism. However, this notion has been challenged by a recent study of Herman et al. (2009), who reported that a visual context (stable/flicking target) can serve as an effective contextual cue for saccade adaptation, although an earlier attempt with target color had been unsuccessful (Deubel, 1995). It may be that the ability to modify saccade gains based on the target visual properties reflects a much higher level and more sophisticated cognitive type of adaptation.
Another difference among these studies is the trial structure of contextual paradigm: two different adaptations in two contexts has been alternated randomly across successive trials (Alahyane & Pelisson, 2004; Deubel, 1995), in blocks of fixed length (Shelhamer & Clendaniel, 2002a,b), or short blocks of irregular length (Herman et al., 2009). Although random context presentation may slow contextual learning since half of the adaptation trials would be preceded by trials with different or opposite error signals, one study has shown robust adaptation in humans (Alahyane & Pelisson, 2004). In monkeys, we chose to alternate the gain-increase and gain-decrease adaptations in blocks (30 trials), because adaptation in monkeys is much slower than in humans and also in this way undesired saccades when switching the contexts (between two vertical eye positions) can be avoided. With this protocol, our monkeys exhibit context-dependent learning though it is not complete. Further study will be required to determine how the trial structure affects the extent and time course of context-specific adaptation.
Context-specific adaptation process is a true neuronal plasticity
One might question whether our monkeys used a cognitive strategy to alter their saccades in different contexts since the target locations were predictable. Several lines of evidence argue against the idea that their contextual adaptation was strategic. First, the adaptation developed gradually over thousands of trials and generally gain-decrease adaptation was more developed than gain-increase adaptation. This is also the case with conventional saccade adaptation and supports the idea that our data reflect true adaptation, not a cognitive strategy. Second, as adaptation proceeds, switching of adapted gains upon each change in context occurred immediately. Thus, an initial saccade was not necessary to provide retinal error feedback in order to trigger saccades of appropriate gain. Third, contextual adaptation was weakened by the interference between the gain-increase and gain-decrease adaptations, suggesting that volitional control of this process is unlikely. Finally, there was some residual amplitude change the day after an experiment, despite the fact that monkey had normal visual experience in between. If contextual adaptation were strategic, we would not expect the strong retention after the animal had spent 24 h in their natural visual environment. Similar retention of gain change induced by conventional saccade adaptation has also been found in humans (Alahyane & Pelisson, 2005) and in monkeys (Noto, Watanabe, & Fuchs, 1999). It may appear surprising that the adaptation can be retained over days, despite the subjects having received visual feedback from many saccades made during their daily activities. One possible explanation is that the amplitude of saccades generated naturally is different from that of adapted saccades during the experiments. It has been reported that most naturally occurring saccades in humans are less than 15° (Bahill, Adler, & Stark, 1975). Another possible factor would be that internally triggered saccades have little effect on de-adaptation of visually triggered saccades performed in the laboratory. Taken together, these results suggest that contextual-specific adaptation cannot be controlled voluntarily, but involves a true adaptive process of reorganization in the brain.
Neuronal substrate of context-specific adaptation
The neuronal substrates of conventional (non-contextual) saccadic adaptation have been extensively investigated. A variety of neurophysiological studies demonstrated that the cerebellum is critically involved in saccadic adaptation (Barash et al., 1999; Catz et al., 2008; Optican & Robinson, 1980; Robinson et al., 2002; Soetedjo et al., 2008; Takagi et al., 1998). However, much less is known about the neuronal site of context-specific adaptation. In a monkey reaching study with prism adaptation, it has been shown that pointing responses can be differentially adapted for each viewing eye. This capability was lost after focal lesions of the dorsal vermal and paravermal cerebellar cortex, but in the same animals non-contextual adaptation to a prism was spared, suggesting that these regions of cerebellum are required in context-dependent motor learning (Lewis & Tamargo, 2001). Based on these results, the cerebellum is a possible candidate area for context-specific saccadic adaptation.
Recently, Lee and Schweighofer (2009) proposed that a parallel model with a fast and a slow process can explain the dual or multiple motor adaptations. In their model, the fast process contains only a single state and the slow process contains multiple states switched via context cues. In the case of conventional saccadic adaptation, a similar two-timescale process has been observed in a gain adaptation paradigm (Ethier, Zee, & Shadmehr, 2008), and in a cross-axis adaptation paradigm (Chen-Harris, Joiner, Ethier, Zee, & Shadmehr, 2008). In human patients with cerebellar degeneration including the vermis and hemispheres, a recent study demonstrated that the fast process of adaptation was absent, but the slow process was less impaired (Xu-Wilson, Chen-Harris, Zee, & Shadmehr, 2009). This suggest that the damage to the cerebellar cortex mostly affects the fast process of adaptation, and supports the idea that the two processes derive from different neural mechanisms and possibly have different anatomical substrates. Similarly, in a monkey lesion study, Barash et al. (1999) proposed that a fast and a slow process that adjust the saccade gain depend on the cerebellar cortex and nuclei separately. Context-specific adaptation involves recruitment of different adaptation modules for different contexts and increases the complexity of its underlying neural circuitry. The precise role played by separate cerebellar loci, or other parts of the brain, in contextual adaptation, especially in learning, storage and retrieval of different adapted states, need to be elucidated. To this important and topical question about how the brain learns new behaviors, the monkey model of contextually driven saccade adaptation can provide critical information.
Context-specific adaptation of saccade gain exists in monkeys.
Switching of gains is evident on the first saccades with each change in context.
Retention of aftereffect indicates a true adaptive process of brain reorganization.
Acknowledgments
This study was supported by the National Institutes of Health (R01-EY01849); the Albert Pennick fund; Leon Levy Foundation; and by the Arnold-Chiari Foundation. Dr. Tian is a Paul and Betty Cinquegrana Scholar. C. Bridges, A. Lasker, and D. Roberts provided technical support. The authors are grateful to M. Shelhamer for a critical review and discussion of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Alahyane N, Fonteille V, Urquizar C, Salemme R, Nighoghossian N, Pelisson D, et al. Separate neural substrates in the human cerebellum for sensory-motor adaptation of reactive and of scanning voluntary saccades. Cerebellum. 2008;7:595–601. doi: 10.1007/s12311-008-0065-5. [DOI] [PubMed] [Google Scholar]
- Alahyane N, Pelisson D. Eye position specificity of saccadic adaptation. Investigative Ophthalmology & Visual Science. 2004;45:123–130. doi: 10.1167/iovs.03-0570. [DOI] [PubMed] [Google Scholar]
- Alahyane N, Pelisson D. Long-lasting modifications of saccadic eye movements following adaptation induced in the double-step target paradigm. Learning Memory. 2005;12:433–443. doi: 10.1101/lm.96405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahill AT, Adler D, Stark L. Most Naturally Occurring Human Saccades Have Magnitudes of 15 Degrees or Less. Investigative Ophthalmology. 1975;14:468–469. [PubMed] [Google Scholar]
- Barash S, Melikyan A, Sivakov A, Zhang M, Glickstein M, Thier P. Saccadic dysmetria and adaptation after lesions of the cerebellar cortex. Journal of Neuroscience. 1999;19:10931–10939. doi: 10.1523/JNEUROSCI.19-24-10931.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein AM, Bunday KL, Reynolds R. What the “Broken Escalator” Phenomenon Teaches Us about Balance. Basic and Clinical Aspects of Vertigo and Dizziness. 2009;1164:82–88. doi: 10.1111/j.1749-6632.2009.03870.x. [DOI] [PubMed] [Google Scholar]
- Catz N, Dicke PW, Thier P. Cerebellar-dependent motor learning is based on pruning a Purkinje cell population response. Proceedings of the National Academy of Sciences USA. 2008;105:7309–7314. doi: 10.1073/pnas.0706032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi V, vanGisbergen JAM. Specificity of saccadic adaptation in three-dimensional space. Vision Research. 1997;37:1367–1382. doi: 10.1016/s0042-6989(96)00266-0. [DOI] [PubMed] [Google Scholar]
- Chen-Harris H, Joiner WM, Ethier V, Zee DS, Shadmehr R. Adaptive control of saccades via internal feedback. Journal of Neuroscience. 2008;28:2804–2813. doi: 10.1523/JNEUROSCI.5300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KD, Kim HJ, Cho BM, Kim JS. Saccadic adaptation in lateral medullary and cerebellar infarction. Experimental Brain Research. 2008;188:475–482. doi: 10.1007/s00221-008-1375-z. [DOI] [PubMed] [Google Scholar]
- Deubel H. Is saccade adaptation context-specific? In: Findlay JM, Walker R, Kentridge RW, editors. Eye movement research: mechanisms, processes and applications. Amsterdam: Elsevier Science; 1995. pp. 177–187. [Google Scholar]
- Deubel H, Wolf W, Hauske G. Adaptive gain control of saccadic eye movements. Human Neurobiology. 1986;5:245–253. [PubMed] [Google Scholar]
- Ethier V, Zee DS, Shadmehr R. Spontaneous recovery of motor memory during saccade adaptation. Journal of Neurophysiology. 2008;99:2577–2583. doi: 10.1152/jn.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Harwood MR, Wallman J. Saccade Adaptation Specific to Visual Context. Journal of Neurophysiology. 2009;101:1713–1721. doi: 10.1152/jn.91076.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp J, Fuchs AE. The characteristics and neuronal substrate of saccadic eye movement plasticity. Progress in Neurobiology. 2004;72:27–53. doi: 10.1016/j.pneurobio.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Lee JY, Schweighofer N. Dual Adaptation Supports a Parallel Architecture of Motor Memory. Journal of Neuroscience. 2009;29:10396–10404. doi: 10.1523/JNEUROSCI.1294-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RF, Tamargo RJ. Cerebellar lesions impair context-dependent adaptation of reaching movements in primates. Experimental Brain Research. 2001;138:263–267. doi: 10.1007/s002210100719. [DOI] [PubMed] [Google Scholar]
- Mcgonigle BO, Flook J. Long-Term Retention of Single and Multistate Prismatic Adaptation by Humans. Nature. 1978;272:364–366. doi: 10.1038/272364a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. Parametric adjustment in saccadic eye movements. Perception & Psychophysics. 1967;2:359–362. [Google Scholar]
- Miller JM, Anstis T, Templeton WB. Saccadic plasticity: parametric adaptive control by retinal feedback. Journal of Experimental Psychology: Human Perception and Performance. 1981;7:356–366. doi: 10.1037//0096-1523.7.2.356. [DOI] [PubMed] [Google Scholar]
- Noto CT, Watanabe S, Fuchs AF. Characteristics of simian adaptation fields produced by behavioral changes in saccade size and direction. Journal of Neurophysiology. 1999;81:2798–2813. doi: 10.1152/jn.1999.81.6.2798. [DOI] [PubMed] [Google Scholar]
- Oohira A, Zee DS. Disconjugate ocular motor adaptation in rhesus monkey. Vision Research. 1992;32:489–497. doi: 10.1016/0042-6989(92)90241-a. [DOI] [PubMed] [Google Scholar]
- Optican LM, Robinson DA. Cerebellar-dependent adaptive control of primate saccadic system. Journal of Neurophysiology. 1980;44:1058–1076. doi: 10.1152/jn.1980.44.6.1058. [DOI] [PubMed] [Google Scholar]
- Reynolds RF, Bronstein AM. The broken escalator phenomenon - Aftereffect of walking onto a moving platform. Experimental Brain Research. 2003;151:301–308. doi: 10.1007/s00221-003-1444-2. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Shelhamer M, Wong A. A new “wireless” search-coil system. Proceedings of the 2008 symposium on Eye tracking research & applications. 2008:197–204. [Google Scholar]
- Robinson FR, Fuchs AF, Noto CT. Cerebellar influences on saccade plasticity. Annals of the New York Academy of Sciences. 2002;956:155–163. doi: 10.1111/j.1749-6632.2002.tb02816.x. [DOI] [PubMed] [Google Scholar]
- Shelhamer M, Aboukhalil A, Clendaniel R. Context-specific adaptation of saccade gain is enhanced with rest intervals between changes in context state. Annals of the New York Academy of Sciences. 2005;1039:166–175. doi: 10.1196/annals.1325.016. [DOI] [PubMed] [Google Scholar]
- Shelhamer M, Clendaniel RA. Context-specific adaptation of saccade gain. Experimental Brain Research. 2002a;146:441–450. doi: 10.1007/s00221-002-1199-1. [DOI] [PubMed] [Google Scholar]
- Shelhamer M, Clendaniel R. Sensory, motor, and combined contexts for context-specific adaptation of saccade gain in humans. Neuroscience Letters. 2002b;332:200–204. doi: 10.1016/s0304-3940(02)00951-5. [DOI] [PubMed] [Google Scholar]
- Soetedjo R, Kojima Y, Fuchs AF. Complex spike activity in the oculomotor vermis of the cerebellum: a vectorial error signal for saccade motor learning? Journal of Neurophysiology. 2008;100:1949–1966. doi: 10.1152/jn.90526.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube A, Fuchs AF, Usher S, Robinson FR. Characteristics of saccadic gain adaptation in rhesus macaques. Journal of Neurophysiology. 1997;77:874–895. doi: 10.1152/jn.1997.77.2.874. [DOI] [PubMed] [Google Scholar]
- Takagi M, Abe H, Hasegawa T, Usui T, Hasebe H, Miki A, et al. Context-specific adaptation of pursuit initiation in humans. Investigative Ophthalmology & Visual Science. 2000;41:3763–3769. [PubMed] [Google Scholar]
- Takagi M, Zee DS, Tamargo R. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. Journal of Neurophysiology. 1998;80:1911–1930. doi: 10.1152/jn.1998.80.4.1911. [DOI] [PubMed] [Google Scholar]
- Tian J, Ethier V, Shadmehr R, Fujita M, Zee DS. Some perspectives on saccade adaptation. Annals of the New York Academy of Sciences. 2009;1164:166–172. doi: 10.1111/j.1749-6632.2009.03853.x. [DOI] [PubMed] [Google Scholar]
- Tian J, Zee DS, Walker MF. Rotational and translational optokinetic nystagmus have different kinematics. Vision Research. 2007;47:1003–1010. doi: 10.1016/j.visres.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Noto CT, Fuchs AF. Flexibility of saccade adaptation in the monkey: different gain states for saccades in the same direction. Experimental Brain Research. 2000;130:169–176. doi: 10.1007/s002219900220. [DOI] [PubMed] [Google Scholar]
- Welch RB, Bridgeman B, Anand S, Browman KE. Alternating Prism Exposure Causes Dual Adaptation and Generalization to A Novel Displacement. Perception & Psychophysics. 1993;54:195–204. doi: 10.3758/bf03211756. [DOI] [PubMed] [Google Scholar]
- Xu-Wilson M, Chen-Harris H, Zee DS, Shadmehr R. Cerebellar Contributions to Adaptive Control of Saccades in Humans. Journal of Neuroscience. 2009;29:12930–12939. doi: 10.1523/JNEUROSCI.3115-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]