Abstract
Mutations in PLA2G6, which encodes calcium-independent phospholipase A2 group VIA (iPLA2-VIA), underlie the autosomal recessive disorder infantile neuroaxonal dystrophy (INAD). INAD typically presents in the first year of life, and leads to optic atrophy and psychomotor regression. We have examined PLA2G6 expression in early human embryonic development by in situ hybridization. At Carnegie Stage (CS) 19 (approximately 7 post conception weeks [PCW]), strong expression is evident in the ventricular zone (VZ) of midbrain and forebrain suggestive of expression in neural stem and progenitor cells. At CS23 (8 PCW) expression is also detectable in the VZ of the hindbrain and the subventricular zone (SVZ) of the developing neocortex, ganglionic eminences and diencephalon. By 9 PCW strong expression in the post-mitotic cells of the cortical plate can be seen in the developing neocortex. In the eye, expression is seen in the lens and retina at all stages examined. PLA2G6 expression is also evident in the alar plate of the spinal cord, dorsal root ganglia, the retina and lens in the eye and and several non-neuronal tissues, including developing bones, lung, kidney and gut. These findings suggest a role for PLA2G6 in neuronal proliferation throughout the developing brain and in maturing neurons in the cortical plate and hindbrain. Although widespread PLA2G6 expression is detected in neuronal tissues, the pattern shows dynamic changes with time and indicates that INAD pathogenesis may begin prior to birth.
Keywords: PLA2G6, INAD, neurodegeneration, development, in-situ hybridization
1. INTRODUCTION
Infantile neuroaxonal dystrophy (INAD) is an autosomal recessive disease with early onset and rapid progression of hypotonia, hyperreflexia and tetraparesis. INAD belongs to a larger class of neuroaxonal dystrophies, which include pantothenate-kinase associated neurodegeneration (PKAN), idiopathic neurodegeneration with brain iron accumulation (NBIA), and Schindler disease. Within this group, INAD has the earliest onset, with motor and cognitive skill regression presenting at a median age of 15 months [14, 18]. Optic atrophy is also commonly observed. Historically, INAD was diagnosed from histological evidence of neuroaxonal spheroids in peripheral nerves [24]. The detection of cerebellar atrophy and iron accumulation in the globus pallidus by MRI is also diagnostic of INAD, although iron accumulation often is not observed until later in the disease [14, 18, 23].
In 2006, the causative gene for INAD was identified as PLA2G6, which encodes a calcium-independent phospholipase (A2 group IVA) [23]. PLA2G6 mutations are found in the vast majority of INAD patients and have also been described in individuals previously diagnosed with idiopathic NBIA [14], as well as individuals with adult-onset dystonia-parkinsonism [29]. Phospholipases A2 comprise a large family of enzymes that catalyze the hydrolysis of sn-2 ester bonds of glycerophospholipids, producing free fatty acids and lysophospholipids [12]. Arachidonic acid and other fatty acids released by iPLA2-VIA can initiate apoptosis, inflammation, and cell growth [4, 36]. The lysophospholipid remaining in the cell membrane can also trigger cellular processes, including chemotaxis and fusion of biological membranes [2, 10].
The early presentation of INAD suggests that iPLA2-VIA may have a developmental role. Malik and colleagues [20] showed that the Pla2g6 knockout mouse [5] develops neurological impairments by 13 months and accumulates neuroaxonal spheroids similar to those in human INAD patients. In addition, four month old Pla2g6 knockout mice have decreased brain docosahexaenoic acid (DHA) metabolism and signaling [6]. In a second INAD mouse model, point mutations within Pla2g6 result in motor dysfunction and neuroaxonal spheroids as early as 7 weeks of age [39]. These studies, however, did not examine Pla2g6 expression during mouse development. Pla2g6 expression has been shown in mouse sagittal sections at embryonic day 14.5 as part of the high-throughput Eurexpress project [38]. At this stage, the strongest expression seen in the brain is in the alar plate of the developing hindbrain with prominent expression also in an analogous region of the midbrain. Pla2g6 also appears to be expressed weakly in the developing diencephalon and telencephalon of the forebrain. Expression was also detected in spinal cord and the bones of the developing skull, face, ribcage and limbs (EMAGE entry 18166; genex.hgu.mrc.ac.uk).
To better understand the potential role for PLA2G6 in neuronal development and pathogenic mechanisms underlying disease, we examined PLA2G6 expression by in-situ hybridization across several stages of human embryonic and early fetal development.
2. MATERIALS AND METHODS
2.1 Human tissue collection and processing
Human embryonic and fetal tissues were obtained from the MRC-Wellcome Trust Human Developmental Biology Resource (www.hdbr.org), Institute of Human Genetics, Newcastle University. The samples were collected with appropriate maternal consents and ethical approval by the Newcastle and North Tyneside Research Ethics Committee. Tissue sections from 7 samples were analysed: Carnegie Stage (CS) 19 (~7 PCW, n=2), CS23 (8PCW, n=3) and 9 PCW (n=2). The stage of embryonic development (CS19 and CS23) was determined by assessment of external morphology as described [9, 25]. For fetal samples (9PCW) age was estimated from measurements of foot length and heel to knee length. These were compared with a standard growth chart [15].
2.2 In situ hybridization
Three fragments of the cDNA for human PLA2G6; 453 bp of PLA2G6 exon 2- exon4 (probe 1), 517 bp of PLA2G6 exon 11–15 (probe2) and 557 bp of the PLA2G6 3’UTR (probe3) were amplified from Homo sapiens PLA2G6 mRNA (Accession: CU013143) and cloned into pCR-BluntII-TOPO (Invitrogen). The construct sequences were verified and prepared using HiSpeed Plasmid Midi kit (Qiagen). To create the PLA2G6 antisense probe, the plasmid was linearized with SpeI and amplified from the T7 promoter. To create the PLA2G6 sense control probe, the plasmid was linearized with Not1 and amplified from the Sp6 promoter. Probes were labeled with digoxygenin (DIG) using the DIG-RNA labeling kit (Roche Applied Science) according to manufacturer’s instructions. All probes were tested and PLA2G6 probe 3 was selected for the studies subsequently described.
In situ hybridizations were performed as previously described [22] with some modifications. Briefly, sections were dewaxed in xylene, gradually hydrated in decreasing ethanol concentrations before incubation in Proteinase K (20µg/ml) at room temperature (RT), followed by fixation in 4% paraformaldehyde in PBS. Background was reduced by treating with 0.1M Triethanolamine pH 8. Sections were air dried and probe added (300ng labeled probe per 100ul of Dig Easy Hyb mix (Roche)) at 68°C overnight. The next day sections were washed once in 5× SSC then once in 2× SSC at 60°C then incubated with Anti-digoxigenin AP Fab fragments (Roche) diluted 1:1000 at 4°C overnight. Sections were then washed and expression detected using NBT/BCIP (20ul/ml Roche) in 0.1M Tris/0.1M NaCl (pH 9.5) in the dark at RT. Developing was stopped by rinsing slides first in 0.1M Tris/0.1M NaCl (pH 9.5) then in distilled H2O. Sections were mounted using Aquamount and analyzed using a Zeiss Axioplan 2 microscope. Images were captured with a Zeiss Axiovision 4 imaging system.
3. RESULTS
In order to characterize the potential role of PLA2G6 in fetal development, we examined PLA2G6 expression by in situ hybridization during human embryonic (CS19 and CS23) and early fetal (9 PCW) development.
3.1 PLA2G6 expression in the brain
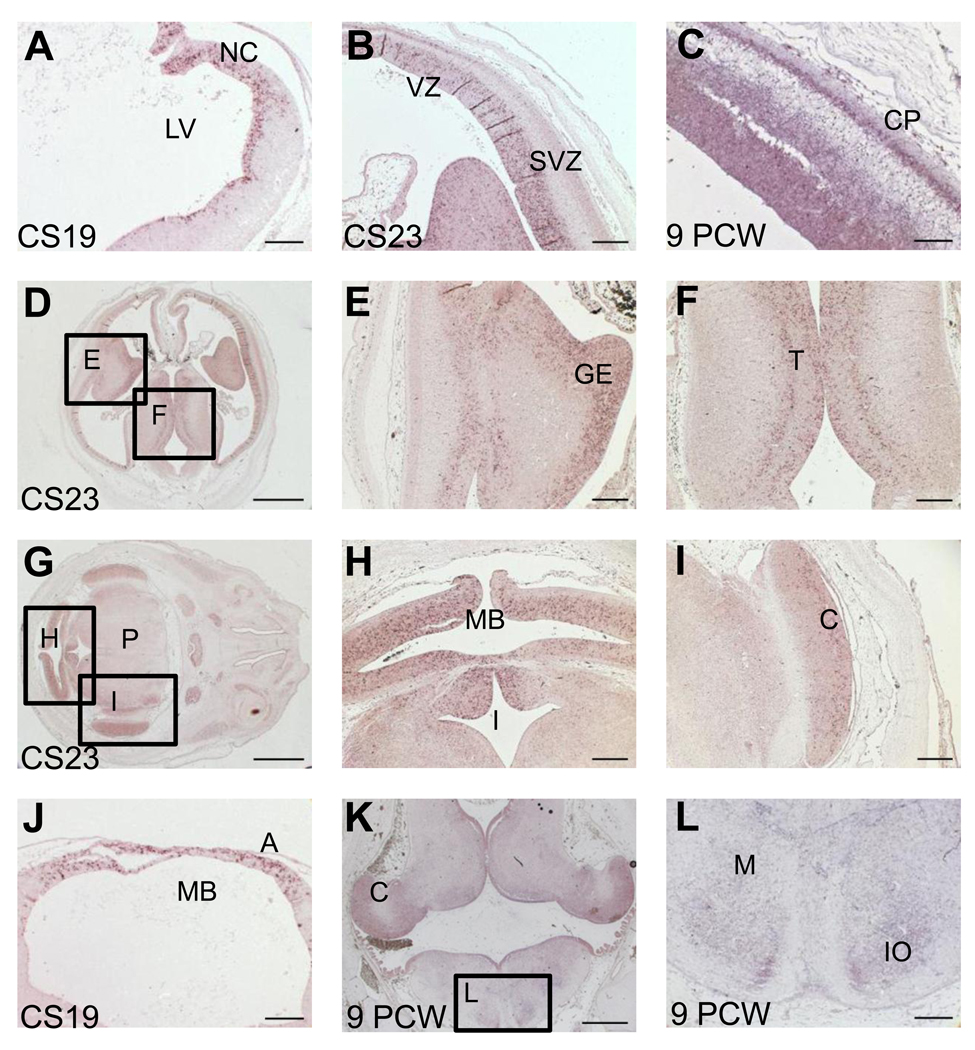
At CS19, PLA2G6 expression was evident in the VZ of the pallium neuroepithelium (NEP) in the developing telencephalon (Figure 1A). The NEP is a pluripotent pseudostratified tissue of neural stem cells that extends from the frontal pole to the spinal cord and is the source of all neurons and neuroglia of the developing nervous system [7]. By CS23, PLA2G6 expression is detected in both the VZ and SVZ of the pallium, while by 9PCW strong signal is also detected in the differentiating neurons of the cortical plate (Figure 1B and C respectively). Strong expression is also seen in proliferative zones in other regions of the developing forebrain such as the ganglionic eminences (Figure 1B, D, E) and thalamus (Figure 1D, F).
Figure 1.
PLA2G6 expression in human brain at CS19, CS23 and 9 PCW. Panels A–L show images of in situ hybridization with antisense probes for PLA2G6 to sections at CS19 (A, J), CS23 (B, D–I) and 9PCW (C, K, L). Signal is detected as a purple stain. A)–C) PLA2G6 staining in developing neocortex. At CS19 (A), expression can be seen close to the lateral ventricle (VZ), by CS23 (B) expression is in the VZ and SVZ and by 9PCW (C) expression is also seen in the CP. D) Low magnification image showing developing neocortex, ganglionic eminences and thalamus. The boxed areas are shown at higher magnification in panels E and F. G) Low magnification image showing developing midbrain, isthmus, cerebellum and pons. The boxed areas are shown at higher magnification in panels H and I. J) PLA2G6 expression is seen in the alar plate of the developing midbrain. K) PLA2G6 expression in the hindbrain is seen in the developing cerebellum, the VZ of medulla and neurons in developing nuclei (IO). Boxed area is seen at higher magnification in panel L. No signal was detected using sense control probes (supplemental figure 1). C- cerebellum, CP- cortical plate; GE- ganglionic eminences, IO- inferior olive, I- isthmus, LV- lateral ventricle, M- medulla, MB- midbrain, NC- neocortex, P= pons, SVZ- subventricular zone, T- thalamus, VZ- ventricular zone. Scale bars are: 100µm in A,C,J,L; 200µm in B,E,F,H,I; 1000µm in D,G,K.
In the midbrain, PLA2G6 expression is detectable in the VZ of the alar plate at CS19 (Figure 1J), CS23 (Figure 1H) and persists through to 9PCW (data not shown). In contrast, expression in the hindbrain is barely detectable at CS19 (data not shown). Yet at CS23 (Figure 1H and I) and 9PCW (Figure 1K and L), PLA2G6 is expressed in the VZ in the isthmus, cerebellum, pons and medulla oblongata and clearly present in differentiating neurons of developing nuclei in the medulla (Fig 1K and L). PLA2G6 expression is also seen in the telencephalic choroid plexus, a glycogen-rich epithelial tissue formed by proliferative stem cells that may play a part in anaerobic metabolism during early development [7] (data not shown).
3.2 PLA2G6 expression in the eye and spinal cord
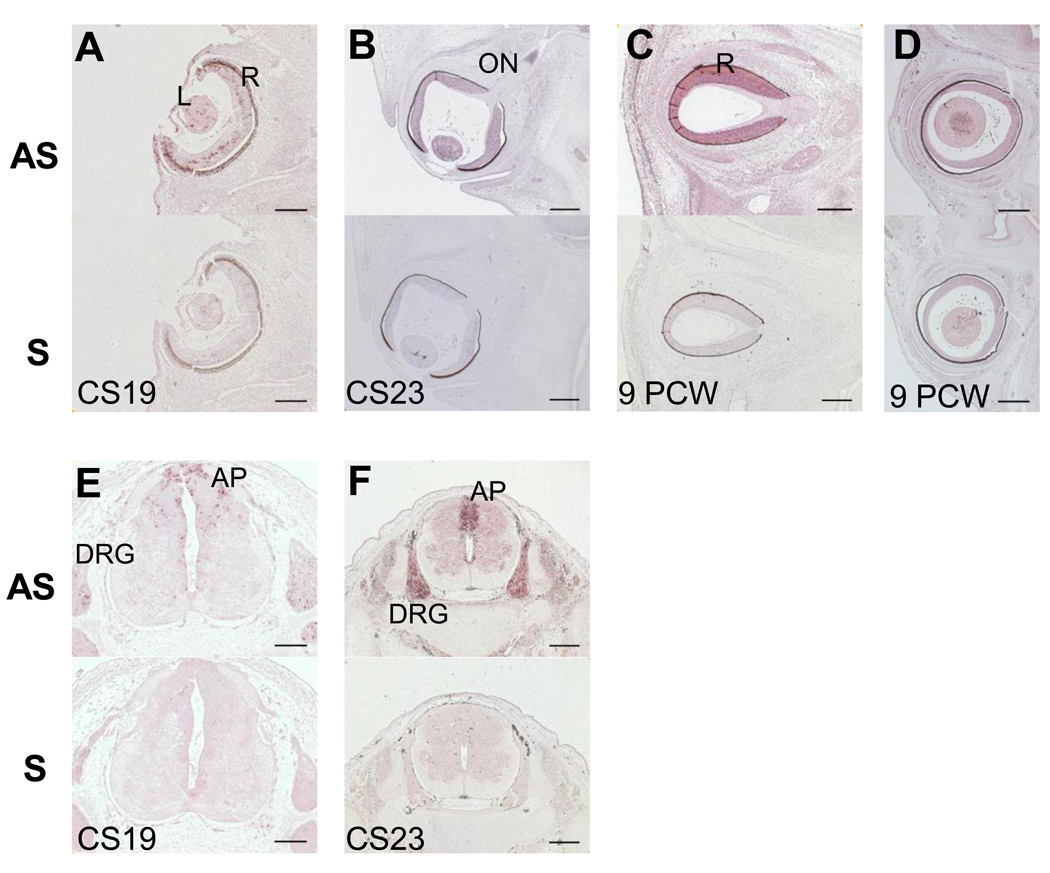
PLA2G6 expression was found in the lens and developing neurosensory retina at CS19, CS23 and PCW9 (Figure 2). Expression in the retina was sparse initially but became stronger and more widespread throughout the developing retina by 9PCW. Interestingly, in one CS23 sample, we also observed strong staining in the optic nerve (Figure 2B). The optic nerve comprises axons from retinal ganglion cells and glial precursors: astrocyte precursors arising from the optic stalk and oligodendrocyte precursors from the preoptic area [27, 35]. However, the optic nerve only begins to be myelinated at five months of gestation [13]. PLA2G6 expression was detected in the spinal cord, principally in the alar plate, as well as in the dorsal root ganglia at CS19 and CS23. Spinal cord expression was not analyzed at 9PCW; however, we did observe it throughout the spinal cord and in dorsal root ganglia at 13PCW (data not shown).
Figure 2.
PLA2G6 expression in human eye and spinal cord at CS19 (A,E) CS23 (B, F) and 9PCW (C,D). Each panel has 2 images, the top one showing in situ hybridization using antisense (AS) probes and the lower one using sense (S) probes for PLA2G6. No signal was detected using sense control probes A) PLA2G6 staining in retina and lens at CS19. B) PLA2G6 staining in lens, retina and optic nerve at CS23. C–D) PLA2G6 staining in retina at PCW9. E) PLA2G6 staining in dorsal root ganglia at CS19. F) PLA2G6 staining in dorsal root ganglia at 9PCW A- alar plate, AS- antisense, DRG- dorsal root ganglia, L- lens, ON- optic nerve, R-retina, S-sense, Scale bars are: 100µm in A,E; 200µm in B,C,D,F.
3.3 PLA2G6 in non-neuronal tissues
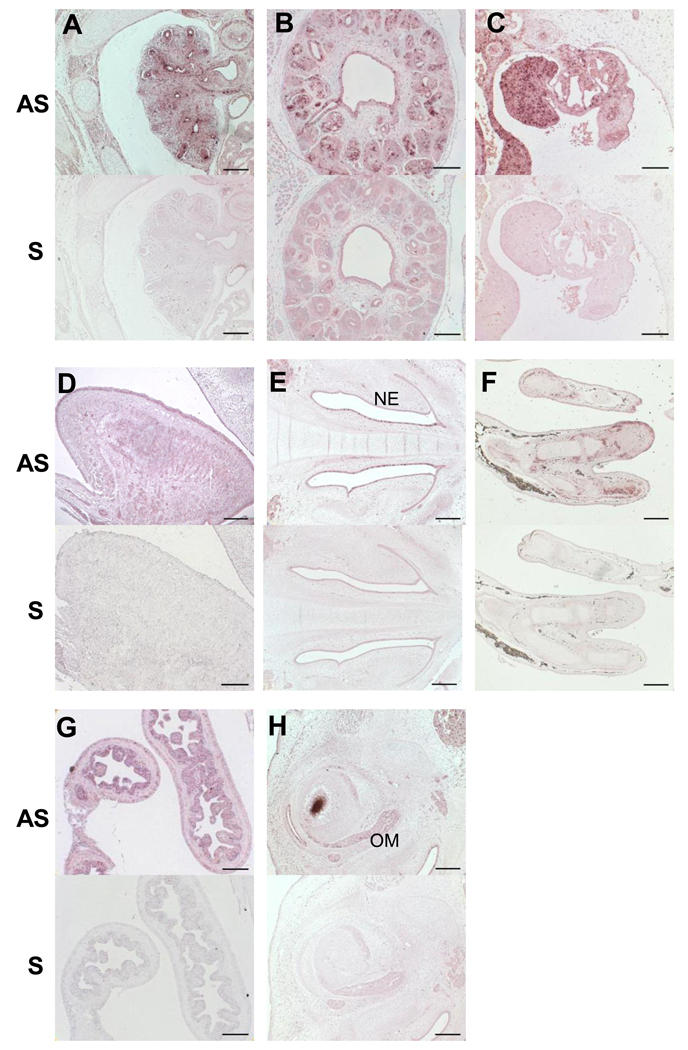
Results from RT-PCR experiments in a battery of RNAs from different tissues at 9PCW suggested that PLA2G6 was expressed widely in non-neuronal tissues (data not shown). This was confirmed by tissue in situ hybridisation as shown in Figure 3. PLA2G6 expression was detected in lung (Figure 3A), kidney (both metanephros and mesonephros Figure 3B and C respectively), gonad (Figure 3C), muscles in the tongue and surrounding the eye (Figure 3 D and 3H), nasal epithelium (Figure 3E), surrounding the developing bones (Figure 3F) and in the intestine (Figure 3G).
Figure 3.
Expression of PLA2G6 in non-neuronal tissues. Each panel has 2 images, the top one showing in situ hybridization using AS probes and the lower one using S probes for PLA2G6. No signal was detected using sense control probes. Panels A, C, E and F are CS23 sections while Panels B, D and G sections at 9PCW. A) Lung B) Metanephros C) Gonad and mesonephros D) Tongue E) Nasal area F) Upper limb G) Intestine H) Eye muscle. AS- antisense, NE- nasal epithelium, OM=- ocular muscles, S- sense. Scale bars are: 100µm in B,C,D,G; 200µm in A,E,F,H.
4. DISCUSSION
The present study was carried out to characterize PLA2G6 expression in the developing human embryonic and fetal nervous system. We found that PLA2G6 has a dynamic expression pattern both in terms of the location of expression and the differentiation state of expressing cells. PLA2G6 is expressed in forebrain and midbrain before it is detectable in hindbrain. Throughout the developing brain, PLA2G6 is expressed in proliferative zones: VZ in all regions, as well as the SVZ in the developing telencephalon including the ganglionic eminences. In the diencephalon there is strong expression in the thalamus and hypothalamus. This expression pattern is in contrast to the only available comparison data derived from studies in developing mouse; in murine studies VZ expression in the alar plate of the midbrain and hindbrain has been described, but none is seen in the VZ of the forebrain (diencephalon or telencephalon) and there is only weak expression in the outer cortical plate/mantle layer in the developing neocortex (EMAGE entry ID 18166). However, this mouse data is only from a single stage and does not capture the dynamic changes in expression pattern seen in human as described above. In our studies of the human forebrain, PLA2G6 was also detected in the differentiated neurons of the cortical plate in the developing cerebral neocortex. Similarly, expression is seen in differentiated neurons in the hindbrain; for example, in the inferior olives of the medulla. At the stages examined, there is no PLA2G6 expression in differentiated neurons of the midbrain.
Broad expression of iPLA2 in the brain has also been reported in adult mammals. In adult monkey brain, high iPLA2 expression was detected in the cerebral neocortex, hippocampus, cerebellum and brain stem, with lower expression in the thalamus and hypothalamus [26]. Within the basal ganglia, the caudate nucleus, putamen and nucleus accumbens were densely labeled while the globus pallidus, subthalamic nucleus and substantia nigra pars compacta were lightly labeled [26]. Within these tissues, iPLA2 expression was predominately localized to the nuclear envelope of neurons, dendritic spines or axon terminals, while expression was sparse or absent in myelinated axons, large diameter dendrites, glial cells and endothelial cells [26]. iPLA2 expression has also been reported in rat cerebellum, with strong expression in the nuclei of Purkinje cells and granule and stellate cells [34]. These findings are consistent with an ongoing role for iPLA2 in signal transduction and lipid metabolism in a variety of CNS cell types In the adult eye, iPLA2-VIA expression has previously been reported as very strong in the retinal pigment epithelium, strong in the optic nerve axons and cornea, and moderate in the iris, ciliary body and lens [17]. Interestingly, iPLA2-VIA is proposed to regulate RPE proliferation and migration, and may play a role in proliferative vitreoretinopathy [16]. Our data indicates that PLA2G6 is expressed in retina and lens as early as CS19 (approx 7 PCW). In addition, we observed expression in the sheath of the optic nerve in one sample. Although retinopathy is not common in PLA2G6-associated disease, optic atrophy is typical [14]. Indeed, Kurian et al. observed reduced volume in the optic chiasm and optic nerves on radiographical imaging in eight out of ten PLA2G6 mutation-positive patients [18], consistent with an important postnatal role for PLA2G6 in maintaining optic nerve integrity.
A survey of non-neuronal embryonic tissues revealed broad PLA2G6 expression. Previously, PLA2G6 was detected in all 23 adult human tissues examined by Northern blot [19]. In our study, strong expression was detected surrounding the developing bones of the arm and in the lung, kidney, gut and muscles of the tongue and surrounding the eye. At the stages examined, we did not detect strong expression in the developing bones of the face and skull. Again, this result is in contrast with the available data in developing mouse. However, the latter data are limited and differences between the species may be temporal rather than spatial. In other studies, iPLA2-VIA is shown to be expressed in numerous cell lines including macrophages [1], pancreatic cells [31], astrocytes [30] and renal cells [11]. Broad PLA2G6 expression is consistent with its role in membrane phospholipid maintenance and divergent signaling pathways [3].
Several disorders with predominantly neurodevelopmental defects are caused by genes with a widespread expression pattern during human development [21, 37]. In addition, degenerative neurological disorders can be caused by mutations in a widely expressed gene, exemplified by Rett syndrome and pantothenate kinase-associated neurodegeneration [32, 40]. Similarly, in several trinucleotide repeat disorders, expanded polyglutamine proteins are ubiquitously expressed but cause selective neurodegeneration in specific brain regions [28].
Our results suggest that PLA2G6 is positioned to play a role in early neuronal development. Disruption of this process may contribute to the widespread neurological problems observed in INAD. In fact, embryonic and fetal PLA2G6 expression suggests that INAD pathogenesis could initiate in-utero. Several INAD mouse models are available to further investigate the effect of PLA2G6 mutations on embryonic development [8, 20, 33, 39]. Certainly, in-utero PLA2G6 function should be considered when treatment strategies for INAD are in development.
Supplementary Material
Supplemental Figure 1 No signal is detected with PLA2G6 sense control probes. Panels A–L show images of in situ hybridization with sense probes for PLA2G6 to sections at CS19 (A, J), CS23 (B, D–I) and 9PCW (C, K, L). A)–C) Developing neocortex. D) Low magnification image showing developing neocortex, ganglionic eminences and thalamus. The boxed areas are shown at higher magnification in panels E and F. G) Low magnification image showing developing midbrain, isthmus, cerebellum and pons. The boxed areas are shown at higher magnification in panels H and I. J) Developing midbrain. K) Developing cerebellum and medulla. Boxed area is seen at higher magnification in L. A- alar plate C- cerebellum, CP- cortical plate; GE- ganglionic eminences, IO- inferior olive, I- isthmus, LV- lateral ventricle, M- medulla, MB- midbrain, NC- neocortex, P= pons, SVZ- subventricular zone, T- thalamus, VZ- ventricular zone. Scale bars are: 100µm in A,C,J,L; 200µm in B,E,F,H,I; 1000µm in D,G,K.
Acknowledgements
This work was supported by National Institute of Child Health and Human Development (to S.H.), the Neurodegeneration with Brain Iron Accumulation (NBIA) Disorders Association (to S.H), the Association Internationale De Dystrophie Neuro Axonale Infantile (AIDNAI) (to S.H.) and the Huebner Family Pediatric Neurobiology of Disease Fellowship (to B.P.). The human embryonic and fetal material was provided by the Joint MRC-Wellcome Trust Human Developmental Biology Resource (http://www.hdbr.org) at the IHG, Newcastle-upon-Tyne, UK.
Abbreviations
- INAD
infantile neuroaxonal dystrophy
- NBIA
neurodegeneration with brain iron accumulation
- PCW
post weeks conception
- PKAN
pantothenate-kinase associated neurodegeneration
- SVZ
subventricular zone
- VZ
ventricular zone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Ackermann EJ, Kempner ES, Dennis EA. Ca(2+)-independent cytosolic phospholipase A2 from macrophage-like P388D1 cells. Isolation and characterization. J Biol Chem. 1994;269:9227–9233. [PubMed] [Google Scholar]
- 2.Balboa MA, Saez Y, Balsinde J. Calcium-independent phospholipase A2 is required for lysozyme secretion in U937 promonocytes. J Immunol. 2003;170:5276–5280. doi: 10.4049/jimmunol.170.10.5276. [DOI] [PubMed] [Google Scholar]
- 3.Balsinde J, Balboa MA. Cellular regulation and proposed biological functions of group VIA calcium-independent phospholipase A2 in activated cells. Cell Signal. 2005;17:1052–1062. doi: 10.1016/j.cellsig.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Balsinde J, Perez R, Balboa MA. Calcium-independent phospholipase A2 and apoptosis. Biochim Biophys Acta. 2006;1761:1344–1350. doi: 10.1016/j.bbalip.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Bao S, Miller DJ, Ma Z, Wohltmann M, Eng G, Ramanadham S, Moley K, Turk J. Male mice that do not express group VIA phospholipase A2 produce spermatozoa with impaired motility and have greatly reduced fertility. J Biol Chem. 2004;279:38194–38200. doi: 10.1074/jbc.M406489200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basselin M, Rosa AO, Ramadan E, Cheon Y, Chang L, Chen M, Greenstein D, Wohltmann M, Turk J, Rapoport SI. Imaging decreased brain docosahexaenoic acid metabolism and signaling in iPLA2{beta} (VIA)-deficient mice. J Lipid Res. 2010 doi: 10.1194/jlr.M008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayer SA. The Human Brain during the Late First Trimester. Taylor & Francis. 2006 [Google Scholar]
- 8.Bouley DM, McIntire JJ, Harris BT, Tolwani RJ, Otto GM, DeKruyff RH, Hayflick SJ. Spontaneous murine neuroaxonal dystrophy: a model of infantile neuroaxonal dystrophy. J Comp Pathol. 2006;134:161–170. doi: 10.1016/j.jcpa.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bullen P, Wilson D. The Carnegie staging of human embryos: a practical guide. In: Strachan T, Lindsay S, Wilson D, editors. Molecular genetics of early human development. Oxford: BIOS Scientific Publishers; 1997. pp. 27–50. [Google Scholar]
- 10.Carnevale KA, Cathcart MK. Calcium-independent phospholipase A(2) is required for human monocyte chemotaxis to monocyte chemoattractant protein 1. J Immunol. 2001;167:3414–3421. doi: 10.4049/jimmunol.167.6.3414. [DOI] [PubMed] [Google Scholar]
- 11.Cummings BS, Gelasco AK, Kinsey GR, McHowat J, Schnellmann RG. Inactivation of endoplasmic reticulum bound Ca2+-independent phospholipase A2 in renal cells during oxidative stress. J Am Soc Nephrol. 2004;15:1441–1451. doi: 10.1097/01.asn.0000127923.57438.ec. [DOI] [PubMed] [Google Scholar]
- 12.Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- 13.Edward DP, Kaufman LM. Anatomy, development, and physiology of the visual system. Pediatr Clin North Am. 2003;50:1–23. doi: 10.1016/s0031-3955(02)00132-3. [DOI] [PubMed] [Google Scholar]
- 14.Gregory A, Westaway SK, Holm IE, Kotzbauer PT, Hogarth P, Sonek S, Coryell JC, Nguyen TM, Nardocci N, Zorzi G, Rodriguez D, Desguerre I, Bertini E, Simonati A, Levinson B, Dias C, Barbot C, Carrilho I, Santos M, Malik I, Gitschier J, Hayflick SJ. Neurodegeneration associated with genetic defects in phospholipase A(2) Neurology. 2008;71:1402–1409. doi: 10.1212/01.wnl.0000327094.67726.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hern WM. Correlation of fetal age and measurements between 10 and 26 weeks of gestation. Obstet Gynecol. 1984;63:26–32. [PubMed] [Google Scholar]
- 16.Kolko M, Kiilgaard JF, Wang J, Poulsen KA, Andreasen JR, la Cour M, Nissen MH, Heegaard S, Bazan NG, Prause JU. Calcium-independent phospholipase A2 regulates retinal pigment epithelium proliferation and may be important in the pathogenesis of retinal diseases. Exp Eye Res. 2009;89:383–391. doi: 10.1016/j.exer.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Kolko M, Wang J, Zhan C, Poulsen KA, Prause JU, Nissen MH, Heegaard S, Bazan NG. Identification of intracellular phospholipases A2 in the human eye: involvement in phagocytosis of photoreceptor outer segments. Invest Ophthalmol Vis Sci. 2007;48:1401–1409. doi: 10.1167/iovs.06-0865. [DOI] [PubMed] [Google Scholar]
- 18.Kurian MA, Morgan NV, MacPherson L, Foster K, Peake D, Gupta R, Philip SG, Hendriksz C, Morton JEV, Kingston HM, Rosser EM, Wassmer E, Gissen P, Maher ER. Phenotypic spectrum of neurodegeneration associated with mutations in the PLA2G6 gene (PLAN) Neurology. 2008;70:1623–1629. doi: 10.1212/01.wnl.0000310986.48286.8e. [DOI] [PubMed] [Google Scholar]
- 19.Larsson Forsell PK, Kennedy BP, Claesson HE. The human calcium-independent phospholipase A2 gene multiple enzymes with distinct properties from a single gene. Eur J Biochem. 1999;262:575–585. doi: 10.1046/j.1432-1327.1999.00418.x. [DOI] [PubMed] [Google Scholar]
- 20.Malik I, Turk J, Mancuso DJ, Montier L, Wohltmann M, Wozniak DF, Schmidt RE, Gross RW, Kotzbauer PT. Disrupted membrane homeostasis and accumulation of ubiquitinated proteins in a mouse model of infantile neuroaxonal dystrophy caused by PLA2G6 mutations. Am J Pathol. 2008;172:406–416. doi: 10.2353/ajpath.2008.070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyake N, Chilton J, Psatha M, Cheng L, Andrews C, Chan WM, Law K, Crosier M, Lindsay S, Cheung M, Allen J, Gutowski NJ, Ellard S, Young E, Iannaccone A, Appukuttan B, Stout JT, Christiansen S, Ciccarelli ML, Baldi A, Campioni M, Zenteno JC, Davenport D, Mariani LE, Sahin M, Guthrie S, Engle EC. Human CHN1 mutations hyperactivate alpha2-chimaerin and cause Duane's retraction syndrome. Science. 2008;321:839–843. doi: 10.1126/science.1156121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moorman AF, Houweling AC, de Boer PA, Christoffels VM. Sensitive nonradioactive detection of mRNA in tissue sections: novel application of the whole-mount in situ hybridization protocol. J Histochem Cytochem. 2001;49:1–8. doi: 10.1177/002215540104900101. [DOI] [PubMed] [Google Scholar]
- 23.Morgan NV, Westaway SK, Morton JE, Gregory A, Gissen P, Sonek S, Cangul H, Coryell J, Canham N, Nardocci N, Zorzi G, Pasha S, Rodriguez D, Desguerre I, Mubaidin A, Bertini E, Trembath RC, Simonati A, Schanen C, Johnson CA, Levinson B, Woods CG, Wilmot B, Kramer P, Gitschier J, Maher ER, Hayflick SJ. PLA2G6, encoding a phospholipase A2, is mutated in neurodegenerative disorders with high brain iron. Nat Genet. 2006;38:752–754. doi: 10.1038/ng1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nardocci N, Zorzi G, Farina L, Binelli S, Scaioli W, Ciano C, Verga L, Angelini L, Savoiardo M, Bugiani O. Infantile neuroaxonal dystrophy: clinical spectrum and diagnostic criteria. Neurology. 1999;52:1472–1478. doi: 10.1212/wnl.52.7.1472. [DOI] [PubMed] [Google Scholar]
- 25.O'Rahilly R, Müller F, Streeter GL. Developmental stages in human embryos : including a revision of Streeter's "Horizons" and a survey of the Carnegie collection. Washington, D.C.: Carnegie Institution of Washington; 1987. [Google Scholar]
- 26.Ong WY, Yeo JF, Ling SF, Farooqui AA. Distribution of calcium-independent phospholipase A2 (iPLA 2) in monkey brain. J Neurocytol. 2005;34:447–458. doi: 10.1007/s11068-006-8730-4. [DOI] [PubMed] [Google Scholar]
- 27.Ono K, Yasui Y, Rutishauser U, Miller RH. Focal ventricular origin and migration of oligodendrocyte precursors into the chick optic nerve. Neuron. 1997;19:283–292. doi: 10.1016/s0896-6273(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 28.Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 29.Paisan-Ruiz C, Bhatia KP, Li A, Hernandez D, Davis M, Wood NW, Hardy J, Houlden H, Singleton A, Schneider SA. Characterization of PLA2G6 as a locus for dystonia-parkinsonism. Ann Neurol. 2009;65:19–23. doi: 10.1002/ana.21415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson B, Knotts T, Cummings BS. Involvement of Ca2+-independent phospholipase A2 isoforms in oxidant-induced neural cell death. Neurotoxicology. 2007;28:150–160. doi: 10.1016/j.neuro.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Ramanadham S, Hsu FF, Zhang S, Jin C, Bohrer A, Song H, Bao S, Ma Z, Turk J. Apoptosis of insulin-secreting cells induced by endoplasmic reticulum stress is amplified by overexpression of group VIA calcium-independent phospholipase A2 (iPLA2 beta) and suppressed by inhibition of iPLA2 beta. Biochemistry. 2004;43:918–930. doi: 10.1021/bi035536m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahbazian MD, Antalffy B, Armstrong DL, Zoghbi HY. Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlate with neuronal maturation. Hum Mol Genet. 2002;11:115–124. doi: 10.1093/hmg/11.2.115. [DOI] [PubMed] [Google Scholar]
- 33.Shinzawa K, Sumi H, Ikawa M, Matsuoka Y, Okabe M, Sakoda S, Tsujimoto Y. Neuroaxonal dystrophy caused by group VIA phospholipase A2 deficiency in mice: a model of human neurodegenerative disease. J Neurosci. 2008;28:2212–2220. doi: 10.1523/JNEUROSCI.4354-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shirai Y, Ito M. Specific differential expression of phospholipase A2 subtypes in rat cerebellum. J Neurocytol. 2004;33:297–307. doi: 10.1023/B:NEUR.0000044191.83858.f7. [DOI] [PubMed] [Google Scholar]
- 35.Small RK, Riddle P, Noble M. Evidence for migration of oligodendrocyte--type-2 astrocyte progenitor cells into the developing rat optic nerve. Nature. 1987;328:155–157. doi: 10.1038/328155a0. [DOI] [PubMed] [Google Scholar]
- 36.Sun GY, Shelat PB, Jensen MB, He Y, Sun AY, Simonyi A. Phospholipases A2 and Inflammatory Responses in the Central Nervous System. Neuromolecular Med. 2009 doi: 10.1007/s12017-009-8092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tischfield MA, Baris HN, Wu C, Rudolph G, Van Maldergem L, He W, Chan WM, Andrews C, Demer JL, Robertson RL, Mackey DA, Ruddle JB, Bird TD, Gottlob I, Pieh C, Traboulsi EI, Pomeroy SL, Hunter DG, Soul JS, Newlin A, Sabol LJ, Doherty EJ, de Uzcategui CE, de Uzcategui N, Collins ML, Sener EC, Wabbels B, Hellebrand H, Meitinger T, de Berardinis T, Magli A, Schiavi C, Pastore-Trossello M, Koc F, Wong AM, Levin AV, Geraghty MT, Descartes M, Flaherty M, Jamieson RV, Moller HU, Meuthen I, Callen DF, Kerwin J, Lindsay S, Meindl A, Gupta ML, Jr, Pellman D, Engle EC. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell. 140:74–87. doi: 10.1016/j.cell.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Visel A, Thaller C, Eichele G. GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res. 2004;32:D552–D556. doi: 10.1093/nar/gkh029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wada H, Yasuda T, Miura I, Watabe K, Sawa C, Kamijuku H, Kojo S, Taniguchi M, Nishino I, Wakana S, Yoshida H, Seino K. Establishment of an improved mouse model for infantile neuroaxonal dystrophy that shows early disease onset and bears a point mutation in Pla2g6. Am J Pathol. 2009;175:2257–2263. doi: 10.2353/ajpath.2009.090343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou B, Westaway SK, Levinson B, Johnson MA, Gitschier J, Hayflick SJ. A novel pantothenate kinase gene (PANK2) is defective in Hallervorden- Spatz syndrome. Nat Genet. 2001;28:345–349. doi: 10.1038/ng572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 No signal is detected with PLA2G6 sense control probes. Panels A–L show images of in situ hybridization with sense probes for PLA2G6 to sections at CS19 (A, J), CS23 (B, D–I) and 9PCW (C, K, L). A)–C) Developing neocortex. D) Low magnification image showing developing neocortex, ganglionic eminences and thalamus. The boxed areas are shown at higher magnification in panels E and F. G) Low magnification image showing developing midbrain, isthmus, cerebellum and pons. The boxed areas are shown at higher magnification in panels H and I. J) Developing midbrain. K) Developing cerebellum and medulla. Boxed area is seen at higher magnification in L. A- alar plate C- cerebellum, CP- cortical plate; GE- ganglionic eminences, IO- inferior olive, I- isthmus, LV- lateral ventricle, M- medulla, MB- midbrain, NC- neocortex, P= pons, SVZ- subventricular zone, T- thalamus, VZ- ventricular zone. Scale bars are: 100µm in A,C,J,L; 200µm in B,E,F,H,I; 1000µm in D,G,K.