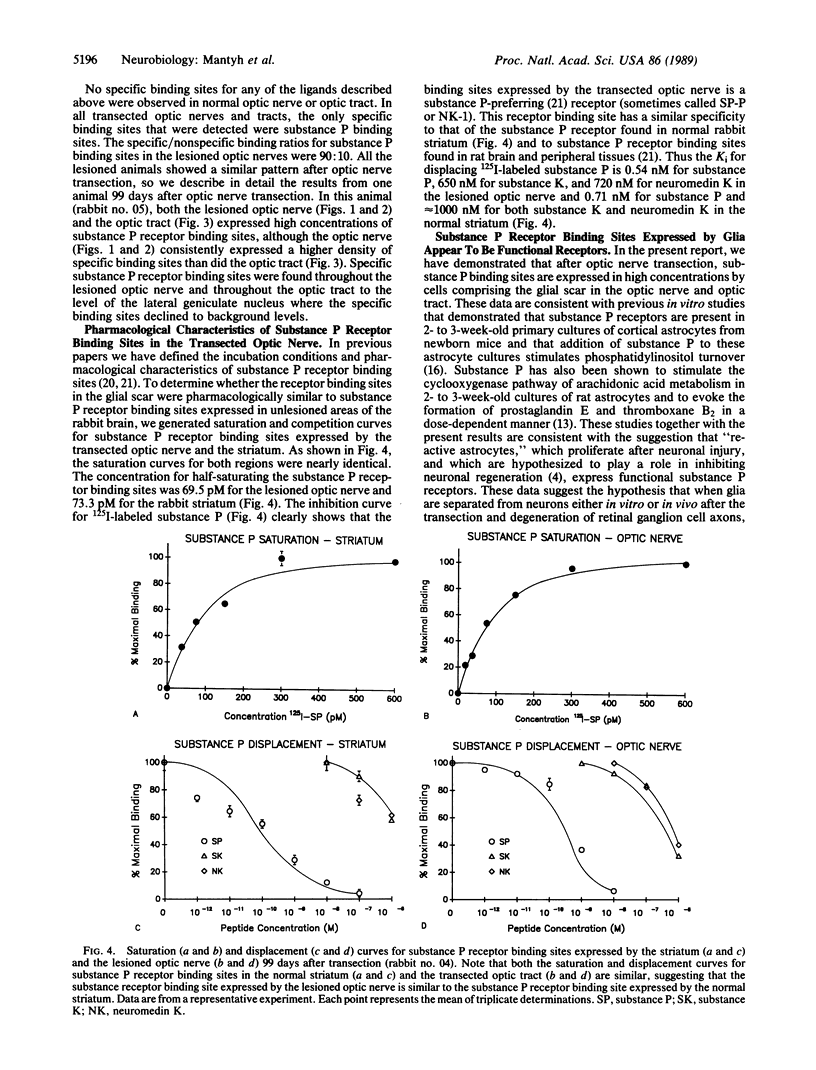
Abstract
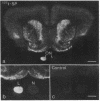
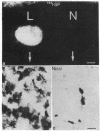
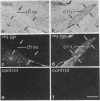
In vitro studies have demonstrated that glia can express functional receptors for a variety of neurotransmitters. To determine whether similar neurotransmitter receptors are also expressed by glia in vivo, we examined the glial scar in the transected optic nerve of the albino rabbit by quantitative receptor autoradiography. Receptor binding sites for radiolabeled calcitonin gene-related peptide, cholecystokinin, galanin, glutamate, somatostatin, substance P, and vasoactive intestinal peptide were examined. Specific receptor binding sites for each of these neurotransmitters were identified in the rabbit forebrain but were not detected in the normal optic nerve or tract. In the transected optic nerve and tract, only receptor binding sites for substance P were expressed at detectable levels. The density of substance P receptor binding sites observed in this glial scar is among the highest observed in the rabbit forebrain. Ligand displacement and saturation experiments indicate that the substance P receptor binding site expressed by the glial scar has pharmacological characteristics similar to those of substance P receptors in the rabbit striatum, rat brain, and rat and canine gut. The present study demonstrates that glial cells in vivo express high concentrations of substance P receptor binding sites after transection of retinal ganglion cell axons. Because substance P has been shown to regulate inflammatory and immune responses in peripheral tissues, substance P may also, by analogy, be involved in regulating the glial response to injury in the central nervous system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguayo A. J., David S., Bray G. M. Influences of the glial environment on the elongation of axons after injury: transplantation studies in adult rodents. J Exp Biol. 1981 Dec;95:231–240. doi: 10.1242/jeb.95.1.231. [DOI] [PubMed] [Google Scholar]
- Blalock J. E., Bost K. L., Smith E. M. Neuroendocrine peptide hormones and their receptors in the immune system. Production, processing and action. J Neuroimmunol. 1985 Nov;10(1):31–40. doi: 10.1016/0165-5728(85)90032-3. [DOI] [PubMed] [Google Scholar]
- Chneiweiss H., Glowinski J., Prémont J. Vasoactive intestinal polypeptide receptors linked to an adenylate cyclase, and their relationship with biogenic amine- and somatostatin-sensitive adenylate cyclases on central neuronal and glial cells in primary cultures. J Neurochem. 1985 Mar;44(3):779–786. doi: 10.1111/j.1471-4159.1985.tb12883.x. [DOI] [PubMed] [Google Scholar]
- David S., Miller R. H., Patel R., Raff M. C. Effects of neonatal transection on glial cell development in the rat optic nerve: evidence that the oligodendrocyte-type 2 astrocyte cell lineage depends on axons for its survival. J Neurocytol. 1984 Dec;13(6):961–974. doi: 10.1007/BF01148596. [DOI] [PubMed] [Google Scholar]
- Evans T., McCarthy K. D., Harden T. K. Regulation of cyclic AMP accumulation by peptide hormone receptors in immunocytochemically defined astroglial cells. J Neurochem. 1984 Jul;43(1):131–138. doi: 10.1111/j.1471-4159.1984.tb06688.x. [DOI] [PubMed] [Google Scholar]
- Eysel U. T., Peichl L. Regenerative capacity of retinal axons in the cat, rabbit, and guinea pig. Exp Neurol. 1985 Jun;88(3):757–766. doi: 10.1016/0014-4886(85)90086-x. [DOI] [PubMed] [Google Scholar]
- Greenamyre J. T., Young A. B., Penney J. B. Quantitative autoradiographic distribution of L-[3H]glutamate-binding sites in rat central nervous system. J Neurosci. 1984 Aug;4(8):2133–2144. doi: 10.1523/JNEUROSCI.04-08-02133.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung H. P., Heininger K., Schäfer B., Toyka K. V. Substance P and astrocytes: stimulation of the cyclooxygenase pathway of arachidonic acid metabolism. FASEB J. 1988 Jan;2(1):48–51. doi: 10.1096/fasebj.2.1.2446942. [DOI] [PubMed] [Google Scholar]
- Latov N., Nilaver G., Zimmerman E. A., Johnson W. G., Silverman A. J., Defendini R., Cote L. Fibrillary astrocytes proliferate in response to brain injury: a study combining immunoperoxidase technique for glial fibrillary acidic protein and radioautography of tritiated thymidine. Dev Biol. 1979 Oct;72(2):381–384. doi: 10.1016/0012-1606(79)90127-1. [DOI] [PubMed] [Google Scholar]
- Liuzzi F. J., Lasek R. J. Astrocytes block axonal regeneration in mammals by activating the physiological stop pathway. Science. 1987 Aug 7;237(4815):642–645. doi: 10.1126/science.3603044. [DOI] [PubMed] [Google Scholar]
- Lopez-Rivas A., Mendoza S. A., Nånberg E., Sinnett-Smith J., Rozengurt E. Ca2+-mobilizing actions of platelet-derived growth factor differ from those of bombesin and vasopressin in Swiss 3T3 mouse cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5768–5772. doi: 10.1073/pnas.84.16.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh C. R., Gates T. S., Zimmerman R. P., Welton M. L., Passaro E. P., Jr, Vigna S. R., Maggio J. E., Kruger L., Mantyh P. W. Receptor binding sites for substance P, but not substance K or neuromedin K, are expressed in high concentrations by arterioles, venules, and lymph nodules in surgical specimens obtained from patients with ulcerative colitis and Crohn disease. Proc Natl Acad Sci U S A. 1988 May;85(9):3235–3239. doi: 10.1073/pnas.85.9.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh C. R., Mantyh P. W. Differential localization of cholecystokinin-8 binding sites in the rat vs. the guinea pig brain. Eur J Pharmacol. 1985 Jul 11;113(1):137–139. doi: 10.1016/0014-2999(85)90356-5. [DOI] [PubMed] [Google Scholar]
- Mantyh P. W., Gates T., Mantyh C. R., Maggio J. E. Autoradiographic localization and characterization of tachykinin receptor binding sites in the rat brain and peripheral tissues. J Neurosci. 1989 Jan;9(1):258–279. doi: 10.1523/JNEUROSCI.09-01-00258.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh P. W., Pinnock R. D., Downes C. P., Goedert M., Hunt S. P. Correlation between inositol phospholipid hydrolysis and substance P receptors in rat CNS. 1984 Jun 28-Jul 4Nature. 309(5971):795–797. doi: 10.1038/309795a0. [DOI] [PubMed] [Google Scholar]
- Maurer R., Reubi J. C. Brain somatostatin receptor subpopulation visualized by autoradiography. Brain Res. 1985 Apr 29;333(1):178–181. doi: 10.1016/0006-8993(85)90142-8. [DOI] [PubMed] [Google Scholar]
- Melander T., Hökfelt T., Nilsson S., Brodin E. Visualization of galanin binding sites in the rat central nervous system. Eur J Pharmacol. 1986 May 27;124(3):381–382. doi: 10.1016/0014-2999(86)90247-5. [DOI] [PubMed] [Google Scholar]
- Miller R. H., Abney E. R., David S., Ffrench-Constant C., Lindsay R., Patel R., Stone J., Raff M. C. Is reactive gliosis a property of a distinct subpopulation of astrocytes? J Neurosci. 1986 Jan;6(1):22–29. doi: 10.1523/JNEUROSCI.06-01-00022.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S., Pearce B. Functional receptors for neurotransmitters on astroglial cells. Neuroscience. 1987 Aug;22(2):381–394. doi: 10.1016/0306-4522(87)90342-3. [DOI] [PubMed] [Google Scholar]
- Nilsson J., von Euler A. M., Dalsgaard C. J. Stimulation of connective tissue cell growth by substance P and substance K. Nature. 1985 May 2;315(6014):61–63. doi: 10.1038/315061a0. [DOI] [PubMed] [Google Scholar]
- Perrone M. H., Lepore R. D., Shain W. Identification and characterization of substance P receptors on LRM55 glial cells. J Pharmacol Exp Ther. 1986 Aug;238(2):389–395. [PubMed] [Google Scholar]
- Raff M. C., Abney E. R., Cohen J., Lindsay R., Noble M. Two types of astrocytes in cultures of developing rat white matter: differences in morphology, surface gangliosides, and growth characteristics. J Neurosci. 1983 Jun;3(6):1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougon G., Noble M., Mudge A. W. Neuropeptides modulate the beta-adrenergic response of purified astrocytes in vitro. Nature. 1983 Oct 20;305(5936):715–717. doi: 10.1038/305715a0. [DOI] [PubMed] [Google Scholar]
- Shaffer M. M., Moody T. W. Autoradiographic visualization of CNS receptors for vasoactive intestinal peptide. Peptides. 1986 Mar-Apr;7(2):283–288. doi: 10.1016/0196-9781(86)90226-3. [DOI] [PubMed] [Google Scholar]
- Torrens Y., Beaujouan J. C., Saffroy M., Daguet de Montety M. C., Bergström L., Glowinski J. Substance P receptors in primary cultures of cortical astrocytes from the mouse. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9216–9220. doi: 10.1073/pnas.83.23.9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp F. A., Henke H., Petermann J. B., Tobler P. H., Janzer R., Hökfelt T., Lundberg J. M., Cuello C., Fischer J. A. Calcitonin gene-related peptide and its binding sites in the human central nervous system and pituitary. Proc Natl Acad Sci U S A. 1985 Jan;82(1):248–252. doi: 10.1073/pnas.82.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas-Pérez M. P., Vidal-Sanz M., Bray G. M., Aguayo A. J. Influences of peripheral nerve grafts on the survival and regrowth of axotomized retinal ganglion cells in adult rats. J Neurosci. 1988 Jan;8(1):265–280. doi: 10.1523/JNEUROSCI.08-01-00265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock J. V., Blum A., Walder J., Walder R. Eosinophils from granulomas in murine schistosomiasis mansoni produce substance P. J Immunol. 1988 Aug 1;141(3):961–966. [PubMed] [Google Scholar]