Abstract
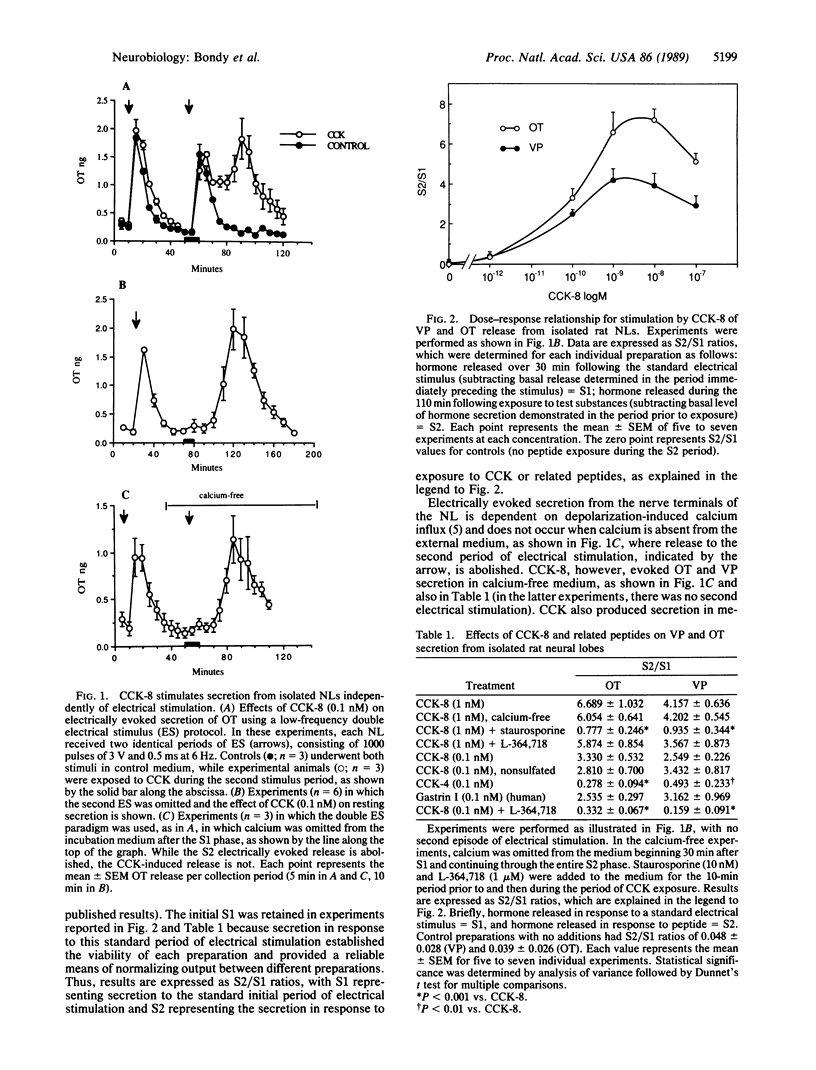
Cholecystokinin (CCK) and its receptors are abundantly represented in the central nervous system. However, a specific role or mechanism of action for CCK in this context has not been established. CCK coexists with oxytocin in magnocellular neurons of the hypothalamic-neurohypophysial system, sharing common neurosecretory vesicles with oxytocin in the neural lobe of the pituitary. The neural lobe, which consists primarily of oxytocin- and vasopressin-containing axons and nerve terminals and their surrounding glia, provides a relatively simple model system allowing for the study of the regulation of neurosecretion at the nerve terminal level, free from the complex array of synaptic effects present throughout the rest of the central nervous system. In this paper, we demonstrate the presence of high-affinity CCK binding sites in the rat neural lobe and show that activation of these receptors by the sulfated octapeptide, CCK-8, and related peptides causes potent secretion of oxytocin and vasopressin from the isolated nerve terminals. The secretagogue action of CCK-8, which is blocked by a CCK receptor antagonist (L-364,718), is independent of electrical stimulation and extracellular calcium and is blocked by an inhibitor of protein kinase C. Thus, the action of CCK on the neural lobe provides an example of peptide ligand-induced neurosecretion apparently mediated by second messengers rather than depolarization-induced calcium influx.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bondy C. A., Gainer H., Russell J. T. Dynorphin A inhibits and naloxone increases the electrically stimulated release of oxytocin but not vasopressin from the terminals of the neural lobe. Endocrinology. 1988 Apr;122(4):1321–1327. doi: 10.1210/endo-122-4-1321. [DOI] [PubMed] [Google Scholar]
- Bondy C. A., Gainer H., Russell J. T. Effects of stimulus frequency and potassium channel blockade on the secretion of vasopressin and oxytocin from the neurohypophysis. Neuroendocrinology. 1987 Sep;46(3):258–267. doi: 10.1159/000124829. [DOI] [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J. Biochemical and pharmacological characterization of an extremely potent and selective nonpeptide cholecystokinin antagonist. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4923–4926. doi: 10.1073/pnas.83.13.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley J. N. Comparative distribution of cholecystokinin and other neuropeptides. Why is this peptide different from all other peptides? Ann N Y Acad Sci. 1985;448:1–8. doi: 10.1111/j.1749-6632.1985.tb29900.x. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. STIMULUS-SECRETION COUPLING IN A NEUROSECRETORY ORGAN: THE ROLE OF CALCIUM IN THE RELEASE OF VASOPRESSIN FROM THE NEUROHYPOPHYSIS. J Physiol. 1964 Jul;172:1–18. doi: 10.1113/jphysiol.1964.sp007399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. D., Jensen R. T. Receptors and cell activation associated with pancreatic enzyme secretion. Annu Rev Physiol. 1986;48:103–117. doi: 10.1146/annurev.ph.48.030186.000535. [DOI] [PubMed] [Google Scholar]
- Hammond C., Paupardin-Tritsch D., Nairn A. C., Greengard P., Gerschenfeld H. M. Cholecystokinin induces a decrease in Ca2+ current in snail neurons that appears to be mediated by protein kinase C. 1987 Feb 26-Mar 4Nature. 325(6107):809–811. doi: 10.1038/325809a0. [DOI] [PubMed] [Google Scholar]
- Higashida H. Acetylcholine release by bradykinin, inositol 1,4,5-trisphosphate and phorbol dibutyrate in rodent neuroblastoma cells. J Physiol. 1988 Mar;397:209–222. doi: 10.1113/jphysiol.1988.sp016996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis R. B., Snyder S. H. Distinct cholecystokinin receptors in brain and pancreas. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6917–6921. doi: 10.1073/pnas.77.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley P. D., Lightman S. L., Forsling M. L., Todd K., Goedert M., Rehfeld J. F., Emson P. C. Localization and actions of cholecystokinin in the rat pituitary neurointermediate lobe. Endocrinology. 1984 May;114(5):1902–1911. doi: 10.1210/endo-114-5-1902. [DOI] [PubMed] [Google Scholar]
- Martin R., Geis R., Holl R., Schäfer M., Voigt K. H. Co-existence of unrelated peptides in oxytocin and vasopressin terminals of rat neurohypophyses: immunoreactive methionine-enkephalin-, leucine-enkephalin- and cholecystokinin-like substances. Neuroscience. 1983;8(2):213–227. doi: 10.1016/0306-4522(83)90061-1. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Petersen O. H. Cholecystokinin activation of single-channel currents is mediated by internal messenger in pancreatic acinar cells. Nature. 1982 Nov 4;300(5887):61–63. doi: 10.1038/300061a0. [DOI] [PubMed] [Google Scholar]
- Moran T. H., Robinson P. H., Goldrich M. S., McHugh P. R. Two brain cholecystokinin receptors: implications for behavioral actions. Brain Res. 1986 Jan 1;362(1):175–179. doi: 10.1016/0006-8993(86)91413-7. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F., Hansen H. F., Larsson L. I., Stengaard-Pedersen K., Thorn N. A. Gastrin and cholecystokinin in pituitary neurons. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1902–1905. doi: 10.1073/pnas.81.6.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisine T., Jensen R. Cholecystokinin-8 stimulates adrenocorticotropin release from anterior pituitary cells. J Pharmacol Exp Ther. 1986 Mar;236(3):621–626. [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Williams J. A., McChesney D. J., Calayag M. C., Lingappa V. R., Logsdon C. D. Expression of receptors for cholecystokinin and other Ca2+-mobilizing hormones in Xenopus oocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4939–4943. doi: 10.1073/pnas.85.13.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]