Abstract
Objective
To determine whether intra- and periarticular cyst-like lesions of the knee are associated with incident knee pain and incident radiographic knee osteoarthritis (OA).
Design
The Multicenter Osteoarthritis (MOST) Study is a cohort of individuals who have or are at high risk for knee OA. Using a nested case-control study design, we investigated the associations of cyst-like lesions (Baker’s, meniscal and proximal tibiofibular joint (PTFJ) cysts, and prepatellar and anserine bursitides) with (a) incident pain at 15- or 30-month follow-up and (b) incident radiographic OA at 30-month follow-up. Baseline cyst-like lesions were scored semiquantitatively using the Whole Organ Magnetic Resonance Imaging Score (WORMS). Conditional logistic regression models were used to assess the relation between these lesions and the outcomes, adjusting for potential confounding factors (i.e. cartilage loss, meniscal damage, bone marrow lesions, synovitis and joint effusion, which were also scored using WORMS).
Results
Incident knee pain study included 157 cases and 336 controls. Prevalence of meniscal and PTFJ cysts in the case group was twice that in the control group (9 (6%) vs. 9 (3%) and 9 (6%) vs. 10 (3%), respectively). Incident radiographic OA study included 149 cases and 298 controls. Prevalence of grade 2 Baker’s cysts and PTFJ cysts in the case group was approximately 4 times that in the control group (16 [11%] vs. 9 [3%] and 6 [4%] vs. 3 [1%], respectively). However, none of the cyst-like lesions was associated with incident pain or radiographic OA after fully adjusted logistic regression analyses and correction of p-values for multiple comparisons.
Conclusion
None of the analyzed lesions was an independent predictor of incident knee pain or radiographic OA. Intra- and periarticular cyst-like lesions are likely to be a secondary phenomenon seen in painful or OA-affected knees, rather than a primary trigger for incident knee pain or radiographic OA.
Keywords: Cysts, Bursitis, Magnetic Resonance Imaging, Osteoarthritis, Knee, Pain
Introduction
Benign cystic lesions are frequently encountered during routine magnetic resonance imaging (MRI) of the knee. Most intra- and periarticular cystic lesions are encapsulated fluid collections exhibiting low signal intensity on T1-weighted images and high signal intensity on T2-weighted images.1 These represent a wide variety of entities that include meniscal and Baker’s (popliteal) cysts; intraarticular and extraarticular ganglia; intraosseous cysts at the insertion of the cruciate ligaments and meniscotibial attachments; and cysts adjacent to the proximal tibiofibular joint.1–5
Cyst-like lesions arising from bursae include anserine, prepatellar, superficial and deep infrapatellar, suprapatellar, iliotibial, and tibial collateral ligament bursitis.1–5 Discrete fluid collections within bursae can be detected by knee MRI, but may not necessarily be clinically significant due to their high prevalence in asymptomatic subjects.6–7
Of the cystic lesions mentioned above, Baker’s cysts are the most frequently encountered.8 Other lesions are seen less frequently, e.g. meniscal cysts,9 proximal tibiofibular joint (PTFJ) cysts,6 prepatellar bursitis6 and anserine bursitis,7 all of which are observed in about 3–5% of both symptomatic and asymptomatic knees. To date, it is not known if intra- and periarticular cyst-like lesions (including bursitides, which are discrete fluid collections with cyst-like appearances) are related to risk for incident pain or radiographic OA. It is conceivable that these lesions might be related in that they sometimes provide the sole or most prominent evidence of pathology in tissues where pathology is linked to OA occurrence (e.g., meniscal cysts). In other cases (e.g. prepatellar bursitis), the cysts are extra-articular and, while they could cause pain, they are almost certainly unrelated to pathology inside the joint, and thus they would not be expected to increase the risk of OA but might be related to knee pain. Prediction of incident pain or incident knee OA on the basis of these lesions would be helpful with respect to preventive or early treatment.
In the present study, we focus on 5 types of cystic lesions that are commonly assessed semiquantitatively in large epidemiologic OA studies (two within the joint capsule: Baker’s cyst and meniscal cysts, and three outside the joint: PTFJ cysts, prepatellar bursitis and anserine bursitis). PTFJ cysts and anserine bursitis are associated with knee pain6 and prepatellar bursitis with radiographic knee OA.6 Baker’s cysts are associated with degenerative joint disease8 and advanced stages of OA10 but not pain.6, 11 Although a strong cross-sectional association between meniscal damage (with which meniscal cysts are usually associated9) and knee OA has been reported,12 there has been no report of a study evaluating the direct relationship between meniscal cysts and incident radiographic OA.
The aims of this study are to examine whether the presence of these cyst-like lesions at baseline is associated longitudinally with incident knee pain and incident radiographic knee OA.
Materials and Methods
Study Design and Subjects
The Multicenter Osteoarthritis (MOST) Study is a prospective cohort study of 3,026 individuals aged 50–79 years with or at high risk of knee OA. Those considered at high risk included persons who were overweight or obese, those with knee pain, aching or stiffness on most of the last 30 days, a history of knee injury that made it difficult to walk for at least 1 week, or previous knee surgery.
Subjects were recruited from two US communities, Birmingham, Alabama and Iowa City, Iowa through mass mailing of letters and study brochures, supplemented by media and community outreach campaigns. The study protocol was approved by the Institutional Review Boards at the University of Iowa, University of Alabama, Birmingham, University of California, San Francisco and Boston University Medical Campus.
Subjects were excluded from MOST if they screened positive for rheumatoid arthritis, had ankylosing spondylitis, psoriatic arthritis, Reiter’s syndrome, had renal insufficiency that required hemo- or peritoneal dialysis, a history of cancer (except for non-melanoma skin cancer), had or planned to have bilateral knee replacement surgery, were unable to walk without assistance, or were planning to move out of the area in the next 3 years.
In the present study, using a nested case-control study design, we investigated the prevalence of periarticular cysts and bursitides and the relation between these lesions and (a) incident pain and (b) incident radiographic OA among knees that were examined both at baseline and follow-up.
For the incident pain study, there were 157 case knees and 336 control (total 493) knees (one knee per subject). If a subject developed pain in only one knee, that knee was included as a case knee. One knee was randomly chosen if incident pain was present bilaterally. Of the 493 knees, 398 (81%) were recruited at 15 months, and an additional 95 (19%) were recruited at 30 months. Thus, this study is based on combined data of the 15 and 30 month follow-up visits. Those subjects who developed incident pain at 15 months did not have the 30-month visit included because they already had symptoms. It was therefore not possible to do our analyses for incident knee pain all based on the 30-month follow-up alone. Also, it was not possible to do our analyses based only on the 15-month follow-up due to lack of sufficient number of meniscal cysts to perform meaningful statistical calculations. Control knees were selected randomly from among the knees examined by MRI at baseline and for which the subject indicated absence of pain at both time points.
All subjects were asked a question regarding knee pain as follows: “During the past 30 days, have you had pain, aching, or stiffness in your knee on most days?” This question was posed to subjects both by phone interview and during a clinic visit 1 month thereafter. If the subject answered no regarding the presence of pain, aching, or stiffness in the knee at each of these time points at baseline, that knee was considered eligible for analysis in the incident pain study. At 15 and 30 months’ follow-up, the same question regarding knee pain was posed to subjects, both in a phone interview and at a clinic visit. If the subject answered yes to the question at both of these, the knee with new pain was considered to be a case knee. Because this question was intended to identify pain, aching, or stiffness “on most days,” and because the 2 time points were, on average, 1 month apart, we characterized a positive response at both time points as “consistent frequent knee pain.” Furthermore, although our question included the symptoms of stiffness and aching, we labeled a positive response as related to pain.13 In addition, subjects completed surveys on medication use, including nonsteroidal anti-inflammatory drugs or other analgesic drugs, at the baseline and follow-up clinic examination. Subjects also completed the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), a survey of joint pain, stiffness, and limitation of physical function,14 for each knee at both the baseline and follow-up visits.
For the incident radiographic OA study, there were 149 case knees and 298 control (total 447) knees. Eligibility for case knees was based on either of two qualifications: no radiographic OA (grade 0 or 1) at baseline but incident radiographic OA (grade 2 or higher) according to the Kellgren-Lawrence (K-L) grading system,15 or having any osteophyte grade 2 or higher, or any osteophyte grade 1 or higher plus joint space narrowing grade 2 or higher in the tibiofemoral joint using the Osteoarthritis Research Society International (OARSI) grading16 at 30-month follow-up. If a subject only had one knee which fulfilled the above criteria, that knee was used as a case knee. In case of subjects having bilateral knees fulfilling the above, one knee was randomly chosen. For the control knees, one knee per subject was randomly selected from knees without radiographic OA at baseline and follow-up. For both studies, cases and controls were matched to the clinic site and follow-up visit.
Radiographs
All subjects underwent baseline and follow-up weight-bearing postero-anterior fixed flexion view knee radiographs using the SynaFlexer positioning frame (Synarc, San Francisco, CA).17–18
A musculoskeletal radiologist (who is not a co-author) and a rheumatologist (DTF) with over 10 years experience each in reading study films and over 30 years of clinical experience graded all baseline postero-anterior films independently according to the K-L grading, blinded to case/control status and clinical data. Disagreements among the readers were adjudicated by a panel of three readers (DTF, aforementioned radiologist and another rheumatologist who is not a co-author). The weighted kappa coefficient of inter-observer reliability for the K-L readings, was 0.79, which was calculated from the baseline readings of 4,297 knee radiographs before adjudication in the MOST study including subsamples used in our analyses.
Full-limb radiographs of both legs were obtained at baseline using the method described previously.19 The mechanical axis was defined as the angle formed by the intersection of a line from the center of the head of the femur to the center of the tibial spines and a line from the center of the talus to the center of the tibial spines.19 The interobserver intraclass correlation coefficient for the mechanical axis was 0.99 (p<0.0001). Varus alignment was defined as an angle <179°, 179 to 181° was considered neutral and valgu s alignment was defined as an angle >181°.
MRI Acquisition
Baseline MRI scans were obtained with a 1.0T MR system (OrthOne™, ONI Medical Systems, Wilmington, MA) with a circumferential transmit-receive extremity coil using a fat-suppressed (FS) fast spin echo (FSE) proton density-weighted (PD-w) sequence in two planes: sagittal, repetition time (TR) = 4,800 ms, echo time (TE) = 35 ms, 3 mm slice thickness, 0 mm interslice gap, 30 slices, 288x192 matrix, number of excitations (NEX) = 2, 140x140 mm field of view (FOV), echo train length (ETL) = 6; and axial, (TR = 4,680 ms, TE = 13 ms, 3 mm slice thickness, 0 mm interslice gap, 26 slices, 288x192 matrix, NEX = 2, 140x140 mm FOV, ETL = 10. Also, a short-tau inversion recovery (STIR) sequence in the coronal plane was obtained (TR = 8,448 ms, TE = 17 ms, TI = 100 ms, 3 mm slice thickness, 0 mm interslice gap, 34 slices, 256x192 matrix, two NEX, 140x140 mm FOV, ETL = 8).
MRI Assessment
Semiquantitative assessment of baseline cysts and bursitides using the Whole Organ Magnetic Resonance Imaging Score (WORMS) criteria20 was performed by two musculoskeletal radiologists (AG and FWR) with 11 and 7 years of experience in standardized semiquantitative analysis of knee OA. Baker’s cyst was graded from 0–3 (0 = none, 1 = small, 2 = medium, 3 = large). Meniscal cysts (medial, lateral and combined), PTFJ cysts, prepatellar bursitis and anserine bursitis were scored 0 or 1 (0 = none, 1 = present). Cartilage status (grade 0–6), meniscal status (grade 0–4), bone marrow lesions, synovitis and effusion (grade 0–3) were also scored using WORMS.20 The two radiologists were blinded to case/control status and clinical data and readings were performed independently. The weighted kappa coefficient of inter-observer reliability (performed on a random sample of 30 knees read by both readers) for the readings of Baker’s cysts (comparing 0–3 scores) was 0.82. Similarly, the non-weighted kappa coefficients for the readings of medial/lateral meniscal cysts, and prepatellar bursitis (comparing 0 or 1 scores) were 1.00/0.73 and 0.48 and respectively.
Knees with typical radiologic signs of traumatic bone contusions, osteonecrosis, fracture or malignant bone infiltration were excluded from the analysis. However, of all analyzed MRIs only one knee showed a subacute tibial depression fracture at follow-up and was excluded.
Statistical Analysis
We evaluated the association of prevalent cyst-like lesions (score >0) at baseline with the presence of incident knee pain and incident radiographic OA (as defined earlier) using conditional logistic regression models adjusting for age, sex, race, body mass index (BMI), and mechanical knee alignment. Adjustments for baseline K-L grade were also made for the incident pain study. To assess whether cyst-like lesions at baseline were associated with incident radiographic OA or pain, additional adjustments for possible confounders were performed, i.e. bone marrow lesions, synovitis, joint effusion, meniscal damage and cartilage loss. For incident pain study, we examined change in pain medication use as an additional covariate. Although Baker’s cysts were expected to show co-linearity with joint effusion, Spearman correlation between these was not significant and thus we adjusted for joint effusion when analyzing Baker’s cysts. The presence of meniscal tear is thought to lead to meniscal cyst formation. Having found a significant positive correlation, we did not adjust for meniscal damage when analyzing meniscal cysts.
Because we performed multiple comparisons in our analyses (i.e. seven cyst-like lesions: Baker’s, meniscal and PTFJ cysts, and prepatellar and anserine bursitides), Bonferroni correction for p-value was made and thus p<0.007 was considered statistically significant.
All statistical calculations were performed using SAS® software (Version 9.1 for Windows, SAS Institute, Cary, NC).
Results
Incident pain Study
For the case group (n=157), the mean (SD) age of subjects was 62.8 (8.2) years and the mean BMI was 29.7 (4.6) kg/m2. Subjects were predominantly female (108, 69%) and white (137, 87%), and 64 (41%) had K-L grade ≥2 at baseline. The proportion of women and those with radiographic OA (K-L grade grade ≥2) were higher in the case group than the control group. Prevalence of cartilage loss (WORMS grade≥2) and meniscal damage (WORMS grade≥1) in the case group were 141 (90%) and 68 (43%), respectively, and were higher than the control group. Prevalence of BML, synovitis and joint effusion was similar between two groups. Malalignment was seen more frequently in the case group than in the control group (table 1).
Table 1.
Demographics of case and control groups at baseline in the present study
| Case knees | Control knees | p-value$ | |
|---|---|---|---|
| Incident knee pain | |||
| N | 157 | 336 | |
| Age, mean (SD) | 62.8 (8.2) | 61.5 (8.1) | 0.095 |
| Women, no. (%) | 108 (69) | 200 (60) | 0.048* |
| White, no. (%) | 137 (87) | 292 (86) | 0.91 |
| BMI, mean (SD) | 29.7 (4.6) | 29.7 (4.5) | 0.99 |
| ROA (K-L grade≥2), no. (%) | 64 (41) | 75 (22) | <.0001* |
| BML (grade≥1)†, (%) | 117 (75) | 225 (67) | 0.090 |
| Cartilage loss (grade≥2)†, (%) | 141 (90) | 271 (81) | 0.011* |
| Meniscal damage (grade≥1)†, (%) | 68 (43) | 99 (30) | 0.0025* |
| Synovitis (grade≥1)†, (%) | 107 (68) | 213 (63) | 0.30 |
| Effusion (grade≥1)†, (%) | 63 (40) | 112 (33) | 0.14 |
| Malalignment** | |||
| Varus (<179°), no. (%) | 71 (45) | 138 (41) | 0.028* |
| Neutral (179–181°), no. (%) | 45 (29) | 139 (41) | |
| Valgus (>181°), no. (%) | 39 (25) | 57 (17) | |
| Incident radiographic OA | |||
| N | 149 | 298 | |
| Age, mean (SD) | 61.7 (7.7) | 61.5 (7.7) | 0.79 |
| Women, % | 92 (62) | 177 (59) | 0.60 |
| White, % | 120 (81) | 258 (86) | 0.11 |
| BMI, mean (SD) | 31.0 (5.4) | 28.9 (4.5) | <.0001* |
| BML (grade≥1)†, (%) | 104 (70) | 174 (58) | 0.056 |
| Cartilage loss (grade≥2)†, (%) | 144 (97) | 232 (78) | <.0001* |
| Meniscal damage (grade≥1)†, (%) | 71 (48) | 55 (18) | <.0001* |
| Synovitis (grade≥1)†, (%) | 72 (48) | 87 (29) | 0.0003* |
| Effusion (grade≥1)†, (%) | 99 (66) | 122 (41) | <.0001* |
| Malalignment | |||
| Varus (<179°), no. (%) | 77 (52) | 129 (43) | 0.059 |
| Neutral(179–181°), no. (%) | 44 (30) | 118 (40) | |
| Valgus (>181°), no. (%) | 25 (17) | 51 (17) | |
Abbreviations: N = total number of knees in case and control groups; SD = standard deviation; BMI = body mass index; ROA = radiographic osteoarthritis; BML = bone marrow lesion; K-L grade = Kellgren-Lawrence grade.
denotes statistically significant differences (p<0.05).
scored using the Whole Organ Magnetic Resonance Imaging Score (scale 0–3 for BML and synovitis and effusion; scale 0–6 for cartiage; and scale 0–4 for meniscus.)
calculated by chi-square test or 2-sample t-test, as appropriate.
Baker’s cysts (WORMS grade ≥1) were the most prevalent lesion in the case (63/157, 40%) and control (126/336, 37%) groups, but were not associated with incident pain at any grade. There were 7 (4%) lateral meniscal cysts in the case group and 4 (1%) in the control group, and the fully adjusted odds ratio (aOR) [95%CI] for incident pain was 4.3 [1.2–15.4]. However, statistical significance was lost after correction of p-values for multiple comparisons. Association of medial or combined meniscal cysts with incident pain was also not present. PTFJ cysts were twice as likely to be seen in the case group as the control group (6% vs. 3%), but were not associated with incident pain (aOR 1.6 [0.6, 4.3]). Prepatellar bursitis was more prevalent than anserine bursitis, in both case and control knees. Neither of these was associated with incident pain (table 2). Additional analyses adjusting for changes in pain medication yielded results similar to those of fully adjusted analyses described above (not shown).
Table 2.
Prevalence of periarticular cystic lesions and cyst-like bursitis at baseline and their association with incident knee pain
| WORMS Grade | Incident knee pain |
||||
|---|---|---|---|---|---|
| Prevalence (%)at baseline |
OR (95% CI) |
||||
| Cases (N=157) | Controls (N=336) | Adjusted # | Fully Adjusted ## | ||
| Baker’s Cyst† | 0 | 93 (60) | 210 (63) | 1.0 (ref) | 1.0 (ref) |
| 1 | 50 (32) | 103 (30) | 1.1 (0.7, 1.6) | 1.0 (0.7, 1.6) | |
| 2 | 11 (7) | 19 (6) | 1.0 (0.5, 2.3) | 0.9 (0.4, 2.1) | |
| 3 | 2 (1) | 4 (1) | 0.7 (0.1, 4.0) | 0.6 (0.1, 3.7) | |
| Meniscal Cyst$ | |||||
| Medial | 0 | 155 (99) | 330 (98) | 1.0 (ref) | 1.0 (ref) |
| 1 | 2 (1) | 6 (2) | 1.0 (0.2, 5.2) | 0.9 (0.2, 4.7) | |
| Lateral | 0 | 150 (96) | 332 (99) | 1.0 (ref) | 1.0 (ref) |
| 1 | 7 (4) | 4 (1) | 4.4* (1.2, 15.9) | 4.3** (1.2, 15.4) | |
| Combined | 0 | 148 (94) | 327 (97) | 1.0 (ref) | (ref) |
| 1 | 9 (6) | 9 (3) | 2.8 (1.0, 7.3) | 2.6 (1.0, 7.0) | |
| PTFJ Cyst†† | 0 | 148 (94) | 321 (97) | 1.0 (ref) | 1.0 (ref) |
| 1 | 9 (6) | 10 (3) | 1.6 (0.6, 4.2) | 1.6 (0.6, 4.3) | |
| Prepatellar bursitis††† | 0 | 106 (68) | 241 (72) | 1.0 (ref) | 1.0 (ref) |
| 1 | 49 (32) | 94 (28) | 1.1 (0.7, 1.7) | 1.1 (0.7, 1.7) | |
| Anserine bursitis | 0 | 135 (86) | 290 (86) | 1.0 (ref) | 1.0 (ref) |
| 1 | 22 (14) | 46 (14) | 0.7 (0.4, 1.3) | 0.7 (0.4, 1.2) | |
Abbreviations: PTFJ = proximal tibiofibular joint; N = total number of subjects in case and control groups; SD = standard deviation; OR = odds ratio; CI = confidence interval; WORMS = Whole Organ Magnetic Resonance Imaging Score
missing value in one case knee;
missing value in five control knees;
missing value in two case knees and one control knee
p=0.022 and
p=0.032. Although 95% CI does not include 1.0, these were not considered statistically significant after Bonferroni correction for multiple comparisons (level of significance was determined to be p<0.007).
Odds ratio were calculated after making adjustments as follows:
adjusting for age, sex, race, BMI, alignment, and K-L grade at baseline
adjusting for age, sex, race, BMI, alignment, K-L grade, and other MRI features at baseline, i.e., maximum score of each feature, including BML, synovitis, joint effusion, meniscal damage, and cartilage loss
Not adjusted for meniscal damage in the fully-adjusted model
Incident Radiographic OA Study
For the case group (n=149), the mean (SD) of demographic parameters were as follows: age 61.7(7.7) years, BMI 31.0 (5.4) kg/m2, 92 (62%) women and 120 (81%) white. Prevalence of cartilage loss, meniscal damage, synovitis and joint effusion in the case group were 144 (97%), 71 (48%), 72 (48%) and 99 (66%), respectively, and were higher than the control group. Varus alignment of the knee joint was commonly seen in both case and control groups but there were no statistically significant differences in malalignment between the two groups (table 1).
Prevalence of grade 2 Baker’s cysts (Figure 1) were higher in the case (16/149, 11%) than the control group (9/298, 3%), and the aOR for incident radiographic OA was 3.1 [1.1, 8.6], but its significance was lost after correction of p-values. Prevalence of grade ≥1 Baker’s cysts were present in 66 (44%) of case knees at baseline, but were less frequent in control knees (97/298, 32%). Six (4%) PTFJ cysts were present in the case group and 3 (1%) in the control group, but association with incident radiographic OA was not significant after full adjustments (aOR 2.6 [0.5, 12.2]). Meniscal cysts, prepatellar bursitis and anserine bursitis were not associated with incident radiographic OA after full adjustments (table 3).
Figure 1.
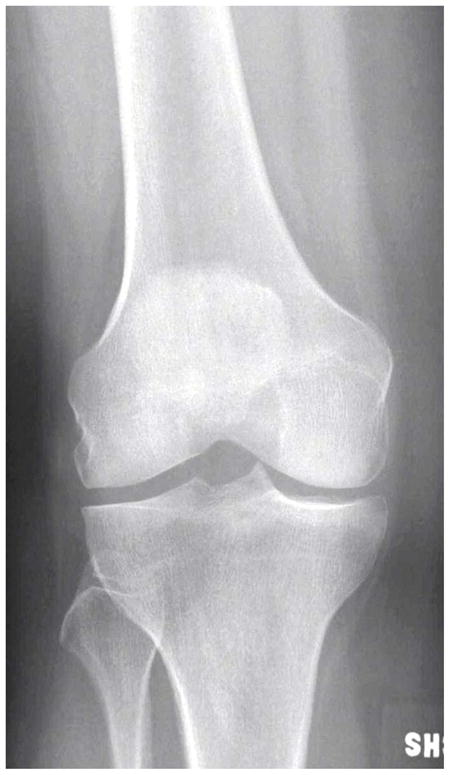
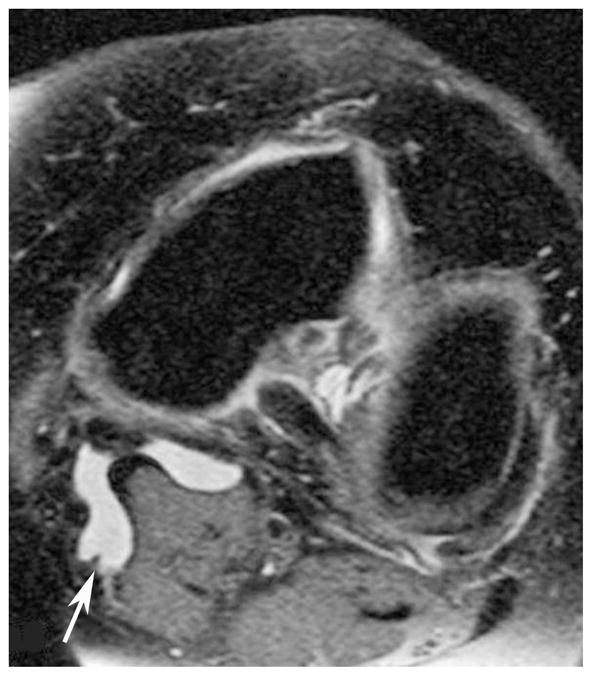
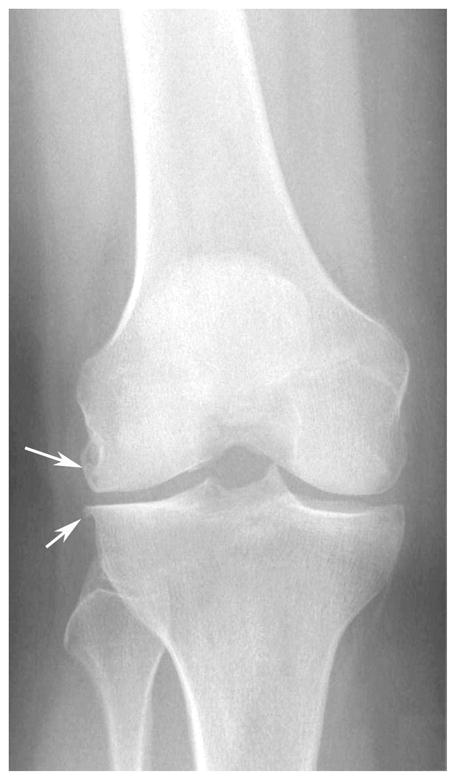
(a) AP X-ray at baseline shows KL=0 of both medial and lateral right femorotibial joints. (b) Baseline axial fat-suppressed proton density-weighted MR image shows grade 2 Baker’s cyst (arrow). (c) AP X-ray at 30 months shows joint space narrowing of the lateral femorotibial joint and definite lateral tibial osteophytes (arrows) with K-L grade = 2.
Table 3.
Prevalence of periarticular cystic lesions and cyst-like bursitis at baseline and their association with incident radiographic OA
| WORMS Grade | Incident radiographic OA |
||||
|---|---|---|---|---|---|
| Prevalence (%)at baseline |
OR (95% CI) |
||||
| Cases (N=149) | Controls (N=298) | Adjusted # | Fully Adjusted ## | ||
| Baker’s Cyst | 0 | 83 (56) | 201 (68) | 1.0 (ref) | 1.0 (ref) |
| 1 | 47 (31) | 84 (28) | 1.2 (0.8, 2.0) | 1.2 (0.7, 2.0) | |
| 2 | 16 (11) | 9 (3) | 4.1 (1.7, 10.0)* | 3.1 (1.1, 8.6)** | |
| 3 | 3 (2) | 4 (1) | 1.6 (0.3, 7.7) | 0.5 (0.1, 3.3) | |
| Meniscal Cyst$ | |||||
| Medial$$ | 0 | 149 (100) | 294 (99) | n/a | n/a |
| 1 | 0 (0) | 4 (1) | n/a | n/a | |
| Lateral$$ | 0 | 147 (99) | 298 (100) | n/a | n/a |
| 1 | 2 (1) | 0 (0) | n/a | n/a | |
| Combined | 0 | 147 (99) | 294 (99) | 1.0 (ref) | (ref) |
| 1 | 2 (1) | 4 (1) | 0.8 (0.1, 4.6) | 0.6 (0.1, 4.2) | |
| PTFJ Cyst† | 0 | 143 (96) | 294 (99) | 1.0 (ref) | 1.0 (ref) |
| 1 | 6 (4) | 3 (1) | 4.5 (1.1, 18.8)*** | 2.6 (0.5, 12.2) | |
| Prepatellar bursitis†† | 0 | 124 (85) | 265 (89) | 1.0 (ref) | 1.0 (ref) |
| 1 | 22 (15) | 33 (11) | 1.1 (0.6, 2.2) | 1.5 (0.7, 3.1) | |
| Anserine bursitis | 0 | 143 (96) | 293 (98) | 1.0 (ref) | 1.0 (ref) |
| 1 | 6 (4) | 5 (2) | 1.5 (0.4, 5.9) | 0.7 (0.1, 3.8) | |
Abbreviations: OA = osteoarthritis; PTFJ = proximal tibiofibular joint; N = total number of subjects in case and control groups; SD = standard deviation; OR = odds ratio; CI = confidence interval; WORMS = Whole Organ Magnetic Resonance Imaging Score
missing value in one control knee;
missing value in three case knees
p=0.0017,
p=0.031 and
p=0.038. Although 95% CI does not include 1.0, the results of ** and *** were not considered statistically significant after Bonferroni correction for multiple comparisons (level of significance was determined to be p<0.007).
Odds ratio were calculated after making adjustments as follows:
adjusting for age, sex, race, BMI and alignment at baseline
adjusting for age, sex, race, BMI, alignment, and other MRI features at baseline, i.e., maximum score of each feature, including BML, synovitis, joint effusion, meniscal damage, and cartilage loss
Not adjusted for meniscal damage in the fully-adjusted model
Calculation of odds ratios was not possible
Discussion
In the present study, we assessed the longitudinal relationship of Baker’s, meniscal and PTFJ cysts, as well as prepatellar and anserine bursitides with incident pain and incident radiographic OA. Statistically significant associations were not found in any of the analyzed lesions after full adjustment for potential confounders and correction of p-value for multiple comparisons.
A Baker’s cyst is not a true cyst, but a fluid collection in the semimembranosus-medial gastrocnemius bursa. Increased intraarticular pressure due to joint effusion causes extravasation of joint fluid through the posteromedial capsule between the medial head of the gastrocnemius and the semimembranosus tendon into the bursa located between these muscles.1 In this longitudinal study, we did not find significant associations between Baker’s cyst of any grade and development of incident pain or radiographic OA. Baker’s cysts can be asymptomatic, so their presence alone is unlikely to be the cause of OA-related pain. Rather, pain can be caused by the presence of a large joint effusion11, 21 and synovitis,21–22 both of which are features of OA. As a Baker’s cyst is an extension of the knee joint capsule, co-linearity between Baker’s cysts and joint effusion was expected but was not present and thus adjustment of our logistic regression model for joint effusion was made. However, not adjusting for joint effusion did not alter the findings of the present study. Based on results of a recent study which reported that joint effusion predicts cartilage loss in knees without OA,23 one could speculate that joint effusion might be a risk factor for incident OA. Such an association, however, has not been shown so far.
We did not observe a significant association between PTFJ cysts and incident pain or radiographic OA. The probable cause of PTFJ lesions is increased pressure in the knee joint leading to an out-pouching of the tibiofibular joint capsule which then herniates to form the synovial cyst.24 The lesions are therefore thought to be more common in patients with chronic knee effusions. It has been reported that PTFJ communicates with the knee joint capsule in 10% of adults.4, 25 Whether such communication was present congenitally or acquired following an increase in intra-articular pressure, perhaps due to active synovitis or joint injury, has not been determined. In the present study, PTFJ cysts were much rarer than Baker’s cysts (19 vs. 189 in incident symptom study, and 9 vs. 163 in incident radiographic OA study, i.e. prevalence of PTFJ cysts was 6–10% of that of Baker’s cysts). These numbers support the previously published data, but why only 10% communicates with Baker’s cysts is unknown. If these two lesions share the same pathogenesis, one would expect to see more of PTFJ cysts in patients who have Baker’s cysts, but this did not seem to be the case. It is hoped that future studies will reveal the exact pathogenesis of PTFJ cysts.
A meniscal cyst is a focal collection of synovial fluid located within or adjacent to the meniscus. Various theories concerning the etiology of meniscal cysts have been proposed, but the most widely held view is that they form as a result of extrusion of synovial fluid through an adjacent meniscal tear.9 In the incident pain study the baseline prevalence of medial meniscal cysts was 1% (2/157) while that of lateral meniscal cysts was 4% (7/157) in the case knees, contrary to a previous study9 that reported a higher prevalence of medial than lateral lesions. This may be explained by a much smaller sample size than in the previous study. A strong cross-sectional association between meniscal damage and the presence of knee OA has been reported.12 Since meniscal cysts are usually associated with an adjacent meniscal tear9 which may be painful, it would have been unsurprising to find an association between meniscal cysts and incident knee pain. However, our data did not demonstrate such an association. Moreover, a recent report concluded that in middle-aged and older adults, any association between meniscal damage and the development of frequent knee pain seemed to occur because both pain and meniscal damage are related to OA, and not because of a direct link between the two26. Thus, neither meniscal cysts nor meniscal damage itself seems to be directly associated with incident knee pain.
As for prepatellar and anserine bursitides, we did not find significant associations with incident pain or incident radiographic OA. Given the location of these cysts outside the knee joint, we expected the null association with knee OA, but periarticular lesions like these have been linked to knee pain before21 and we expected that they might be sources of pain. To date, there are few reports describing the prevalence of these lesions and their association with pain and radiographic OA. Prepatellar bursitis is commonly seen among both symptomatic and asymptomatic older individuals with radiographic knee OA.6 Furthermore, high-intensity signal changes are commonly observed anteriorly to the patella and the patella tendon and often represent a non-specific finding of little clinical relevance. True prepatellar bursitis should be assessed by contrast-enhanced MRI to distinguish edema from bursitis, which was not available in our study. Anserine bursitis may be detected in conjunction with knee OA, but its association with degeneration is controversial.1 Chronic anserine bursitis is thought to be most common in elderly patients who have degenerative disease or rheumatoid arthritis.3 However, a recent case control study found no association between prevalent anserine bursitis and radiographic knee OA.27 Taking these findings into account we speculate that, although these bursitides are commonly seen in OA patients, their pathogenesis is not directly linked to OA disease processes.
A major limitation of our study is that we had two time points for follow-up in the incident pain study. As described earlier, it was not feasible to perform analyses based on either 15- or 30-month follow-up only. Using only one time point for follow-up would have simplified the study design and interpretation of the results. Another limitation of our study is that the prevalence of meniscal and PTFJ cysts was very low at baseline, and only a small number of lesions were available for the analysis (between 6 and 19 in total). More samples are needed to produce more statistically robust data. Thus, from our data alone, it may be difficult to draw a definitive conclusion concerning the longitudinal association between meniscal cysts and incident OA-related pain. However, the low prevalence in this large sample size may indicate that these lesions may not be clinically appropriate to predict incident knee pain. Lastly, the way we defined incident pain may have affected the outcome of our analysis. To qualify for incident knee pain, subjects must have had no knee pain at baseline and consistent knee pain at follow-up. However, those subjects who reported no frequent knee pain at baseline often noted some knee pain on the baseline WOMAC questionnaire. Thus, it must be noted that “incident knee pain” in such subjects are not necessarily completely new pain and may represent an increase in the frequency and severity of the knee pain.
In conclusion, this was the first study to assess longitudinal relationships of intra- and periarticular cyst-like lesions of the knee with incident pain and radiographic OA. The analyzed lesions, i.e. Baker cysts, meniscal cysts, PTFJ cyst, prepatellar bursitis and anserine bursitis were not associated with incident pain or radiographic OA. Although intra- and periarticular cyst-like lesions are commonly observed on MRI, and have previously been shown to be cross-sectionally associated with knee pain and radiographic OA, results of the present study suggest they are more likely to be a secondary phenomenon that occurs in painful or OA-affected knees, rather than a primary trigger for knee pain or radiographic OA.
Acknowledgments
We would like to thank the participants and staff of the MOST study at the clinical sites in Birmingham, AL and Iowa City, IA and at the Coordinating Center at University of California San Francisco, San Francisco, CA.
Funding sources
The MOST study is supported by National Institutes of Health (NIH) grants from the National Institute on Aging to Drs Lewis (U01-AG-18947), Torner (U01-AG-18832), Nevitt (U01-AG-19069), and Felson (U01-AG-18820). The contents of this paper have been approved by the MOST committee to be published.
Footnotes
Author contributions:
(a) Conception and design, acquisition of data, analysis and interpretation of data: AG, DH, FWR, JN, MKJ, MY, JAL, JCT, CEL, BS, DTF, MCN
(b) Drafting the article or revising it critically for important intellectual content: AG, DH, FWR, JN, MKJ, MY, JAL, JCT, CEL, BS, DTF, MCN
(c) Final approval of the version to be published: AG, DH, FWR, JN, MKJ, MY, JAL, JCT, CEL, BS, DTF, MCN
(d) Guarantor of the integrity of the study: AG (guermazi@bu.edu)
Competing interests
Ali Guermazi is the president of Boston Imaging Core Lab LLC (BICL), Boston, MA, a company providing radiological image assessment services. He is a consultant to MerckSerono, Facet Solutions, Genzyme and Stryker.
Frank Roemer is a shareholder of BICL.
None of the other authors have declared any possible conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crema MD, Roemer FW, Marra MD, Guermazi A. MR imaging of intra- and periarticular soft tissues and subchondral bone in knee osteoarthritis. Radiol Clin N Am. 2009;47:687–701. doi: 10.1016/j.rcl.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Beall DP, Ly JQ, Wolff JD, Sweet CF, Kirby AB, Murphy MP, et al. Cystic masses of the knee: magnetic resonance imaging findings. Curr Probl Diagn Radiol. 2005;34:143–159. doi: 10.1067/j.cpradiol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy CL, McNally EG. The MRI appearance of cystic lesions around the knee. Skeletal Radiol. 2004;33:187–209. doi: 10.1007/s00256-003-0741-y. [DOI] [PubMed] [Google Scholar]
- 4.Janzen DL, Peterfy CG, Forbes JR, Tirman PF, Genant HK. Cystic lesions around the knee joint: MR imaging findings. AJR Am J Roentgenol. 1994;163:155–161. doi: 10.2214/ajr.163.1.8010203. [DOI] [PubMed] [Google Scholar]
- 5.Mara MD, Crema MD, Chung MC, Roemer FW, Hunter DJ, Zaim S, et al. MRI features of cystic lesions around the knee. Knee. 2008;15:423–438. doi: 10.1016/j.knee.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Hill CL, Gale DR, Chaisson CE, Skinner K, Kazis L, Gale ME, et al. Periarticular lesions detected on magnetic resonance imaging: Prevalence in knees with and without symptoms. Arthritis Rheum. 2003;48:2836–2844. doi: 10.1002/art.11254. [DOI] [PubMed] [Google Scholar]
- 7.Tschirch FT, Schmid MR, Pfirrmann CW, Romero J, Hodler J, Zanetti M. Prevalence and size of meniscal cysts, ganglionic cysts, synovial cysts of the popliteal space, fluid-filled bursae, and other fluid collections in asymptomatic knees on MR imaging. AJR Am J Roentgenol. 2003;180:1431–1436. doi: 10.2214/ajr.180.5.1801431. [DOI] [PubMed] [Google Scholar]
- 8.Miller TT, Staron RB, Koenigsberg T, Levin TL, Feldman F. MR imaging of Baker cysts: association with internal derangement, effusion, and degenerative arthropathy. Radiology. 1996;201:247–250. doi: 10.1148/radiology.201.1.8816552. [DOI] [PubMed] [Google Scholar]
- 9.Campbell SE, Sanders TG, Morrison WB. MR imaging of meniscal cysts: incidence, location, and clinical significance. AJR Am J Roentgenol. 2001;177:409–413. doi: 10.2214/ajr.177.2.1770409. [DOI] [PubMed] [Google Scholar]
- 10.Link TM, Steinbach LS, Ghosh S, Ries M, Lu Y, Lane N, et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology. 2003;226:373–381. doi: 10.1148/radiol.2262012190. [DOI] [PubMed] [Google Scholar]
- 11.Kornaat PR, Bloem JL, Ceulemans RYT, Riyazi N, Rosendaal FR, Nelissen RG, et al. Osteoarthritis of the knee: association between clinical features and MR imaging findings. Radiology. 2006;239:811–817. doi: 10.1148/radiol.2393050253. [DOI] [PubMed] [Google Scholar]
- 12.Englund M, Guermazi A, Gale D, Hunter DJ, Aliabadi P, Clancy M, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Eng J Med. 2008;359:1108–1115. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felson DT, Niu J, Guermazi A, Roemer FW, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56:2986–2992. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 14.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to anti-rheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 15.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthritis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15 (Suppl 1):A1–A56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Kothari M, Guermazi A, von Ingersleben G, Miaux Y, Sieffert M, Block JE, et al. Fixed-flexion radiography of the knee provides reproducible joint space width measurements in osteoarthritis. Eur Radiol. 2004:14. doi: 10.1007/s00330-004-2312-6. [DOI] [PubMed] [Google Scholar]
- 18.Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semiflexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32:128–132. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 19.Sharma L, Song J, Felson DT, et al. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188–195. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 20.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Hill CL, Gale DG, Chaisson CE, Skinner K, Kazis L, Gale ME, et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol. 2001;28:1330–1337. [PubMed] [Google Scholar]
- 22.Chatzopoulos D, Moralidis E, Markou P, Makris V, Arsos G. Baker’s cysts in knees with chronic osteoarthritis pain: a clinical, ultrasonographic, radiographic and scintigraphic evaluation. Rheumatol Int. 2008;29:141–146. doi: 10.1007/s00296-008-0639-z. [DOI] [PubMed] [Google Scholar]
- 23.Roemer FW, Guermazi A, Felson DT, Niu J, Nevitt MC, Crema MD, et al. Do baseline synovitis and effusion predict structural progression in subjects without radiographic osteoarthritis? Results from the Multicenter Osteoarthritis (MOST) Study. Osteoarthritis Cartilage. 2009;17(Suppl 1):S210. [Google Scholar]
- 24.Jerome D, McKendry R. Synovial cyst of the proximal tibiofibular joint. J Rheumatol. 2000;27:1096–1098. [PubMed] [Google Scholar]
- 25.Steiner E, Steinbach LS, Schnarkowski P, Tirman PF, Genant HK. Ganglia and cysts around joints. Radiol Clin North Am. 1996;34:395–425. xi–xii. [PubMed] [Google Scholar]
- 26.Englund M, Niu J, Guermazi A, Roemer FW, Hunter DJ, Lynch JA, et al. Effect of meniscal damage on the development of frequent knee pain, aching or stiffness. Arthritis Rheum. 2007;56:4048–4054. doi: 10.1002/art.23071. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez-Nemegyei J. Risk factors for pes anserinus tendinitis/bursitis syndrome: a case control study. J Clin Rheumatol. 2007;13:63–65. doi: 10.1097/01.rhu.0000262082.84624.37. [DOI] [PubMed] [Google Scholar]


