Abstract
Excessive alcohol use leads to neurodegeneration in several brain structures including the hippocampal dentate gyrus and the entorhinal cortex. Cognitive deficits that result are among the most insidious and debilitating consequences of alcoholism. The object exploration task (OET) provides a sensitive measurement of spatial memory impairment induced by hippocampal and cortical damage. In this study, we examine whether the observed neurotoxicity produced by a 4-day binge ethanol treatment results in long-term memory impairment by observing the time course of reactions to spatial change (object configuration) and non-spatial change (object recognition). Wistar rats were assessed for their abilities to detect spatial configuration in the OET at 1 week and 10 weeks following the ethanol treatment, in which ethanol groups received 9–15 g/kg/day and achieved blood alcohol levels over 300 mg/dl. At 1 week, results indicated that the binge alcohol treatment produced impairment in both spatial memory and non-spatial object recognition performance. Unlike the controls, ethanol treated rats did not increase the duration or number of contacts with the displaced object in the spatial memory task, nor did they increase the duration of contacts with the novel object in the object recognition task. After 10 weeks, spatial memory remained impaired in the ethanol treated rats but object recognition ability was recovered. Our data suggest that episodes of binge-like alcohol exposure result in long-term and possibly permanent impairments in memory for the configuration of objects during exploration, whereas the ability to detect non-spatial changes is only temporarily affected.
Keywords: memory, learning, recognition, hippocampus, entorhinal
INTRODUCTION
Chronic alcohol use ultimately leads to neurodegenerative processes in specific regions of the brain (Fadda & Rossetti, 1998). In rats, the development of neuronal changes in the brain appear to be correlated with the amount and duration of ethanol consumed (Walker, Barnes, Zornetzer, Hunter, & Kubanis, 1980). However, the pattern of alcohol consumption seems to be an important predictor of brain damage as well, since smaller doses consumed in a pattern that produced correspondingly higher blood alcohol levels have been shown to be potentially more harmful than larger ones in developing rats (Bonthius & West, 1990). Accordingly, it is well established that large doses of alcohol administered over a short period of time (3–4 days), a model mimicking a single cycle of binge drinking in human alcoholics (Majchrowicz, 1975), reliably produces neurotoxicity in corticolimbic areas including the olfactory bulb, the hippocampal dentate gyrus (DG), and the entorhinal cortex [EC; (Cippitelli, Damadzic, Frankola, Goldstein, Thorsell, Singley, Eskay, & Heilig, 2010; Collins, Corso, & Neafsey, 1996; Collins, Zou, & Neafsey, 1998; Crews, Braun, Hoplight, Switzer, & Knapp, 2000; Hamelink, Hampson, Wink, Eiden, & Eskay, 2005; Obernier, White, Swartzwelder, & Crews, 2002; Zou, Martinez, Neafsey, & Collins, 1996)].
The structural changes induced by excessive alcohol may result in the development of functional cognitive deficits including learning and memory impairments (Pfefferbaum, Sullivan, Rosenbloom, Mathalon, & Lim, 1998; White, 2003). There is evidence that human alcoholics show deficits in spatial memory tasks (Bowden & Mccarter, 1993) and abnormal response perseveration (Oscar-Berman, Hutner, & Bonner, 1992). Similar deficits are reproduced in a variety of animal models of alcohol exposure (Assuncao, Santos-Marques, De Freitas, Carvalho, Andrade, Lukoyanov, & Paula-Barbosa, 2007; Carvalho, Pereira, Pires, Ferraz, Romano-Silva, Oliveira-Silva, & Ribeiro, 2006; Matthews, Simson, & Best, 1995; Schulteis, Archer, Tapert, & Frank, 2008). This is consistent with the observation that alcohol bingeing procedure caused deficits in relearning performance of rats subjected to the reversal learning task of the Morris water maze, as well as prominent neuronal cell loss in the dentate gyrus of hippocampus and the entorhinal cortex (Cippitelli et al., 2010; Obernier et al., 2002).
Entorhinal cortex and hippocampus are closely interconnected regions, known to be involved in spatial learning and memory (Aggleton, Vann, Oswald, & Good, 2000). Their reciprocal connections, in which the EC provides cortical input to the hippocampus and from hippocampus to neocortical areas, suggest a close functional link. Indeed, it is generally believed that the EC is critical for normal hippocampal function and it is involved in the translation of hippocampal-dependent memory to long-term memory localized to the neocortex (Eichenbaum, Otto, & Cohen, 1994). Along with this, evidence indicates that lesions in the hippocampus result in robust and reliable spatial reference memory deficits as assessed in the eight-arm radial maze (Jarrard, 1993) and in the Morris water maze (Morris, Garrud, Rawlins, & Okeefe, 1982), and that lesions of EC produces similar outcomes in the same tasks (Nagahara, Otto, & Gallagher, 1995; Schenk & Morris, 1985). The relative contribution of EC to learning and memory is debated, however, with some studies showing that spatial learning can proceed normally without the EC, suggesting that this area is not required for normal hippocampal function (Bannerman, Yee, Lemaire, Wilbrecht, Jarrard, Iversen, Rawlins, & Good, 2001; Burwell, Saddoris, Bucci, & Wiig, 2004; Galani, Weiss, Cassel, & Kelche, 1998; Pouzet, Welzl, Gubler, Broersen, Veenman, Feldon, Rawlins, & Yee, 1999).
Through their spontaneous exploratory behavior, rats are thought to gather information about the surrounding environment (Renner & Seltzer, 1991). Thus, exploratory behavior has been used to assess the ability of rats to integrate spatial features and to build up a spatial representation of a new environment (Save, Poucet, Foreman, & Buhot, 1992). The object exploration task (OET), which models a spontaneous encoding of the geometric arrangement of distinct objects, has been demonstrated to provide a sensitive measurement of deficits in spatial memory induced by hippocampal and cortical damage since both hippocampal (Save et al., 1992) and entorhinal cortex lesions (Parron & Save, 2004) disrupted detection of the spatial change of a previously acquired configuration. In addition, it was suggested that hippocampal-entorhinal connections were more important for identification of a novel geometric arrangement of objects than for place navigation (Parron, Poucet, & Save, 2006).
Here we report on whether alcohol-induced neurodegeneration is accompanied by long-term spatial and non spatial memory impairment. First, we replicated prior findings that a single cycle of binge-like alcohol exposure leads to neurotoxicity within circuitry that subserves spatial memory. Then, we used the object exploration task as a paradigm to investigate the time course of reactions to spatial change (object configuration) and non-spatial change (object recognition).
MATERIAL AND METHODS
Animals
Forty-five male Wistar rats (Charles River, Wilmington, MA), weighing 300–350 g at the beginning of the experiments, were pair-housed with water and food available ad libitum. The animals were maintained in a temperature and humidity-controlled vivarium on a reverse 12-hour light/dark cycle (lights off at 8:00AM). All animal care was performed according to NIH guidelines. Before the onset of the experiments, the animals were handled daily for three days and arbitrarily assigned to three groups: one (N=8, 4 ethanol exposed and 4 non-ethanol exposed) was exposed to the binge alcohol procedure and used for histochemical analysis of fluoro-jade B; a second group (N=19, 10 ethanol exposed, 9 non-ethanol exposed) underwent behavioral assessment at 1 week following binge alcohol treatment; a third group (N=18, 9 ethanol exposed, 9 non-ethanol exposed) underwent behavioral assessment 10 weeks following the binge alcohol treatment. Thus, each animal experienced the OET just once in order to avoid the possible confound of other time-dependent processes.
Binge alcohol treatment
Wistar rats were subjected to a gavage procedure which induced alcohol dependence, as previously described by Majchrowicz (Majchrowicz, 1975). The gavage solution consisted of 14.7% Nestle Infant Formula powder (w/v) and 6% sucrose (w/v) in water, mixed either with alcohol to an alcohol concentration of 20% (v/v), or tap water (Cippitelli et al., 2010). Alcohol-treated animals (EtOH) were given a priming dose of 5 g/kg. Additional alcohol was administered every 8 h for 4 consecutive days based on the animals' estimated blood alcohol level, as determined using a six-point intoxication scale (Majchrowicz, 1975). This treatment model titrates the dose according to the animal's estimated blood alcohol level, thereby maintaining intoxicating levels of blood alcohol while minimizing mortality (Obernier et al., 2002). Each animal received between 9–15 g/kg alcohol per day throughout the study. Control animals received equal volumes of the sucrose-Nestle powder water solution instead of the alcohol solution.
Blood alcohol Levels (BALs)
Blood samples were taken from the rat tail vein two hrs after the last gavage treatment. Blood was collected in 300μl vials containing EDTA dipotassium salt (Sarstedt, Nümbrecht, Germany) and stored at −20°C. Quantitative gas chromatography was used to assess blood alcohol concentrations.
Fluoro-Jade B (FJB) Staining
Fluoro-Jade B was purchased from Histochem, Inc. (Jefferson, Arkansas), and used as a marker of degenerating neurons as described by Schmued and Hopkins (Schmued & Hopkins, 2000). FJB staining was used because it is equally sensitive but methodologically simpler and more reliable than classical amino-cupric silver staining. It has been established that the results obtained by these two methods are highly correlated (Obernier, Bouldin, & Crews, 2002). In brief, 3 hours after the last alcohol gavage, rats were anesthetized and perfused with 4% paraformaldehyde. Horizontal 20-μm cryosections were obtained, allowing visualization in the same section of both entorhinal cortex and ventral hippocampus containing the dentate gyrus. Sections were mounted directly on gelatin coated slides and stained for FJB according to the manufacturer's protocol. Dry slides were cleared in xylene and cover-slipped with Cytoseal (Richard-Allan Scientific, Kalamazoo, Michigan). Alternate sets of sections were stained with cresyl violet to verify basic histology. Cell density analysis was performed on a Leica DMLB microscope using an FITC filter set and BioQuant imaging software (R&M Biometrics, Nashville, Tennessee). Six horizontal sections containing the hippocampus and the EC, both left and right side, were analyzed for degenerating cells between 5.82 to 6.10 mm ventral from bregma (Paxinos & Watson, 1998). Results for EC degeneration are depicted as counts per square mm by dividing the total number of degenerating cells found in 48 examined microscope fields, equivalent to 16.8 square millimeter (single field area was 0.35 square millimeter × 4 fields per side × 2 sides per section × 6 sections per animal, for a total of 16.8 square millimeter) with a 20× microscope objective. Degenerating granule cells of the entire dentate gyrus (DG) were measured using a semiautomatic stereology system (Bioquant, Nashville, Tennessee). Data for EC and DG degenerating cells are presented as number per square millimeter.
Object Exploration Task
Following binge alcohol treatment, all animals were left for five days in their home cages until symptoms of ethanol withdrawal dissipated (Obernier et al., 2002). The rats were then assessed for performance in the object exploration task. The experiment was completed in 4 days so that the assessment would correspond to the 1 week time point. OET performance of the second group of animals was evaluated under identical experimental conditions 9 weeks later (10 week time point). Once again, the behavioral evaluation lasted 4 days.
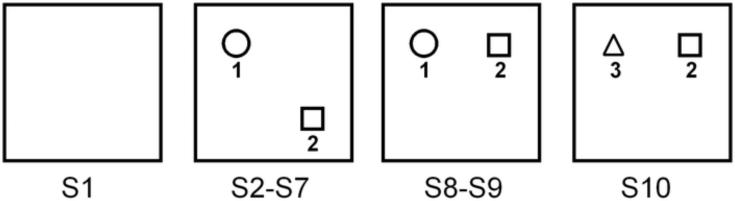
The OET field consisted of a square box with an open top, painted black, 75 cm wide × 50 cm tall. The arena was dimly illuminated and surrounded by a black curtain so that the environment was visually uniform. The test consisted of ten consecutive 4 minute sessions, each followed by 4 minute periods in which the rats were returned to their cages, as previously described by Parron and colleagues (Parron et al., 2006). A schematic representation of the object exploration task is shown in Figure 1. In session 1 (S1), the animal was placed in the empty open field. This first session served as a familiarization stage to prevent anxiety and to assess locomotor activity. In sessions 2 through 7 (S2–S7), the animal was exposed to a diagonal configuration of two objects in the field and became habituated to this new environment. In session 8 (S8), object 2 (obj. 2) was displaced and moved to a linear configuration in relation to object 1 (obj.1) to test recognition of spatial change. Session 9 (S9) was a repetition of session 8. In session 10 (S10), object 1 was replaced by a novel object, object 3 (obj. 3), to test recognition of the new, non displaced object. All objects were located at the same distance from the wall of the field, and the field was cleaned with water and dried after every session.
Figure 1.
Schematic representation of the object exploration task and geometric arrangement of objects. S1, familiarization session (absence of objects); S2–S7, habituation sessions (open circle: object1; open square: object2); S8–S9, spatial change sessions (open circle: object1; open square: object2); S10, non-spatial change session (open triangle: object3; open square: object2).
Each session was recorded by a video camera suspended above the field and interfaced with a computerized tracking system using Ethovision®3 software (Noldus Information Technology, Wageningen, Netherlands). For each session, the total distance moved was measured, the number of contacts made with an object was calculated, and the duration of contacts was determined with a stopwatch. A contact was defined as any time the rat touched his nose or hands on the object to actively explore it, not just as a passing sniff as it passed by the object while walking around the field.
Statistics
Data are reported as mean values ± standard error. Because their variances were not homogenous, degeneration data were examined using nonparametric Mann-Whitney U tests for pairwise comparisons. Behavioral data did not violate homogeneity of variance criteria, and were analyzed using two-way mixed-model analysis of variance (ANOVA), where the within subjects variable was session (S1 locomotor activity, S2–S7 total objects exploration, S8 spatial change, and S9–S10 non-spatial change) and the between subjects variable was the experimental group (Control, EtOH). When a statistically significant threshold (p<0.05) was reached, ANOVA was followed by Newman Keuls post hoc tests for pairwise comparisons.
RESULTS
Blood alcohol levels
Average BALs across all treatment groups at the end of the binge alcohol procedure were around 300 mg/dl (309.3±14.4 mg/dl in the group of animals used for histochemical analysis of fluoro-jade B, 289.1±18.0 mg/dl in the group used for behavioral assessment following 1 week from the exposure, and 319.3±22.7 mg/dl in the 10 weeks assessment group). Blood alcohol levels in animals given gavage with control solution were undetectable.
Binge alcohol-induced neurodegeneration in the DG and the EC
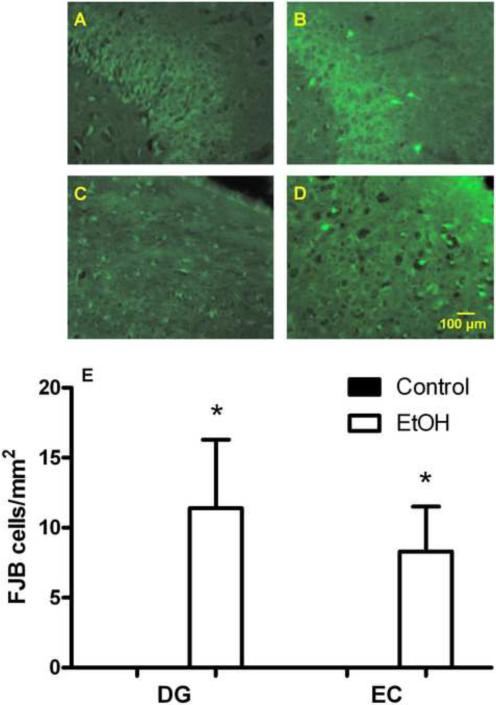
Binge alcohol treatment induced substantial neuronal cell death throughout the hippocampal cortical circuits of the brain. The most affected regions included the olfactory bulb, the hippocampal DG, as well as the perirhinal and the entorhinal cortices. Within these regions, FJB labeling was predominantly detected in the granular cell layer of the DG and in layer III of the EC. Thus, FJB stained cells were quantified in these two regions. No degeneration was found in animals exposed to the non-alcohol solution. Nonparametric comparisons revealed a significant effect of alcohol exposure both in the DG (p<0.05) and in the EC (p<0.05; Figure 2A–E).
Figure 2.
Binge alcohol-induced neurotoxicity in the hippocampal dentate gyrus (DG) and the entorhinal cortex (EC). Sections were stained by Fluoro-Jade B (FJB) to visualize neurodegeneration. Panels (A), (B), (C), and (D) show representative sections visualizing labeled neurons in both the DG, (A) and (B), and the EC, (C) and (D) of animals without (A) and (C) or with ethanol exposure (B) and (D). Panel (E) shows quantitation of the histological data demonstrating alcohol-induced neurodegeneration (*p<0.05, difference from control group). No neurodegeneration was found in animals exposed to the non-alcohol solution. Data are given as the mean number of FJB positive cells/mm2±SEM; solid bars: control animals not exposed to alcohol; open bars: alcohol treated animals. For detailed statistics, see Results.
Binge alcohol exposure does not alter habituation to the OET
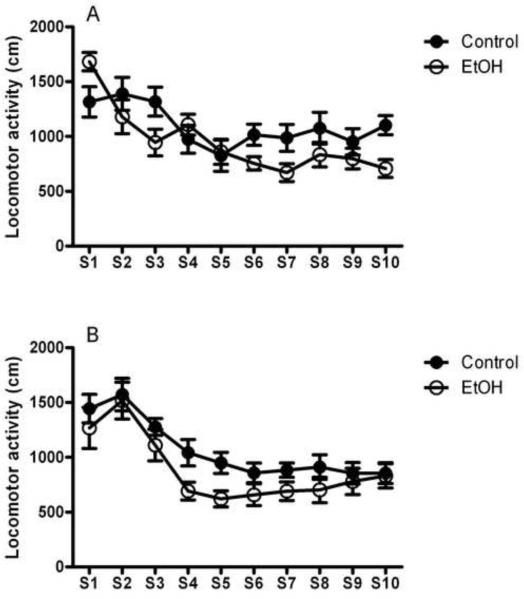
Figure 3 shows the time course data for distance traveled throughout all ten sessions of the task. In the groups tested 1 week following completion of the binge procedure, ANOVA showed a significant main effect of session [F(9,153)=11.0, p<0.0001] and a group x session interaction [F(9,153)=3.4, p<0.001], but no main effect of group [F(1,17)=1.7, NS], suggesting that, despite different patterns of locomotor activity, both the EtOH and Control groups displayed habituation to the task (Figure 3A). Among animals tested 10 weeks following binge exposure, EtOH and Control animals showed habituation to the same degree, as indicated by a significant main effect of session [F(9,144)=21.4, p<0.0001], in the absence of either a main effect of group [F(1,16)=2.4, NS] or a group x session interaction [F(9,144)=0.7, NS; Figure 3B]. No differences between groups were observed when analyzing the distance traveled in the center of the field across all sessions of the task (Supplemental Figure S1).
Figure 3.
Binge alcohol treatment does not alter locomotor activity throughout the 10 sessions (S1–S10) of the object exploration task. Alcohol exposed (EtOH, open circle) and non-exposed (Control, solid circle) animals showed similar habituation to the task following 1 week (A) and 10 weeks (B) from the exposure. Data are given as the mean distance traveled expressed in cm ± SEM. For detailed statistics, see Results.
Analysis of the total number of contacts and their duration from sessions 2 to 7, a further dependent measure indicating habituation, substantially paralleled results of locomotor activity (Table 1). Upon testing 1 week after binge exposure, analysis of object exploration in terms of number of contacts and duration revealed that both EtOH and Control animals displayed habituation from session 2 to 7. There were main effects of session for number of contacts [F(5,85)=6.7, p<0.0001] and duration of contacts [F(5,85)=2.8, p<0.05]), but no main effect of group for either measure (number of contacts: F(1,17)=1.9, NS; duration of contacts: F(1,17)=2.8, NS) nor any interaction of group x session (number of contacts: F(5,85)=1.9, NS; duration of contacts: F(5,85)=1.5, NS). Results were very similar at 10 weeks. There were main effects of session for both number of contacts [F(5,80)=29.7, p<0.0001] and duration of contacts [F(5,80)=21.6, p<0.0001]), but no main effect of group for either measure (number of contacts: F(1,16)=0.0, NS; duration of contacts: F(1,16)=1.1, NS) nor any interaction of session x group (number of contacts: F(5,80)=0.7, NS; duration of contacts: F(5,80)=0.5, NS).
Table 1.
Alcohol exposed (EtOH) and non-exposed (Control) animals experienced similar object exploration as showed by the time course of the total duration and total number of contacts with objects from sessions 2 to 7 (S2–S7) at time points 1 week and 10 weeks following binge alcohol treatment. Data are given as the mean number and duration of contacts expressed in seconds ± SEM. For detailed statistics, see Results.
| Total contacts | Duration of contacts (s) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 week | 10 weeks | 1 week | 10 weeks | |||||
| Control | EtOH | Control | EtOH | Control | EtOH | Control | EtOH | |
| S2 | 13.8±2.7 | 9.1±1.0 | 9.8±1.5 | 10.1±0.9 | 39.3±7.0 | 21.4±3.6 | 25.1±4.6 | 31.0±4.4 |
| S3 | 10.7±2.7 | 6.0±1.2 | 5.1±0.6 | 5.4±0.9 | 38.0±10.6 | 17.8±4.1 | 16.9±2.3 | 17.8±2.6 |
| S4 | 7.7±2.0 | 8.2±1.0 | 4.3±1.0 | 4.1±0.9 | 26.9±6.8 | 24.8±4.1 | 10.6±2.4 | 16.0±4.9 |
| S5 | 7.8±2.2 | 5.4±0.7 | 3.1±0.8 | 2.8±0.6 | 27.0±7.3 | 16.2±2.5 | 6.8±1.5 | 9.6±2.6 |
| S6 | 8.1±2.1 | 7.2±1.4 | 3.8±0.9 | 2.7±0.6 | 27.4±7.8 | 28.0±6.0 | 9.4±2.2 | 9.3±4.2 |
| S7 | 7.3±1.7 | 3.2±0.9 | 1.1±0.4 | 2.6±0.5 | 19.6±4.1 | 10.9±3.0 | 3.1±1.4 | 7.3±2.2 |
Binge alcohol exposure produces long-term spatial memory impairment
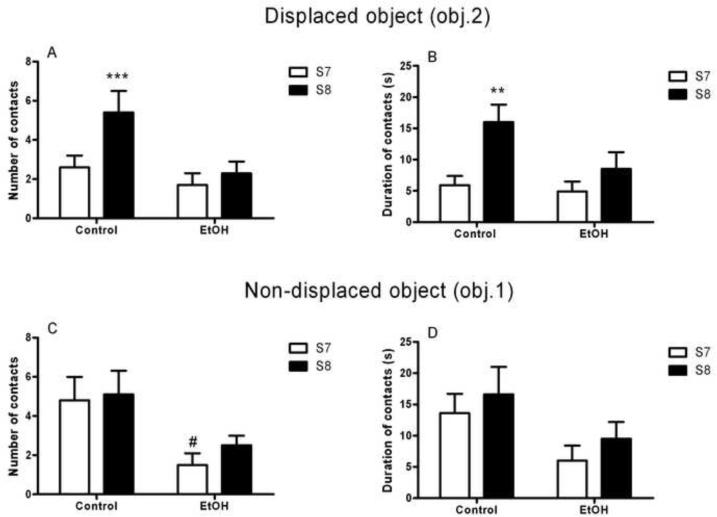
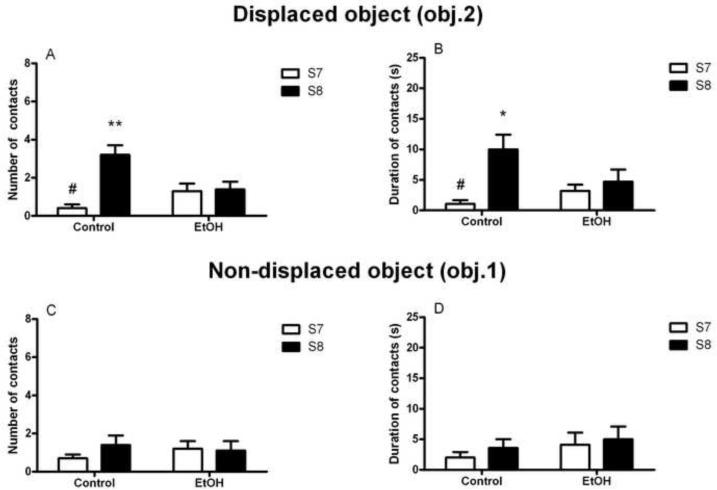
Reaction to spatial change was assessed by a comparison between the number of contacts and the time spent exploring obj. 2 before (session 7) and after (session 8) it was displaced to another location of the open field. The binge alcohol treatment produced impairment in spatial memory detected on testing at the 1 week time point. As indicated by a main effect of session (S7 vs. S8; F(1,17)=16.5, p<0.001]), the number of contacts with the displaced object (obj. 2) was modified by the spatial change, and this modification differed between the EtOH and Control groups as evidenced by a significant group x session interaction [F(1,17)=7.1, p<0.05]. Post hoc analysis revealed that the number of contacts increased from S7 to S8 in Control animals (p<0.001), but did not increase in EtOH animals (Figure 4A). A similar pattern was seen when analyzing the duration of contacts with the displaced object as the dependent variable, where a main effect of session [F(1,17)=14.3, p<0.001] was accompanied by a trend for a group x session interaction [F(1,17)=3.2, p=0.09]. Post hoc analysis revealed a significant difference in the duration of contacts with obj. 2 between Control and EtOH animals (p<0.01) following displacement of the object (Figure 4B).
Figure 4.
Binge-like ethanol exposure impaired the ability to detect the spatial change 1 week following the binge alcohol treatment. When object 2 (obj. 2) was displaced to a new location, non-exposed rats (Control) more than doubled the number of contacts (A) as well as the duration of contacts (B) with the object from session 7 (S7, white bars) to session 8 (S8, black bars) whereas both measures were unaltered in alcohol exposed (EtOH) rats. Number (C) and duration (D) of contacts with the non-displaced object, object1 (obj.1), did not change between S7 and S8 in either group. Values are given as the mean (±SEM) number and duration of contacts with both the displaced and non-displaced object. **p<0.01, ***p<0.001, difference from Control performance in S7; #p<0.05, difference from Control performance in S7. For detailed statistics, see Results.
This difference in exploratory behavior was selective for the displaced object (obj. 2) since neither number of contacts (exploration [F(1,17)=2.9, NS]; interaction group x exploration [F(1,17)=0.7, NS]) nor duration of contacts (exploration [F(1,17)=4.4, NS]; interaction group x exploration [F(1,17)=0.0, NS]) was significantly different between S7 and S8 for the non-displaced object (obj.1) in either treatment group. However, a main effect of group was found when analyzing the number of contacts with the non-displaced object [F(1,17)=6.0, p<0.05]. Post hoc analysis showed a significant difference between EtOH and Control animals (p<0.05) in session 7. This suggests an increase in general exploratory behavior towards the non-displaced object in Control rats when compared with EtOH animals. The duration of contacts with the non-displaced object, however, was not different between groups ([F(1,17)=2.9, NS]; Figure 4C–D).
Binge-like alcohol exposure produced similar impairment in detecting the spatial change at 10 weeks following treatment. On testing at this time point, ANOVA revealed a main effect of session (S7 vs. S8) on the number of contacts with the displaced object [F(1,16)=13.0, p<0.001], indicating an effect of spatial change. This effect of spatial change again differed between the treatment groups as shown by a significant group x session interaction [F(1,16)=8.0, p<0.05]. Post hoc analysis revealed that the number of contacts increased between S7 and S8 in Control animals (p<0.01) but did not increase in EtOH animals (Figure 5A). Results for duration of contacts essentially paralleled those for number of contacts with the displaced object: a main effect of session [F(1,16)=9.1, p<0.01] was accompanied by a trend for a group x session interaction [F(1,16)=4.3, p=0.05]. Post hoc analysis showed a significant difference in performance of the Control animals (p<0.05) but no change in the EtOH group (Figure 5B). On post hoc analysis, a significant difference (p<0.05) in number as well as duration of contacts in S7 between Control and EtOH animals was also observed, indicating a decreased degree of exploration for controls in this session.
Figure 5.
Binge-like ethanol exposure impaired the ability to detect the spatial change 10 weeks following the binge alcohol treatment. When object 2 (obj.2) was displaced to a new location, non-exposed rats (Control) greatly increased the number of contacts (A) as well as the duration of contacts (B) with obj. 2 from session 7 (S7, white bars) to session 8 (S8, black bars) whereas both measures were unaltered in alcohol exposed (EtOH) rats. Number (C) and duration (D) of contacts with the non displaced object, object1 (obj.1), did not change between S7 and S8 in either group. Values are given as the mean (±SEM) number and duration of contacts at both the displaced and non-displaced object. *p<0.05, **p<0.01, difference from Control performance in S7; #p<0.05, difference from Control performance in S7. For detailed statistics, see Results.
Once again, increased exploratory behavior in Control rats was selective for the displaced object since the number (group [F(1,16)=0.0, NS]; exploration [F(1,16)=0.8, NS]; interaction group x exploration [F(1,16)=1.5, NS]) and the duration of contacts with the non-displaced object (group [F(1,16)=0.6, NS]; exploration [F(1,16)=0.7, NS]; interaction group x exploration [F(1,16)=0.1, NS]) did not differ between sessions 7 and 8 in either treatment group (Figure 5C–D).
Binge alcohol exposure produces short-term object recognition impairment
In session 10, a familiar object (obj. 1) was replaced by a novel object (obj. 3) while obj. 2 was maintained in the same location as in sessions 8 and 9. Greater exploration of the novel object reflects non-spatial processes such as recognition, which we consider a negative behavioral control for the spatial change performance.
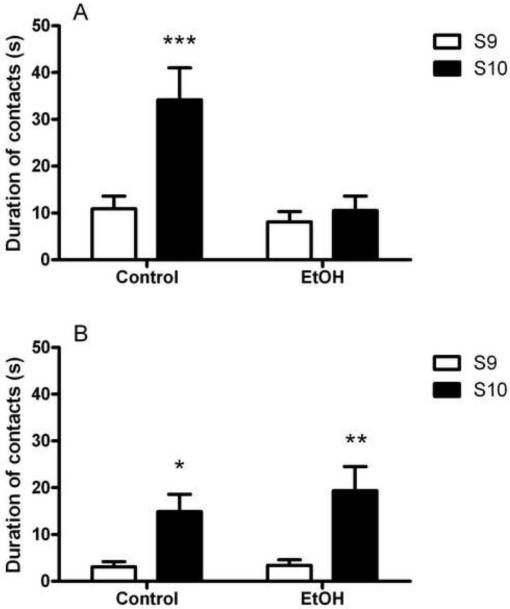
Figure 6A shows exploratory activity directed towards the novel object on testing at 1 week, expressed as duration of contacts. ANOVA revealed a significant main effect of group [F(1,17)=9.1, p<0.001], a significant main effect of session (S9 vs. S10; [F(1,17)=12.4, p<0.001]) and an interaction of group x session [F(1,17)=8.2, p<0.05]. Post hoc analyses indicated that Control rats were able to detect the new object, as evidenced by an increase in duration of contacts (p<0.001; Figure 5A), while the EtOH rats did not.
Figure 6.
Binge-like ethanol exposure impaired the ability to detect the non-spatial change at 1 week (A) but not 10 weeks (B) following the alcohol treatment. (A) When a novel object, object 3, replaced object 1, non-exposed rats (Control) greatly increased the duration of contacts with the novel object compared with the old one from session 9 (S9, white bars) to session 10 (S10, black bars), whereas duration of contacts was unaltered in alcohol exposed (EtOH) rats. (B) Both Control and EtOH groups detected the non-spatial change as shown by the increased duration of contacts with the novel object in S10 vs. S9. Values are given as the mean (±SEM) duration of contacts (expressed in seconds) with the novel object. *p<0.05, difference from Control performance in S9; **p<0.01, difference from EtOH performance in S9. For detailed statistics, see Results.
In contrast to the results obtained 1 week following the binge treatment, on testing at 10 weeks there was no significant main effect of group [F(1,16)=0.4, NS] and no interaction between group and session [F(1,16)=0.5, NS]. There was a main effect of session [F(1,16)=21.1, p<0.001], suggesting that both EtOH and Control groups were able to detect the novel object. Post hoc analyses revealed that the duration of contacts of EtOH animals with obj. 3 (p<0.01) surprisingly increased from S9 to S10 in a greater fashion compared with Control (p<0.05; Figure 6B).
The number of contacts was a less sensitive measure for the detection of non-spatial change since none of the groups exhibited a greater exploration of the novel object relative to the familiar object (data not shown).
DISCUSSION
We show here that binge-like alcohol exposure produces neuronal death in the EC and hippocampal DG, and that this is associated with long-term and possibly permanent impairment of memory for a novel geometric configuration of objects during exploration. In contrast, the ability to detect non-spatial changes, manifested as recognition of a novel object, was only temporarily affected.
Rats exposed to a 4-day binge ethanol treatment showed neurotoxicity in selected brain regions. Cellular damage occurred due to neuronal death during alcohol intoxication and was highlighted by the fluoro-jade B staining. In agreement with previous findings (Cippitelli et al., 2010; Collins et al., 1996; Collins et al., 1998; Crews et al., 2000; Hamelink et al., 2005; Obernier et al., 2002; Zou et al., 1996), the hippocampal dentate gyrus granular cell layer and pyramidal neurons in layer III of the entorhinal cortex were especially affected. Damage to these brain areas is particularly important because of their involvement in cognition including learning and memory formation. Since excessive alcohol consumption is known to produce spatial learning and memory impairments in humans (Bowden and Mccarter, 1993; Oscar-Berman et al., 1992) and experimental animals (Matthews et al., 1995; Obernier et al., 2002), the object exploration task was used in the present study to investigate the habituation and the time course of reactions to spatial and non-spatial changes occurring in a previously experienced environment in animals that had undergone a binge-like alcohol exposure.
The results show that when repeatedly subjected to the same spatial configuration of objects, alcohol exposed as well as non-exposed rats displayed habituation to the test situation in terms of decreased locomotor activity and decreased exploration directed to the objects following both 1 week and 10 weeks from the exposure (S2–S7). This general decrease of exploratory activity may indicate a normal encoding of the environmental features (Galani et al., 1998; Save et al., 1992). Place learning and memory are thought to be mediated by the hippocampus, suggesting that the integrity of this structure is needed for normal acquisition of reference memory (O'Keefe & Nadel, 1978). Indeed, it has been demonstrated that animals with hippocampal lesions showed spatial reference memory deficits in the eight-arm radial maze (Jarrard, 1993) as well as in the Morris water maze (Morris et al., 1982). Rats with hippocampal lesions consistently failed to show any sign of habituation to the object exploration task, whereas entorhinal cortex lesions did not impair performance in this task (Galani et al., 1998; Parron et al., 2006). However, when aspiration lesions of the hippocampus were applied, patterns of habituation similar to that seen in control rats were observed in the OET (Save et al., 1992), suggesting that the nature and selectivity of the lesions is critical to assess the spatial patterns of behavior in these experimental paradigms. Although alcohol-induced impairments in spatial learning and memory are thought to parallel those induced by lesions (Matthews & Silvers, 2004), our data indicate that alcohol-induced neurotoxicity in the DG and the EC may not compromise the ability to normally encode the surrounding environment such as the geometric arrangement of objects in a open arena. This observation is consistent with previous work showing that binge-like ethanol exposure did not affect acquisition of the spatial reference memory task of the Morris water maze and furthermore, that alcohol treated rats and controls displayed similar swim speed and similar locomotor activity in the open field (Cippitelli et al., 2010; Obernier et al., 2002). Thus, it is likely that selective and bilateral hippocampal lesions affect the acquisition of reference memory more than cell death in the hippocampal DG does. Our results also show that patterns of locomotor activity 1 week, but not 10 weeks, following alcohol treatment were different between alcohol and non-alcohol exposed rats, although relative performance in every single session of the task did not change, including performance in session 1 where locomotor activity was assessed in the absence of objects. Such differences may be due to increased thigmotaxis (i.e., increased distance traveled near the walls of the open field) that has been previously demonstrated in binge alcohol exposed rats. The re-learning deficits observed in these animals in the reversal learning task of the Morris water maze have been closely associated with different navigation patterns reflecting increased perseverative behavior as well as decreased cognitive flexibility (Cippitelli et al., 2010). Conversely, it is unlikely that the observed difference in locomotor patterns is secondary to an alcohol withdrawal state since our behavioral assessment started five days following the alcohol treatment, a time point in which animals experiencing a binge-like exposure are known to be free from overt symptoms of ethanol withdrawal (Cippitelli et al., 2010; Obernier et al., 2002; Schulteis et al., 2008).
After habituation, one of the two familiar objects was displaced to a different location and the ability of the animals to detect the spatial change was examined (S7–S8). Renewal of exploration normally occurs after a rearrangement of the familiar objects in intact animals. One week following the alcohol bingeing we found that non-alcohol exposed rats exhibited increased as well as selective exploration towards the displaced object, since the number and duration of contacts with the non-displaced object did not change. In contrast, alcohol exposed animals did not increase their exploration of the displaced object, indicating impaired spatial information processing. Neither group changed their exploration of the non-displaced object. Both groups were then tested for their reaction to a non-spatial change task, that is, when a novel object replaced a familiar one without any spatial rearrangement (S9–S10). The results revealed that the spatial damage observed in the alcohol exposed animals 1 week following binge treatment was extended to an impaired ability to detect the novel object, indicating that binge alcohol exposure produces cognitive deficits that, at this stage, may affect both spatial and non-spatial learning. It may be argued that alcohol exposed animals failed to investigate the novel object because of the development of fear, which is thought to counteract exploratory activity (Myhrer, 1988). However, the fact that no group differences were seen in locomotor activity throughout the task, and no differences in object exploration were observed during session 2 (when both objects were novel), in our view make that interpretation less likely.
Data from lesions studies suggest that the detection of a novel configuration of objects in the OET is fully dependent on entorhinal-hippocampal connectivity, since hippocampal lesions (Galani et al., 1998; Save et al., 1992) as well as contralateral lesions of the EC (Parron et al., 2006) caused impaired encoding of the geometric arrangement of objects. Furthermore, hippocampal place cell function was demonstrated to depend on direct inputs from the EC (Brun, Otnass, Molden, Steffenach, Witter, Moser, & Moser, 2002), highlighting the importance of this circuit for spatial information processing. On the other hand, lesions of EC and the subiculum led to deficits in reacting to the non-spatial change (Galani et al., 1998), indicating an important role of these structures in novel object recognition. Therefore, the observed alcohol-induced neurodegeneration in the DG and the EC may ultimately impair the interaction between hippocampus and entorhinal cortex in mediating responses to spatial change, whereas the neurotoxicity in the EC may account for the deficits in the non-spatial task. This possibility is supported by the theory that hippocampal deficits may lead to the loss of the neural system that provides the animal with a cognitive or spatial map of its environment, whereas non-spatial memory can be independent of the hippocampus in some specific tasks and is likely to be dependent on the function of other brain regions (Nadel, 1991; O'Keefe and Nadel, 1978).
When the OET assessment was repeated with a new cohort of animals under the same experimental conditions at 10 weeks, the spatial deficit remained, while performance in the non-spatial task was no longer impaired. Thus, alcohol bingeing induced a long-term and possibly permanent impairment in spatial learning and memory whereas non-spatial performance was only transiently affected, since alcohol exposed rats recovered the ability to detect the novel object. Consistent with this, previous studies using the binge model showed that alcohol exposed animals were impaired in their reversal learning but not in the ability to perform a non-spatial or visually cued task (Cippitelli et al., 2010; Obernier et al., 2002). Also, acute ethanol administration was shown to impair spatial memory in a dose-dependent manner but not to alter a model of non-spatial memory such as the stimulus/response task (Matthews et al., 1995).
A possible mechanism for recovery of the ability to detect non-spatial change may be associated with brain regeneration. Alcohol inhibits the ongoing genesis of neurons and glia during intoxication (Crews & Nixon, 2009). In particular, it has been shown that binge-like alcohol exposure blocked neuronal stem cells proliferation, contributing to mechanisms of neurodegeneration (Nixon & Crews, 2002). However, during the first week of abstinence following a 4-day binge ethanol treatment in rats, a burst of brain cell proliferation in hippocampal and cortical structures was shown to occur (Nixon & Crews, 2004), and in humans very long abstinence has been reported to resolve most of the neurocognitive deficits associated with alcoholism (Fein, Torres, Price, & Di, V, 2006; Oscar-Berman, Shagrin, Evert, & Epstein, 1997; Parsons & Nixon, 1993). Therefore, it is possible that during alcohol bingeing the proliferation and maturation of stem cells is delayed, producing impairment of behavioral functions, while a long period of abstinence may increase neurogenesis thereby improving and, in part, re-establishing cognitive abilities, as demonstrated by the alcohol exposed animals tested for their ability to detect non-spatial change at 10 weeks following the exposure. However, reversal of non-spatial memory could be also due to neuromodulatory effects in other brain regions implicated in learning and memory, such as the medial prefrontal cortex (mPFC) which is central to the executive-attention processes of the working memory system (Kane & Engle, 2002). In fact, although no neurodegeneration was detected in the mPFC (Supplemental Figure S2), it is possible that binge-like alcohol exposure affects its functionality (i.e. cell impairment which cannot be labeled by FJB staining) without producing neuronal death. Evidence that working memory and other neuropsychological deficits improve with abstinence (Meyerhoff, Blumenfeld, Truran, Lindgren, Flenniken, Cardenas, Chao, Rothlind, Studholme, & Weiner, 2004; Parks, Dawant, Riddle, Hartmann, Dietrich, Nickel, Price, & Martin, 2002; Sullivan, Rosenbloom, & Pfefferbaum, 2000) strengthen this possibility.
In conclusion, we replicate here prior findings that a single cycle of binge-like alcohol exposure leads to neurotoxicity in brain structures associated with spatial learning and memory. We then extend these findings by presenting novel data from the object exploration task demonstrating impairments in spatial as well as non-spatial cognitive abilities as a result of binge-like alcohol exposure. By evaluating performance in this task at two different time points, we were also able to demonstrate the differential time course of spatial and non-spatial memory impairments. Together, these data illustrate that the object exploration task is a useful experimental paradigm to assess spatial learning and memory damage following alcohol exposure in the rat.
Supplementary Material
ACKNOWLEDGEMENTS
We are thankful to Karen Smith and Dr. Annika Thorsell for careful revision of the paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aggleton JP, Vann SD, Oswald CJ, Good M. Identifying cortical inputs to the rat hippocampus that subserve allocentric spatial processes: a simple problem with a complex answer. Hippocampus. 2000;10:466–474. doi: 10.1002/1098-1063(2000)10:4<466::AID-HIPO13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Assuncao M, Santos-Marques MJ, De Freitas V, Carvalho F, Andrade JP, Lukoyanov NV, Paula-Barbosa MM. Red wine antioxidants protect hippocampal neurons against ethanol-induced damage: A biochemical, morphological and behavioral study. Neuroscience. 2007;146:1581–1592. doi: 10.1016/j.neuroscience.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Yee BK, Lemaire M, Wilbrecht L, Jarrard L, Iversen SD, Rawlins JN, Good MA. The role of the entorhinal cortex in two forms of spatial learning and memory. Experimental Brain Research. 2001;141:281–303. doi: 10.1007/s002210100868. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Alcohol-Induced Neuronal Loss in Developing Rats - Increased Brain-Damage with Binge Exposure. Alcoholism-Clinical and Experimental Research. 1990;14:107–118. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Bowden SC, Mccarter RJ. Spatial Memory in Alcohol-Dependent Subjects - Using A Push-Button Maze to Test the Principle of Equiavailability. Brain and Cognition. 1993;22:51–62. doi: 10.1006/brcg.1993.1024. [DOI] [PubMed] [Google Scholar]
- Brun VH, Otnass MK, Molden S, Steffenach HA, Witter MP, Moser MB, Moser EI. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296:2243–2246. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Saddoris MP, Bucci DJ, Wiig KA. Corticohippocampal contributions to spatial and contextual learning. Journal of Neuroscience. 2004;24:3826–3836. doi: 10.1523/JNEUROSCI.0410-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho FM, Pereira SRC, Pires RGW, Ferraz VP, Romano-Silva MA, Oliveira-Silva IF, Ribeiro AM. Thiamine deficiency decreases glutamate uptake in the prefrontal cortex and impairs spatial memory performance in a water maze test. Pharmacology Biochemistry and Behavior. 2006;83:481–489. doi: 10.1016/j.pbb.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Frankola K, Goldstein A, Thorsell A, Singley E, Eskay RL, Heilig M. Alcohol-Induced Neurodegeneration, Suppression of Transforming Growth Factor-beta, and Cognitive Impairment in Rats: Prevention by Group II Metabotropic Glutamate Receptor Activation. Biol Psychiatry. 2010;67:823–830. doi: 10.1016/j.biopsych.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Collins MA, Corso T, Neafsey EJ. Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic “binge” intoxication with ethanol: possible explanation for olfactory deficts in alcoholics. Alcoholism Clinical & Experimental Research. 1996;20:284–292. doi: 10.1111/j.1530-0277.1996.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Collins MA, Zou JY, Neafsey EJ. Brain damage due to episodic alcohol exposure in vivo and in vitro: furosemide neuroprotection implicates edema-based mechanism. FASEB Journal. 1998;12:221–230. doi: 10.1096/fasebj.12.2.221. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcoholism-Clinical and Experimental Research. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of Neurodegeneration and Regeneration in Alcoholism. Alcohol and Alcoholism. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. 2 Functional Components of the Hippocampal Memory System. Behavioral and Brain Sciences. 1994;17:449–472. [Google Scholar]
- Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Progress in Neurobiology. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Fein G, Torres J, Price LJ, Di S, V. Cognitive performance in long-term abstinent alcoholic individuals. Alcohol Clin.Exp.Res. 2006;30:1538–1544. doi: 10.1111/j.1530-0277.2006.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galani R, Weiss I, Cassel JC, Kelche C. Spatial memory, habituation, and reactions to spatial and nonspatial changes in rats with selective lesions of the hippocampus, the entorhinal cortex or the subiculum. Behavioural Brain Research. 1998;96:1–12. doi: 10.1016/s0166-4328(97)00197-6. [DOI] [PubMed] [Google Scholar]
- Hamelink C, Hampson A, Wink DA, Eiden LE, Eskay RL. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J Pharmacol Exp Ther. 2005;314:780–788. doi: 10.1124/jpet.105.085779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE. On the Role of the Hippocampus in Learning and Memory in the Rat. Behavioral and Neural Biology. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon.Bull Rev. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Silvers JR. The use of acute ethanol administration as a tool to investigate multiple memory systems. Neurobiology of Learning and Memory. 2004;82:299–308. doi: 10.1016/j.nlm.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Simson PE, Best PJ. Acute ethanol impairs spatial memory but not stimulus/response memory in the rat. Alcohol Clin.Exp.Res. 1995;19:902–909. doi: 10.1111/j.1530-0277.1995.tb00965.x. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, Chao LL, Rothlind J, Studholme C, Weiner MW. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcohol Clin Exp Res. 2004;28:650–661. doi: 10.1097/01.ALC.0000121805.12350.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, Okeefe J. Place Navigation Impaired in Rats with Hippocampal-Lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Myhrer T. Exploratory behavior and reaction to novelty in rats with hippocampal perforant path systems disrupted. Behavioral Neuroscience. 1988;102:356–362. doi: 10.1037//0735-7044.102.3.356. [DOI] [PubMed] [Google Scholar]
- Nadel L. The hippocampus and space revisited. Hippocampus. 1991;1:221–229. doi: 10.1002/hipo.450010302. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Otto T, Gallagher M. Entorhinal-perirhinal lesions impair performance of rats on two versions of place learning in the Morris water maze. Behavioral Neuroscience. 1995;109:3–9. doi: 10.1037//0735-7044.109.1.3. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. Journal of Neurochemistry. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. Journal of Neuroscience. 2004;24:9714–9722. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The hippocampus as a cognitive map. Clarendon Press; Oxford: 1978. [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcoholism-Clinical and Experimental Research. 2002;26:547–557. [PubMed] [Google Scholar]
- Obernier JA, White AM, Swartzwelder HS, Crews FT. Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacology, Biochemistry and Behavior. 2002;72:521–532. doi: 10.1016/s0091-3057(02)00715-3. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Hutner N, Bonner RT. Visual and Auditory Spatial and Nonspatial Delayed-Response Performance by Korsakoff and Non-Korsakoff Alcoholic and Aging Individuals. Behavioral Neuroscience. 1992;106:613–622. doi: 10.1037//0735-7044.106.4.613. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Shagrin B, Evert DL, Epstein C. Impairments of brain and behavior: the neurological effects of alcohol. Alcohol Health Res World. 1997;21:65–75. [PMC free article] [PubMed] [Google Scholar]
- Parks MH, Dawant BM, Riddle WR, Hartmann SL, Dietrich MS, Nickel MK, Price RR, Martin PR. Longitudinal brain metabolic characterization of chronic alcoholics with proton magnetic resonance spectroscopy. Alcohol Clin Exp Res. 2002;26:1368–1380. doi: 10.1097/01.ALC.0000029598.07833.2D. [DOI] [PubMed] [Google Scholar]
- Parron C, Poucet B, Save E. Cooperation between the hippocampus and the entorhinal cortex in spatial memory: a disconnection study. Behavioural Brain Research. 2006;170:99–109. doi: 10.1016/j.bbr.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Parron C, Save E. Comparison of the effects of entorhinal and retrosplenial cortical lesions on habituation, reaction to spatial and non-spatial changes during object exploration in the rat. Neurobiology of Learning and Memory. 2004;82:1–11. doi: 10.1016/j.nlm.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Parsons OA, Nixon SJ. Neurobehavioral sequelae of alcoholism. Neurol Clin. 1993;11:205–218. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. Academic Press; San Diego: 1998. [Google Scholar]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon H, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Archives of General Psychiatry. 1998;55:905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- Pouzet B, Welzl H, Gubler MK, Broersen L, Veenman CL, Feldon J, Rawlins JN, Yee BK. The effects of NMDA-induced retrohippocampal lesions on performance of four spatial memory tasks known to be sensitive to hippocampal damage in the rat. European Journal of Neuroscience. 1999;11:123–140. doi: 10.1046/j.1460-9568.1999.00413.x. [DOI] [PubMed] [Google Scholar]
- Renner MJ, Seltzer CP. Molar characteristics of exploratory and investigatory behavior in the rat (Rattus norvegicus) J.Comp Psychol. 1991;105:326–339. doi: 10.1037/0735-7036.105.4.326. [DOI] [PubMed] [Google Scholar]
- Save E, Poucet B, Foreman N, Buhot MC. Object exploration and reactions to spatial and nonspatial changes in hooded rats following damage to parietal cortex or hippocampal formation. Behavioral Neuroscience. 1992;106:447–456. [PubMed] [Google Scholar]
- Schenk F, Morris RG. Dissociation between components of spatial memory in rats after recovery from the effects of retrohippocampal lesions. Experimental Brain Research. 1985;58:11–28. doi: 10.1007/BF00238949. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Research. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Archer C, Tapert SF, Frank LR. Intermittent binge alcohol exposure during the periadolescent period induces spatial working memory deficits in young adult rats. Alcohol. 2008;42:459–467. doi: 10.1016/j.alcohol.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000;24:611–621. [PubMed] [Google Scholar]
- Walker DW, Barnes DE, Zornetzer SF, Hunter BE, Kubanis P. Neuronal loss in hippocampus induced by prolonged ethanol consumption in rats. Science. 1980;209:711–713. doi: 10.1126/science.7394532. [DOI] [PubMed] [Google Scholar]
- White AM. What happened? Alcohol memory blackouts, and the brain. Alcohol Research & Health. 2003;27:186–196. [PMC free article] [PubMed] [Google Scholar]
- Zou JY, Martinez DB, Neafsey EJ, Collins MA. Binge ethanol-induced brain damage in rats: Effect of inhibitors of nitric oxide synthase. Alcohol Clin.Exp Res. 1996;20:1406–1411. doi: 10.1111/j.1530-0277.1996.tb01141.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.