Abstract
Brief tonal stimuli and spoken sentences were utilized to examine whether adolescents (aged 14;3-18;1) with specific language impairments (SLI) exhibit atypical neural activity for rapid auditory processing of non-linguistic stimuli and linguistic processing of verb-agreement and semantic constraints. Further, we examined whether the behavioral and electrophysiological indices for rapid auditory processing were correlated with those for linguistic processing. Fifteen adolescents with SLI and 15 adolescents with normal language met strict criteria for displaying consistent diagnoses from kindergarten through the eighth grade. The findings provide evidence that auditory processing for non-linguistic stimuli is atypical in a significant number of adolescents with SLI compared to peers with normal language and indicate that reduced efficiency in auditory processing in SLI is more vulnerable to rapid rates (200 ms ISI) of stimuli presentation (indexed by reduced accuracy, a tendency for longer RTs, reduced N100 over right anterior sites, and reduced amplitude P300). Many adolescents with SLI displayed reduced behavioral accuracy for detecting verb-agreement violations and semantic anomalies, along with less robust P600s elicited by verb-agreement violations. The results indicate that ERPs elicited by morphosyntactic aspects of language processing are atypical in many adolescents with SLI. Additionally, correlational analyses between behavioral and electrophysiological indices of processing non-linguistic stimuli and verb-agreement violations suggest that the integrity of neural functions for auditory processing may only account for a small proportion of the variance in morphosyntactic processing in some adolescents.
Keywords: auditory processing, verb-agreement violations, semantic anomalies, specific language impairment, ERPs, adolescents, verbs, morpho-syntax, lexical, semantic
1. Introduction
Children with specific language impairment (SLI) exhibit significant difficulties in areas of language such as morphosyntax and lexical development (e.g., Leonard, 1998; McGregor, Newman, Reilly, & Capone, 2002). Although deficits in language are the primary and most obvious characteristics for many of these children, it is becoming increasingly clear that more subtle deficits in cognitive processes essential for language learning may contribute to these patterns of marked language difficulties. Several different cognitive processes have been shown to be atypical in children with SLI and it is possible that the cumulative effects of these fundamental weaknesses have a profound impact on these children's ability to acquire language in a typical manner. Earlier studies indicate that some of these primary cognitive processes may involve auditory processing, including rapid temporal processing of non-linguistic stimuli (e.g., Basu, Krishnan, & Weber-Fox, 2010; Benasich & Tallal, 2001; Friedrich, Weber, & Friederici, 2004; McArthur, Atkinson & Ellis, 2009; McArthur & Bishop, 2005; Neville, Coffey, Holcomb, & Tallal, 1993; Tallal, 1980; 2000; Wible, Nicol, & Kraus, 2004; 2005), selective auditory attention (Stevens, Sanders, & Neville, 2006), speed of processing for non-linguistic as well as linguistic information (Miller, Leonard, & Finneran, 2008; Miller, Kail, Leonard, & Tomblin, 2001; Miller, Leonard, Kail, Zhang, Tomblin, & Francis, 2006), and processing capacity/working memory (Ellis Weismer, Evans, & Hasketh, 1999; Ellis Weismer, Plante, Jones, & Tomblin, 2005; Leonard, Ellis Weismer, Miller, Francis, Tomblin, & Kail, 2007).
Much of the work characterizing SLI has been conducted with preschool and young school-age children (e.g., Leonard, 1998). However, studies indicate that SLI is often longstanding (e.g., Tomblin, Freese, & Records, 1992) and older children and adolescents with a history of SLI are at risk for reading disability (Catts, Bridges, Little, & Tomblin, 2008). Even though the overt symptoms of SLI change with age, some of the same details of language can persist as problems into adolescence and beyond. For example, many adolescents with SLI show an insensitivity to certain grammatical morpheme errors during sentence processing tasks (Miller, Leonard, & Finneran, 2008; Leonard, Miller, & Finneran, 2009).
Given that auditory processing constitutes a fundamental ability, it would be natural to assume that deficits in auditory processing were responsible for the morphosyntactic deficits that are also observed in the SLI population. However, recent evidence from twin studies suggests that the relationship between auditory processing and language ability may be less direct. In one study, Bishop, Bishop, Bright, James, Delaney, and Tallal (1999) administered a rapid auditory processing task employing non-linguistic stimuli as well as a nonword repetition task to monozygotic and same-sex dizygotic twins. Weaknesses on the nonword repetition task showed high estimates of group heritability. On the other hand, weaknesses in rapid auditory processing were more influenced by shared environment than by genetic factors. In a subsequent twin study, Bishop, Adams, and Norbury (2006) found that weaknesses in “grammatical computation” also had a genetic basis, yet this type of heritable ability was separable from ability on nonword repetition tasks. Central to the grammatical computation problem was a limitation in the use of verb-related grammatical forms such as third person singular –s and past tense –ed, and difficulty understanding the relations expressed in particular morphosyntactic constructions. Despite the distinct sources of these types of problems, Bishop and her colleagues found that many children exhibited deficits in more than one of these areas. These findings raise the possibility that weaknesses observed on measures of auditory processing and those seen on measures of grammatical ability are not causally related. Rather, any correlations observed between these different measures will be rather modest, and attributable to the comorbidity of these two types of weaknesses in the SLI population, one arising from a shared genetic source (grammatical computation deficit) and one showing low heritability (auditory processing deficit). We examine this issue in the present study.
The current study utilizes electrophysiological measures of both non-linguistic auditory processing and linguistic processing. Event-related brain potentials (ERPs) provide a functional measure of neural activity with very fine temporal resolution (Nunez, 1995) and allow the examination of specific operations of non-linguistic and linguistic processing (e.g., Friederici, 1997; King & Kutas, 1995; Kutas & Hillyard, 1980; Mangun & Hillyard, 1991; Neville, Nicol, Barss, Forster, & Garrett, 1991; Hagoort, Brown, & Groothusen, 1993; Osterhout & Holcomb, 1992; Polich & Kok, 1995; Weber-Fox, Hart, & Spruill, 2006). In this study, we focus on adolescents with SLI and their typically developing same-age peers. Although SLI is a longstanding problem, it is not known whether the conditions originally responsible for the language deficit are still present. For example, an early neuromaturational delay could lead to an initial period of slow language development such that by adolescence, age-level language ability has not yet been achieved. However, it is not clear if, at this point, neurological evidence of the language deficit still remains. Examination of the adolescents' underlying brain functions as reflected in ERPs can be extremely informative in this regard and therefore ideal for capturing group differences that may not be evident from behavioral measures alone. ERPs provide another level of analysis that may be more sensitive to differences in the processing abilities, and further, can shed light about underlying aspects of processing (e.g., post-lexical syntactic re-analysis, ease of lexical access). ERPs, combined with behavioral measures, are utilized in the current study to more closely examine neural functions underlying: (1) non-linguistic auditory processing in an odd-ball paradigm that elicits both early perceptual cortical potentials related to sensory processing (N100, P200) and later cognitive potentials indexing updates in working memory (N200, P300); (2) morphosyntactic processing, using verb-agreement violations in natural speech, that elicit a well-known late positivity (P600 or syntactic positive shift) thought to index recognition of a morphosyntactic violation and the listener's attempts to recover the meaning of a sentence (e.g., Osterhout & Holcomb, 1992; Osterhout & Mobley, 1995); and (3) lexical/semantic processing, utilizing unexpected verbs in natural speech, that elicit an N400 thought to index ease of lexical access and integration (e.g., Kutas & Hillyard, 1980; Federmeier, Segal, Lombrozo, & Kutas, 2000). Below we summarize findings from earlier electrophysiological studies of children with SLI.
1.1. Non-linguistic Auditory Processing and SLI
One of the first electrophysiological studies of brain activity in children with SLI employed both non-linguistic and linguistic stimuli (Neville et al., 1993). For the non-linguistic auditory processing condition, ERPs were elicited by brief tonal stimuli in children aged 8-10 years. ERPs elicited by brief tones with shorter interstimulus intervals (ISIs) resulted in reduced N100 amplitudes in the children with SLI who also performed poorly on a rapid auditory sequencing task. Further evidence that brain functions for non-linguistic auditory processing may be atypical for children with SLI was reported for subgroups of children with SLI who demonstrated atypical early cortical potentials (N1-P2-N2) elicited by tonal pairs (McArthur & Bishop 2004; 2005; McArthur, Atkinson, & Ellis, in press). A study of cortical potentials in children with SLI aged 10-19 years and matched controls suggested that the neural responses of the group with SLI were less mature for tonal pairs presented with short ISIs (Bishop & McArthur, 2004). A recent study of auditory processing in children with SLI (aged 7-17) indicates that spectral information of consonant-vowel (CV) syllables is also processed less accurately by this population and further, that amplitudes of N2-N4 peaks for these stimuli correlated with the severity of language deficits (Čeponienè, Cummings, Wulfeck, Ballantyne, & Townsend, 2009).
Recent electrophysiological evidence indicates that differences in auditory processing in children with SLI are not limited to cortical regions. A recent study of children with and without SLI aged 4-10 years revealed atypical brainstem neural responses for temporal auditory processing for the children with SLI (Basu, Krishnan, & Weber-Fox, 2010). Measures of the sustained frequency following response (FFR) elicited by fast tonal sweeps revealed degraded FFR phase-locked neural activity in seven of the 10 children with specific language impairment (SLI). In addition, the onset auditory brainstem responses (ABRs) of these children had longer latencies for waves II and V and an enhanced prolongation of wave III at higher click rates, indicating a greater susceptibility to neural adaptation (Basu et al., 2010). These results were consistent with findings of atypical brainstem and cortical auditory processes in children with more generally defined language impairment and language-based learning deficits (Wible, Nicol, & Kraus, 2004; 2005). The findings from these electrophysiological studies indicate that neural functions for non-linguistic auditory processing may be less efficient in at least a subgroup of children with SLI.
Recent findings also suggest that auditory processing skills may be correlated with later language ability. In a behavioral study of infants that employed a head turn paradigm, Benasich and Tallal (2002) found that early deficits in discriminating rapid auditory cues are predictive of language delays at age 3 years. In addition, electrophysiological measures of auditory processing related to duration discrimination for syllables at 4 months of age were found to correlate with language impairments at age 4 years (Weber, Hahne, Friedrich, & Friederici, 2004). Thus, there are strong indications that fundamental auditory processing abilities may play a key role in later language ability and deficits in auditory processing may contribute to language impairments (Benasich & Tallal, 2002; Weber et al., 2004).
1.2. Grammatical Processing and SLI
In a study of language processing and SLI, ERPs were elicited by closed-class words (function words such as “the”, “and”, “by”, “he”) in a visual sentence processing task in which words were presented one word per second (Neville et al., 1993). The ERPs of a comparison group of typically developing children aged 8-10 years revealed a left anterior negativity. In contrast, for the children with SLI, who were selected based on poor performance on a test of syntax, the anterior negativity was larger in amplitude over the right hemisphere. This ERP pattern in the subgroup of children with SLI was opposite to that observed for the children with typical language skills and a subgroup of children with SLI who demonstrated adequate morphosyntactic skills (Neville et al., 1993). A reversed hemispheric asymmetry was also observed for children with SLI aged 8-14 years in an auditory paradigm (Shafer, Schwartz, Morr, Kessler, & Kurtzberg, 2000). In that study, ERPs were elicited by one closed-class word, “the”, which occurred multiple times embedded in a story.
Processing of morphosyntactic violations was examined in a subgroup of children with SLI who exhibited especially weak grammatical skills (Fonteneau & van der Lely, 2008). In that study, ERPs were elicited by auditory sentences containing violations in morphosyntactic dependencies (e.g., “Who did Joe see someone?”). The ERPs of the children and young adults with SLI (aged 10-21 years) were compared to aged-matched controls and a group of younger controls matched on language test scores. Results indicated that the early left anterior negativity was absent in the SLI group. Instead, the ERPs of this group contained a posterior, later negativity that resembled an N400 more often associated with a semantic anomaly. The later positivity (P600) was, however, similar in the SLI group and comparison groups. In another study of school aged children with SLI, a P600 comparable to that of typically developing children was also reported (Sabisch, Hahne, Glass, von Suchodoletz, & Friederici, 2009). In that study, the P600 was elicited by word category violations and prosodic incongruities. In addition, an early bilateral negativity was absent for the group of children with SLI, however, a later, left anterior negativity was present (Sabisch et al., 2009). The right anterior negativity was attributed to prosodic processing, and thus it was suggested that access to prosodic information, potentially important for development of morphosyntactic processing abilities was atypical in children with SLI (Sabisch et al, 2009).
Thus, converging evidence suggests that brain activity elicited by closed-class words which carry grammatical information (unlike open-class words which primarily convey referential meaning) is characterized by atypical hemispheric asymmetry in children with SLI. Further, violations of morphosyntactic dependencies elicit ERPs in children with SLI that do not contain an early component associated with automatic morphosyntactic parsing, but rather include a later negativity associated with lexical access difficulty as well as a typical late positivity associated with listeners' attempts to recover the meaning of the sentence. And finally, atypical morphosyntactic processing in children with SLI may also be associated with reduced access to prosodic information as indexed by right hemisphere anterior negativity.
1.3. Lexical/Semantic Processing and SLI
An ERP study of 8- to 10-year-old children with SLI revealed that ease of lexical integration for semantic anomalies may differ for this population compared to their typically developing peers (Neville et al., 1993). In that study, visual sentences were presented one word at a time on a monitor at a rate of one word per second. The ERPs elicited by open-class words (e.g., nouns, verbs, adjectives such as table, run, tall) in the sentences (excluding the final words) exhibited a larger negativity (N400) over occipital regions for the children with SLI compared to their typically developing peers, suggesting increased effort for lexical integration for this semantically rich word class. In addition, half of the sentences ended with an anomalous noun. The children with SLI identified the correct and anomalous sentences with less accuracy compared to the control children; however, accuracy levels were nevertheless rather high (89% vs. 98%). The anomalous nouns elicited an increased N400 compared to the highly constrained, high cloze probability endings for both the children with and without SLI. However, the amplitude of the N400 for anomalous nouns was larger for the group with SLI. These findings suggested the children with SLI had a greater reliance on context for word recognition, reflected as increased effort for lexical integration.
In a study of a subgroup of individuals with SLI aged 10-21 who exhibited striking limitations in grammatical ability, the N400s elicited by semantic violations presented in auditory sentences (e.g., “Barbie bakes the people in the kitchen”) were similar for the group with SLI and a group of age-matched controls (Fonteneau & van der Lely, 2008). Another study of children with SLI (9-10 years old) also examined effects of lexical/semantic processing, with sentences presented auditorially via a loudspeaker (Sabisch, Hahne, Glass, von Suchodoletz, & Friederici, 2006). This study focused on German-speaking children. Similar to the Neville et al. (1993) study, all the semantic anomalies occurred on the final word of the sentences. However, in this case, the words were sentence-final verbs (conforming to one of the syntactic constructions of German). In that study, the children with SLI also performed with decreased accuracy compared to their typically developing peers, but again with a fairly high level of accuracy (83% vs. 91% for control sentences and 92% vs. 96% for anomalous sentences). The ERP results of this study indicated that the children with SLI exhibited a relatively large N400 to the control verbs, without an increase for the anomalous verbs. Thus, the N400 effect was not significant across conditions for the children with SLI. In contrast, the children with typical language development exhibited a significant N400 increase to the anomalous verbs compared to the appropriate verbs. The findings from that study indicated that the semantic representations of verbs may be weaker for children with SLI (Sabisch et al., 2006). A recent study by Cummings and Čeponienè (2010) examined semantic processing in 7 -15 year old children with language impairment with nonverbal performance IQs of 80 or higher and typically developing children with nonverbal performance IQs of 85 or higher. The N400 elicited by words that mismatched picture stimuli were delayed in peak latency in the children with language impairment compared to their age matched peers (Cummings & Čeponienè, 2010) suggesting that language impairment may be associated with less efficient neural connections for lexical integration.
1.4. Current Focus
In summary, the current study builds on previous electrophysiological findings reported for elementary-school-age children with SLI that indicate the neural activations elicited by linguistic and non-linguistic stimuli are different for this population. The current study compares brain activity from a group of adolescents with SLI with that of their typically developing peers for both non-linguistic rapid auditory processing and linguistic processing. The brief tonal stimuli utilized in the current study are presented at a short (200 ms) and long (1000 ms) ISI to explore whether a rate deficit for non-linguistic auditory processing continues to pose a challenge for adolescents with persistent SLI. The sentence stimuli employed in the current study focuses on processing of verbs as the critical comparison across conditions. This design, utilizing natural speech stimuli, allows for examining both morphosyntactic and semantic constraints on the same word class, verbs, and at the same point during sentence processing to explore whether differences in linguistic processing in SLI may be observed across diverse aspects of linguistic processing in adolescence. Evidence suggests that the well-documented problem with verb-related morphology (e.g., third person singular –s verb inflections) seen in children with SLI may not be limited to the formal grammatical functions of the morphemes but may instead reflect a broader problem with how verbs combine with other elements in the sentence (Leonard, 1998). Like nouns, verbs are conceptually rich and provide semantic information in a sentence, but in addition, they provide relational information for integrating grammatical inflections and argument structures across the sentence (Hirsh-Pasek & Golinkoff, 2006; Langacker, 1987; Osterhout, Kim, & Kuperberg, in press). Verb processing engages distinctive neural activations compared to nouns as indexed by measures of ERPs and fMRI in both children and adults (Federmeier et al., 2000; Weber-Fox et al., 2006; Yokoyama, Miyamoto, Riera, Kim, Akitsuki, Iwata et al., 2006). Finally, the current design allows for examining the relationship between performance and ERPs elicited in the rapid auditory processing task and sentence processing task to further explore the relationship between rapid auditory processing and linguistic processing in adolescents. We expect only a modest relationship between ERPs associated with rapid auditory processing and those associated with sentence processing. Although deficits in these two areas can co-occur, the recent genetic evidence reviewed earlier indicates that these two ability areas are separable.
Method
2.1. Participants and Screening Procedures
Participants were 30 adolescents between the ages of 14;3 and 18;1 years. All participants were right handed as determined by the Edinburgh Inventory for Assessment of Handedness (Oldfield, 1971) and were native English speakers with no reported history of neurological or hearing impairments. The participants were a subset of the children who were involved in a large-scale investigation of the prevalence of language impairment from urban, suburban, and rural areas in Iowa (Tomblin, Records, Buckwalter, Zhang, Smith, & O'Brien, 1997). This subset of participants formed two comparison groups, each of which displayed consistent diagnoses across kindergarten, second, fourth, and eighth grades. The diagnosis requirement for inclusion was that the eighth grade diagnosis was consistent with at least 2 of the 3 prior testing points (kindergarten, second, and fourth grades). The two groups consisted of participants with normal language development (ND) and those with SLI. These adolescents participated in two experiments to examine their neural activity elicited by a non-linguistic, rapid auditory processing task and that associated with natural auditory sentence processing. The ND and SLI groups were balanced for age (ND: Mean = 15.9 (SE = .31), SLI: Mean = 16.0 (SE = .32)) and gender (10 males and 5 females in each group).
Comprehensive language and non-verbal intelligence testing was performed for these participants at multiple points in development; they were evaluated while in kindergarten, second, fourth, and eighth grades. The test batteries were adjusted for age at each grade level while maintaining comparable measures of language and non-verbal abilities. Testing measures at the eighth grade level included the Peabody Picture Vocabulary Test – Revised (PPVT-R; Dunn & Dunn, 1981) to evaluate receptive vocabulary, the Comprehensive Receptive and Expressive Vocabulary Test (CREVT; Wallace & Hammill, 1994) to assess expressive vocabulary, the Concepts and Directions and Recalling Sentences subtests of the Clinical Evaluation of Language Fundamentals – Third Edition (CELF-3; Semel, Wiig, & Secord, 1994) to evaluate receptive and expressive grammatical skills, and passages from the Qualitative Reading Inventory – 3 (QRI-3; Leslie & Caldwell, 2001) to assess narrative comprehension and production skills. Composite z-scores were calculated based on the entire language testing battery, with each assessment measure weighted equally. The z-scores were based on norms from the entire sample of children from the Iowa project (see Tomblin et al., 1997), and were corrected to account for the large proportion of children with SLI relative to prevalence in the general population. The participants who scored greater than 1.25 SD below the mean for their age group on at least two composite language battery scores were considered as demonstrating language impairment. Non-verbal intelligence was evaluated using the Wechsler Intelligence Scale for Children – Third Edition (WISC-III; Wechsler, 1991). Performance scale subtests included Block Design and Picture Completion. The ND and SLI groups' performance IQs measured in the 8th grade met criteria for matching, with a mean of 98.4 for the ND group and 97.7 for the SLI group, F (1, 28) = .071, p = .792 (Mervis & Klein-Tasman, 2004). All participants exhibited normal hearing in each ear as confirmed by hearing screenings at a level of 20 dB HL at 500, 1000, and 2000 Hz presented via headphones.
2.2. Stimuli
2.2.1. Rapid Auditory Processing Task
Stimuli for eliciting event-related brain potentials mediating rapid auditory processing were pure tones of 1000 Hz (standard) and 2000 Hz (target) presented via headphones in a standard odd-ball paradigm. In order to evaluate whether neural processing related to the side of sound presentation may have differed between groups, 33% of all tones were presented to the right earphone, 33% to the left earphone, and 34% bilaterally and participants were asked to attend to all the stimuli. Previous studies have indicated that compared with typical language development, developmental language disorders may be associated with differences in hemispheric asymmetries in white matter volumes of the planum polare on the superior temporal gyrus (Jäncke, Siegenthaler, Preis, & Steinmetz, 2007) and peak latencies of the N1 to right-ear pure tone stimuli (Tonnquist-Uhlèn, Borg, Persson, & Spens, 1996). Because there were no group effects related to the location of sound presentation, the ERPs elicited by the left, right, and bilateral presentation were combined for this report. Two ISIs were employed; a long ISI condition (1000 ms) and a short ISI condition (200 ms). All tones were 50 ms in duration with rise/fall times of 5 ms. A total of 900 tones were presented, with a ratio of 80:20 standard (1000 Hz) to target (2000 Hz) tones. Tones to the left and right ears were presented at 90 dB SPL and central tones at 85 dB SPL to equate loudness for the binaural presentation. The perceived loudness for these brief tones was a comfortable listening level. The stimuli were presented in a pseudo-randomized order such that target tones were not immediately followed by another target tone. And, the sequence of stimulus presentation was identical for every participant. The total run time for the tone presentation was approximately 10 minutes.
2.2.2. Sentence Processing Task
The stimuli used to elicit ERPs mediating processing of auditory speech were 180 naturally spoken sentences produced by a female speaker with normal prosody digitized at a rate of 16 kHz (Weber-Fox & Hampton, 2008). For each word up to and including the critical word, the visual display of the auditory waveform was used to pick the onsets and offsets and then perceptually checked to make sure the word was not clipped (using Cool Edit Pro software). The wave files for each word, converted to sound files, were then presented (via the Neuroscan STIM program) with a 50 ms ISI interjected between each word up to and including the word following the critical verb comparison. Thus, onsets of each word were clearly discernable while maintaining a natural sounding rate, rhythm, and prosody. The sentence stimuli consisted of three conditions in which the verb was the critical word. The conditions included a control (e.g., “Every day, the children pretend to be superheroes”), a verb agreement violation (e.g., “Every day, the children *pretends to be superheroes”), and a semantically unexpected verb (e.g., “Every day, the children *rust to be superheroes”). A complete set of the sentence stimuli appears in Appendix A. These sentences were designed so that all the words leading up to the critical word (the verb) were identical across the three conditions. Also, the sentences were counterbalanced in two ways: (1) half of the noun subjects in the sentences were singular and half were plural; and (2) each word that served as a control verb also served as a semantically unexpected verb in another sentence. For the semantic condition, the critical word (the verb) does not produce a frank anomaly in many cases (see Appendix A), but rather an unexpected verb. However, as the sentence continues, it becomes clear that the meaning of the verb is incorrect. The ERP measure at the point of the verb therefore does not reflect the additional semantic information provided by the completion of the sentence. So, the ERPs elicited by the verbs across the three conditions reflect processing of identical information leading up to the verb, and the additional information following the verb does not confound a comparison of the ERP measures across the three conditions. For the morphosyntactic condition the violation (presence or absence of third person singular -s) occurs at the very end of the verb, so that any detection of a verb-agreement violation would not be processed until after the verb-stem.
2.3. Electroencephalographic Recordings
Electrical activity at the scalp was recorded from electrodes secured in an elastic cap (Quikcap, Compumedics Neuroscan). Twenty-eight electrodes (Ag-AgCl) were positioned over homologous locations of the two hemispheres according to the criteria of the International 10-10 system (American Electroencephalographic Society, 1994). Locations were as follows: lateral sites F7/F8, FT7/FT8, T7/T8, TP7/TP8, P7/P8; mid-lateral sites FP1/FP2, F3/F4, FC3/FC4, CP3/CP4, P3/P4, O1/O2; and midline sites FZ, FCZ, CZ, CPZ, PZ, OZ. Recordings were referenced to linked electrodes placed on the left and right mastoids. Horizontal eye movement was monitored via electrodes placed over the left and right outer canthi. Electrodes over the left inferior and superior orbital ridge were used to monitor vertical eye movement. All electrode impedances were adjusted to 5 kΩ or less. The electrical signals were amplified within a bandpass of .1 and 100 Hz and digitized on-line (Neuroscan 4.0) at a rate of 500 Hz. A total of 8 subjects (4 ND, 4 SLI) had bad electrode sites that included 1 to 3 of the following sites: P8, T8, FT7, FT8, O1, OZ, O2. However, none of these sites were utilized in the statistical analyses described below.
2.4. Procedures
Once appropriate impedance levels were obtained, the participants were seated comfortably in a sound-attenuating room and positioned 160-cm from a 47.5-cm monitor. For the rapid auditory processing task, a fixation crosshair was provided on a monitor and participants were asked to blink as little as possible. The fixation point remained on the screen for the duration of the tonal presentation. Participants were instructed to listen to the tones presented via headphones and to press a response key as rapidly as possible each time they heard the higher tone (2000 Hz target). Response hands were balanced between right and left hands within diagnostic category and sex. A practice session of 20 tones acquainted the participant to the task. Upon successful completion of the practice session, the 900 tone stimuli presentation began.
For the sentence processing task, participants were instructed to listen to each of the sentences and judge whether the sentence was a good English sentence and made sense. They were also asked to refrain from blinking during trials. Each trial was initiated by the participant with a button press. A fixation point appeared on the screen and, following a delay of 1000 ms, the sentence was presented binaurally through headphones at 70-75 dB SPL. Following the presentation of the sentence, there was a delay of 500 ms, followed by a “Yes/No?” presented on the screen to cue the participant to press the “Yes” button if the sentence was a good English sentence and made sense. Otherwise, they were instructed to press “No”. The response hands corresponding to the “Yes” and “No” buttons were counterbalanced across participants, sex, and within each of the groups. The sentence stimuli were presented in 5 blocks with 36 sentences in each block. The sentences were pseudo-randomized so that each condition was equally represented in all blocks.
2.5 Data Analyses
2.5.1. Behavioral measures
Accuracy and reaction time (RT) for detecting the target tones were measured. Correct responses to target tones were counted if they occurred between 100 and 1000 ms after the tone onset to eliminate spurious button presses. RT was computed for correct button presses only. Statistical comparisons were made using a mixed effects ANOVA with repeated measures including a between-group comparison (ND, SLI), and a within-subjects factor of condition (200 ms ISI, 1000 ms ISI).
Sentence judgment accuracy was also obtained from signals generated by the response pad. Sentence judgment accuracies for each participant in each condition were compared using a mixed effects ANOVA with repeated measures that included a between-group factor (ND, SLI) and a within-subjects factor of condition (control, verb-agreement violation, semantic). In addition, the A, which is an adjusted measure of sensitivity, was computed to take into account a participant's bias toward accepting a sentence as correct (Zhang & Mueller, 2005). A values range from .5 to 1 which corresponds to the range of responding at chance level to perfect discrimination. The A values for each participant were calculated for the verb-agreement violation condition and the semantic condition (each of these incorporating performance on the control sentences). The A values were compared using a mixed effects ANOVA with repeated measures that included a between-group factor (ND, SLI) and a within-subjects factor of condition (verb-agreement, semantic).
2.5.2. ERP measures
Trials with excessive eye movement or other forms of artifact were excluded from further analyses of the ERP responses by visually inspecting the continuous EEG records of each participant (as described in Luck, 2005, p. 156). For the auditory processing experiment, the number of accepted trials for the standard tones did not differ between groups, for the short (ND-293.2 (range: 251-325), SLI-289.5 (range 237-327)) or long (ND - 291.9 (range 258-336), SLI - 288.9 (range 223-336) ISI conditions, F (1, 28) <1. The number of accepted trials for the target tones also did not differ between groups for the short (ND - 59.1 (range: 37-76), SLI - 56.1(range 32-76)) or long (ND - 59.3 (range 43-77), SLI - 58.5 (range 34-75)) ISI conditions, F (1, 28) < 1. For the sentence processing experiment, the number of accepted trials for the control, verb agreement, and semantic conditions were also similar for the two groups (ND – 39.9 (range 21-55), 37.7 (range 17-53), 37.4 (14-56); SLI – 35.0 (range 19-55), 37.4 (range 15-58), 34.8 (range 16-54), F (1, 28) < 1) and across the three sentence processing conditions (control – 37.4, verb-agreement – 37.5, semantic – 36.1, F (2, 56) = 1.31, H-F p = .28). The accepted trials were averaged by condition for each participant. The average number of usable trials in each sentence condition for each group were adequate, with means slightly higher than previous studies of auditory sentence processing in children and adolescents (Hahne, Eckstein, & Friederici, 2004; Sabisch, Hahne, Glass, von Suchodoletz, & Friederici, 2006). However, some of the averages fell short on the number of trials and resulted in noisy waveforms. The percentage of cases out of the total number of averages (15 participants × 3 conditions = 45), that fell below 20 trials per average was 3% for the ND group and 4% for the SLI group.
ERP averages for the rapid auditory processing experiment were computed 100 ms prior to the onset of the tone to 400 ms post onset for the standard tones and to 700 ms post-onset for the target tones. Because half of the standard tones were followed by a short ISI (200 ms) tone we did not analyze a longer epoch for the ERPs elicited by the standards. However, the longer epochs (700 ms post-onset) are illustrated (referred to later in the results section 3.2) as a comparison to the ERPs elicited by the target tones. The ERP averages for the sentence processing paradigm were triggered 100 ms prior to the verb onset in each sentence and included 1500 ms post-stimulus onset. In both experiments, the ERP data from the 100 ms interval prior to the tone and verb onsets served as the baseline activity. The peak latencies of ERP components were computed in relation to the trigger point (0 ms) that marked the tone and verb onsets. The peak latencies and peak and mean amplitudes were automatically detected using Neuroscan 4.2 software within specified temporal windows that captured the ERP components elicited in these paradigms, as described below.
Our method for selecting the temporal windows for measuring ERP peak latencies and amplitudes elicited by the tones was to examine the grand averages of both the ND and SLI groups and centering the windows on the peaks. For this data set we found that the same temporal windows were appropriate for each group's grand averages. As a second step, we examined each individual record to make sure the windows captured the peaks of interest for each participant. The peak amplitudes (maximal negative or positive value within the temporal window, Neuroscan 4.2) and latencies (time of the maximal negative or positive value relative to time 0 ms, within the temporal window, Neuroscan 4.2) for the N100 elicited by the standard and the target tones were measured within the temporal window of 50-200 ms post-stimulus onset. The temporal window of 150-250 ms post-stimulus onset was used to measure the peak amplitude and latency of the P200 elicited by the 1000 ms standard tones. The P200 peak in the positive range (i.e., crossed 0 μV) was not reliably observed for the 200 ms ISI condition so these measures are not reported for the short ISI condition, however no group differences in amplitude or latency were found within the P200 temporal window. For target tones, the N200 peak latency and amplitude was measured between 200-350 ms post-stimulus onset and the P300 peak and mean amplitudes (mean value across the temporal window) and peak latencies were measured between 280 and 650 ms post-stimulus onset.
Statistical analyses of ERP measures for both the standard and target tones in the short and long ISI conditions were performed using mixed effects ANOVAs with repeated measures including a between-group factor (ND, SLI) and 2 within-subjects factors of hemisphere (left, right), and electrode sites. The subset of electrode sites used for comparisons was based on visual inspection of the elicited waveforms to determine where reliable peaks could be measured and based on the distribution findings from previous studies (Polich & Kok, 1995). The subset of electrode sites selected for the repeated measures ANOVAs for each component of interest were as follows: F3/4, FC3/4, and C3/4 for the N100; C3/4, and CP3/4 for the P200; F3/4 and FC3/4 for the N200; and C3/4, CP3/4, and P3/4 for the P300. The corresponding midline sites for each component (N100: FZ, FCZ, CZ; P200: CZ, CPZ; N200: FZ, FCZ; P300: CZ, CPZ, PZ) were included in separate repeated measures ANOVAs excluding the within-subjects factor of hemisphere.
The method for selecting the temporal windows for measuring ERP peak latencies and amplitudes elicited in the sentence conditions was to examine the grand averages of both the ND and SLI groups and centering the windows around the regions of maximal activity. For this data set we found that the same temporal windows were appropriate for each group's grand averages. As a second step, we examined each individual record to make sure the windows captured the peaks of interest for each participant. For ERPs elicited by the verbs in each of the three sentence conditions, the mean amplitudes (mean value across the temporal window, Neuroscan 4.2) of the Anterior Negativity (AN) and N400 were measured within the temporal window of 350-550 ms. For the later occurring positivity (P600), the mean amplitudes were measured within the temporal window of 900-1300 ms post-stimulus onset. The onsets for the natural speech stimuli were characterized by gradual envelopes and did not elicit clear ERP peaks for reliable measurement. Therefore the peak latency measures for these broad cognitive potentials are not reported.
ERP mean amplitudes were compared with mixed effects ANOVAs with repeated measures. For analyses of the ERP responses elicited at lateral and mid-lateral electrode sites, the ANOVAs with repeated measures included a between-subjects factor of group (ND, SLI) and 3 within-subjects factors including condition (control vs. verb-agreement violation, and control vs. unexpected semantic condition), hemisphere (left, right), and electrode site. The electrode sites included in the analysis for the N400 and P600 included the central parietal sites where these components are most clearly distributed (FC3/4, C3/4, CP3/4, P3/4). For measures of the AN, frontal and frontal-temporal electrode sites were included (F3/4, FC3/4). For analyses of ERPs elicited at midline sites, the repeated measures ANOVAs included a between-subjects factor of group (ND, SLI) and 2 within-subjects factors including condition (control, verb-agreement violation, semantic), and electrode site (N400 and P600: FCZ, CZ, CPZ, PZ; AN: FZ, FCZ). Significance values were set at p < .05.
For all repeated measures with greater than one degree of freedom in the numerator, the Huynh-Feldt (H-F) adjusted p-values were used to determine significance (Hays, 1994). The effect sizes, indexed by the partial-eta squared statistic (ηp2), are reported for all significant effects. Interactions involving Group (H-F p < .05) were followed-up with step-down ANOVAs for each group to determine effects specific to the ND and SLI participants. The repeated measures ANOVAs were identical in design as described above without the between-subjects factor of group.
Regression analyses were conducted to explore the relationship between rapid auditory processing and processing of the verb -agreement violation, computed separately for the ND and SLI groups. Previous studies have found that fundamental auditory processing skills correlated with later language ability (Benasich & Tallal; Weber et al., 2004). The current correlations examine both behavioral and corresponding electrophysiological relationships between the non-linguistic and linguistic processing domains. Correlations were computed between accuracy of performance on the short ISI target tone detection task, and the accuracy in the grammaticality judgment for the verb-agreement violations (A values). In addition, correlations were computed between the corresponding ERP mean amplitudes of the P300 elicited by the short ISI target tones and the P600 elicited by the verb-agreement violation, both averaged across CZ, CPZ, and PZ. To test whether possible relationships to rapid auditory processing were specific to the verb-agreement condition, we also computed the correlations for the accuracy in short ISI target tone detection and A values for detecting unexpected lexical items. The correlations for the corresponding ERP mean amplitudes of the P300 elicited by the short ISI target tones and the N400 elicited by the unexpected verbs in the semantic condition, both averaged across CZ, CPZ, and PZ were also computed. The Pearson correlations r and 1-tailed p values are reported for each of these four comparisons.
2. Results
3.1 Target Tone Detection Accuracy and RT
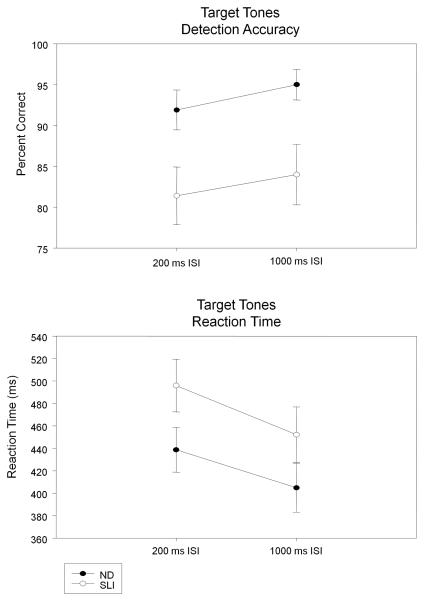
The mean accuracies for detecting target tones in the 200 ms and 1000 ms ISI conditions are plotted for the ND and SLI groups in Figure 1 (top panel). As is evident in this illustration, both groups detected the majority of target tones; however, the ND group performed with greater accuracy than the SLI group, in both the short ISI (F (1, 28) = 5.97, p = .02, ηp2 = .176) and long ISI (F (1, 28) = 7.05, p = .01, ηp2 = .201) conditions. False alarm rates (button presses to standard tones) were low for both groups (Mean %: ND 5.0, SLI 6.8), F (1, 28) = 1.85, p = .19.
Figure 1.
ND and SLI Mean (SE) group accuracies (top panel) and reaction times (bottom panel) for detecting target tones in the 200 ms and 1000 ms ISI conditions.
The RTs for detecting the target tones in the 200 ms and 1000 ms ISI conditions are also plotted for the ND and SLI groups in Figure 1 (bottom panel). The RTs of the SLI group tended to be slower compared to the ND group in the short ISI condition, F (1, 28) = 3.47, p = .07, ηp2 = .110. As can be seen in Figure 1, both groups exhibited faster RTs for the long ISI (1000 ms) condition, with no significant group effect for the long ISI condition, F (1, 28) = 2.07, p = .16.
3.2. ERPs Elicited by Standard and Target Tones
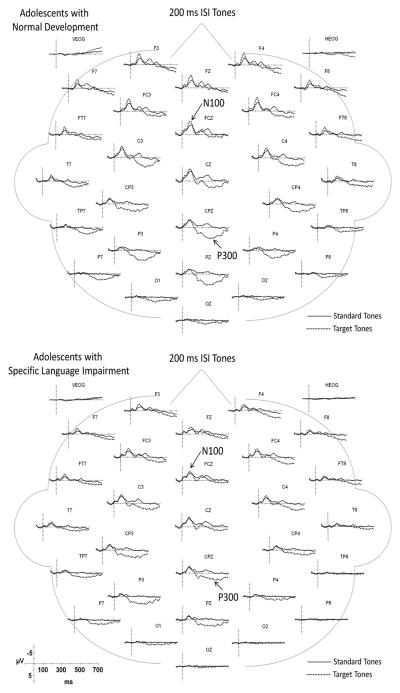
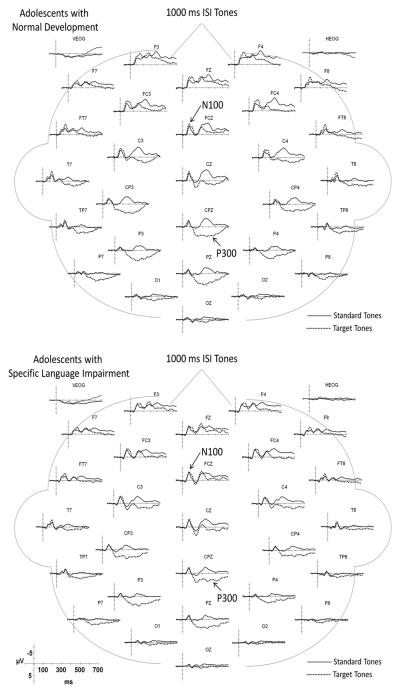
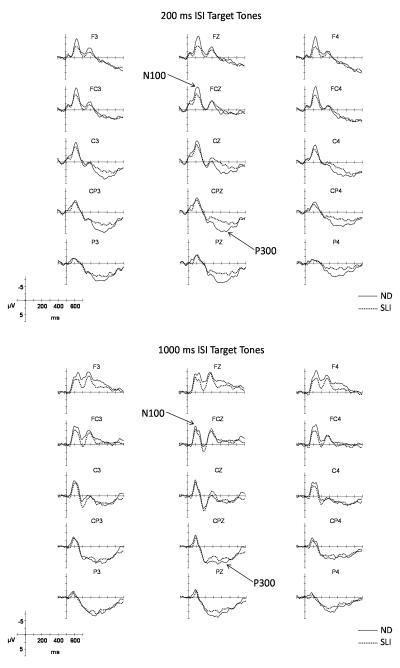
Illustrations of the ERPs elicited by the standard and target tones over the full montage of electrode sites are overlaid in Figure 2 for the 200 ms ISI condition and in Figure 3 for the 1000 ms ISI condition. The N100 peaks which are robust in the grand averages were also visible in the ERPs of each participant. In Figure 4, the grand average ERPs elicited by the target tones for electrode sites used in the statistical analyses are overlaid for the ND and SLI groups to illustrate group differences. The ERPs elicited by the short (200 ms) and long (1000 ms) ISI conditions are illustrated in the left and right panels, respectively. The waveforms in the solid lines represent the grand average ERPs for the ND group; the dashed lines are those for the SLI group. Below, we summarize the similarities and differences in the ERP components in the waveforms of the ND and SLI groups for each standard and target tones.
Figure 2.
Grand average ERPs elicited by standard and target tones in the 200 ms ISI condition for the ND (top panel) and SLI (bottom panel) groups. Negative potentials are plotted upward in this and in all subsequent ERP figures.
Figure 3.
Grand average ERPs elicited by standard and target tones in the 1000 ms ISI condition for the ND (top panel) and SLI (bottom panel) groups.
Figure 4.
ND and SLI superimposed grand average ERPs elicited by the target tones in the 200 ms (top panel) and 1000 ms ISI (bottom panel) conditions for the electrode sites used for statistical analyses.
3.2.1. Standard Tones
No group differences were observed for the peak amplitudes or peak latencies of the N100 elicited by the 200 ms or the N100 and P200 elicited in the 1000 ms ISI standard tones (Table 1).
Table 1.
Standard Tones Statistical Results involving Group Effects on the Peak Amplitudes and Peak Latencies of ERPs elicited in the 200 ms and 1000 ms ISI conditions.
| Mid-lateral | Midline | |||||||
|---|---|---|---|---|---|---|---|---|
| Effect | F (1, 28) | p | ηp2 | F (1, 28) | p | ηp2 | ||
| Amplitude | ||||||||
| 200 ms ISI | N100 | Group | 1.25 | .27 | 1.33 | .26 | ||
| 1000 ms ISI | N100 | Group | <1 | <1 | ||||
| P200 | Group | <1 | 1.46 | .24 | ||||
| Latency | ||||||||
| 200 ms ISI | N100 | Group | 3.19 | .08 | 1.43 | .24 | ||
| 1000 ms ISI | N100 | Group | 1.19 | .28 | <1 | |||
| P200 | Group | <1 | <1 | |||||
3.2.2. Target Tones
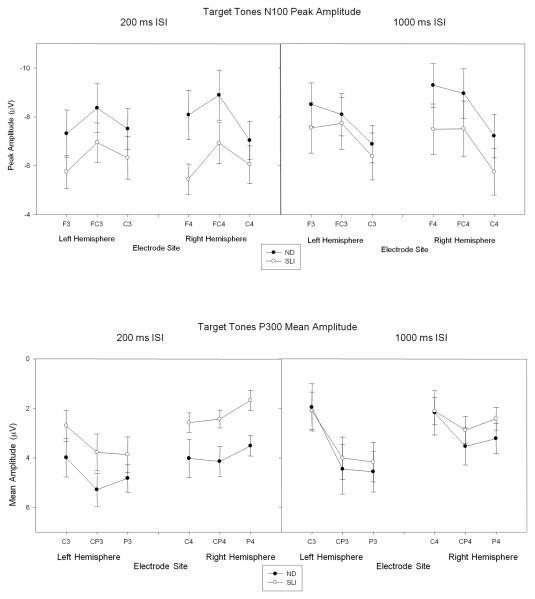
The amplitude of the N100 elicited in the 200 ms ISI condition was reduced in amplitude over the anterior right hemisphere in the SLI group (See Table 2). The reduced N100 in the SLI group can be observed in the ERPs illustrated in Figure 4. A reduced-amplitude N100 in the SLI group was not observed for the long, 1000 ms ISI condition. Figure 5 (top panel) illustrates the peak amplitudes of the N100 for each group for the 200 ms ISI condition and 1000 ms ISI conditions. No other group differences were observed for measures of peak amplitudes or peak latencies of the N100 and N200 elicited by the 200 ms or 1000 ms ISI target tones (Table 2).
Table 2.
Target Tones Statistical Results involving Group Effects on the Peak Amplitudes and Peak Latencies of ERPs elicited in the 200 ms ISI and 1000 ms ISI conditions.
| Mid-lateral | Midline | |||||||
|---|---|---|---|---|---|---|---|---|
| Effect | F (1, 28) | p | ηp2 | F (1, 28) | p | ηp2 | ||
| Amplitude | ||||||||
| 200 ms ISI | N100 | Group | 1.98 | .17 | 1.73 | .20 | ||
| HXEXG | 4.20 | .03* | .130 | |||||
| N200 | Group | <1 | <1 | |||||
| P300 | Group | 4.44 | .04* | .137 | 6.18 | .02* | .181 | |
| 1000 ms ISI | N100 | Group | <1 | <1 | ||||
| N200 | Group | <1 | <1 | |||||
| P300 | Group | <1 | <1 | |||||
| Latency | ||||||||
| 200 ms ISI | N100 | Group | 1.99 | .17 | <1 | |||
| N200 | Group | <1 | 1.96 | .17 | ||||
| P300 | ||||||||
| 1000 ms ISI | N100 | Group | <1 | <1 | ||||
| N200 | Group | 2.26 | .14 | <1 | ||||
| P300 | ||||||||
Note. H = Hemisphere, E = electrode site, G = Group
Figure 5.
ND and SLI Mean (SE) group N100 peak amplitudes and P300 mean amplitudes elicited by the 200 ms ISI and 1000 ms target tones for electrode sites over the left and right hemispheres.
The mean amplitude of the P300 elicited by the target tones in the short, 200 ms ISI condition was reduced for the SLI group (See Table 2). The reduced amplitude P300 can be observed in Figure 5 (bottom panel) which illustrates the ERPs elicited by the target tones presented with a 200 ms ISI. No group differences in the peak latency of the P300 were observed (Table 2.)
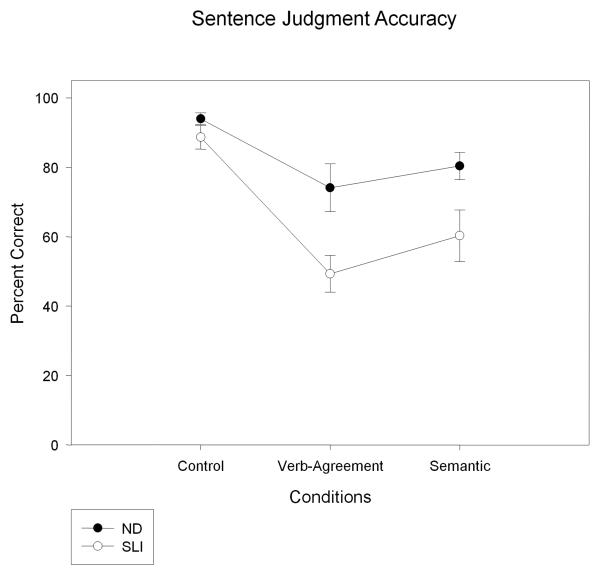
3.3. Sentence Judgment Accuracy
Figure 6 illustrates the mean behavioral accuracy of the ND and SLI groups in judging the sentence stimuli. As can be seen in this figure, the SLI group was less accurate compared to the ND group in determining whether the stimulus sentences made sense and were grammatically correct, Group F (1, 28) = 20.11, p < .01, ηp2 = .418. As Figure 6 illustrates, the difference between the ND and the SLI group was least pronounced for the control sentence and most pronounced for the verb-agreement condition. The mean A (SE) values for the verb-agreement condition were .91 (.013) for the ND group and .79 (.016) for the SLI group. The mean A (SE) values for the semantic condition were .92 (.013) for the ND group and .83 (.020) for the SLI group. These adjusted sensitivity scores also resulted in group differences, F (1, 28) = 16.77, p < .01 ηp2 = .375. The performance accuracy on the verb-agreement and semantic condition was not significantly different, F (1, 28) = 1.83, p = .19.
Figure 6.
Sentence judgment accuracies (means (SE)) are plotted for the control, verb-agreement, and semantic conditions for the ND and SLI groups.
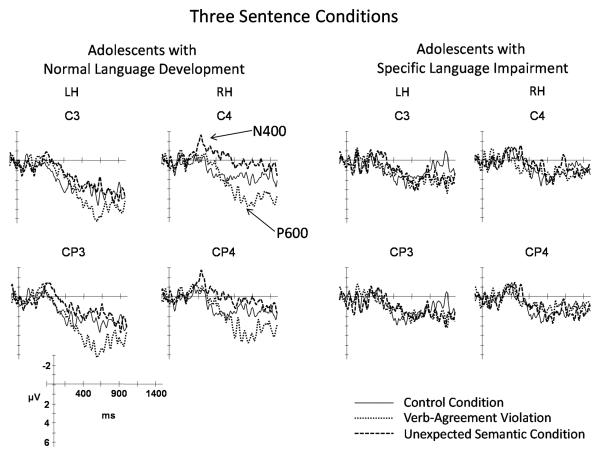
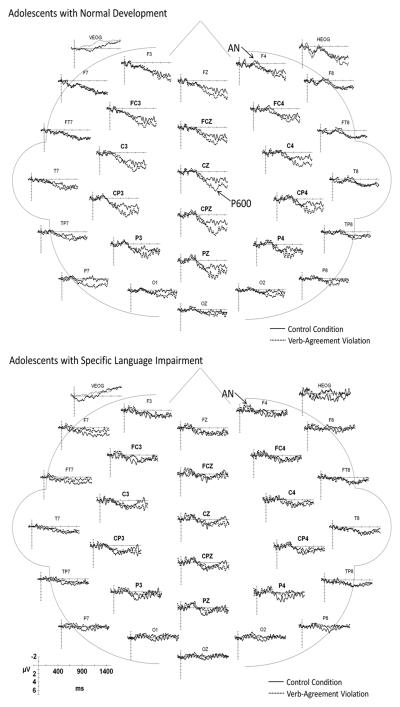
3.4 Processing Verb-Agreement Violations
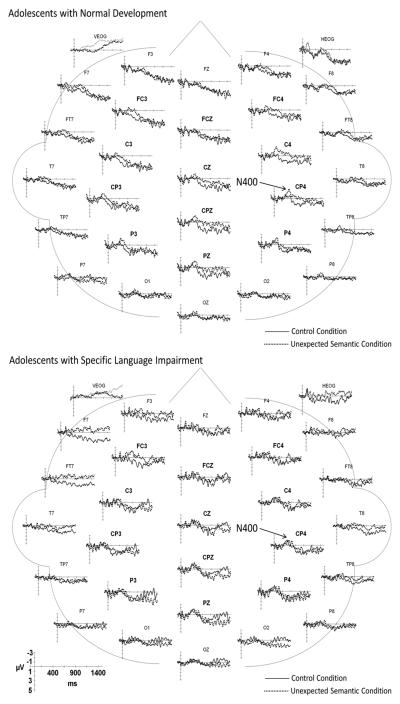
Illustrations of the ERPs elicited by each of the three sentence conditions are overlaid for each group in Figure 7. The ERPs elicited by the verbs in the control and verb-agreement violation conditions for the electrode sites included in the statistical analyses are illustrated in Figure 8 for the ND and SLI groups. The key components distinguishing the waveforms elicited by the verb-agreement violations relative to the control sentences are indicated on the figures. The similarities and differences in the effects of verb-agreement violations on the elicited waveforms for the two groups are summarized below.
Figure 7.
Grand average ERPs elicited by the 3 sentence conditions superimposed for the ND (left panel) and SLI (right panel) groups. Negative potentials are plotted upward.
Figure 8.
Grand average ERPs elicited by the control and verb-agreement conditions for the ND (top) and SLI (bottom) groups. Negative potentials are plotted upward.
3.4.1. Anterior Negativity (AN)
At anterior mid-lateral electrode sites, the mean amplitude of the AN was greater for the verb-agreement violations relative to the control verbs for the ND and SLI groups combined. Condition effects did not interact with group and there were no main effects of group (See Table 3).
Table 3.
Statistical Results involving Group and Condition Effects for the Comparison between the ERPs elicited by Verb-Agreement Violations and the Control Verbs.
| Mid-lateral | Midline | |||||||
|---|---|---|---|---|---|---|---|---|
| Effect | F (1, 28) | p | ηp2 | F (1, 28) | p | ηp2 | ||
| Anterior Negativity | ||||||||
| Amplitude | Group | 1.09 | .30 | 2.68 | .11 | |||
| Cond | 6.94 | .01* | .199 | 3.34 | .08 | |||
| C × G | <1 | <1 | ||||||
| Late Positivity (P600) | ||||||||
| Amplitude | Group | 6.87 | .01* | .197 | 7.99 | .01* | .222 | |
| Cond | 4.67 | .04* | .143 | 10.00 <. | 01* | .263 | ||
| C × G | 5.38 | .03* | .161 | 3.73 | .06 | |||
| Step-Down | ND | Cond | 12.03 | <.01* | .462 | 13.56 | <.01* | .492 |
| SLI | Cond | <1 | <1 | |||||
Note. C = Condition, G = group
3.4.2. Late Positivity (LP, P600)
Verb-agreement violations elicited an increase in late positivity relative to the control verbs for both mid-lateral sites and midline sites (Table 3). The overall amplitude of the LP was reduced in the adolescents with SLI compared to those with ND over mid-lateral and midline sites (Table 3). Step-down ANOVAs revealed that the verb-agreement violations elicited a robust increase in the LP amplitude for the ND group over both the mid-lateral and midline sites (Table 3). In contrast, the increase in late positivity for the verb-agreement violations in the SLI group was less robust and did not reach significance over mid-lateral or midline sites.
3.5. Processing Verbs with Reduced Semantic Expectation
The ERPs elicited by the verbs in the control sentences and those with reduced semantic expectation are illustrated in Figure 9 for the ND and SLI groups. The statistical analyses of the measures of mean amplitude of the N400 are summarized below.
Figure 9.
Grand average ERPs elicited by the control and semantic conditions for the ND (top) and SLI (bottom) groups. Negative potentials are plotted upward.
3.5.1. N400
The N400 at central-parietal sites elicited by the verbs with reduced semantic expectation was larger in mean amplitude compared to the control verbs at mid-lateral and midline sites. There were no effects of group on the amplitude of the N400 (Table 4).
Table 4.
Statistical Results involving Group and Condition Effects for the Comparison between the ERPs elicited by Semantically Unexpected Verbs and the Control Verbs.
| Mid-lateral | Midline | ||||||
|---|---|---|---|---|---|---|---|
| Effect | F (1, 28) | p | ηp2 | F (1, 28) | p | ηp2 | |
| N400 | |||||||
| Amplitude | Group | <1 | <1 | ||||
| Cond | 4.52 | .04* | .139 | 2.14 | .15 | ||
| C × G | <1 | <1 | |||||
Note. C = Condition, G = Group
3.6. Relationships between behavioral and ERP indices of rapid auditory processing and sentence processing
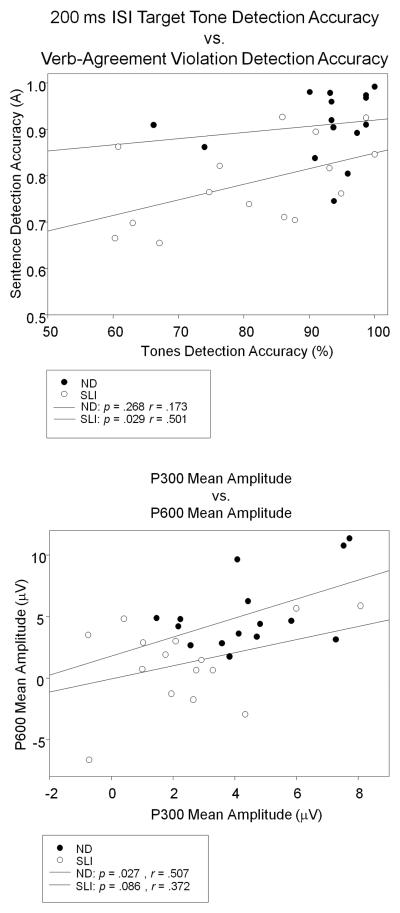
For the ND group, the accuracy for target tone detection for the short ISI condition was not significantly correlated with the A measure for detection accuracy of verb-agreement violations, r = .173, p = .27. However, this relationship was significant for the SLI group, r = .501, p = .03 (See Figure 10). The amplitude of the P300 elicited by the short ISI target tones was correlated with the amplitude of the Late Positivity elicited by the verb-agreement violations for the ND group, r = .507 p = .03, but failed to reach significance for the SLI group, r = .372, p = .09. The mean amplitudes of the P300 elicited by target tones presented after a short ISI and the Late Positivity elicited by verb-agreement violations are plotted for the ND and SLI participants in Figure 10, bottom panel. In contrast, the regression between target tone detection and the A measure of accuracy for unexpected lexical items did not reach significance for either group, ND r = .170, p = .27, SLI r = .209, p = .23. Similarly the amplitudes of the corresponding ERPs (P300, N400) were not significantly correlated for either group, ND r =. 383, p = .08, SLI r = −.212, p = .22. The scatter plots in Figure 10 highlight the degree of overlap of individual subject's behavioral and electrophysiological measures in the ND and SLI groups and show that some adolescents with SLI may fall in the normal range on these measures. In the case of the P600 amplitude for example, it can be seen that 7 adolescents with SLI displayed P600 amplitudes that overlapped with those of the ND group, although all were at the smaller end of the range in amplitudes. Also, Figure 10 illustrates that the P300 amplitudes elicited in adolescents with SLI were within the range of the ND group for 10 individuals, with 8 of those clustering at the lower end of the range.
Figure 10.
Scatter plot illustrating the relationships between 200 ms ISI target tone and verb-agreement violation detection accuracy (top panel) and between mean amplitudes of the P300 elicited by the target tones and the P600 elicited by verb agreement violations (bottom panel) for the ND and SLI participants.
4. Discussion
Tone and sentence stimuli were utilized to examine whether adolescents with SLI continue to exhibit atypical neural activity for rapid auditory and linguistic processing, and to examine whether the behavioral and electrophysiological indices for tone processing are correlated with those for linguistic processing. The SLI and ND groups in the current study met strict criteria for displaying consistent diagnoses across kindergarten, second, fourth, and eighth grades. As can be seen in Figure 10, there was considerable heterogeneity in each of the two groups. Clearly, not all adolescents with SLI were functioning in an atypical manner. Nevertheless, the current findings provide strong evidence that auditory processing for non-linguistic stimuli is atypical in a significant number of adolescents with SLI compared to their peers with normal language development. Further, the findings indicate that reduced efficiency in auditory processing in SLI is more vulnerable to rapid rates of stimuli presentation. The behavioral and electrophysiological findings for the verb-agreement condition also indicates that atypical sentence processing extends into adolescence. Converging evidence from behavioral and electrophysiological measures indicated that, as a group, adolescents with SLI did not process the verb-agreement stimuli as effectively as their peers with normal language development. Additionally, behavioral performance for processing tones with a short ISI correlated with those for verb-agreement violations in the SLI group (but not the ND group), and electrophysiological indices for processing tones with a short ISI correlated with those for verb-agreement violations in the ND group but only tended to in the SLI group. The small to moderate correlations suggest that the integrity of neural functions for auditory processing may only account for a small proportion of the variance in morphosyntactic processing in some adolescents.
4.1. Decreased Efficiency for Rapid Auditory Processing in SLI
The adolescents with SLI demonstrated reduced behavioral accuracy for detecting brief target tones in an odd-ball paradigm. Further, the amplitude of the N100s elicited by the target tones was reduced for the adolescents with SLI over anterior right hemisphere in the short ISI condition. In addition, the longer latency, endogenous P300 component elicited by the target tones was reduced in the adolescents with SLI in the short ISI condition. The current findings are consistent with previous reports indicating that language impairment may be associated with atypical neural functions for rapid auditory processing in some children with SLI (Neville et al., 1993; McArthur & Bishop, 2004; 2005; McArthur, Atkinson, & Ellis, in press) and confirm that atypical patterns continue well into adolescence (Bishop & McArthur, 2004). An important finding of the current study is that the N100 component, which is an early cortical index of perceptual processes (Luck, 2005), was reduced in the SLI group for target tones in the short (200 ms) ISI condition, however, the quantitative differences in N100 amplitude across the two groups for the long (1000 ms) ISI condition did not reach significance. The amplitudes of ERPs are in part related to the degree of synchronization of populations of neurons (Nunez, 1995). Rapid stimulus presentation rate impacts the ERP amplitude due to the recovery cycle of populations of neurons (i.e., their readiness to respond again) (Polich, 1990). Thus, the current findings are consistent with an earlier study of processing tonal pairs with short ISIs (Bishop & McArthur, 2004) and suggest that the recovery rates of neuronal activity for encoding auditory signals presented at rapid rates may be inefficient in adolescents with SLI compared to their peers with normal language development.
It is unknown what underlying mechanisms contribute to inefficient cortical encoding of rapidly presented acoustic signals in SLI. However, evidence from MRI studies suggests that some children with SLI display subtle neuroanatomical abnormalities in a variety of cortical and white matter regions (See Dick, Richardson, & Saccuman, 2008 for review). Of particular interest for the current findings is that atypical asymmetries in the planum temporal, an area important for processing acoustic signals, have been reported in several studies of children with language impairments (e.g., Gauger, Lombardino, & Leonard, 1997; Herbert, Ziegler, & Deutsch, O'Brien, Kennedy, Filipek, et al., 2005; Plante, Swisher, Vance & Rapcsak, 1991). It is possible that the differences in auditory processing indexed by ERPs in the current study reflect these subtle differences in cortical neuroanatomy (Friederici, 2006). More work needs to be done in this area to discern how subtle and heterogeneous neuroanatomical abnormalities reported for children with SLI relate to specific functions (e.g. rapid auditory processing) that may contribute to disordered language development (e.g., Dick et al., 2008)
Another possible contributor to inefficient neural encoding of rapid auditory signals at the cortical level may be related to effects of degraded neural signals originating at the level of the brainstem. Both neural onset responses (indexed by ABRs) and frequency phase-locking integrity (indexed by FFRs) have been shown to be degraded in children with language impairment (Basu et al, 2010; Wible et al., 2004; 2005). The findings indicate that SLI may be associated with disrupted neural activity in the brainstem that is necessary for encoding rapid frequency changes and also with increased susceptibility to desynchronizing factors related to faster rates of stimulus presentation (Basu et al., 2010, Wible et al., 2004; 2005). Several studies have indicated that the integrity of auditory brainstem function impacts the representations of acoustic features (e.g., temporal encoding) at the cortical level (Abrams, Nicol, Zecker, & Kraus, 2006; Banai, Nicol, Zecker, & Kraus, 2005; Wible, Nicol, & Kraus, 2005).
The later endogenous potential, the P300, elicited by the target tones, particularly for the short ISI condition, was also reduced in amplitude in the SLI group. Because the neural signals for earlier perceptual encoding of auditory signals (as indexed by the N100 elicited by the target tones) were reduced for the SLI group, it is likely that the representations of the acoustic features of the stimuli were less robust. Therefore, it is possible that the working memory traces for making comparisons between subsequent tones were weaker. It has been hypothesized that the P300 component reflects a summation of a variety of cognitive events including focusing on novel information and updating working memory (Knight & Nakada, 1998; Polich & Kok, 1995). The current findings indicating less efficient updates in working memory for a simple target tone detection task are in agreement, in a broader sense, with reports for other tasks and domains that indicate working memory limitations may be a contributing factor to SLI (Ellis Weismer et al., 2005; Leonard et al., 2007).
4.2. Atypical Neural Functions for Processing Verb-Agreement Violations in SLI
Behavioral accuracy for detecting verb agreement violations was reduced for the adolescents with SLI compared to their typically developing peers. These findings are consistent with behavioral measures in adolescents with language impairment that indicate more subtle, but continued deficits with tense and agreement morphosyntax (Leonard, Miller, & Finneran, 2009). Also, the group differences in ERPs for the verb-agreement violations were greater compared to those for the semantic condition. These findings are consistent with the literature on younger children with SLI showing that grammatical deficits are usually more severe than lexical deficits, in both comprehension and production. For example, Deevy and Leonard (2004) matched children with SLI and typically developing children on scores of a receptive vocabulary test and then compared the two groups on their comprehension of certain types of wh-questions (e.g., “Who is the happy brown dog washing?”). The children with SLI proved to be significantly less accurate than their receptive vocabulary-matched peers on such questions. Leonard, Miller, and Gerber (1999) examined the production of verb morphology (e.g., past tense –ed, copula and auxiliary is, are) in children with SLI as a function of their expressive vocabulary. Relative to a group of typically developing children, these children's production of verb morphology lagged behind expectations based on the number of different verbs they used.
ERP indices indicated that as a group, the adolescents with SLI exhibited less robust late positivities for verb-agreement violations. The lack of a significant late positivity effect for the SLI group is in contrast with a previous study of morphosyntactic processing in a group of older children and adolescents with especially severe grammatical impairments (Fonteneau & van der Lely, 2008). In that study, a robust late positivity (P600) was elicited by violations in structural syntactic dependencies (e.g., “Who did Barbie push the clown into the wall?”) However, in addition, a negativity resembling an N400 rather than a LAN was observed in the ERPs of the SLI group, suggesting greater difficulty in lexical integration.
There are a number of potentially important differences between the Fonteneau and van der Lely (2008) study and the current study to consider in interpreting the differences in ERP findings. First, the groups of participants with SLI in the two studies were not selected using the same criteria. Fonteneau and van der Lely selectively recruited those individuals with SLI whose grammatical deficits constituted their greatest weakness. In the present study, the selection criteria were broader. Second, in the current study, the group of SLI adolescents performed well below their peers for identifying verb-agreement violations. Regression analyses revealed a significant relationship between behavioral accuracy and the amplitude of the late positivity for the SLI group. The accuracy of grammaticality judgments was not reported in the Fonteneau and van der Lely (2008) study, so it is not known how the level of accuracy compared to the current study or how performance was related to the ERP patterns observed in that study. Third, the types of morphosyntactic violations tested in the two studies differed. The morphosyntactic marker for the verb-agreement violation as compared to morphosyntactic dependency is less perceptually salient, possibly contributing to greater difficulty in detecting the error. Also, the verb-agreement violation in the current study may have created less of a need to “recover the meaning of the sentence”. This could have contributed to a reduced or absent late positivity in many of the participants in the SLI group. In addition, the verb-agreement violations are only marked with an erroneous presence or inappropriate absence of the third person singular inflection –s. Because this does not change the meaning of the verb itself, the violation apparently did not affect the ease of lexical access and integration, and thus no N400 was observed. Lastly, the participants in the current study were required to monitor for both verb-agreement violations and semantic anomalies. In the Fonteneau and van der Lely study, morphosyntactic and semantic stimuli were presented in two separate experiments. It is possible that the design of the current study may have imposed a greater load for processing and for making grammaticality judgments. The ERP indices of the current study provide evidence that neural processes indexing morphosyntactic re-analysis are weak in adolescents with SLI when encountering verb-agreement violations.
To be comprehensive, the current study employed two types of verb-agreement violations – one in which an agreement inflection appeared that should not have been present (commission), and one in which an agreement inflection was missing from an obligatory context (omission). The latter type of error occurs in the speech of children with SLI through the preschool period and into school age (Rice, Wexler, & Hershberger, 1998), whereas the former type of error is much less frequent (Rice & Wexler, 1996). The current study was not designed to examine differences in processing for these two kinds of verb-agreement violations and there were not enough trials to examine ERPs separately for the two cases. A future study to examine whether ERP findings differ according to the type of verb-agreement error in SLI is needed. Based on the behavioral evidence (Leonard et al., 2009) it is possible that processing differences between ND and SLI groups would be larger for cases of omission than for cases of commission.
4.3. Behavioral Indices for Semantically Unexpected Verbs May Indicate Less Robust Representations in SLI
Behavioral accuracy for detecting semantic anomalies was substantially reduced for the adolescents with SLI compared to their typically developing peers. In earlier studies, the level of accuracy for detecting semantic anomalies was fairly high in children with LI, though nevertheless lower than that of typically developing peers (Neville et al., 1993; Sabisch et al., 2006). It is possible that because the unexpected verbs did not constitute a frank semantic anomaly, and because the correct verbs were not highly primed (i.e., low cloze probability), the detection of incorrect sentences in the present study was more ambiguous. In addition, the participants were also monitoring the sentences for verb-agreement violations, imposing a greater load on their meta-linguistic skills. The fact that the ND group in the present study also performed with reduced accuracy compared to the control groups in previous studies (Neville et al., 1993, Sabisch et al., 2006) confirms that the semantic task was not as straight forward and may have imposed a greater processing load on the participants. The current findings indicate that when challenged to a greater extent, the semantic processing system in adolescents with SLI is vulnerable and functions less effectively compared to peers with normal language development.
The electrophysiological evidence however did not yield significant differences between groups. It was noted that although a significant N400 effect was observed, the magnitude was quite small even in the ND group. The small N400 effect in the current study may be in part due to the nature of the stimuli. Since the unexpected verbs did not pose frank semantic violations at that point in the sentence, they may have been lexically integrated with relatively greater ease compared to typical semantic anomalies. Given the very small amplitude changes and the variability across a relatively small number of subjects, we may not have enough power to detect potential group differences in the neural patterns elicited by these type of unexpected semantic stimuli. A limitation of our study is that the behavioral indices for semantic processing included additional information provided by the rest of the sentence, whereas the electrophysiological indices only reflect lexical integration of the unexpected verb. Our behavioral findings are more consistent with the idea that semantic representations for verbs may be weaker in SLI (Sabisch et al., 2006) and is consistent with the suggestion that processing verbs may be particularly challenging for children with SLI (Leonard, 1998). However, our electrophysiological findings are more consistent with earlier studies of processing semantically anomalous nouns which reported robust N400 effects in children with SLI (Fonteneau & van der Lely, 2008; Neville et al., 1993).
4.4. Possible Relationships between Non-Linguistic Auditory and Linguistic Processing
The present results indicated that performance in detecting target tones in the short ISI condition was significantly correlated with performance accuracy for detecting verb-agreement violations in the SLI group, and further that the amplitude of the P300 elicited by short ISI target tones was correlated with the amplitude of the Late Positivity elicited by the verb-agreement violations in the ND group, but only a trend in the SLI group. These relationships were not significant for the tone and semantic processing conditions. The findings allow for the possibility that specific aspects of non-linguistic processing (e.g., rapid auditory processing) could play a small role in language processing. The observation that processing factors such as those studied here might have a somewhat greater effect on morphosyntax is generally consistent with earlier findings that this area of language is most fragile. For example, the grammatical profile of children with SLI (e.g., failure to detect verb morphemes such as –s and –ed) can be replicated in normally functioning adults during tasks in which sentences must be produced or comprehended under difficult listening conditions (e.g., Kilborn, 1991). Computer simulation studies have produced the same SLI profile by degrading the input (e.g., Hoeffner & McClelland, 1993; Joanisse, 2004). It seems reasonable to assume that difficulties in processing rapidly presented auditory stimuli could not only slow the initial acquisition of language but also make the online processing of already-acquired language more difficult for individuals with SLI.
On the other hand, in the present study, auditory processing did not account for a large proportion of the variability in sentence processing. Quite possibly these two abilities were modestly correlated due to their comorbidity, rather than to any causal relationship held between them. Recall that previous twin studies found that weaknesses in grammatical computation were heritable but that weaknesses in rapid auditory processing seemed attributable to shared environment rather than to genetic factors.
The finding of a significant (though rather weak) relationship, between both behavioral (SLI group) and electrophysiological measures of rapid auditory processing and morphosyntactic processing adds support to the potential value of developing infant auditory processing measures that might serve as early identifiers of risk for language impairment. Previous reports have indicated that basic auditory processing abilities may play a role in the course of language development and suggest that deficits in these basic auditory abilities may contribute to language impairment (Benasich & Tallal, 2002; Weber et al., 2004). However, even when causality cannot be assumed, the presence of a limitation in auditory processing ability might serve to flag children who are more likely to have a language impairment, due to the documented comorbidity of these two type of problems. The current findings suggest that simple auditory stimuli presented with short and long ISIs (with appropriate parameters for infants) in a standard odd-ball paradigm may be useful for developing an efficient tool for assessing risk for language impairment.
5. Limitations of the current study
A limitation of the current study is that while the mean number of trials for each of the sentence processing conditions was adequate and similar for the two groups and across conditions, the number of trials in some cases fell short and resulted in noisy ERP waveforms. This is important in considering the reliability of the findings for the sentence processing ERPs in general, and especially in the case of the N400 elicited by the current stimuli. Because the N400 is relatively small in amplitude, group differences may have gone undetected given the background noise in the waveforms. A possible methodological detail that may have contributed to the relatively large number of blink artifacts was the use of a small cross for visual fixation. This proved to be an inadequate visual fixation for some of the adolescents. Our current work with children utilizes more interesting visual stimuli that accompany auditory sentences for a more effective visual fixation.
A related limitation was that the accuracy levels for detecting the verb-agreement violations and semantic violations for the adolescents with SLI resulted in too few trials for an analysis of the correct trials only. This raises the question of whether the amplitude of the P600 for the adolescents with SLI may have been within the range of the ND if only correct trials were analyzed. To begin to address this question, we correlated the performance accuracy for verb-agreement (A statistic) with the amplitude of the P600 over central parietal sites for the SLI group. The results indicated significant, but modest, correlations for the SLI group for midline sites (CZ, CPZ, PZ, r = .45 −.54, p = .02-.04) and right hemisphere sites (CP4 and P4, r = .45-.56, p = .02-.04) . These correlations provide some evidence that a relatively modest proportion of the variance in the P600 amplitude for this group may be accounted for by their performance accuracy. In contrast, correlations between the A measures for the semantic task and N400 amplitudes were not significant for the SLI group at any electrode site. Taken together, these preliminary analyses suggest that a follow-up study of verb-agreement that results in a sufficient number of trials for a correct-only analyses is needed. A possible approach for accomplishing this may be to include only syntactic stimuli (exclude semantic violations) to reduce the cognitive load and, at the same time, permit the inclusion of more trials for this condition to be accomplished within a reasonable timeframe.
6. Conclusions
This study extends our knowledge of the electrophysiological correlates of auditory and linguistic processing in several ways. First, the findings provided an important replication that subtle problems in processing at both the behavioral and the electrophysiological level can be detected in individuals with SLI who are well into their adolescent years. Second, at both the behavioral and electrophysiological level, relationships between non-linguistic auditory processing and sentence processing were stronger when the deviations from typical sentences involved grammatical rather than semantic changes. The findings of this study should prompt additional research with individuals with SLI across the age range. If the types of subtle deficits observed here can be traced to earlier points in development, further justification will be provided for the clinical application of processing measures as a means of identifying young children at risk for language impairment.
Another important aspect of the ERP findings is that some of the adolescents with SLI exhibited ERP amplitudes that were within the range of the adolescents with ND. It will be important for future studies to examine individual differences for these electrophysiological measures and to determine how the heterogeneity in ERP patterns relates to functional language performance.
Acknowledgments
This research was funded by the National Institute Deafness and Other Communication Disorders, National Institutes of Health, P50 DC02746. We would like to thank Wendy Fick for the ERP data collection and Marlea O'Brien for the coordination and recruitment of participants. Also, special thanks to John Spruill for help with stimulus development and piloting.
Appendix A
Correct verb (verb-agreement violation, reduced semantic expectation)
Every day, the horses gallop (gallops, sing) to the top of the hill.
Every day, the canaries sing (sings, gallop) at the top of their lungs.
Every day, the secretary types (type, grazes) many legal documents.
Every day, the cow grazes (graze, types) along the fence line.
Every day, the ballerina dances (dance, submerges) on her pointed toe shoes.
Every day, the submarine submerges (submerge, dances) to a depth of five hundred feet.
Every day, the beauticians style (styles, slither) at least 30 heads.
Every day, the snakes slither (slithers, style) through the fallen leaves.
Every day, the lieutenant salutes (salute, meows) his captain during routine inspections.
Every day, the cat meows (meow, salutes) when he thinks he is going to be fed.
Every day, the grandmothers knit (knits, peck) scarves and sweaters for their friends and families.
Every day, the chickens peck (pecks, knit) at the dirt for bits of dried corn.
Every day, the earrings dangle (dangles, cry) from her earlobes.
Every day, the babies cry (cries, dangle) when they are hungry.
Every day, the customers gossip (gossips, swoop) about the hot news in town.
Every day, the eagles swoop (swoops, gossip) down to hunt for their food.
Every day, the mailman delivers (deliver, growls) our letters and packages with care.
Every day, the dog growls (growl, delivers) when someone passes his yard.
Every day, the teachers assign (assigns, gnaw) at least a little homework.
Every day, the mice gnaw (gnaws, assign) on the old boxes stored there.
Every day, the children pretend (pretends, rust) to be super-heroes.
Every day, the cars rust (rusts, pretend) a little bit more.
Every day, the senator votes (vote, perches) on important issues.
Every day, the bird perches (perch, votes) at the top of our tree.
Every day, the owner rents (rent, backfires) his rooms for reasonable rates.
Every day, the engine backfires (backfire, rents) on my way to work.
Every day, the farmers plow (plows, buzz) their corn and soybean fields.
Every day, the bees buzz (buzzes, plow) happily as they make honey.
Every day, the president plans (plan, blasts) his next course of action.
Every day, the wind blasts (blast, plans) the lakefront residents.
Every day, the officers arrest (arrests, shimmer) people who break the law.
Every day, the pennies shimmer (shimmers, arrest) in the sunlight.
Every day, the sirens blare (blares, sew) to signal emergencies.
Every day, the seamstresses sew (sews, blare) custom designed gowns.
Every day, the telephones ring (rings, scurry) constantly until closing time.
Every day, the squirrels scurry (scurries, ring) under the rose bushes.
Every day, the tree grows (grow, plays) taller and more beautiful.
Every day, the theater plays (play, grows) seven different movies.
Every day, the hikers climb (climbs, sparkle) closer to the mountain's peak.
Every day, the minerals sparkle (sparkles, climb) in the midday sun.
Every day, the girls giggle (giggles, roar) about the funny cartoons.
Every day, the lions roar (roars, giggle) around dinner time.
Every day, the seagull flies (fly, melts) over the waves.
Every day, the wax melts (melt, flies) onto the table.
Every day, the boys plan (plans, overflow) the next football game.
Every day, the rivers overflow (overflows, plan) the low lands.
Every day, the lifeguard watches (watch, sprouts) the swimmers.
Every day, the plant sprouts (sprout, watches) new leaves.
Every day, the gardener digs (dig, drips) in the flower bed.
Every day, the water drips (drip, digs) into the sink.
Every day, the pen leaks (leak, wrinkles) in my pocket.
Every day, the shirt wrinkles (wrinkle, leaks) in the dryer.
Every day, the musicians tune (tunes, rumble) their instruments.
Every day, the trucks rumble (rumbles, tune) down the highway.
Every day, the tooth aches (ache, deflates) when I eat sweets.
Every day, the tire deflates (deflate, aches) a little bit more.
Every day, the rose wilts (wilt, swings) in the hot sunshine.
Every day, the door swings (swing, wilts) shut by the draft.
Every day, the chefs prepare (prepares, flicker) hundreds of meals.
Every day, the lights flicker (flickers, prepare) in the window.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams DA, Nicol T, Zecker SG, Kraus N. Auditory brainstem timing predicts cerebral asymmetry for speech. Journal of Neuroscience. 2006;26:11131–11137. doi: 10.1523/JNEUROSCI.2744-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Electroencephalographic Society Guideline thirteen: Guidelines for standard electrode placement nomenclature. Journal of Clinical Neurophysiology. 1994;11:111–113. [PubMed] [Google Scholar]
- Banai K, Nicol T, Zecker SG, Kraus N. Brainstem timing: Implications for cortical processing and literacy. Journal of Neuroscience. 2005;25:9850–9857. doi: 10.1523/JNEUROSCI.2373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu M, Krishnan A, Weber-Fox C. Brainstem correlates of temporal auditory processing in children with specific language impairment. Developmental Science. 2010;13:77–91. doi: 10.1111/j.1467-7687.2009.00849.x. [DOI] [PubMed] [Google Scholar]
- Benasich AA, Tallal P. Infant discrimination of rapid auditory cues predict later language impairment. Behavioural Brain Research. 2002;136:31–49. doi: 10.1016/s0166-4328(02)00098-0. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, Adams C, Norburby CF. Distinct genetic influences on grammar and phonological short-term memory deficits: Evidence from 6-year-old twins. Genes, Brain, and Behavior. 2006;5:158–169. doi: 10.1111/j.1601-183X.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, Bishop SJ, Bright P, James C, Delaney T, Tallal P. Different origin of auditory and phonological problems in children with language impairment: Evidence from a twin study. Journal of Speech, Language, and Hearing Research. 1999;42:155–168. doi: 10.1044/jslhr.4201.155. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, McArthur GM. Immature cortical responses to auditory stimuli in specific language impairment: Evidence from ERPs to rapid tone sequences. Developmental Science. 2004;7:F11–F18. doi: 10.1111/j.1467-7687.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- Catts HW, Bridges MS, Little TD, Tomlin BJ. Reading achievement growth in children with language impairments. Journal of Speech, Language, and Hearing Research. 2008;51:1569–1579. doi: 10.1044/1092-4388(2008/07-0259). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čeponienè R, Cummings A, Wulfeck B, Ballantyne A, Townsend J. Spectral vs. temporal auditory processing in specific language impairment: A developmental ERP study. Brain and Language. 2009;110:107–120. doi: 10.1016/j.bandl.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings A, Čeponienè R. Verbal and nonverbal semantic processing in children with developmental language impairment. Neuropsychologia. 2010;48:77–85. doi: 10.1016/j.neuropsychologia.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deevy P, Leonard L. The comprehension of wh-questions in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 2004;47:802–815. doi: 10.1044/1092-4388(2004/060). [DOI] [PubMed] [Google Scholar]
- Dick F, Richardson F, Saccuman MC. Using magnetic resonance imaging to investigate developmental language disorders. In: Norbury CF, Tomblin JB, Bishop DVM, editors. Understanding Developmental Language Disorders; From Theory to Practice. Psychological Press; New York, NY: 2008. [Google Scholar]
- Dunn L, Dunn L. Peabody Picture Vocabulary Test-Revised. American Guidance Service; Circle Pines, MN: 1981. [Google Scholar]
- Ellis Weismer S, Plante E, Jones M, Tomblin JB. A functional magnetic resonance imaging investigation of verbal working memory in adolescents with specific language impairment. Journal of Speech, Language, and Hearing Research. 2005;48:405–425. doi: 10.1044/1092-4388(2005/028). [DOI] [PubMed] [Google Scholar]
- Ellis Weismer S, Evans J, Hesketh L. An examination of verbal working memory capacity in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 1999;42:1249–1260. doi: 10.1044/jslhr.4205.1249. [DOI] [PubMed] [Google Scholar]
- Federmeier KD, Segal JB, Lombrozo T, Kutas M. Brain responses to nouns, verbs, and class-ambiguous words in context. Brain. 2000;123:2552–2566. doi: 10.1093/brain/123.12.2552. [DOI] [PubMed] [Google Scholar]
- Fonteneau E, van der Lely HKJ. Electrical brain responses in language-impaired children reveal grammar-specific deficits. PLoS ONE. 2008;3:e1832. doi: 10.1371/journal.pone.0001832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD. Neurophysiological aspects of language processing. Nature. 1997;4:64–72. [PubMed] [Google Scholar]
- Friederici AD. The neural basis of language development and its impairment. Neuron. 2006;52:941–952. doi: 10.1016/j.neuron.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Friedrich M, Weber C, Friederici AD. Electrophysiological evidence for delayed mismatch response in infants at-risk for specific language impairment. Psychophysiology. 2004;41:772–782. doi: 10.1111/j.1469-8986.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- Gauger LM, Lombardino LJ, Leonard CM. Brain morphology in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 1997;40:1272–1284. doi: 10.1044/jslhr.4006.1272. [DOI] [PubMed] [Google Scholar]
- Hahne A, Eckstein K, Friederici A. Brain signatures of syntactic and semantic processes during children's language development. Journal of Cognitive Neuroscience. 2004;16:1302–1318. doi: 10.1162/0898929041920504. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O'Brien LM, Kennedy DN, Filipek PA, et al. Brain asymmetries in autism and developmental language disorder: A nested whole brain analysis. Brain. 2005;128:213–226. doi: 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Brown C, Groothusen J. The syntactic positive shift (SPS) as an ERP measure of syntactic processing. Language and Cognitive Processes. 1993;8:439–483. [Google Scholar]
- Hays WL. Statistics. Fifth Edition Harcourt Brace College Publishers; Forth Worth, TX: 1994. [Google Scholar]
- Hirsh-Pasek K, Golinkoff RM. Action Meets Word: How Children Learn Verbs. Oxford University Press; New York, NY: 2006. [Google Scholar]
- Hoeffner J, McClelland J. Can a perceptual processing deficit explain the impairment of inflectional morphology in developmental dysphasia? A computational investigation; Proceedings of the 25th Annual Child Language Research Forum; Stanford University Press; 1993. [Google Scholar]
- Jäncke L, Siegenthaler Th., Preis S, Steinmetz H. Decreased white-matter density in a left sided fronto-temporal network in children with developmental language disorder: Evidence for anatomical anomalies in a motor-language network. Brain and Language. 2007;102:91–98. doi: 10.1016/j.bandl.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Joanisse M. Specific language impairments in children: Phonology, semantics, and the English past tense. Current Directions in Psychological Science. 2004;13:156–160. [Google Scholar]
- Kilborn K. Selective impairment of grammatical morphology due to induced stress in normal listeners: Implications for aphasia. Brain and Language. 1991;41:275–288. doi: 10.1016/0093-934x(91)90156-u. [DOI] [PubMed] [Google Scholar]
- King J, Kutas M. Who did what and when? Using word- and clause-level ERPs to monitor working memory usage in reading. Journal of Cognitive Neuroscience. 1995;7:376–395. doi: 10.1162/jocn.1995.7.3.376. [DOI] [PubMed] [Google Scholar]
- Knight RT, Nakada T. Cortico-limbic circuits and novelty: A review of EEG and blood flow data. Reviews in the Neurosciences. 1998;9:57–70. doi: 10.1515/revneuro.1998.9.1.57. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading between the lines: event-related brain potentials during natural sentence processing. Brain and Language. 1980;11:354–373. doi: 10.1016/0093-934x(80)90133-9. [DOI] [PubMed] [Google Scholar]
- Langacker RW. Nouns and verbs. Language. 1987;63:53–94. [Google Scholar]
- Leonard LB. Children with Specific Language Impairment. MIT Press; Cambridge, MA: 1998. [DOI] [PubMed] [Google Scholar]
- Leonard LB, Ellis Weismer S, Miller CA, Francis DJ, Tomblin JB, Kail RV. Speech of processing, working memory, and language impairment in children. Journal of Speech, Language, and Hearing Research. 2007;50:408–428. doi: 10.1044/1092-4388(2007/029). [DOI] [PubMed] [Google Scholar]
- Leonard LB, Miller CA, Finneran DA. Grammatical morpheme effects on sentence processing by school-aged adolescents with specific language impairment. Language and Cognitive Processes. 2009;24:450–478. doi: 10.1080/01690960802229649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard L, Miller C, Gerber E. Grammatical morphology and the lexicon in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 1999;42:678–689. doi: 10.1044/jslhr.4203.678. [DOI] [PubMed] [Google Scholar]
- Leslie L, Caldwell J. Qualitative Reading Inventory – 3. Allyn & Bacon; Boston, MA: 2001. [Google Scholar]
- Luck SJ. An Introduction to the Event-Related Potential Technique. MIT Press; Cambridge, MA: 2005. [Google Scholar]
- Mangun GR, Hillyard SA. Modulations of sensory-evoked brain potentials indicate changes in perceptual processing during visual spatial priming. Journal of Experimental Psychology: Human Perception and Performance. 1991;17:1057–1074. doi: 10.1037//0096-1523.17.4.1057. [DOI] [PubMed] [Google Scholar]
- McArthur GM, Atkinson CM, Ellis D. Atypical brain responses to sounds in children with specific language and reading impairments. Developmental Science. doi: 10.1111/j.1467-7687.2008.00804.x. In press. [DOI] [PubMed] [Google Scholar]
- McArthur GM, Bishop DVM. Which people with specific language impairment have auditory processing deficits? Cognitive Neurophysiology. 2004;21:79–94. doi: 10.1080/02643290342000087. [DOI] [PubMed] [Google Scholar]
- McArthur GM, Bishop DVM. Speech and non-speech processing people with specific language impairment: A behavioral and electrophysiological study. Brain and Language. 2005;94:260–273. doi: 10.1016/j.bandl.2005.01.002. [DOI] [PubMed] [Google Scholar]
- McGregor KK, Newman RM, Reilly RM, Capone NC. Semantic representation and naming in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 2002;45:998–1014. doi: 10.1044/1092-4388(2002/081). [DOI] [PubMed] [Google Scholar]
- Mervis CB, Klein-Tasman BP. Methodological issues in group-matching designs: α Levels for control variable comparisons and measurements characteristics of control and target variables. Journal of Autism and Developmental Disorders. 2004;34:7–17. doi: 10.1023/b:jadd.0000018069.69562.b8. [DOI] [PubMed] [Google Scholar]
- Miller CA, Kail R, Leonard LB, Tomblin JB. Speed of processing in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 2001;44:416–433. doi: 10.1044/1092-4388(2001/034). [DOI] [PubMed] [Google Scholar]
- Miller CA, Leonard LB, Finneran D. Grammaticality judgments in adolescents with and without language impairment. International Journal of Communication Disorders. 2008;43:346–360. doi: 10.1080/13682820701546813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Leonard LB, Kail RV, Zhang X, Tomblin JB, Francis DJ. Response time in 14-year-olds with language impairment. Journal of Speech, Language, and Hearing Research. 2006;49:712–748. doi: 10.1044/1092-4388(2006/052). [DOI] [PubMed] [Google Scholar]
- Neville HJ, Coffey SA, Holcomb PJ, Tallal P. The neurobiology of sensory and language processing in language-impaired children. Journal of Cognitive Neuroscience. 1993;5:235–253. doi: 10.1162/jocn.1993.5.2.235. [DOI] [PubMed] [Google Scholar]
- Neville HJ, Nicol J, Barss A, Forster KI, Garrett MF. Syntactically based sentence processing classes: Evidence from event-related brain potentials. Journal of Cognitive Neuroscience. 1991;3:151–165. doi: 10.1162/jocn.1991.3.2.151. [DOI] [PubMed] [Google Scholar]
- Nippold M. Later Language Development: School-Age Children, Adolescents, and Young Adults. Third Edition Pro-Ed; Austin, TX: 2007. [Google Scholar]
- Nunez PL. Neocortical dynamics and human EEG rhythms. Oxford University Press; New York: 1995. [Google Scholar]
- Oldfield RC. The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Osterhout L, Kim A, Kuperberg GR. The neurobiology of sentence comprehension. In: Spivey M, Joannisse M, McRae K, editors. The Cambridge Handbook of Psycholinguistics. Cambridge University Press; Cambridge, United Kingdom: In press. [Google Scholar]
- Osterhout L, Holcomb PJ. Event-related brain potentials elicited by syntactic anomaly. Journal of Memory and Language. 1992;31:2–22. [Google Scholar]
- Osterhout L, Mobley LA. Event-related brain potentials elicited by failure to agree. Journal of Memory and Language. 1995;34:739–773. [Google Scholar]
- Plante E, Swisher L, Vance R, Rapcsak S. MRI findings in boys with specific language impairment. Brain & Language. 1991;41:52–66. doi: 10.1016/0093-934x(91)90110-m. [DOI] [PubMed] [Google Scholar]
- Polich J. Probability and inter-stimulus interval effects on the P300 from auditory stimuli. International Journal of Psychophysiology. 1990;10:163–170. doi: 10.1016/0167-8760(90)90030-h. [DOI] [PubMed] [Google Scholar]
- Polich J, Kok A. Cognitive and biological determinants of P300 – An integrative review. Biological Psychology. 1995;41:113–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Rice ML, Wexler K. Toward tense as a clinical marker of specific language impairment in English-speaking children. Journal of Speech, Language, and Hearing Research. 1996;39:1239–1257. doi: 10.1044/jshr.3906.1239. [DOI] [PubMed] [Google Scholar]
- Rice ML, Wexler K, Hershberger S. Tense over time: The longitudinal course of tense acquisition in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 1998;41:1412–1431. doi: 10.1044/jslhr.4106.1412. [DOI] [PubMed] [Google Scholar]
- Sabisch B, Hahne A, Glass E, von Suchodoletz W, Friederici AD. Lexical-semantic processes in children with specific language impairment. NeuroReport. 2006;17:1511–1514. doi: 10.1097/01.wnr.0000236850.61306.91. [DOI] [PubMed] [Google Scholar]
- Sabisch B, Hahne A, Glass E, von Suchodoletz W, Friederici AD. Children with specific language impairment: The role of prosodic processes in explaining difficulties in processing syntactic information. Brain Research. 2009;1261:37–44. doi: 10.1016/j.brainres.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig E, Secord W. Clinical Evaluation of Language Fundamentals-3. Psychological Corporation; San Antonio, TX: 1994. [Google Scholar]
- Shafer VL, Schwartz RG, Morr ML, Kessler KL, Kurtzberg D. Deviant neurophysiological asymmetry in children with language impairment. NeuroReport. 2000;11:3715–3718. doi: 10.1097/00001756-200011270-00025. [DOI] [PubMed] [Google Scholar]
- Stevens C, Sanders L, Neville H. Neurophysiological evidence for selective auditory attention deficits in children with specific language impairment. Brain Research. 2006;1111:143–152. doi: 10.1016/j.brainres.2006.06.114. [DOI] [PubMed] [Google Scholar]
- Tallal P. Experimental studies of language learning impairments: from research to remediation. In: Bishop DVM, Leonard LB, editors. Speech and language impairments in children. Psychology Press; Hove, UK: 2000. pp. 131–156. [Google Scholar]
- Tomblin BJ, Freese PR, Records NL. Diagnosing specific language impairment in adults for the purpose of pedigree analysis. Journal of Speech and Hearing Research. 1992;35:832–843. doi: 10.1044/jshr.3504.832. [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Records NL, Buckwalter P, Zhang X, Smith E, O'Brien M. Prevalence of specific language impairment in kindergarten children. Journal of Speech, Language, and Hearing Research. 1997;40:1245–1260. doi: 10.1044/jslhr.4006.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin JB, Zhang X. Language patterns and etiology in children with specific language impairment. In: Tager-Flusberg H, editor. Neurodevelopmental Disorders. MIT Press; Cambridge, MA: 1999. [Google Scholar]
- Tonnquist-Uhlèn I, Borg E, Persson HE, Spens KE. Topography of auditory evoked cortical potentials in children with severe language impairment: the N1 component. Electroencephalography and Clinical Neurophysiology. 1996;100:250–260. doi: 10.1016/0168-5597(95)00256-1. [DOI] [PubMed] [Google Scholar]
- Wallace G, Hammill D. Comprehensive Receptive and Expressive Vocabulary Test. Pro-Ed; Austin, TX: 1994. [Google Scholar]
- Weber C, Hahne A, Friedrich M, Friederici AD. Discrimination of word stress in early infant perception: Electrophysiological evidence. Cognitive Brain Research. 2004;18:149–161. doi: 10.1016/j.cogbrainres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Weber-Fox C, Hampton A. Stuttering and natural speech processing of semantic and syntactic constraints on verbs. Journal of Speech, Language, and Hearing Research. 2008;51:1058–1071. doi: 10.1044/1092-4388(2008/07-0164). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Fox C, Hart LJ, Spruill J., III Effects of grammatical categories on children's visual language processing: Evidence from event-related brain potentials. Brain and Language. 2006;98:26–39. doi: 10.1016/j.bandl.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children III. Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- Wible B, Nicol T, Kraus N. Atypical brainstem representation of onset and formant structure of speech sounds in children with language-based learning problems. Biological Psychology. 2004;67:299–317. doi: 10.1016/j.biopsycho.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Wible B, Nicol T, Kraus N. Correlation between brainstem and cortical auditory processes in normal and language-impaired children. Brain. 2005;128:417–423. doi: 10.1093/brain/awh367. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Miyamoto T, Riera J, Kim J, Akitsuki Y, Iwata K, et al. Cortical mechanisms involved in the processing of verbs: An fMRI study. Journal of Cognitive Neuroscience. 2006;18:1304–1313. doi: 10.1162/jocn.2006.18.8.1304. [DOI] [PubMed] [Google Scholar]
- Zhang J, Mueller ST. A note on ROC analysis and non-parametric estimate of sensitivity. Psychometrika. 2005;70:203–212. [Google Scholar]