Abstract
Objectives
To evaluate the association of USA300 genotype with outcomes in persons with MRSA bacteremia and examine the epidemiology of MRSA bacteremia over time.
Methods
Population-based surveillance for MRSA bacteremia was performed in 8-county Atlanta from 2005–2008. Cases of MRSA bacteremia were classified as healthcare-associated with hospital-onset (HAHO), healthcare-associated with community-onset (HACO), or community-associated (CA) disease. A survival analysis was performed on a nested cohort of cases with isolates characterized by pulse field gel electrophoresis (PFGE).
Results
4344 MRSA bacteremia cases were identified; 2579 (59.4%) HACO, 1144 (26.3%) HAHO; and 601 (13.8%) CA. Overall incidence rates of MRSA bacteremia declined from 33.9/100,000 in 2005 to 24.8/100,000 in 2008. Rates were highest in persons ≥ 65 years, blacks, males, and persons with AIDS. In multivariate analysis of 1104 cases, USA300 genotype was associated with increased in-hospital mortality (HR 1.63, 95% CI 1.19–2.23). USA300 strains were also associated with increased mortality when compared to USA100 strains (HR 1.79, 95% CI 1.24–2.58).
Conclusions
MRSA bacteremia incidence declined over 4 years but CA disease rates remained stable. Persons with HIV, the elderly, and blacks were disproportionately affected. Bacteremia due to USA300 MRSA strains was associated with increased mortality, suggesting that USA300 strains may be more virulent.
Keywords: Methicillin-Resistant, Staphylococcus aureus, USA300, Community-Associated
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) bacteremia is associated with high mortality and places a substantial burden on healthcare systems [1]. Most cases are acquired in the hospital or in persons with well-defined healthcare risk factors. A surveillance study in the U.S. found S. aureus (SA) to be the causative agent in 20% of all cases of nosocomial bacteremia and the proportion of isolates that were MRSA increased from 22% to 57% from 1995–2002 [2]. A 2005 population-based study estimated the burden of invasive MRSA disease in the U.S. to be 94,360 cases, most of which was healthcare-associated (HA) bacteremia [3].
In the last decade, MRSA infection has emerged in the community setting as a separate epidemic among persons with no recent hospitalization history or other established risk factors for MRSA infection. In 1999, a report in the United States described 4 fatal cases of community-associated MRSA (CA-MRSA) in otherwise healthy children [4]. This report heralded the rising epidemic of CA-MRSA infections and was followed by reports of outbreaks among American Indian and Alaska Natives [5], sports teams [6], prison inmates [7], child care attendees [8], military trainees [9], and men who have sex with men [10].
CA-MRSA isolates were more likely to be susceptible to non-β-lactam antimicrobial agents and to possess the staphylococcal chromosomal cassette (SCC) mec type IV and genes encoding for Panton-Valentine leukocidin (PVL) toxin [11]. Molecular typing using pulse field gel electrophoresis (PFGE) demonstrated that one PFGE type, USA300, accounted for most CA-MRSA disease in the U.S, particularly skin and soft tissue infections [12]. Other PFGE types of community origin include USA400, USA1000, and USA1100. Strains causing HA-MRSA disease have more often been PFGE types USA100, USA200, and USA500 [13]. However, a number of recent reports have linked isolates possessing genotypic features typical of CA-MRSA strains with healthcare-associated infection [14, 15].
While the majority of CA-MRSA infections are localized skin and soft tissue infections, CA-MRSA has also been associated with severe invasive disease [4, 11]. A U.S. population-based study found that CA-MRSA was responsible for 13.7% of all invasive MRSA cases [3]. The predominance of USA300 strains in CA-MRSA disease and the association with severe disease have prompted a search for unique virulence factors that may be contributing to disease. PVL, the arginine catabolic element (ACME), and phenol-soluble modulins (PSM) have been proposed as factors contributing to the pathogenecity of USA300 strains [16]. While epidemiologic and experimental data support the pathogenecity of USA300, there have been no clinical studies evaluating an association of USA300 with mortality. The main objective of this study was to evaluate the association of USA300 genotype with increased mortality in persons with MRSA bacteremia. Additionally, trends in the incidence and epidemiology of MRSA bacteremia over time were examined.
METHODS
Patient Selection
Patients were identified through the Active Bacterial Core Surveillance Program of the Georgia Emerging Infections Program (GA EIP). Since 2004 the GA EIP has performed prospective, population-based, laboratory-based surveillance for all invasive MRSA isolates in Georgia Health District 3 (HD3), the 8-county Atlanta metropolitan area. The study period was from January 1, 2005 through December 31, 2008. Cases were defined as MRSA isolated from blood in a resident of HD3. Case finding was active and laboratory-based and included review of clinical microbiology laboratory printouts from all HD3 hospitals and reference laboratories.
Study personnel retrospectively reviewed medical records using a standard case report form to abstract data on demographics, clinical characteristics, and outcomes. Cases were classified into 3 categories: healthcare-associated with hospital-onset (HAHO), healthcare-associated with community-onset (HACO) or community-associated (CA) [3]. Healthcare-association was defined as having one or more of the following: presence of a central venous catheter (CVC) at presentation or a history of MRSA infection or colonization, surgery, dialysis, or long-term care facility residence within the prior year. Healthcare-associated cases were further classified as community-onset (cases with a culture obtained as an outpatient or < 48 hours after admission) and hospital-onset (cases with culture obtained >48 hours after admission). Community-associated cases were those without documented healthcare risk factors.
Clinical syndromes, comorbidities, and the presence of septic shock were recorded from the medical record. The presence of a CVC was not routinely recorded for HAHO cases. Hospital variables were created for each hospital that treated ≥10% of the study population. Persistent invasive MRSA infection was defined as a repeat positive blood culture 7– 30 days from the initial culture. Recurrent invasive MRSA was defined as a positive culture result obtained for the same case ≥30 days after the initial culture. Patient outcomes were recorded as either survival to discharge or death prior to discharge. Mortality represented crude in-hospital death.
Isolate Collection and Laboratory Testing
All laboratories were asked to submit MRSA blood isolates for further testing. Of the 30 surveillance area laboratories, 11 (37%) contributed isolates. The GA EIP reference laboratory performed PFGE on submitted isolates using restriction endonuclease SmaI; patterns were analyzed using Bionumerics version 4.01 and grouped into pulse field types using Dice coefficients and 80% relatedness [13].
Statistical Analysis
Statistical analyses were performed using SAS, version 9.2 (SAS Institute, Cary, NC). U.S. census bureau bridged-race vintage post census population estimates and Georgia Public Health HIV/AIDS surveillance estimates were used for denominators in calculating incidence rates. Logistic regression was used for trend analysis of incidence rates over the four year surveillance period. For descriptive statistics, differences in categorical variables were tested using χ2 and for continuous variables a two sample t test.
A Cox proportional hazards model was used to perform survival analysis of the cases with PFGE typing to assess risk factors for in-hospital mortality. The main predictor variable was MRSA PFGE type. Other risk factors with possible significance or known to be associated with mortality in MRSA bacteremia were included. Model building and selection was based on the purposeful selection of covariates strategy as previously described [17]. An additional survival analysis was performed comparing only USA 300 to USA 100 isolates. A p value <.05 was considered significant.
IRB
The study was approved by the Emory University and Georgia Department of Human Resources IRBs, the Grady Memorial Hospital Research Oversight Committee, and the VA Research and Development Committee.
RESULTS
Incidence
A total of 4344 cases of MRSA bacteremia were identified from 2005–2008. Most were healthcare-associated, including 2579 (59.4%) HACO infections and 1144 (26.3%) HAHO infections; 601 (13.8%) were CA infections, and 20 (0.5%) were unclassified. Incidence rates significantly decreased over time from 33.9 per 100,000 in 2005 to 24.8 per 100,000 in 2008 (Figure 1). While there was a significant downward trend in rates of HACO and HAHO disease over time, CA MRSA bacteremia rates remained relatively constant (Figure 1). Overall incidence rates per 100,000 were highest in persons 65 years and older (133.0), blacks (45.2), male (33.7), and in persons with AIDS (650.5) (Table 1). Disease rates were higher in blacks than whites across all age groups (data not shown).
Figure 1.
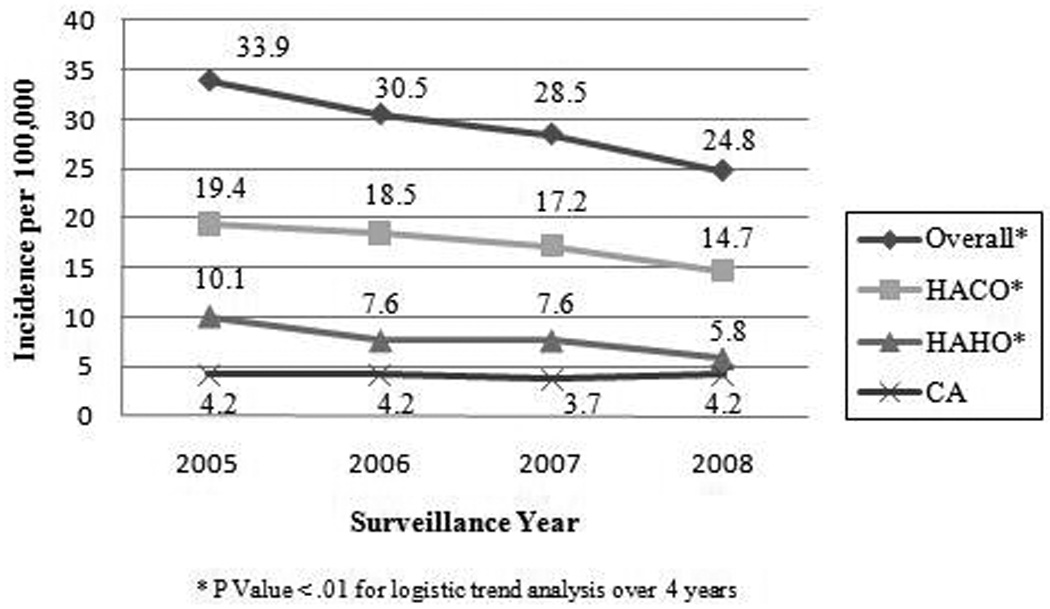
MRSA bacteremia incidence rates by year and epidemiological classification, Atlanta, GA, 2005–2008. Classifications: Healthcare-associated community-onset (HACO); healthcare-associated hospital-onset (HAHO); community-associated (CA). Overall rate includes all HACO, HAHO, and CA cases plus 20 cases with unknown epidemiological classification.
Table 1.
Estimated Methicillin-Resistant Staphylococcus aureus Bacteremia Incidence Rates by Sex, Age, Race, and HIV/AIDS Status, Atlanta, GA, 2005–2008
| Demographic | No. of Cases |
Incidence of MRSA Bacteremia per 100,000 |
||||
|---|---|---|---|---|---|---|
| Surveillance Year |
||||||
| 2005 | 2006 | 2007 | 2008 | Total | ||
| Sex | ||||||
| Male | 2471 | 37.9 | 35.2 | 33.3 | 28.9 | 33.7 |
| Female | 1872 | 30.0 | 25.9 | 23.8 | 20.7 | 25.0 |
| Age, y | ||||||
| ≤1 | 79 | 16.4 | 13.0 | 22.1 | 15.0 | 16.7 |
| 2–17 | 75 | 2.6 | 1.7 | 2.5 | 2.4 | 2.3 |
| 18–34 | 460 | 15.5 | 11.7 | 11.7 | 11.2 | 12.5 |
| 35–49 | 970 | 28.7 | 30.8 | 22.8 | 21.7 | 26.0 |
| 50–64 | 1256 | 51.6 | 51.9 | 52.4 | 42.3 | 49.4 |
| ≥ 65 | 1504 | 172.6 | 135.3 | 125.7 | 103.4 | 133.0 |
| Race | ||||||
| Black | 2558 | 53.3 | 48.6 | 42.4 | 37.2 | 45.2 |
| Othera | 207 | 33.0 | 21.7 | 23.3 | 19.6 | 24.1 |
| White | 1579 | 21.1 | 18.9 | 19.5 | 16.8 | 19.0 |
| HIV/AIDS | ||||||
| HIVb | 118 | 1002.9 | 610.4 | 311.9 | 226.4 | 456.2 |
| AIDS | 321 | 656.6 | 782.8 | 612.6 | 565.6 | 650.5 |
Other includes American Indian, Asian, and Pacific Island;
Not Including AIDS
PFGE
From the overall cohort, 1104 cases (25.4%) had isolates sent for PFGE. A comparison of cases with and without isolates sent for laboratory testing revealed the nested cohort was similar to the overall cohort (Supplemental Table 1). The most common PFGE types were USA300, USA100, USA500, and Iberian (Figure 2).
Figure 2.
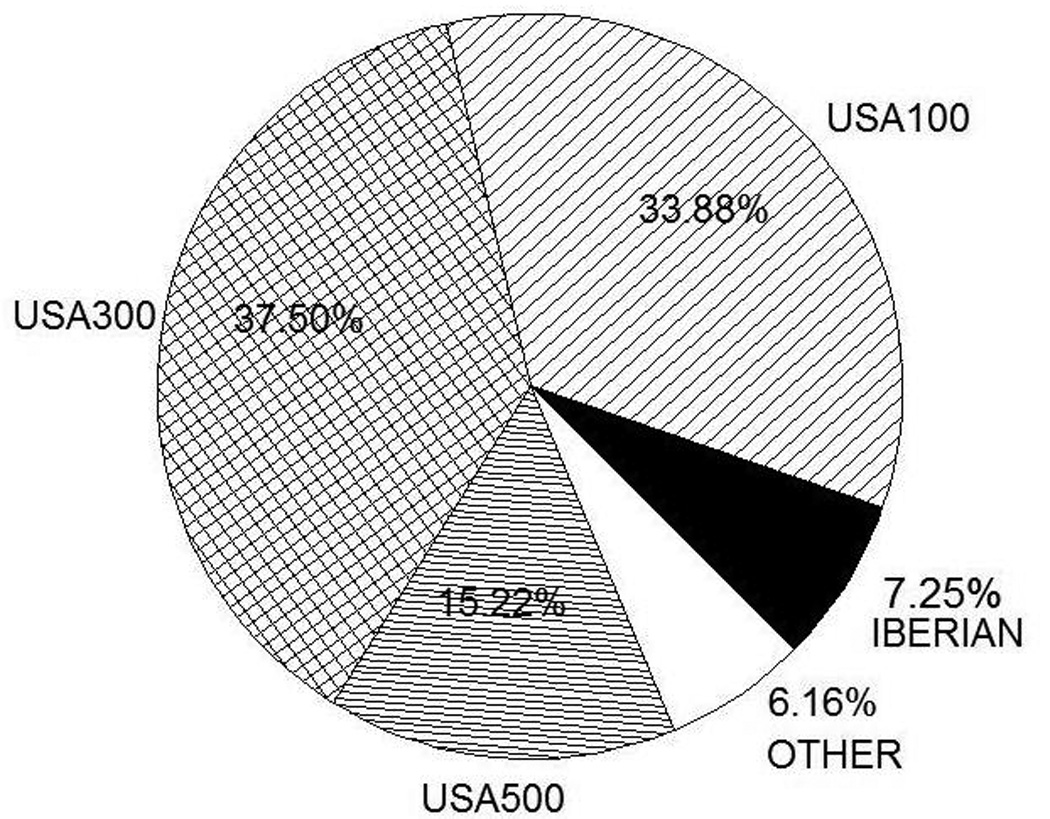
Distribution of PFGE Types in Nested Cohort (N=1104). Other: USA 800 (2.8%), USA700 (1.1%), CAMRSA9, Brazilian, Group D, USA200, USA400, USA600, USA1000, USA1100 (all <1%).
Comparison of USA300 versus Non-USA300
Of the 1104 cases, 414 were USA300 and 690 were non-USA300. Significant differences between the two groups were noted (Table 2). USA300 cases were considerably younger (47.1 versus 57.7 years old, P <.01) and more likely to be male (64.5% versus 54.5, P<.01) and black (73.0% versus 65.9%) Additionally, community-associated cases accounted for a higher proportion of USA300 isolates than non-USA 300 isolates (30.7% versus 7.4%, P<.01) and healthcare-associated cases (HAHO and HACO) accounted for a higher proportion of non-USA300 isolates. USA300 isolates were more often associated with intravenous drug use (3.4% versus 0.1%, P<.01) and chronic liver disease (3.6% vs. 1.5%, P<.05) while non-USA300 isolates were more common in most other chronic medical conditions (Table 2). A similar proportion of USA300 and non-USA300 cases were hospitalized and no differences in the frequency of recurrent or persistent disease were noted. For HAHO cases, those with non-USA300 isolates were hospitalized longer before developing MRSA bacteremia than those with USA300 isolates (33.2 versus 16.1 days, P<.01). The following clinical syndromes were associated with bacteremia from USA300: deep tissue abscess, osteomyelitis, and skin and soft tissue infection. Seventy-two percent of non-USA300 cases had bacteremia without an identified clinical syndrome. In-hospital mortality was 18.5% ; no significant difference was noted between USA300 and non-USA300 cases (16.4 versus 19.7%, P=0.17). However, more USA300 cases died within the first 7 days after positive culture than non-USA300 cases (73.5% versus 49.3%, P <.01), a difference also seen when looking only at HAHO cases (58.8% versus 35.3%, P <.01).
Table 2.
Comparison of Selected Characteristics between USA 300 and Non-USA 300 Methicillin-Resistant Staphylococcus aureus Bacteremia Cases
| Characteristic | Non USA 300 (n=690) | USA 300 (n=414) | P valuee (If <0.05) |
|---|---|---|---|
| Age,Y (mean) | 57.7 | 47.1 | <.01 f |
| Mean Hospital Days before + Culture | 10.5 | 3.7 | <.01 f |
| Mean Hospital Days before + Culture (HAHO) | 33.2 | 16.1 | <.01f |
| Male Sex (%) | 54.5 | 64.5 | <.01 |
| Racea (%) | |||
| Black | 65.9 | 73.0 | <.05 |
| White | 30.4 | 24.4 | <.05 |
| Epidemiological Typeb, c (%) | |||
| HAHO | 29.6 | 17.6 | <.01 |
| Community Onset | 70.0 | 81.4 | <.01 |
| HACO | 62.6 | 50.7 | <.01 |
| CA | 7.4 | 30.7 | <.01 |
| Comorbidities (%) | |||
| Alcoholism | 2.9 | 5.1 | - |
| Intravenous Drug Use | 0.1 | 3.4 | <.01 |
| HIV non AIDS | 2.5 | 4.8 | <.05 |
| AIDS | 9.0 | 11.4 | - |
| Solid Malignancy | 9.4 | 6.0 | <.05 |
| Hematological Malignancy | 2.0 | 0.7 | - |
| Peripheral Vascular Disease | 7.5 | 4.4 | <.05 |
| Congestive Heart Failure | 16.8 | 10.1 | <.01 |
| Coronary Heart Disease | 15.9 | 9.7 | <.01 |
| Cerebrovascular Accident | 14.2 | 5.3 | <.01 |
| Chronic Pulmonary Obstructive Disease | 10.7 | 5.6 | <.01 |
| Diabetes Mellitus | 40.9 | 30.2 | <.01 |
| Chronic Renal Insufficiency | 37.8 | 26.8 | <.01 |
| Hemodialysis in the Last Year | 27.0 | 18.8 | <.01 |
| Hospitalized in last year | 57.4 | 41.8 | <.01 |
| Surgery in Last year | 23.5 | 15.2 | <.01 |
| Long Term Care Facility | 24.4 | 10.6 | <.01 |
| Chronic Liver Disease | 1.5 | 3.6 | <.05 |
| Immunosuppression | 7.5 | 4.6 | - |
| Clinical Characteristics (%) | |||
| Hospitalized | 94.9 | 93.2 | - |
| Persistent Disease | 8.6 | 6.0 | - |
| Relapse | 17.7 | 15.5 | - |
| Clinical Syndromes (%) | |||
| Endocarditis | 3.5 | 4.1 | - |
| Pneumonia | 10.7 | 10.1 | - |
| Septic Arthritis | 2.5 | 1.9 | - |
| Deep Tissue Abscess | 0.9 | 2.4 | <.05 |
| Osteomyelitis | 2.6 | 5.3 | <.05 |
| Surgical Site Infection | 2.8 | 1.9 | - |
| Skin and Soft Tissue Infection | 4.6 | 20.8 | <.01 |
| No associated syndrome | 71.9 | 51.5` | <.01 |
| Severity of Illness (%) | |||
| Septic Shock | 2.9 | 3.9 | - |
| Outcomesd (%) | |||
| Overall Death | 19.7 | 16.4 | - |
| % Overall Death within 7 days | 49.3 | 73.5 | <.01 |
| % Overall Death within 7 days (HAHO cases) | 35.3 | 58.8 | <.01 |
3.3% of overall population was of other race (Asian, American Indian, Hispanic, or Unknown)
Epidemiological classification of disease consisted of healthcare associated (either hospital-onset with a culture collected > 48 hours after hospital admission or community-onset cases with healthcare risk factors and culture collected ≤48 hours after hospital admission) or community associated cases (no healthcare risk factors)
3 cases of non USA300 and 4 cases of USA300 had unknown epidemiological classification.
Death refers to in-hospital mortality
P Value for Chi Square Test unless otherwise stated
P value for two sided, unpaired T test
USA300 vs. Non-USA300 Survival Analysis
USA300 was not associated with mortality in univariate analysis (HR 1.02, 95% CI 0.76, 1.37). The factors significantly associated with death by univariate analysis are shown in Table 3 and included increasing age, white race, the presence of a solid malignancy, peripheral vascular disease, congestive heart failure, coronary heart disease, cerebrovascular accident, chronic obstructive pulmonary disease, chronic liver disease, and long term care facility residence, cases with no identified clinical syndrome, and septic shock. Conversely, syndromes associated with decreased mortality included recurrent MRSA disease, osteomyelitis, and skin and soft tissue infection.
Table 3.
Univariate and Multivariate Analysis of Risk Factors for Mortality in persons with Methicillin-Resistant Staphylococcus aureus bacteremia.
| USA 300 vs. Non-USA 300 (N=1104) |
USA 300 vs. USA 100 (N=788) |
||||
|---|---|---|---|---|---|
| Risk Factor | Univariate Analysis | Multivariate Analysis | Multivariate Analysis | ||
| HRa (95% CI) | HRb (95% CI) | P | HRb (95% CI) | P | |
| USA 300 | 1.02 (0.76–1.37) | 1.63 (1.19–2.23) | .002 | 1.79 (1.24–2.58) | .002 |
| Age, (per year) | 1.03 (1.02–1.04) | 1.04 (1.03–1.05) | <.0001 | 1.04 (1.03–1.05) | <.0001 |
| Hospital days before + culture | 1.00 (1.0–1.0) | 1.004 (1.00–1.01) | .077 | ||
| Female | 0.98 (0.74–1.30) | 0.98 (0.74–1.30) | |||
| White | 1.60 (1.20–2.14) | 1.36 (1.00–1.85) | .050 | ||
| Epidemiological Type | |||||
| Community onset (HACO & CA) | 0.98 (0.72–1.32) | ||||
| Treating Hospital | |||||
| Hospital one | 1.09 (0.76–1.57) | ||||
| Hospital two | 0.91 (0.67–1.23) | ||||
| Hospital three | 0.73 (0.43–1.24) | ||||
| Hospital four | 0.93 (0.60–1.45) | ||||
| Comorbidities | |||||
| Alcoholism | 1.35 (0.73–2.45) | 1.79 (0.95–3.38) | .070 | 2.16 (1.10—4.27) | .026 |
| Intravenous drug use | 0.71 (0.18–2.85) | ||||
| HIV non AIDS | 0.46 (0.15–1.43) | ||||
| AIDS | 1.11 (0.71–1.72) | 2.04 (1.28–3.27) | .003 | ||
| Solid malignancy | 2.08 (1.38–3.12) | ||||
| Hematological malignancy | 1.96 (0.81–4.77) | ||||
| Peripheral vascular disease | 1.91 (1.26–2.88) | ||||
| Congestive heart failure | 1.62 (1.16–2.26) | ||||
| Coronary heart disease | 1.57 (1.10–2.25) | ||||
| Cerebrovascular accident | 1.57 (1.07–2.30) | ||||
| COPD | 1.72 (1.15–2.59) | ||||
| Diabetes mellitus | 1.08 (0.82–1.43) | ||||
| Chronic renal insufficiency | 1.08 (0.81–1.44) | ||||
| Chronic liver disease | 2.23 (1.18–4.22) | 2.48 (1.28–3.27) | .007 | 2.13 (1.02–4.45) | .043 |
| Hemodialysis in the last year | 0.91 (0.65–1.28) | ||||
| Hospitalized in last year | 0.98 (0.74–1.29) | ||||
| Surgery in last year | 1.03 (0.75–1.41) | ||||
| Long term care facility | 1.79 (1.31–2.44) | ||||
| Immunosuppression | 1.14 (0.65–2.00) | ||||
| Clinical Characteristics | |||||
| Persistent disease | 0.76 (0.36–1.57) | ||||
| Relapse | 0.59 (0.39–0.91) | ||||
| Clinical Syndromes | |||||
| Endocarditis | 0.66 (0.29–1.48) | ||||
| Pneumonia | 1.30 (0.89–1.92) | 2.54 (1.44–4.46) | .001 | 2.58 (1.32–5.02) | .006 |
| Septic arthritis/bursitis | 0.16 (0.02–1.17) | ||||
| Deep tissue abscess | 0.26 (0.04–1.84) | ||||
| Osteomyelitis | 0.21 (0.05–0.85) | ||||
| Surgical site infection | 0.59 (0.19–1.86) | ||||
| Skin and soft tissue infection | 0.37 (0.17–0.79) | ||||
| Bacteremia without identified clinical syndrome | 1.79 (1.31–2.46) | 3.26 (2.04–5.20) | <.0001 | 3.18 (1.83–5.53) | <.0001 |
| Severity of Illness | |||||
| Septic shock | 3.99 (2.57–6.20) | 5.07 (3.21–7.99) | <.0001 | 5.47 (3.23–0.28) | <.0001 |
Unadjusted Hazard Ratio
Adjusted Hazard Ratio
After adjusting for confounding variables in multivariate analysis USA300 was associated with increased mortality (HR 1.63, 95% CI 1.19, 2.23). The main confounder of USA300 was age (50% change in HR with addition of age to model). Final multivariate analysis results are shown in Table 3 and a survival graph for USA300 versus non-USA300 isolates is shown in Figure 3A. Other factors in the final model significantly associated with mortality included increasing age (HR 1.04, 95% CI 1.03, 1.05), chronic liver disease (HR 2.48, 95% CI 1.28, 4.81), AIDS (HR 2.04, 95% CI 1.28, 3.27), bacteremia without an identified clinical syndrome (HR 3.26, 95% CI 2.04, 5.20), pneumonia (HR 2.54, 95% CI 1.44, 4.46), and septic shock (HR 5.07, 95% CI 3.21, 7.99).
Figure 3.
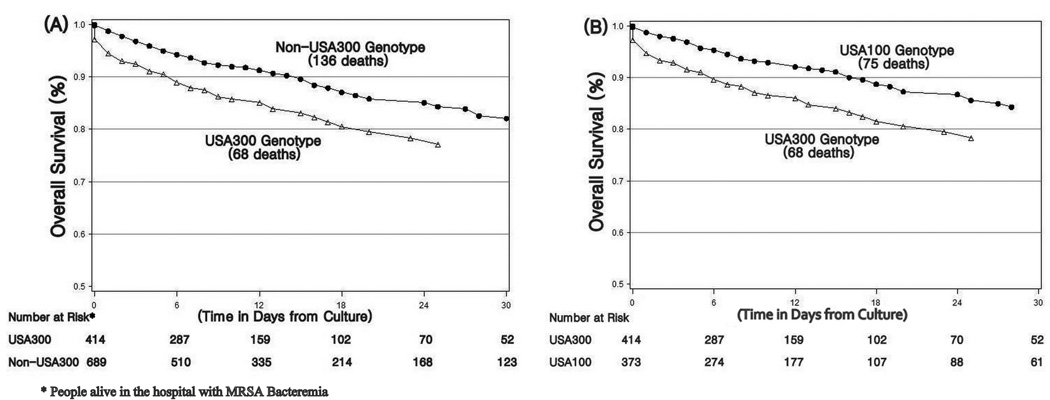
Cox Adjusted Survival Graphs. A, Comparison of USA300 cases vs. Non-USA300 cases. B, Comparison of USA300 cases vs. USA100 cases.
USA300 vs. USA100 Survival Analyses
A secondary survival analysis comparing USA300 vs. USA100 isolates was performed and results are shown in Table 3 and Figure 3B. A stronger association between USA300 and in-hospital mortality was noted when compared to USA100 alone (HR 1.79, 95% CI 1.24, 2.58). Other factors significantly associated with mortality included age, alcoholism, chronic liver disease, pneumonia, bacteremia without an identified syndrome and septic shock.
DISCUSSION
The current study demonstrated a significantly increased risk of death in persons with MRSA bacteremia due to USA300 MRSA strains compared to non-USA300 strains and an even greater increase when compared specifically to USA100 strains. The lack of a significant association between USA300 and increased mortality identified in univariate analysis was explained by the presence of negative confounding by age, a well described risk factor for mortality in persons with MRSA bacteremia [14, 18, 19]. Individuals with USA300 MRSA bacteremia were significantly younger than those with non-USA 300MRSA bacteremia and increasing age was a significant risk factor for mortality.
A number of reports have suggested that CA-MRSA strains, in particular USA300, may have increased virulence as compared to more traditional HA-MRSA strains [16, 20, 21]. Studies in animal models of MRSA bacteremia have demonstrated increased virulence of USA300 strains as compared to HA-MRSA PFGE types and evidence suggests that the underlying mechanism may be the differential expression of key pathogenic determinants [21, 22]. Wang et al. identified a new class of staphylococcal cytolytic peptides, α-type phenol soluble modulins (PSMs), with increased expression in CA-MRSA strains than in HA-MRSA strains, and demonstrated a dramatic effect of PSMs on pathogenecity of USA300 and USA400 strains in mouse abscess and bacteremia models [20]. Li and colleagues, provided additional evidence that the increased virulence of USA300 in a mouse bacteremia model was due in part to the increased expression of virulence factors, including α-type PSMs, rather than the acquisition of new pathogenic factors [21]. The role of PVL, a CA-MRSA associated toxin, and the USA300 unique ACME in MRSA virulence remains unclear [16, 23–25] . In contrast to data from animal models, clinical data comparing the outcomes of serious infections with MRSA USA300 and other MRSA strains is lacking. The subanalysis presented here comparing outcomes of bacteremia from USA300 directly with USA100, the most common HA-MRSA strain in the U.S., eliminated potential confounding by the larger non-USA300 group that included both USA500 isolates (15% of all strains), known progenitors of USA300 that may share virulence characteristics with USA300, and other CA-MRSA strains (<2% of total) [21].
Several other risk factors in multivariate analysis were found to be associated with higher mortality in persons with MRSA bacteremia including septic shock, pneumonia, bacteremia without an identified clinical syndrome, AIDS, and chronic liver disease. Septic shock was most strongly associated with mortality and has been shown to be a risk factor for death in many MRSA bacteremia studies [18, 19, 26]. AIDS, chronic liver disease, and MRSA bacteremia with pneumonia have all also been previously associated with mortality in persons with MRSA bacteremia, likely in part due to immunosuppression-related increased disease severity [14, 26–28]. It is less clear why bacteremia without an identified clinical syndrome was associated with increased mortality. This diagnosis may serve as a marker for increased underlying morbidity since it was more common in individuals with chronic medical diseases or the lack of an identifiable clinical syndrome may have delayed timely diagnosis and treatment.
The current study demonstrated a significant decline in MRSA bacteremia rates over the 4 year surveillance period, but the overall rate of MRSA bacteremia remained high (24.8/100,000 population in 2008). In a population-based study of all invasive MRSA disease (75% bacteremia) in 9 U.S. cities including Atlanta in 2005, rates of MRSA disease were between 19.2 and 116.7 per 100,000 [3]. In contrast, a report from population-based surveillance for SA bacteremia in Calgary, documented a much lower overall rate of MRSA bacteremia of 2.2 per 100,000, but noted rising rates over the 7 year surveillance period [29].
A downward trend in healthcare-associated bacteremia (both HACO and HAHO) was responsible for the overall decline in MRSA bacteremia documented in this study. A recent study evaluating central line-associated blood stream infections in U.S. intensive care units from 1997–2007, showed that the rates of MRSA central line-associated bacteremias in ICUs declined 50% or more since 2001 [30]. These results along with the findings reported here suggest that currently implemented MRSA prevention strategies and other infection control measures in the U.S. may be working to decrease rates of HA-MRSA bacteremia. CA-MRSA bacteremia rates did not decline, suggesting that effective prevention measures for the community setting (and community strains) remain to be identified and/or implemented.
Similar to previous reports [12], the current study documented higher rates of MRSA bacteremia in individuals of black race, regardless of the epidemiologic category. It is unclear whether this represents a true racial predisposition based on genetic or immunological factors or that race serves as a marker for other predisposing factors such as comorbidities or socioeconomic status. Bagger et al. reported an increased rate of MRSA postoperative infection in persons from economically deprived areas [31] and a more recent study demonstrated that higher socioeconomic status was significantly associated with lower rates of SA bacteremia [32].
The current study documented an extremely high rate of MRSA bacteremia in persons with HIV and/or AIDS. Another study evaluating MRSA bacteremia among patients enrolled in a Baltimore HIV outpatient clinic found similarly high rates of MRSA bacteremia[33]. HIV has been shown to be a risk factor for nasal colonization with MRSA and colonization has been demonstrated to be a risk factor for subsequent MRSA infection [34, 35]. While recent data is lacking, a few studies have demonstrated that the neutrophils of HIV infected persons exhibit reduced phagocytosis of S. aureus, an effect that was more pronounced at lower CD4 counts [36, 37]. Our findings highlight the importance of MRSA as an opportunistic pathogen in HIV infected persons and stress the need for vigilance in prevention, early recognition and treatment of MRSA infections in this population.
A number of limitations to our survival analysis should be noted. The survival analysis was performed on a nested cohort that was selected using a convenience sampling method. This sampling method could introduce bias into a risk factor analysis. However, the nested study group was similar to the overall cohort and thus not likely to have adversely impacted the validity of the survival analysis.
Another limitation is the absence of information on timing and receipt of antibiotics, allowing for the introduction of bias if there were differences in the selection and timing of appropriate antibiotic treatment between USA300 and non-USA300 strains. The fact that vancomycin is currently the only standard empiric antibiotic treatment for MRSA bacteremia in the U.S. [38, 39] makes it less likely that significant variability in the initial selection of antibiotics occurred. Additionally, while the timely initiation of effective empiric therapy has been shown to improve the outcome of MRSA bacteremia in some studies, many others have found the delay of proper MRSA treatment did not have a significant effect on outcome [40–43].
Finally, the outcome of mortality represented crude in-hospital mortality that may have been influenced by comorbidities. An extensive list of comorbidities were included as variables in the analysis and time (in days) to death was used rather than overall death as an outcome to control for competing causes of mortality.
This study demonstrated an association of USA300 strains with increased in-hospital mortality compared with non-USA300 strains, particularly USA100, in persons with MRSA bacteremia. In addition, USA300 was responsible for a significant portion of HA-MRSA bacteremias. Future studies confirming these findings and further investigation of the role of microbial virulence mechanisms will be needed to better understand the pathogenesis of USA300 MRSA infections and develop improved strategies for prevention and treatment.
Supplementary Material
Acknowledgements
Financial Support: R.R.K. supported in part by the NIH Fogarty International Center (D43TW007124 and D43TW007124-06S1) and the Atlanta Clinical and Translational Science Institute (NIH/NCRR UL1RR025008). MRSA surveillance was funded by the Emerging Infections Program, CDC. We thank all the participating hospitals and laboratories in Georgia Health District 3 for their support; Scott Fridkin for leadership in the EIP MRSA surveillance program and Brandi Limbago and Greg Fosheim for laboratory support at CDC and Christal Hembree and Brooke Napier for laboratory support at the Georgia EIP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship: All authors have reviewed and approved the manuscript. Additionally, all authors have contributed significantly to this work. No other writing assistance was provided in the preparation of this manuscript.
Article Summary: USA300 genotype is the predominant MRSA strain causing community-associated infections and may be associated with increased virulence. This study demonstrated an association of USA300 strains with increased in-hospital mortality compared with non-USA300 strains, particularly USA100, in persons with MRSA bacteremia.
Conflict of interest: None of the authors have any commercial or other association that may pose a conflict of interest.
REFERENCES
- 1.Shorr AF, Tabak YP, Killian AD, Gupta V, Liu LZ, Kollef MH. Healthcare-associated bloodstream infection: A distinct entity? Insights from a large U.S. database. Crit Care Med. 2006 Oct;34(10):2588–2595. doi: 10.1097/01.CCM.0000239121.09533.09. [DOI] [PubMed] [Google Scholar]
- 2.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004 Aug 1;39(3):309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 3.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007 Oct 17;298(15):1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 4.From the Centers for Disease Control and Prevention. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus--Minnesota and North Dakota, 1997–1999. JAMA. 1999 Sep 22–29;282(12):1123–1125. [PubMed] [Google Scholar]
- 5.Baggett HC, Hennessy TW, Rudolph K, et al. Community-onset methicillin-resistant Staphylococcus aureus associated with antibiotic use and the cytotoxin Panton-Valentine leukocidin during a furunculosis outbreak in rural Alaska. J Infect Dis. 2004 May 1;189(9):1565–1573. doi: 10.1086/383247. [DOI] [PubMed] [Google Scholar]
- 6.Kazakova SV, Hageman JC, Matava M, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005 Feb 3;352(5):468–475. doi: 10.1056/NEJMoa042859. [DOI] [PubMed] [Google Scholar]
- 7.Outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections--Los Angeles County, California, 2002–2003. MMWR Morb Mortal Wkly Rep. 2003 Feb 7;52(5):88. [PubMed] [Google Scholar]
- 8.Adcock PM, Pastor P, Medley F, Patterson JE, Murphy TV. Methicillin-resistant Staphylococcus aureus in two child care centers. J Infect Dis. 1998 Aug;178(2):577–580. doi: 10.1086/517478. [DOI] [PubMed] [Google Scholar]
- 9.Zinderman CE, Conner B, Malakooti MA, LaMar JE, Armstrong A, Bohnker BK. Community-acquired methicillin-resistant Staphylococcus aureus among military recruits. Emerg Infect Dis. 2004 May;10(5):941–944. doi: 10.3201/eid1005.030604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diep BA, Chambers HF, Graber CJ, et al. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann Intern Med. 2008 Feb 19;148(4):249–257. doi: 10.7326/0003-4819-148-4-200802190-00204. [DOI] [PubMed] [Google Scholar]
- 11.Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003 Dec 10;290(22):2976–2984. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 12.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006 Aug 17;355(7):666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 13.McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003 Nov;41(11):5113–5120. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seybold U, Kourbatova EV, Johnson JG, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis. 2006 Mar 1;42(5):647–656. doi: 10.1086/499815. [DOI] [PubMed] [Google Scholar]
- 15.Klevens RM, Morrison MA, Fridkin SK, et al. Community-associated methicillin-resistant Staphylococcus aureus and healthcare risk factors. Emerg Infect Dis. 2006 Dec;12(12):1991–1993. doi: 10.3201/eid1212.060505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diep BA, Otto M. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 2008 Aug;16(8):361–369. doi: 10.1016/j.tim.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosmer DW, Lemeshow S, May S. Applied Survival Anlaysis: Regression Modeling of Time-to-Event Data. 2nd ed. Model Development: Wiley-Interscience; 2008. [Google Scholar]
- 18.Soriano A, Martinez JA, Mensa J, et al. Pathogenic significance of methicillin resistance for patients with Staphylococcus aureus bacteremia. Clin Infect Dis. 2000 Feb;30(2):368–373. doi: 10.1086/313650. [DOI] [PubMed] [Google Scholar]
- 19.Kaech C, Elzi L, Sendi P, et al. Course and outcome of Staphylococcus aureus bacteraemia: a retrospective analysis of 308 episodes in a Swiss tertiary-care centre. Clin Microbiol Infect. 2006 Apr;12(4):345–352. doi: 10.1111/j.1469-0691.2005.01359.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang R, Braughton KR, Kretschmer D, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007 Dec;13(12):1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Diep BA, Villaruz AE, et al. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A. 2009 Apr 7;106(14):5883–5888. doi: 10.1073/pnas.0900743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voyich JM, Braughton KR, Sturdevant DE, et al. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol. 2005 Sep 15;175(6):3907–3919. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- 23.Cremieux AC, Dumitrescu O, Lina G, et al. Panton-valentine leukocidin enhances the severity of community-associated methicillin-resistant Staphylococcus aureus rabbit osteomyelitis. PLoS One. 2009;4(9):e7204. doi: 10.1371/journal.pone.0007204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labandeira-Rey M, Couzon F, Boisset S, et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007 Feb 23;315(5815):1130–1133. doi: 10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- 25.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009 Sep;7(9):629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conterno LO, Wey SB, Castelo A. Risk factors for mortality in Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol. 1998 Jan;19(1):32–37. doi: 10.1086/647704. [DOI] [PubMed] [Google Scholar]
- 27.Kim SH, Park WB, Lee KD, et al. Outcome of Staphylococcus aureus bacteremia in patients with eradicable foci versus noneradicable foci. Clin Infect Dis. 2003 Sep 15;37(6):794–799. doi: 10.1086/377540. [DOI] [PubMed] [Google Scholar]
- 28.Shurland S, Zhan M, Bradham DD, Roghmann MC. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007 Mar;28(3):273–279. doi: 10.1086/512627. [DOI] [PubMed] [Google Scholar]
- 29.Laupland KB, Ross T, Gregson DB. Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000–2006. J Infect Dis. 2008 Aug 1;198(3):336–343. doi: 10.1086/589717. [DOI] [PubMed] [Google Scholar]
- 30.Burton DC, Edwards JR, Horan TC, Jernigan JA, Fridkin SK. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997–2007. JAMA. 2009 Feb 18;301(7):727–736. doi: 10.1001/jama.2009.153. [DOI] [PubMed] [Google Scholar]
- 31.Bagger JP, Zindrou D, Taylor KM. Postoperative infection with meticillin-resistant Staphylococcus aureus and socioeconomic background. Lancet. 2004 Feb 28;363(9410):706–708. doi: 10.1016/S0140-6736(04)15647-X. [DOI] [PubMed] [Google Scholar]
- 32.Huggan PJ, Wells JE, Browne M, Richardson A, Murdoch DR, Chambers ST. Population-based epidemiology of Staphylococcus aureus bloodstream infection in Canterbury, New Zealand. Intern Med J. 2009 Feb 10; doi: 10.1111/j.1445-5994.2009.01910.x. [DOI] [PubMed] [Google Scholar]
- 33.Burkey MD, Wilson LE, Moore RD, Lucas GM, Francis J, Gebo KA. The incidence of and risk factors for MRSA bacteraemia in an HIV-infected cohort in the HAART era. HIV Med. 2008 Nov;9(10):858–862. doi: 10.1111/j.1468-1293.2008.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hidron AI, Kourbatova EV, Halvosa JS, et al. Risk factors for colonization with methicillin-resistant Staphylococcus aureus (MRSA) in patients admitted to an urban hospital: emergence of community-associated MRSA nasal carriage. Clin Infect Dis. 2005 Jul 15;41(2):159–166. doi: 10.1086/430910. [DOI] [PubMed] [Google Scholar]
- 35.Shet A, Mathema B, Mediavilla JR, et al. Colonization and subsequent skin and soft tissue infection due to methicillin-resistant Staphylococcus aureus in a cohort of otherwise healthy adults infected with HIV type 1. J Infect Dis. 2009 Jul 1;200(1):88–93. doi: 10.1086/599315. [DOI] [PubMed] [Google Scholar]
- 36.Pos O, Stevenhagen A, Meenhorst PL, Kroon FP, Van Furth R. Impaired phagocytosis of Staphylococcus aureus by granulocytes and monocytes of AIDS patients. Clin Exp Immunol. 1992 Apr;88(1):23–28. doi: 10.1111/j.1365-2249.1992.tb03033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaumann R, Krosing J, Shah PM. Phagocytosis of Escherichia coli and Staphylococcus aureus by neutrophils of human immunodeficiency virus-infected patients. Eur J Med Res. 1998 Dec 16;3(12):546–548. [PubMed] [Google Scholar]
- 38.Gould FK, Brindle R, Chadwick PR, et al. Guidelines (2008) for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the United Kingdom. J Antimicrob Chemother. 2009 May;63(5):849–861. doi: 10.1093/jac/dkp065. [DOI] [PubMed] [Google Scholar]
- 39.Corey GR. Staphylococcus aureus bloodstream infections: definitions and treatment. Clin Infect Dis. 2009 May 15;48 Suppl 4:S254–S259. doi: 10.1086/598186. [DOI] [PubMed] [Google Scholar]
- 40.Kim SH, Park WB, Lee KD, et al. Outcome of inappropriate initial antimicrobial treatment in patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2004 Aug;54(2):489–497. doi: 10.1093/jac/dkh366. [DOI] [PubMed] [Google Scholar]
- 41.Fang CT, Shau WY, Hsueh PR, et al. Early empirical glycopeptide therapy for patients with methicillin-resistant Staphylococcus aureus bacteraemia: impact on the outcome. J Antimicrob Chemother. 2006 Mar;57(3):511–519. doi: 10.1093/jac/dkl006. [DOI] [PubMed] [Google Scholar]
- 42.Kim SH, Park WB, Lee CS, et al. Outcome of inappropriate empirical antibiotic therapy in patients with Staphylococcus aureus bacteraemia: analytical strategy using propensity scores. Clin Microbiol Infect. 2006 Jan;12(1):13–21. doi: 10.1111/j.1469-0691.2005.01294.x. [DOI] [PubMed] [Google Scholar]
- 43.Ammerlaan H, Seifert H, Harbarth S, et al. Adequacy of antimicrobial treatment and outcome of Staphylococcus aureus bacteremia in 9 Western European countries. Clin Infect Dis. 2009 Oct 1;49(7):997–1005. doi: 10.1086/605555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


