Abstract
Behavioral inhibition (BI) is an adaptive defensive response to threat; however, children that display extreme BI as a stable trait are at risk for development of anxiety disorders and depression. The present study validates a rodent model of BI based on an ethologically relevant predator exposure paradigm. We demonstrate that individual differences in rat BI are stable and trait-like from adolescence into adulthood. Using in situ hybridization to quantify expression of the immediate early genes homer1a and fos as measures of neuronal activation, we show that individual differences in BI are correlated with the activation of various stress-responsive brain regions that include the paraventricular nucleus of the hypothalamus and CA3 region of the hippocampus. Further supporting the concept that threat-induced BI in rodents reflect levels of anxiety, we also demonstrate that BI is decreased by administration of the anxiolytic, diazepam, Finally, we developed criteria for identifying extreme BI animals that are stable in their expression of high levels of BI and also demonstrate that high BI (HBI) individuals exhibit maladaptive appetitive responses following stress exposure. These findings support the use of predator threat as a stimulus and HBI rats as a model to study mechanisms underlying extreme and stable BI in humans.
Keywords: Anxiety, homer, psychological stress, hypothalamus, adolescence
Introduction
Behavioral inhibition (BI) is an adaptive defensive response to threat that has been evolutionarily conserved across species to promote survival (Porges 2003). Individual differences in BI are evident early in life and are moderately stable throughout development (Fox et al. 2005). When children exhibit extreme BI, characterized by prolonged and excessively inhibited responses to strangers and novelty, this trait-like tendency is viewed as a temperamental disposition and is a considerable risk factor for the development of anxiety and stress-related disorders (Caspi et al. 1995; Caspi & Silva 1995). Importantly, it is children with both high and stable levels of BI that are at greatest risk to develop psychopathology, which tends to be social anxiety disorder (Biederman et al. 2001). Because other disorders are comorbid with social anxiety disorder (Hettema et al. 2005; Stein et al. 2001; Zimmermann et al. 2003), it is likely that extreme and stable BI is a broad risk factor for the later development of other anxiety and depressive-related psychopathologies (Gladstone et al. 2005; Goldsmith & Lemery 2000; Hirshfeld et al. 1992).
We have developed a rat model of BI that is elicited by ferret exposure, a naturalistic and ethologically relevant predatorial threat. Our laboratory and others previously demonstrated that exposure to predator stimuli elicit conditioned and unconditioned anxiety- and defensive-like behaviors such as BI, freezing and withdrawal in rats (Campeau et al. 2008; Masini et al. 2006; McGregor et al. 2002; Nanda et al. 2008). In our model, we define BI as combination of threat-induced freezing and hypervigilant behaviors. Eliciting BI by exposure to potentially threatening situations is consistent with methods used in human and non-human primate models of BI (Kagan et al. 1988; Kalin & Shelton 2003). In addition, predatorial threat is an important aspect of the model because rodents tend not habituate to predator exposure (Blanchard et al. 1998), which allows for the performance of longitudinal studies with repeated assessments.
Because BI has trait-like characteristics associated with human and non-human primate anxiety (Fox et al. 2005; Kalin & Shelton 2003), we characterized the stability of BI from adolescence to adulthood in rats and determined the ability of the anxiolytic, diazepam, to modulate BI. Given the importance of the hypothalamic-pituitary-adrenal (HPA) axis (Blanchard et al. 1998; File et al. 1993; Masini et al. 2005; Morrow et al. 2000; Perrot-Sinal et al. 1999) and other stress-responsive regions such as the amygdala and hippocampus in mediating the physiological and behavioral response to predator exposure (Campeau et al. 2008; Dielenberg et al. 2001; Masini et al. 2005; Roseboom et al. 2007), we determined the extent to which activity of these regions is associated with individual differences in BI. We assessed regional activity by quantifying in situ hybridization signals for the immediate early genes homer1a and fos. Finally, we assessed the extent to which rats with extreme BI are vulnerable to the influences of stress on appetitive behaviors, as reductions in appetitive behaviors are common maladaptive characteristics of stress-related psychopathology (Lo Sauro et al. 2008; O’Brien & Vincent 2003).
Methods
Animals
Male and female Sprague-Dawley rats (Charles River, Wilmington, MA) weighing 225–250 g were housed individually in clear, plastic cages in a temperature and light-controlled vivarium (24 °C, lights on from 0700–1900 h). Except where indicated, food and water were available ad libitum. All rats were handled by the experimenters for at least five days prior to testing to minimize handling- and novelty-related stress. Six male ferrets (Marshall Farms, North Rose, NY) were used in these studies and were housed in pairs; food and water were available ad libitum. All facilities are AAALAC approved; the protocols were in accordance with the guidelines of the National Institutes of Health and the Institutional Animal Care and Use Committee of the University of Wisconsin-Madison.
Drug treatment
Diazepam (DZP) was obtained from Sigma-Aldrich (St. Louis, MO) and was dissolved at 1 mg/ml in 40% propylene glycol and 10% ethanol in sterile water, which also served as the vehicle (VEH). To minimize the stress of the injection procedure, beginning three days prior to testing rats received daily intraperitoneal (IP) injections of 0.2 mL of 0.9% saline. On the test day, rats received either VEH or 1 mg/kg DZP given in a volume of 1 mL/kg given IP 30 min prior to behavioral testing. This dose has been shown in numerous publications to produce anxiolytic-like effects in a variety of rodent models (Duzzioni et al. 2008; Jochum et al. 2007; Nicolas & Prinssen 2006; Wilson et al. 2004).
Ferret predator paradigm
Rats were placed into empty standard rat housing cages (length 18” by width 10” by height 8”) without bedding, and cage lids were flipped upside down and clipped into place to allow for an unobstructed side view of the rats. The cage containing the rat was transferred into the ferret colony room and placed directly in front of the six ferrets, approximately 30 cm from the ferret cages, such that the rat could still hear, see and smell the ferrets with no direct physical contact. All ferret exposures were videotaped for 15 min and rat behavior was assessed by a trained reviewer. The behaviors that were scored are described in the “behavioral testing” section below.
Experimental design
In Experiment 1a (n = 15), we examined the extent to which predator threat elicits BI in adolescent male rats. The behavior of adolescent male rats (42 or 43 days old) was first observed at baseline, followed immediately by another baseline measurement (n = 7) or 15 min of ferret exposure (n = 8). Experiment 1b sought to elucidate gender differences in BI response, as well as stability of behavioral responding from adolescence to adulthood. To achieve this, male (n = 19) and female (n = 18) adolescent rats (42 or 43 days old) were exposed to the ferret predator paradigm and re-exposed 28 days later (70 or 71 days old). This experiment was repeated in male rats (n = 24) that were exposed to ferrets twice in adolescence (at 36 and 38 days of age) and twice in adulthood (at 85 and 87 days of age) during the dark phase (testing between 1100–1500 h, lights off from 0900–1700 h). To further establish rodent BI as a model of anxiety-like behavior, in Experiment 2 we employed the use of an anxiolytic drug to pharmacologically validate the model. Rats (n = 8/group) received either vehicle or 1 mg/kg diazepam IP, as described above, and 30 min later were exposed to the predator paradigm. The aim of Experiment 3 (n = 54) was to examine how individual differences in brain reactivity are related to individual differences in BI. Based on our previous studies indicating that homer1a and fos expression are increased in response to ferret exposure in our paradigm (Nanda et al. 2008; Roseboom et al. 2007), we use in situ hybridization to quantify homer1a and fos mRNA levels and then determined the relationship to the expression of BI. Forty-five rats were exposed to the ferret predator paradigm and nine rats remained in their home cage serving as controls. All rats were killed by decapitation 1 h following cessation of ferret exposure and the brains were rapidly removed and placed in isopentane at −20°C to −30°C. The whole brains were stored at −80°C until they were sliced into 20 μm coronal sections for in situ hybridization analysis. Finally, we examined the extent to which extremely inhibited animals exhibit other maladaptive behaviors after the cessation of threat exposure. In Experiment 4, sixty adult rats were initially screened, and extreme and stable groups of low BI (LBI) and high BI (HBI) were identified as those animals that consistently ranked in the bottom or top quartile, respectively, in two tests separated by 48 hours. The eight HBI and seven LBI rats were subjected to the feeding test as described in the next section.
Behavioral testing
Definitions of the behaviors that were scored were previously described in detail (Nanda et al. 2008). Briefly, the durations of the following behaviors were scored: rearing (raising both front paws off the floor of the test cage), grooming (licking and rubbing with paws of any accessible area of skin and fur), locomotion and BI. Locomotion is defined as the duration of movement of all four feet. BI is defined as the sum of freezing (this behavior is scored if a period of at least 3 seconds passes, during which there is an absence of all movement except those required for respiration) and hypervigilance, which is characterized by an absence of locomotion with accompanying minor head movements associated with sensory acquisition (sniffing and vibrissa movement).
The feeding test was performed essentially as previously described (Jochman et al. 2005). Rats were habituated for three consecutive days to nightly food deprivation (from 1600 to 0900 h). During this time, rats were also habituated to the feeding test environment, which consisted of placing the rat in a novel empty housing cage containing wire mesh flooring in a room separate from where the ferret exposure occurred. On the first test day, rats were entered into a 30 min baseline feeding test. On the following day, all rats were given a 15 min direct ferret exposure immediately prior to the feeding test. Direct ferret exposure consisted of exposing the rat to a ferret by placing the rat in a protective wire cage (length 7.75” by width 6” by height 5.5”) within the ferret homecage as previously described (Roseboom et al. 2007). During the feeding test, rats were rated for the amount of time spent eating, and food was weighed before and after testing to determine the amount of food eaten.
In situ hybridization histochemistry
The single-stranded homer1a in situ hybridization probe was obtained by PCR amplification of rat whole brain cDNA and corresponded to bases 5231–5699 of GenBank accession number U92079.1. The fos probe corresponded to bases 1045–1868 of GenBank accession number NM_022197.1. The respective PCR products were subcloned into pCRII-Topo (Invitrogen, Carlsbad, CA, USA). Each plasmid was linearized with the restriction enzyme SpeI (Promega, Madison, WI, USA) and the antisense [α-35S] uracyl tri-phosphate (UTP)-labeled riboprobe was generated by in vitro transcription using the T7 Riboprobe System (Promega) as previously described (Herringa et al. 2004). Coronal sections (20 μM) were fixed in 4% paraformaldehyde and subsequently processed for in situ hybridization as previously described (Hsu et al. 2001).
Quantification of in situ hybridization was as previously described with the specific parameters detailed below (Nanda et al. 2008). Probed sections were exposed to phosphor screens for 4 days and scanned with a Typhoon phosphorimager (GE Healthcare, Piscataway, NJ, USA) at 50 μM resolution. ImageQuant 5.0 software (GE Healthcare) was used to analyze positively hybridized probe signals. The experimenter quantifying the signals was blind to the experimental condition associated with each image. Four sections per animal were analyzed for the paraventricular nucleus of the hypothalamus (PVN), 14 for the basolateral amygdala (BLA), 15 for the medial amygdala (MeA) and 20 each for the dentate gyrus (DG), CA1 and CA3 regions of the hippocampus. Signal intensity was assessed using regions of interest (ROI) with fixed dimensions along the rostral-caudal extent of the PVN (1.2 by 1.55 mm ellipse), BLA (0.9 by 1.35 mm ellipse), MeA (1.3 by 1.9 mm ellipse), DG (0.4 by 0.4 mm square), CA1 (0.45 by 0.55 mm rectangle) and CA3 (0.45 by 0.55 mm rectangle) in each hemisphere. Background signal was calculated by using an identically shaped ROI placed over an adjacent region that showed low levels of hybridization. A background ROI was placed over the thalamus for the BLA and MeA quantification, and a ROI placed over the internal capsule was used for the PVN, DG, CA1 and CA3 quantification. The sections that were used for signal quantification corresponded to the following anterior/posterior co-ordinates relative to Bregma–PVN (−1.5 to −2.0 mm), BLA and MeA (−1.9 to −3.6 mm) and the DG, CA1 and CA3 (−1.7 to −4.1 mm). For the BLA, MeA, DG, CA1 and CA3 regions, the highest average signal volume (signal intensity multiplied by area of ROI) from consecutive sections was determined for each hemisphere in each region using a rolling average. For the BLA and MeA, the rolling averages consisted of three sections, while the DG, CA1 and CA3 rolling averages consisted of five sections. In each rat, the highest average signal volume from the left and right hemispheres were averaged together. For PVN, because the signals for the left and right hemispheres were in close proximity, a single ellipse was drawn that contained signal from both hemispheres. In addition, because there were only 4 sections per animal that contained a PVN signal, the highest signal volume obtained from a single section was used instead of a rolling average.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 5.01 software (GraphPad Software, San Diego, CA, USA). Because some BI data sets were not normally distributed and various transformations failed to normalize the distribution, analyses of BI, with the exception of the effects of gender on BI, were performed using non-parametric methods. Comparisons between two groups were made using Mann-Whitney U-tests and comparisons between multiple groups were made using Kruskal-Wallis H-tests followed by Dunn’s post tests for multiple comparisons. Correlations were made using Spearman’s rank correlation. For analysis of the impact of gender on BI it was possible to normalize the distribution by log transforming the data, and a two-factor ANOVA was performed followed by Bonferroni post tests. In addition, Pearson correlations were performed including co-varying for gender. The feeding data in experiment 4 was normally distributed and was analyzed parametrically using a two-way ANOVA followed by comparing each treatment group individually using Bonferroni post tests.
Results
Experiment 1a: ferret exposure induces behavioral inhibition in adolescent rats
To examine the extent to which animals exhibit BI early in development, we assessed the effects of ferret exposure on adolescent male rats (42 or 43 days old). The study design was such that all rats (n = 15) received a baseline assessment of BI in the absence of the ferret. Baseline testing consisted of placing the rat in an empty rat housing cage and transporting the animal to a separate housing suite. This exposure was followed immediately by another 15 min exposure in the baseline environment (control group; n = 7) or a 15 min ferret exposure (ferret exposed group; n = 8). The control rats were transported in the facility for approximately the length of time that was required to move a rat to the ferret colony room before being placed back in the baseline room for a second exposure. A Kruskal-Wallis H-test analysis of BI over the 15 min sessions revealed significant differences between the control and ferret exposed groups (χ2 = 18.39, df = 3, p < 0.001; Figure 1). Dunn’s post hoc analyses revealed that the ferret exposed group exhibited significantly more BI than the control group during the second test session (p < 0.001). These results show that similar to adult rats, ferret exposure reliably elicits BI in adolescent rats.
Figure 1. Ferret exposure induces behavioral inhibition in adolescent rats.
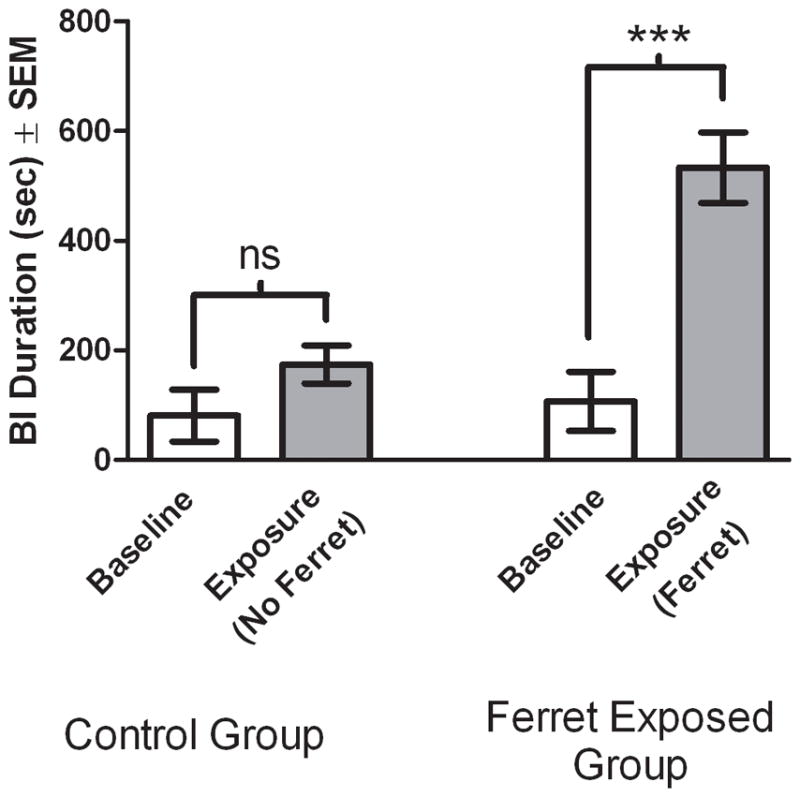
The control rats were exposed to a baseline session followed by exposure to the same environment as baseline, and the experimental group was exposed to baseline followed immediately by exposure to the ferret. In the Ferret Exposed Group, rats exhibited significantly more BI in the presence of the ferret compared to the baseline condition. Bars represent the mean ± SEM. ***, p < 0.001.
Experiment 1b: behavioral inhibition in rats is stable from adolescence to adulthood and within adulthood
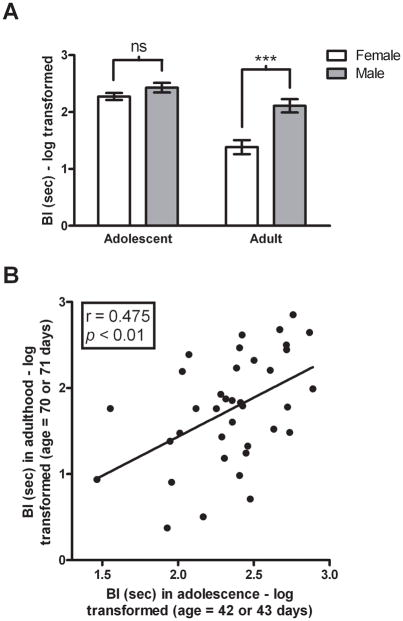
Because BI in children is considered trait-like, and stable extreme BI is a considerable risk factor for the later development of psychopathology, we examined the extent to which individual differences in ferret-induced BI in adolescent rats is predictive of adulthood BI. Thirty-seven adolescent rats (male: n = 19; female: n = 18) 42 or 43 days old were exposed to ferrets for 15 min and were re-exposed 28 days after initial exposure, at ages 70 or 71. A two-way repeated measures ANOVA was performed with gender as the between subjects factor and age (adolescent vs. adult) as the within subjects factor. Significant main effects of both gender (F(1,35) = 13.68, p < 0.001) and age (F(1,35) = 58.09, p < 0.0001) were observed as was a significant gender by age interaction (F(1,35) = 12.98, p < 0.001). The main effects demonstrated that males exhibited more BI than females, and that more BI occurred during adolescence. Post hoc analysis revealed that males exhibited significantly more BI than females in adulthood (t = 5.076, df = 36, p < 0.001; Figure 2A). A correlational analysis revealed that the levels of BI displayed in adolescent male and female rats predicted adult levels (r = 0.475, n = 37, p < 0.01; Figure 2B); a result that remained significant when co-varying for gender (r = 0.43, n = 37, p < 0.01). Similar stability in individual differences from adolescence to adulthood was found in a subsequent study involving 24 male rats assessed during the dark phase of their light dark cycle in which BI was measured twice in adolescence (at 36 and 38 days of age) and twice in adulthood (at 85 and 87 days of age). We found that BI was stable between the two measures taken in adolescence (r = 0.538, n = 24, p < 0.01) and between the two measures taken in adulthood (r = 0.723, n = 24, p < 0.001). Analysis of the average BI scores from adolescence and adulthood also revealed a significant correlation (r = 0.559, n = 24, p < 0.01).
Figure 2. Behavioral inhibition is dimorphic and stable across development.
BI was measured in male (n = 19) and female (n = 18) adolescent rats (age 42 or 43 days) and again in the same rats in adulthood (age 70 or 71 days). (A) Male rats engaged in more BI during adulthood than female rats. Bars represent the mean ± SEM. (B) BI exhibited in adolescent male and female rats is trait-like and maintained across development into adulthood.
Experiment 2: diazepam reduces threat-induced BI
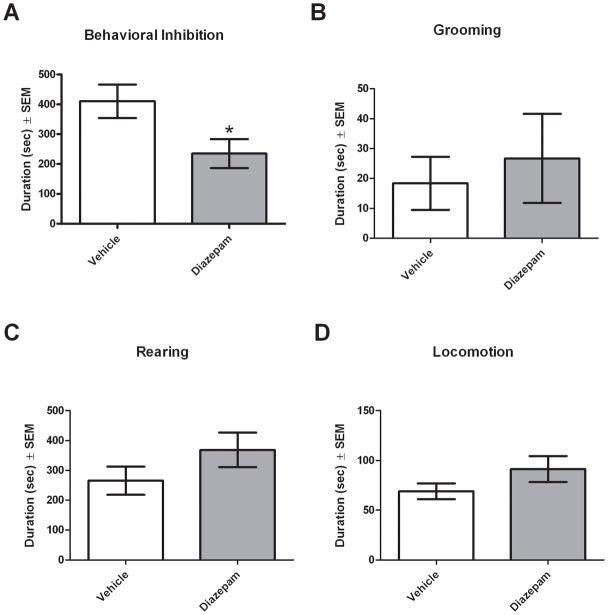
To further validate predator threat-induced BI as a model of anxiety-like behavior, we examined the ability of the anxiolytic diazepam (DZP) to decrease the expression of BI. Adult male rats (n = 8/group) were treated with either vehicle (VEH) or DZP (1 mg/kg) 30 min prior to ferret exposure. Male rats were used because they were found to engage in significantly more BI than female rats in Experiment 1b. As predicted, DZP-treated rats engaged in significantly less BI than VEH-treated rats (U = 13, df = 16, p < 0.05; Figure 3A). The effects of DZP appeared to be selective to BI as no significant differences were observed in the duration of grooming (U = 29, df = 16, p = 0.792; Figure 3B), rearing (U = 20, df = 16, p = 0.235; Figure 3C) or locomotion (U = 24, df = 16, p = 0.442; Figure 3D).
Figure 3. Diazepam administration decreases behavioral inhibition.
Rats were treated with either vehicle or diazepam (1 mg/kg) 30 min prior to ferret exposure. Diazepam-treated animals exhibited significantly less BI than vehicle-treated animals (A). No significant differences in grooming (B), rearing (C) or locomotion (D) between vehicle and diazepam treated groups were observed. Bars represent the mean ± SEM for 8 independent determinations in the vehicle and diazepam treated groups. *, p < 0.05 compared with vehicle.
Experiment 3: Threat-induced hippocampal and PVN activation predicts individual differences in BI
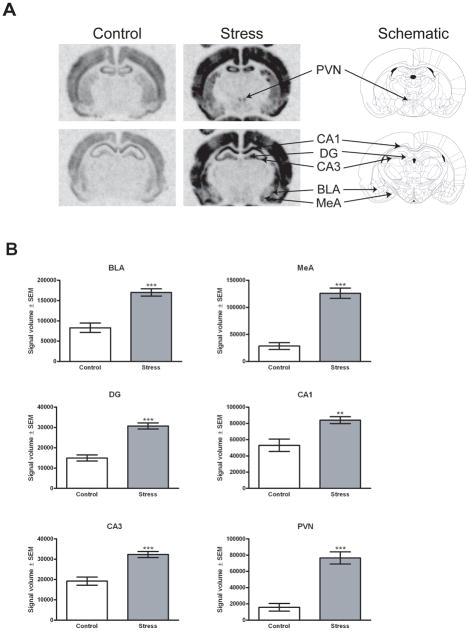
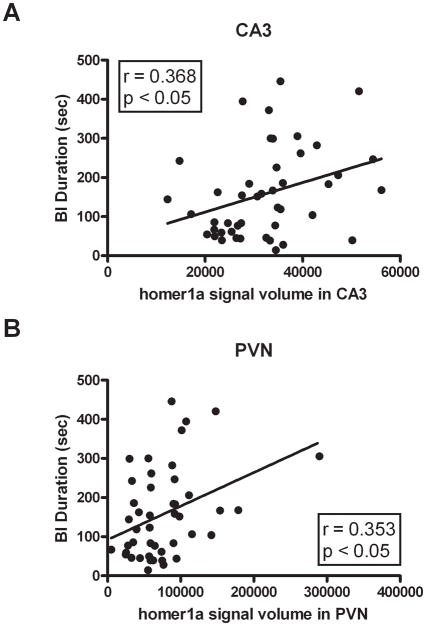
To determine if activation of stress responsive brain regions is predictive of individual differences in BI and anxious temperament, brains from adult male rats sacrificed 1 hr post-ferret exposure (n = 45) were compared to those from rats that remained in their home cage (n = 9) through in situ hybridization analysis of the immediate early genes homer1a and fos. Homer1a mRNA expression was assessed in the amygdala (basolateral amygdala (BLA) and medial amygdala (MeA)), hippocampus (dentate gyrus (DG), CA1 and CA3 regions) and the PVN. Since no homer1a signal was detected in the central amygdala (CeA), this region was not examined. Consistent with data from our previous amygdala microarray studies, predator stress significantly induced homer1a expression in the BLA (U = 32, df = 54, p < 0.0001) and MeA (U = 10, df = 54, p < 0.0001). In addition, significant increases in homer1a mRNA occurred in the DG (U = 32, df = 54, p < 0.0001), CA1 (U = 82, df = 54, p < 0.01), CA3 (U = 57, df = 54, p < 0.001) and PVN (U = 23, df = 54, p < 0.0001; Figure 4B). Furthermore, individual differences in homer1a mRNA expression in the CA3 and PVN were significantly correlated with BI (r = 0.368, n = 45 in the CA3, Figure 5A; r = 0.353, n = 45 in the PVN, p < 0.05 two-tailed, Figure 5B). No significant correlations between homer1a expression and BI were observed in the DG (r = 0.053, n = 45, p = 0.727), BLA (r = 0.217, n = 45, p = 0.168) or MeA (r = 0.184, n = 45, p = 0.227); a trend for a significant correlation was found in the CA1 (r = 0.288, n = 45, p = 0.055). We also assessed the relationship between BI and fos mRNA expression since fos is another immediate early gene that has a different expression pattern. Significant predator stress-induced increases in fos mRNA expression were observed in the BLA (U = 2, df = 54, p < 0.0001), MeA (U = 0, df = 54, p < 0.0001), PVN (U = 3, df = 54, p < 0.0001), CA1 (U = 0, df = 54, p < 0.0001) and CA3 (U = 0, df = 54, p < 0.0001). No significant correlations between fos expression and BI were observed in the BLA (r = 0.219, n = 45, p = 0.149), MeA (r = 0.216, n = 45, p = 0.155), PVN (r = 0.223, n = 45, p = 0.142) and CA1 (r = 0.072, n = 45, p = 0.639), although a trend for a positive correlation existed for the CA3 region (r = 0.259, n = 45, p = 0.086). Similar to homer1a, the lack of a distinct fos mRNA signal within the CeA prevented quantification of this region.
Figure 4. Ferret exposure increases homer1a mRNA levels within the BLA, MeA, DG, CA1, CA3 and PVN.
Forty-five rats were exposed to the ferret and sacrificed 1 h following cessation of exposure. Nine rats served as controls and remained in their home cages until sacrificed. (A) In situ hybridization was used to detect homer1a mRNA in control and stressed rats and the location of the signals for the basolateral amygdala (BLA), medial amygdala (MeA), dentate gyrus (DG), CA1 region of the hippocampus (CA1), CA3 region of the hippocampus (CA3) and paraventricular nucleus of the hypothalamus (PVN) are indicated on the phosphor images from a representative ferret exposed rat and schematic. Schematic images representing Bregma −1.80 mm and Bregma −3.14 mm are adapted from a published atlas (Paxinos & Watson 1998). (B) Ferret exposure significantly increased homer1a expression in the BLA, MeA, DG, CA1, CA3 and PVN. Bars represent the mean quantified signal volume (signal intensity multiplied by ROI area) ± SEM for each group (9 independent determinations for control, 45 independent determinations for stress). **, p < 0.01 compared to control. ***, p < 0.001 compared to control.
Figure 5. homer1a mRNA levels in the CA3 region of the hippocampus and the PVN correlate with levels of behavioral inhibition.
In situ hybridization was used to assess the level of homer1a mRNA in rat brains (n = 45) obtained 1 h following ferret exposure. Regression analyses show that homer1a expression in the CA3 (A) and PVN (B) are significantly correlated with BI.
Experiment 4: Developing criteria to select pathologically extreme rats and demonstrating that they display disrupted appetitive responses
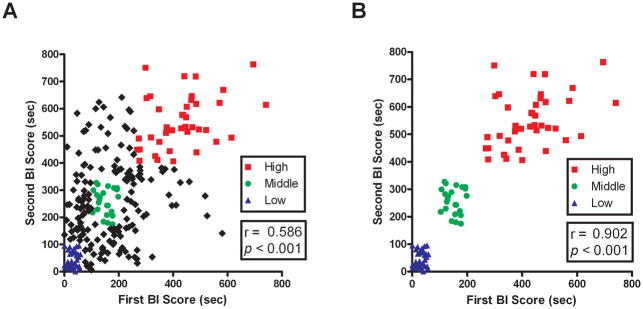
To further develop the BI phenotype as a model of psychopathology, we sought to characterize differences between rats that stably display extremely high or low levels of BI. Since the selection of temperamentally extreme groups of animals requires screening large numbers of individuals, we examined the stability of BI across two ferret exposures separated by 48 hrs in 255 adult male rats. Consistent with earlier findings, BI was stable across the two exposures (r = 0.586, n = 255, p < 0.001; Figure 6A). To identify the animals that displayed extreme and stable levels of BI, the BI durations from each of the 2 tests were ranked by quartiles. Rats in the bottom quartile on both tests were considered to have low and stable levels of BI (LBI), rats consistently in the middle quartiles were considered to have mid-range stable levels of BI (MBI) and rats consistently in the top quartile were considered to have extremely high and stable BI (HBI) (LBI, n = 34; MBI, n = 23; HBI, n = 39). When assessing the stability of the selected LBI, MBI, and HBI groups as expected a strong correlation was found between the first and second tests (r = 0.902, n = 96, p < 0.001; Figure 6B).
Figure 6. The expression of behavioral inhibition is stable trait-like characteristic and rats that express extreme and stable levels of BI can be identified.
(A) Scatter-plot shows the relationship between the level of BI obtained from two exposures separated by 48 hr in a population of rats (n = 255). The duration of BI from the first exposure is significantly correlated to the duration of BI from the second exposure. Rats in the bottom quartile from both tests were labeled LBI (triangles), rats consistently in the middle quartile were labeled MBI (circles) and rats consistently in the top quartile were labeled HBI (squares). (B) Scatter-plot represents only animals in the LBI, MBI and HBI groups. As expected, the correlation of BI between first exposure and second exposure markedly increases when animals are selected for stability.
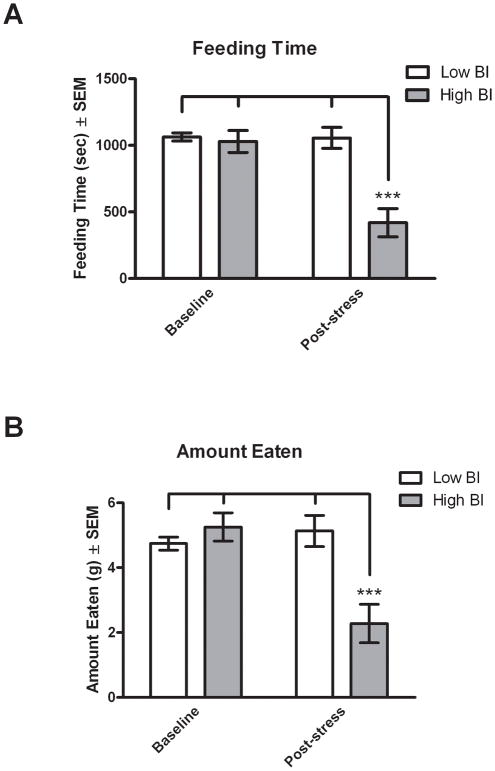
To determine if animals with extreme levels of threat-induced BI have altered appetitive behaviors as an indicator of additional maladaptive responding, HBI and LBI rats were identified and tested in a feeding paradigm. Rats were selected as being extreme and stable using the quartile method described above. Of the sixty rats that were screened, 8 met criteria for LBI (13% of population) and 7 for HBI (12% of population). As a baseline, HBI and LBI rats were food deprived for 17 hrs and were tested for feeding behavior over a 30 min period when they had access to chow in a non-threatening environment (Baseline). The rats were again food deprived and on the next day were exposed to 15 min of direct ferret stress as described in the methods section which was immediately followed by the feeding test occurring in the same non-threatening environment in which the baseline feeding test was performed. A two-way repeated measures ANOVA with temperament (HBI vs. LBI) as the between subjects factor and testing session (baseline vs. post-stress) as the within subjects factor revealed a significant difference in total feeding time between baseline and post-stress testing (F(1,13) = 27.00, p < 0.001) and between LBI and HBI temperaments (F(1,13) = 13.20, p < 0.01; Figure 7A). Importantly, a significant stress by temperament interaction was also observed (F(1,13) = 25.78, p < 0.001). There were no significant differences in feeding between HBI and LBI animals in the baseline condition, but when tested after threat exposure; there was a significant reduction in feeding in the HBI compared to LBI animals. Compared to LBI animals, the HBI animals spent 60% less time feeding after predator exposure (t = 5.803, df = 14, p < 0.001). Similar results were obtained when analyzing the total amount of food eaten, which revealed significant main effects of stress (F(1,13) = 16.26, p < 0.01) and temperament (F(1,13) = 4.726, p < 0.05; Figure 7B) as well as a significant stress by temperament interaction (F(1,13) = 27.49, p < 0.001). HBI animals ate 56% less food post-stress compared to LBI animals (t = 4.552, df = 14, p < 0.001). These findings demonstrate that prior stress exposure differentially affects HBI and LBI rats such that after the offset of the stressor, the HBI rats display a deficit in adaptive appetitive behaviors.
Figure 7. Ferret exposure induces feeding reductions in HBI rats.
Food deprived extreme and stable (HBI and LBI) rats were tested for feeding behavior in a non-threatening environment immediately following ferret exposure. At baseline, no difference was observed between HBI and LBI in both feeding time (A) and amount of food eaten (B). However, following ferret stress, HBI rats spent 60% less time feeding (A) and ate 56% less food than LBI rats (B). Bars represent mean ± SEM. ***, p < 0.001 compared to LBI post-stress.
Discussion
Understanding the molecular mechanisms underlying anxiety-related psychopathology through the use of an ethologically relevant rodent model is crucial for identifying novel drug targets and developing new pharmacological treatments. Our previous rodent work (Nanda et al. 2008; Roseboom et al. 2007) used ferret exposure, an ethologically relevant threat, to study stress-induced behavioral responses and changes in gene expression. In the present studies, we extend this work by establishing a rodent model of BI temperament analogous to human and non-human primate BI. Importantly, we emphasize the selection of stable and extreme animals as at-risk individuals, consistent with what is observed in human children (Biederman et al. 2001).
Because BI in humans is assessed in childhood, we aimed to establish a developmental model to allow for the identification of individuals that will be susceptible to the deleterious effects of stress. Our findings demonstrate that, as in monkeys and humans, rat BI is present early in life and is trait-like. Specifically, we found that BI is stable when repeatedly tested in adolescence and in adulthood; and that the level of BI expressed by an individual in adolescence is predictive of the level of BI in adulthood. To further establish that rodent BI reflects anxiety, we demonstrate that diazepam selectively decreases BI without affecting other behaviors such as rearing, grooming and locomotion. This finding is consistent with other studies using benzodiazepines to validate rodent models of anxiety (Duzzioni et al. 2008; Jochum et al. 2007; Nicolas & Prinssen 2006; Wilson et al. 2004). Additionally, it is noteworthy that we observed an effect of diazepam on BI levels in an unselected population. Given that previous studies show no effect of diazepam in tests of anxiety-like responding in non-anxious animals (do-Rego et al. 2006), it is likely that we would find an even greater effect of diazepam in a population that is enriched with HBI rats.
Human and non-human primate studies have demonstrated involvement of a network of brain systems in mediating anxiety, fear and associated psychopathology (Charney 2003; Kalin et al. 2004; Kalin et al. 2005; McEwen 2007). We assessed the activity of brain regions using the expression of the immediate early genes homer1a and fos. These genes were chosen as they are highly-inducible in response to increases in neuronal activity, with each gene showing somewhat different patterns of activation. Additionally, we have shown that expression of both these genes are induced by our predator stress paradigm (Nanda et al. 2008; Roseboom et al. 2007). We chose to measure mRNA expression by in situ hybridization over immunodetection of protein as induction of mRNA transcription is more proximal to increases in neuronal activity, and therefore may more accurately reflect alterations in cellular activity. In this study, we found similarities between brain regions associated with BI in rats with regions identified in our non-human primate imaging studies (Fox et al. 2008). Using homer1a mRNA expression, we determined individual differences in hippocampal CA3 region activity were predictive of individual differences in BI. Given the importance of the hippocampus in contextual fear conditioning and in memory consolidation and retrieval, it is logical that hippocampal activity would play an important role in regulating the expression of BI (Davidson et al. 2000; Kalin & Shelton 2003; McEwen 2007). Similar to CA3, there was a correlation between CA1 region homer1a expression and BI that approached statistical significance. As both the CA3 and CA1 are activated during acute stress and functionally respond to stress in similar ways (Joels 2009; McEwen 1999), it is not surprising that they exhibit similar activation relative to BI.
We also found a positive correlation between PVN activity and BI. Involvement of the PVN in BI is expected, as BI is associated with increased HPA activity that has been described as a component of anxious temperament in humans (Schmidt et al. 1997). Additionally, rodent studies have also suggested a relationship between HPA axis activity with trait-like anxiety (Landgraf et al. 1999; Veenema et al. 2007). Furthermore, the PVN receives direct and indirect inputs from the hippocampus and amygdala and has a central role in orchestrating the physiological response to stress (Herman et al. 2005; Jankord & Herman 2008).
The lack of a significant correlation between BLA and/or MeA homer1a expression with BI was unexpected given the role of the amygdala in human and non-human primate BI (Fox et al. 2008; Schwartz et al. 2003). Moreover, numerous studies using predator odor as a stimulus have shown that both BLA and MeA play roles in the unconditioned response to predator threat (Blanchard et al. 2005; Li et al. 2004; Muller & Fendt 2006; Takahashi et al. 2005). Lack of a positive finding should be interpreted with caution since only one time point was examined in this study, and other time points may have revealed a significant relation between amygdala activation and BI expression.
We also examined the expression of the immediate early gene fos as an additional marker of neuronal activation (Herrera & Robertson 1996). Similar to previous studies examining the effects of predator or predator odor on fos expression in rats (Campeau et al. 2008; Day et al. 2004; Dielenberg et al. 2001; Masini et al. 2005), we observed significant stress-induced increases in fos expression. However, we did not see significant correlations between BI and fos expression. This lack of finding supports the use of more than one immediate early gene in these types of studies, since different patterns of gene expression often occur in different neuronal populations.
While BI expressed in the presence of a predator is adaptive, excessive BI or altered behavior occurring in the absence of a threatening context is likely a sign of maladaptive emotional responding (Rotenberg & Boucsein 1993). In addition to characterizing the behavioral consequences and neural systems associated with rodent BI, we used our model to identify pathological at-risk individuals. To examine maladaptive pathological responding in relation to our model, we developed criteria to select animals that stably expressed extreme HBI or extreme LBI. To be categorized into the extreme groups, individuals had to stably display levels of BI that were in the upper or lower quartile when tested on two separate occasions. Using these criteria, we found a 12% prevalence of HBI in rats, which is similar to the prevalence of extreme BI reported in human children (Kagan et al. 1988). We then demonstrated that HBI, but not LBI rats, fail to engage in appetitive behaviors when tested in a non-threatening environment following stress exposure. This finding is consistent with human stress-related psychopathology in which disruptions of appetitive behavior is common (Lo Sauro et al. 2008; O’Brien & Vincent 2003). It is of interest that data from our laboratory and others suggest that an overactive corticotropin releasing factor system may mediate stress-induced decreases in appetitive drives (Bakshi et al. 2002; Gosnell et al. 1983; Jochman et al. 2005; Krahn et al. 1986; Negri et al. 1985) and may be important in the pathophysiology of anxiety and depressive disorders (Koob & Heinrichs 1999; Vale et al. 1981). Given the importance of mesolimbic dopamine neurotransmission in regulating appetitive behaviors (Bello & Hajnal 2010; Berridge 2009), it is also possible that alterations in this system may underlie the differential effects of stress on feeding behavior observed in HBI and LBI rodents.
Previous studies establishing rodent models of psychopathology have used rats or mice that are selectively bred for anxiety-associated traits (Liebsch et al. 1998; Touma et al. 2008). Similar to high-anxiety-bred (HAB) and low-anxiety-bred (LAB) rodent models (Liebsch et al. 1998; Muigg et al. 2009), our data suggest HBI animals have impaired coping mechanisms and greater brain reactivity to stressful or novel situations. Other data from our laboratory demonstrates that animals with high levels of BI tend to display increased anxiety-related responses in the elevated plus maze test (unpublished data), and this is consistent with HAB rats (Liebsch et al. 1998). Although selective breeding studies provide insights into the pathophysiology of the anxious temperament (Knapman et al. 2009; Muigg et al. 2009; Touma et al. 2008), this approach differs significantly from our studies which focus on unselected and unmanipulated populations to identify animals that display extreme and stable BI. Furthermore, in relation to rodent anxiety, few studies have examined the issue of stability of BI expression over time (Cavigelli et al. 2007).
In summary, the present studies establish that rodent BI has many similarities to human and non-human primate BI. In the rodent model, BI is evident early in life, is trait-like and responds to anxiolytic treatment. Furthermore, individuals with extreme and stable high BI demonstrate other maladaptive responses. In addition, these studies implicate the PVN and hippocampus in modulating the expression of BI. Taken together, these studies form a solid foundation for further work aimed at identifying the molecular mechanisms that underlie the relationship between BI and stress-induced psychopathology. Moreover, the rodent model can identify novel systems that may serve as both markers for identifying at-risk individuals and as targets for new pharmacological treatments aimed at either preventing or ameliorating stress-induced psychopathology.
Acknowledgments
This work was funded by NIH Grants MH40855 and MH43454, the University of Wisconsin HealthEmotions Research Institute and Meriter Hospital (Madison, WI).
References
- Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. J Neurosci. 2002;22:2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello NT, Hajnal A. Dopamine and binge eating behaviors. Pharmacol Biochem Behav. 2010 doi: 10.1016/j.pbb.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav. 2009;97:537–550. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Hirshfeld-Becker DR, Rosenbaum JF, Herot C, Friedman D, Snidman N, Kagan J, Faraone SV. Further evidence of association between behavioral inhibition and social anxiety in children. Am J Psychiatry. 2001;158:1673–1679. doi: 10.1176/appi.ajp.158.10.1673. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: effects on responsivity to a Cat, Cat odor, and nonpredator threat. Neurosci Biobehav Rev. 2005;29:1243–1253. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Nikulina JN, Sakai RR, McKittrick C, McEwen B, Blanchard DC. Behavioral and endocrine change following chronic predatory stress. Physiol Behav. 1998;63:561–569. doi: 10.1016/s0031-9384(97)00508-8. [DOI] [PubMed] [Google Scholar]
- Campeau S, Nyhuis TJ, Sasse SK, Day HE, Masini CV. Acute and chronic effects of ferret odor exposure in Sprague-Dawley rats. Neurosci Biobehav Rev. 2008;32:1277–1286. doi: 10.1016/j.neubiorev.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Henry B, McGee RO, Moffitt TE, Silva PA. Temperamental origins of child and adolescent behavior problems: from age three to age fifteen. Child Dev. 1995;66:55–68. doi: 10.1111/j.1467-8624.1995.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, Silva PA. Temperamental qualities at age three predict personality traits in young adulthood: longitudinal evidence from a birth cohort. Child Dev. 1995;66:486–498. doi: 10.1111/j.1467-8624.1995.tb00885.x. [DOI] [PubMed] [Google Scholar]
- Cavigelli SA, Stine MM, Kovacsics C, Jefferson A, Diep MN, Barrett CE. Behavioral inhibition and glucocorticoid dynamics in a rodent model. Physiol Behav. 2007;92:897–905. doi: 10.1016/j.physbeh.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS. Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatr Scand Suppl. 2003:38–50. doi: 10.1034/j.1600-0447.108.s417.3.x. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol Bull. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Day HE, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025:139–151. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- de Bartolomeis A, Iasevoli F. The Homer family and the signal transduction system at glutamatergic postsynaptic density: potential role in behavior and pharmacotherapy. Psychopharmacol Bull. 2003;37:51–83. [PubMed] [Google Scholar]
- Dielenberg RA, Hunt GE, McGregor IS. “When a rat smells a cat”: the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001;104:1085–1097. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- do-Rego JC, Viana AF, Le Maitre E, Deniel A, Rates SM, Leroux-Nicollet I, Costentin J. Comparisons between anxiety tests for selection of anxious and non anxious mice. Behav Brain Res. 2006;169:282–288. doi: 10.1016/j.bbr.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Duncan RS, Hwang SY, Koulen P. Effects of Vesl/Homer proteins on intracellular signaling. Exp Biol Med (Maywood) 2005;230:527–535. doi: 10.1177/153537020523000803. [DOI] [PubMed] [Google Scholar]
- Duzzioni M, Calixto AV, Duarte FS, De Lima TC. Modulation of anxiety in rats evaluated in the elevated T-maze: evidence of the relationship between substance P and diazepam. Behav Brain Res. 2008;187:140–145. doi: 10.1016/j.bbr.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Ehrengruber MU, Kato A, Inokuchi K, Hennou S. Homer/Vesl proteins and their roles in CNS neurons. Mol Neurobiol. 2004;29:213–227. doi: 10.1385/MN:29:3:213. [DOI] [PubMed] [Google Scholar]
- File SE, Zangrossi H, Jr, Sanders FL, Mabbutt PS. Dissociation between behavioral and corticosterone responses on repeated exposures to cat odor. Physiol Behav. 1993;54:1109–1111. doi: 10.1016/0031-9384(93)90333-b. [DOI] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-like brain activity during adolescence predicts anxious temperament in primates. PloS one. 2008;3:e2570. doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu Rev Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Gladstone GL, Parker GB, Mitchell PB, Wilhelm KA, Malhi GS. Relationship between self-reported childhood behavioral inhibition and lifetime anxiety disorders in a clinical sample. Depress Anxiety. 2005;22:103–113. doi: 10.1002/da.20082. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Lemery KS. Linking temperamental fearfulness and anxiety symptoms: a behavior-genetic perspective. Biol Psychiatry. 2000;48:1199–1209. doi: 10.1016/s0006-3223(00)01003-9. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Morley JE, Levine AS. A comparison of the effects of corticotropin releasing factor and sauvagine on food intake. Pharmacol Biochem Behav. 1983;19:771–775. doi: 10.1016/0091-3057(83)90078-3. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Robertson HA. Activation of c-fos in the brain. Prog Neurobiol. 1996;50:83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Herringa RJ, Nanda SA, Hsu DT, Roseboom PH, Kalin NH. The effects of acute stress on the regulation of central and basolateral amygdala CRF-binding protein gene expression. Brain Res Mol Brain Res. 2004;131:17–25. doi: 10.1016/j.molbrainres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Prescott CA, Myers JM, Neale MC, Kendler KS. The structure of genetic and environmental risk factors for anxiety disorders in men and women. Arch Gen Psychiatry. 2005;62:182–189. doi: 10.1001/archpsyc.62.2.182. [DOI] [PubMed] [Google Scholar]
- Hirshfeld DR, Rosenbaum JF, Biederman J, Bolduc EA, Faraone SV, Snidman N, Reznick JS, Kagan J. Stable behavioral inhibition and its association with anxiety disorder. J Am Acad Child Adolesc Psychiatry. 1992;31:103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Lombardo KA, Herringa RJ, Bakshi VP, Roseboom PH, Kalin NH. Corticotropin-releasing hormone messenger RNA distribution and stress-induced activation in the thalamus. Neuroscience. 2001;105:911–921. doi: 10.1016/s0306-4522(01)00239-1. [DOI] [PubMed] [Google Scholar]
- Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochman KA, Newman SM, Kalin NH, Bakshi VP. Corticotropin-releasing factor-1 receptors in the basolateral amygdala mediate stress-induced anorexia. Behav Neurosci. 2005;119:1448–1458. doi: 10.1037/0735-7044.119.6.1448. [DOI] [PubMed] [Google Scholar]
- Jochum T, Boettger MK, Wigger A, Beiderbeck D, Neumann ID, Landgraf R, Sauer H, Bar KJ. Decreased sensitivity to thermal pain in rats bred for high anxiety-related behaviour is attenuated by citalopram or diazepam treatment. Behav Brain Res. 2007;183:18–24. doi: 10.1016/j.bbr.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Joels M. Stress, the hippocampus, and epilepsy. Epilepsia. 2009;50:586–597. doi: 10.1111/j.1528-1167.2008.01902.x. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Ann N Y Acad Sci. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol Psychiatry. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapman A, Heinzmann JM, Hellweg R, Holsboer F, Landgraf R, Touma C. Increased stress reactivity is associated with cognitive deficits and decreased hippocampal brain-derived neurotrophic factor in a mouse model of affective disorders. J Psychiatr Res. 2009 doi: 10.1016/j.jpsychires.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Krahn DD, Gosnell BA, Grace M, Levine AS. CRF antagonist partially reverses CRF- and stress-induced effects on feeding. Brain Res Bull. 1986;17:285–289. doi: 10.1016/0361-9230(86)90233-9. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Wigger A, Holsboer F, Neumann ID. Hyper-reactive hypothalamo-pituitary-adrenocortical axis in rats bred for high anxiety-related behaviour. J Neuroendocrinol. 1999;11:405–407. doi: 10.1046/j.1365-2826.1999.00342.x. [DOI] [PubMed] [Google Scholar]
- Li CI, Maglinao TL, Takahashi LK. Medial amygdala modulation of predator odor-induced unconditioned fear in the rat. Behav Neurosci. 2004;118:324–332. doi: 10.1037/0735-7044.118.2.324. [DOI] [PubMed] [Google Scholar]
- Liebsch G, Montkowski A, Holsboer F, Landgraf R. Behavioural profiles of two Wistar rat lines selectively bred for high or low anxiety-related behaviour. Behav Brain Res. 1998;94:301–310. doi: 10.1016/s0166-4328(97)00198-8. [DOI] [PubMed] [Google Scholar]
- Lo Sauro C, Ravaldi C, Cabras PL, Faravelli C, Ricca V. Stress, hypothalamic-pituitary-adrenal axis and eating disorders. Neuropsychobiology. 2008;57:95–115. doi: 10.1159/000138912. [DOI] [PubMed] [Google Scholar]
- Masini CV, Sauer S, Campeau S. Ferret odor as a processive stress model in rats: neurochemical, behavioral, and endocrine evidence. Behav Neurosci. 2005;119:280–292. doi: 10.1037/0735-7044.119.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini CV, Sauer S, White J, Day HE, Campeau S. Non-associative defensive responses of rats to ferret odor. Physiol Behav. 2006;87:72–81. doi: 10.1016/j.physbeh.2005.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Schrama L, Ambermoon P, Dielenberg RA. Not all ‘predator odours’ are equal: cat odour but not 2,4,5 trimethylthiazoline (TMT; fox odour) elicits specific defensive behaviours in rats. Behav Brain Res. 2002;129:1–16. doi: 10.1016/s0166-4328(01)00324-2. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Redmond AJ, Roth RH, Elsworth JD. The predator odor, TMT, displays a unique, stress-like pattern of dopaminergic and endocrinological activation in the rat. Brain Res. 2000;864:146–151. doi: 10.1016/s0006-8993(00)02174-0. [DOI] [PubMed] [Google Scholar]
- Muigg P, Scheiber S, Salchner P, Bunck M, Landgraf R, Singewald N. Differential stress-induced neuronal activation patterns in mouse lines selectively bred for high, normal or low anxiety. PloS one. 2009;4:e5346. doi: 10.1371/journal.pone.0005346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Fendt M. Temporary inactivation of the medial and basolateral amygdala differentially affects TMT-induced fear behavior in rats. Behav Brain Res. 2006;167:57–62. doi: 10.1016/j.bbr.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Nanda SA, Qi C, Roseboom PH, Kalin NH. Predator stress induces behavioral inhibition and amygdala somatostatin receptor 2 gene expression. Genes Brain Behav. 2008;7:639–648. doi: 10.1111/j.1601-183X.2008.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri L, Noviello L, Noviello V. Effects of sauvagine, urotensin I and CRF on food intake in rats. Peptides. 1985;6(Suppl 3):53–57. doi: 10.1016/0196-9781(85)90350-x. [DOI] [PubMed] [Google Scholar]
- Nicolas LB, Prinssen EP. Social approach-avoidance behavior of a high-anxiety strain of rats: effects of benzodiazepine receptor ligands. Psychopharmacology (Berl) 2006;184:65–74. doi: 10.1007/s00213-005-0233-y. [DOI] [PubMed] [Google Scholar]
- O’Brien KM, Vincent NK. Psychiatric comorbidity in anorexia and bulimia nervosa: nature, prevalence, and causal relationships. Clin Psychol Rev. 2003;23:57–74. doi: 10.1016/s0272-7358(02)00201-5. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Perrot-Sinal TS, Ossenkopp KP, Kavaliers M. Brief predator odour exposure activates the HPA axis independent of locomotor changes. Neuroreport. 1999;10:775–780. doi: 10.1097/00001756-199903170-00021. [DOI] [PubMed] [Google Scholar]
- Porges SW. Social engagement and attachment: a phylogenetic perspective. Ann N Y Acad Sci. 2003;1008:31–47. doi: 10.1196/annals.1301.004. [DOI] [PubMed] [Google Scholar]
- Roseboom PH, Nanda SA, Bakshi VP, Trentani A, Newman SM, Kalin NH. Predator threat induces behavioral inhibition, pituitary-adrenal activation and changes in amygdala CRF-binding protein gene expression. Psychoneuroendocrinology. 2007;32:44–55. doi: 10.1016/j.psyneuen.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg VS, Boucsein W. Adaptive versus maladaptive emotional tension. Genet Soc Gen Psychol Monogr. 1993;119:209–232. [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Rubin KH, Sternberg EM, Gold PW, Smith CC, Schulkin J. Behavioral and neuroendocrine responses in shy children. Dev Psychobiol. 1997;30:127–140. doi: 10.1002/(sici)1098-2302(199703)30:2<127::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Stein MB, Fuetsch M, Muller N, Hofler M, Lieb R, Wittchen HU. Social anxiety disorder and the risk of depression: a prospective community study of adolescents and young adults. Arch Gen Psychiatry. 2001;58:251–256. doi: 10.1001/archpsyc.58.3.251. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Kalivas PW, Worley PF. Homer proteins: implications for neuropsychiatric disorders. Curr Opin Neurobiol. 2006;16:251–257. doi: 10.1016/j.conb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Kleschen MJ, Oleson EB, Dehoff MH, Schwarz MK, Seeburg PH, Worley PF, Kalivas PW. Behavioral and neurochemical phenotyping of Homer1 mutant mice: possible relevance to schizophrenia. Genes Brain Behav. 2005;4:273–288. doi: 10.1111/j.1601-183X.2005.00120.x. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Nakashima BR, Hong H, Watanabe K. The smell of danger: a behavioral and neural analysis of predator odor-induced fear. Neurosci Biobehav Rev. 2005;29:1157–1167. doi: 10.1016/j.neubiorev.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Touma C, Bunck M, Glasl L, Nussbaumer M, Palme R, Stein H, Wolferstatter M, Zeh R, Zimbelmann M, Holsboer F, Landgraf R. Mice selected for high versus low stress reactivity: a new animal model for affective disorders. Psychoneuroendocrinology. 2008;33:839–862. doi: 10.1016/j.psyneuen.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Torner L, Blume A, Beiderbeck DI, Neumann ID. Low inborn anxiety correlates with high intermale aggression: link to ACTH response and neuronal activation of the hypothalamic paraventricular nucleus. Horm Behav. 2007;51:11–19. doi: 10.1016/j.yhbeh.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Burghardt PR, Ford KA, Wilkinson MB, Primeaux SD. Anxiolytic effects of diazepam and ethanol in two behavioral models: comparison of males and females. Pharmacol Biochem Behav. 2004;78:445–458. doi: 10.1016/j.pbb.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, Worley PF. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Wittchen HU, Hofler M, Pfister H, Kessler RC, Lieb R. Primary anxiety disorders and the development of subsequent alcohol use disorders: a 4-year community study of adolescents and young adults. Psychol Med. 2003;33:1211–1222. doi: 10.1017/s0033291703008158. [DOI] [PubMed] [Google Scholar]