Abstract
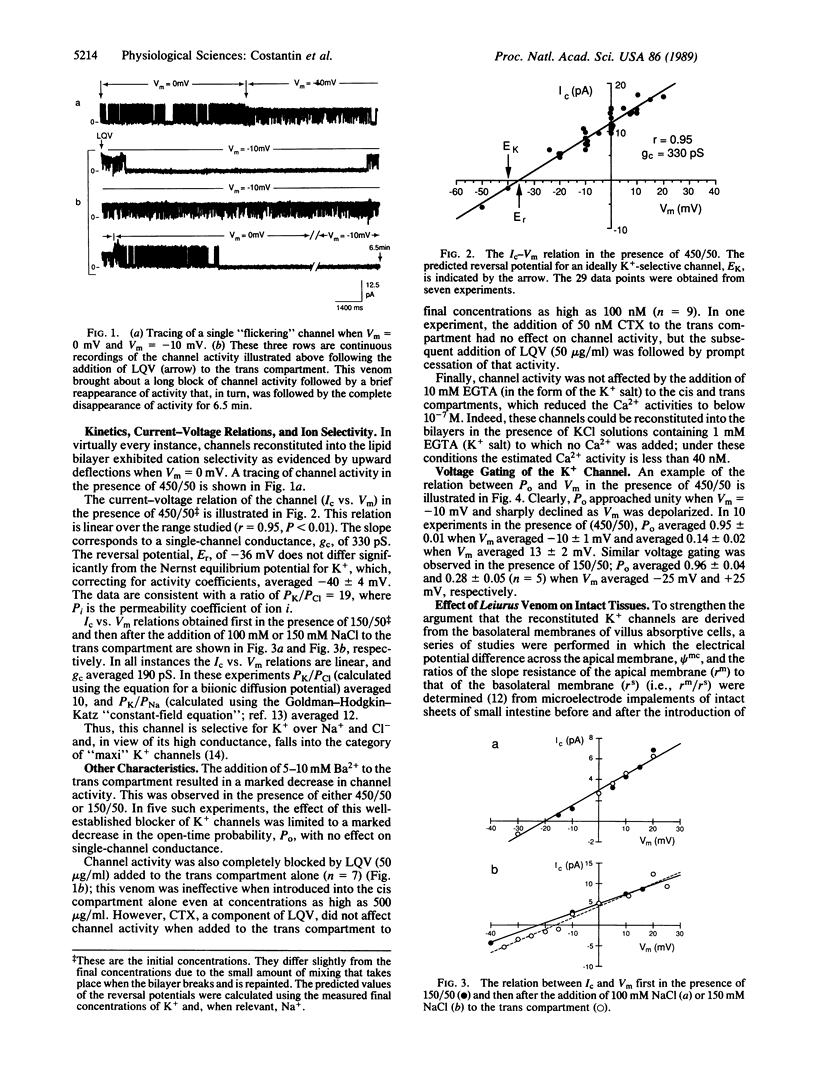
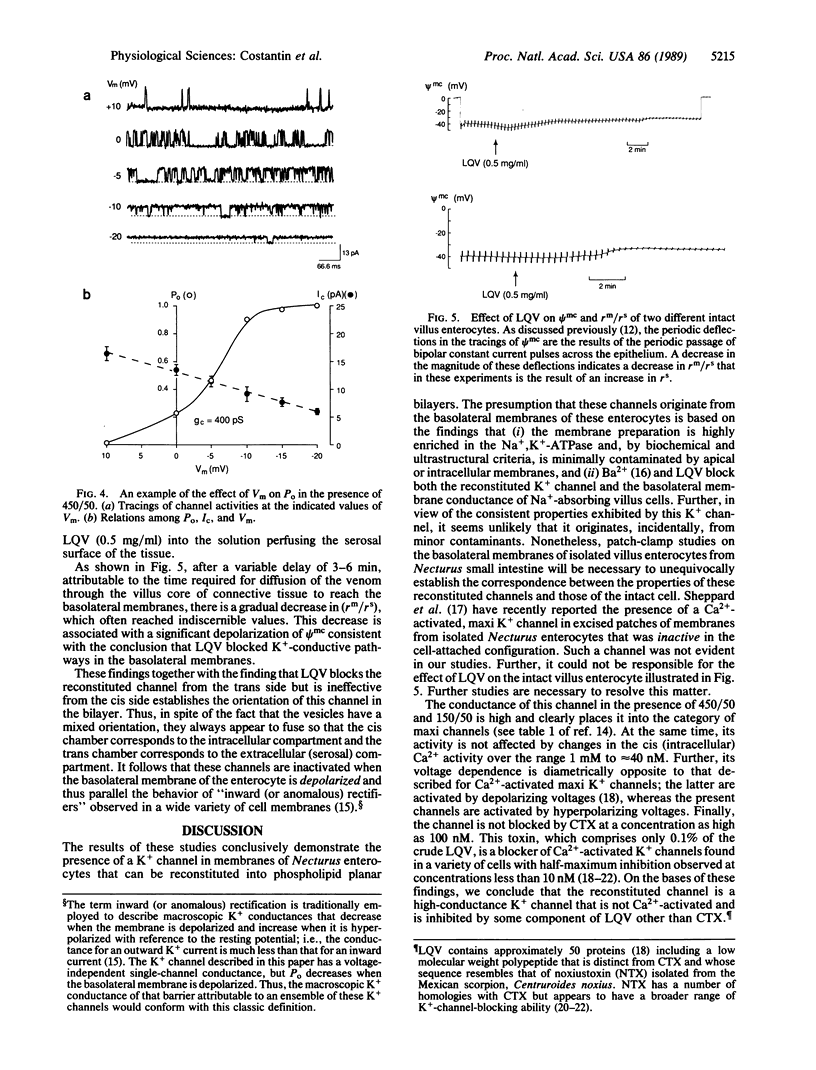
Basolateral membrane vesicles from Necturus enterocytes, highly (greater than 20-fold) enriched in Na+,K+-ATPase, were reconstituted into planar lipid bilayers. The principal channel activity observed is selective for K+ over Na+ and Cl-. This K+ channel is blocked by Ba2+ and Leiurus quinquestriatus venom but is not affected by Ca2+ over the range of 10(-3) to less than 10(-7) M and is not inhibited by charybdotoxin. L. quinquestriatus venom also markedly reduces the conductance of the basolateral membrane of intact villus cells of Necturus small intestine. The open-time probability (Po) of the channel displays a voltage-dependence characteristic of an "inward rectifier"; i.e., the channel inactivates when the basolateral membrane is depolarized and Po increases with increasing hyperpolarization of that barrier. Assuming that similar properties prevail under physiological conditions, this characteristic could provide, in part, an explanation for the parallelism between Na+-pump and K+-leak activities of the basolateral membrane observed in this epithelium. Thus, an increase in rheogenic Na+-pump activity at the basolateral membrane would hyperpolarize that barrier and, in turn, increase the open time of this K+ channel.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. S., MacKinnon R., Smith C., Miller C. Charybdotoxin block of single Ca2+-activated K+ channels. Effects of channel gating, voltage, and ionic strength. J Gen Physiol. 1988 Mar;91(3):317–333. doi: 10.1085/jgp.91.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumendil-Podevin E. F., Podevin R. A. Isolation of basolateral and brush-border membranes from the rabbit kidney cortex. Vesicle integrity and membrane sidedness of the basolateral fraction. Biochim Biophys Acta. 1983 Oct 26;735(1):86–94. doi: 10.1016/0005-2736(83)90263-8. [DOI] [PubMed] [Google Scholar]
- Dubinsky W. P., Monti L. B. Resolution of apical from basolateral membrane of shark rectal gland. Am J Physiol. 1986 Nov;251(5 Pt 1):C721–C726. doi: 10.1152/ajpcell.1986.251.5.C721. [DOI] [PubMed] [Google Scholar]
- Gimenez-Gallego G., Navia M. A., Reuben J. P., Katz G. M., Kaczorowski G. J., Garcia M. L. Purification, sequence, and model structure of charybdotoxin, a potent selective inhibitor of calcium-activated potassium channels. Proc Natl Acad Sci U S A. 1988 May;85(10):3329–3333. doi: 10.1073/pnas.85.10.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasset E., Gunter-Smith P., Schultz S. G. Effects of Na-coupled alanine transport on intracellular K activities and the K conductance of the basolateral membranes of Necturus small intestine. J Membr Biol. 1983;71(1-2):89–94. doi: 10.1007/BF01870677. [DOI] [PubMed] [Google Scholar]
- Gunter-Smith P. J., Grasset E., Schultz S. G. Sodium-coupled amino acid and sugar transport by Necturus small intestine. An equivalent electrical circuit analysis of a rheogenic co-transport system. J Membr Biol. 1982;66(1):25–39. doi: 10.1007/BF01868479. [DOI] [PubMed] [Google Scholar]
- Horisberger J. D., Giebisch G. Voltage dependence of the basolateral membrane conductance in the Amphiuma collecting tubule. J Membr Biol. 1988 Nov;105(3):257–263. doi: 10.1007/BF01871002. [DOI] [PubMed] [Google Scholar]
- Hunter M., Kawahara K., Giebisch G. Potassium channels along the nephron. Fed Proc. 1986 Nov;45(12):2723–2726. [PubMed] [Google Scholar]
- Kawahara K., Hunter M., Giebisch G. Potassium channels in Necturus proximal tubule. Am J Physiol. 1987 Sep;253(3 Pt 2):F488–F494. doi: 10.1152/ajprenal.1987.253.3.F488. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lang F., Messner G., Rehwald W. Electrophysiology of sodium-coupled transport in proximal renal tubules. Am J Physiol. 1986 Jun;250(6 Pt 2):F953–F962. doi: 10.1152/ajprenal.1986.250.6.F953. [DOI] [PubMed] [Google Scholar]
- Langridge-Smith J. E., Field M., Dubinsky W. P. Isolation of transporting plasma membrane vesicles from bovine tracheal epithelium. Biochim Biophys Acta. 1983 Jun 10;731(2):318–328. doi: 10.1016/0005-2736(83)90024-x. [DOI] [PubMed] [Google Scholar]
- Lapointe J. Y., Hudson R. L., Schultz S. G. Current-voltage relations of sodium-coupled sugar transport across the apical membrane of Necturus small intestine. J Membr Biol. 1986;93(3):205–219. doi: 10.1007/BF01871175. [DOI] [PubMed] [Google Scholar]
- Latorre R., Miller C. Conduction and selectivity in potassium channels. J Membr Biol. 1983;71(1-2):11–30. doi: 10.1007/BF01870671. [DOI] [PubMed] [Google Scholar]
- Lau K. R., Hudson R. L., Schultz S. G. Cell swelling increases a barium-inhibitable potassium conductance in the basolateral membrane of Necturus small intestine. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3591–3594. doi: 10.1073/pnas.81.11.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczydlowski E., Lucchesi K., Ravindran A. An emerging pharmacology of peptide toxins targeted against potassium channels. J Membr Biol. 1988 Oct;105(2):95–111. doi: 10.1007/BF02009164. [DOI] [PubMed] [Google Scholar]
- Nagel W. Basolateral membrane ionic conductance in frog skin. Pflugers Arch. 1985;405 (Suppl 1):S39–S43. doi: 10.1007/BF00581778. [DOI] [PubMed] [Google Scholar]
- Nelson B. D. Rat liver acid phosphatase: differences in lysosomal and cytoplasmic forms. Proc Soc Exp Biol Med. 1966 Apr;121(4):998–1001. doi: 10.3181/00379727-121-30947. [DOI] [PubMed] [Google Scholar]
- Sackin H., Palmer L. G. Basolateral potassium channels in renal proximal tubule. Am J Physiol. 1987 Sep;253(3 Pt 2):F476–F487. doi: 10.1152/ajprenal.1987.253.3.F476. [DOI] [PubMed] [Google Scholar]
- Schultz S. G. Homocellular regulatory mechanisms in sodium-transporting epithelia: avoidance of extinction by "flush-through". Am J Physiol. 1981 Dec;241(6):F579–F590. doi: 10.1152/ajprenal.1981.241.6.F579. [DOI] [PubMed] [Google Scholar]
- Sheppard D. N., Giraldez F., Sepúlveda F. V. Kinetics of voltage- and Ca2+ activation and Ba2+ blockade of a large-conductance K+ channel from Necturus enterocytes. J Membr Biol. 1988 Oct;105(1):65–75. doi: 10.1007/BF01871107. [DOI] [PubMed] [Google Scholar]
- Valdivia H. H., Smith J. S., Martin B. M., Coronado R., Possani L. D. Charybdotoxin and noxiustoxin, two homologous peptide inhibitors of the K+ (Ca2+) channel. FEBS Lett. 1988 Jan 4;226(2):280–284. doi: 10.1016/0014-5793(88)81439-x. [DOI] [PubMed] [Google Scholar]


