Abstract
Learned helplessness in animals has been used to model disorders such as depression and post-traumatic stress disorder (PTSD), but there is a lack of knowledge concerning which individual behavioral characteristics at baseline can predict helpless behavior after exposure to inescapable stress. The first aim of this study was to determine behavioral predictors of helplessness using the novel and familiar open-field tests, sucrose consumption, and passive harm-avoidance tasks before learned helplessness training and testing. Individual differences in physiologic responses to restraint stress were also assessed. A cluster analysis of escape latencies from helplessness testing supported the division of the sample population of Holtzman rats into approximately 50% helpless and 50% non-helpless. Linear regression analyses further revealed that increased reactivity to the novel environment, but not general activity or habituation, predicted susceptibility to learned helplessness. During restraint stress there were no mean differences in heart rate, heart rate variability, and plasma corticosterone between helpless and non-helpless rats; however, a lower heart rate during stress was associated with higher activity levels during exploration. Our most important finding was that by using an innocuous screening tool such as the novel and familiar open-field tests, it was possible to identify subjects that were susceptible to learned helplessness.
Keywords: Novelty, open field test, learned helplessness, individual differences, inescapable stress, heart rate
Introduction
Current evidence supports the relationship between individual personality traits and the predisposition to neuropsychiatric disorders such as depression and post-traumatic stress disorder (PTSD) [1–4]. In fact, animals have been selectively bred and used as disease models to characterize behavioral correlates of these conditions [5, 6]. One such model is the congenitally helpless rat, which was selectively bred for a genetic predisposition to helpless behavior as defined by the learned helplessness paradigm (failure to escape shock after a previous exposure to inescapable shock) [7]. Behavioral characteristics of congenitally helpless rats include increased reactivity to spatial novelty, reduced reward sensitivity, and an impaired ability to extinguish conditioned fear [6, 8]. Novelty reactivity is characterized by increased exploratory activity in a novel, but not familiar, open field [9, 10]. Intuitively, increased exploration in a new environment seems to contradict susceptibility to helplessness. However, it is consistent with a predisposition to PTSD, given the association of this disorder with increased novelty seeking, as measured by the Cloninger Tridimensional Personality Questionnaire [4, 11].
Based on data from the congenitally helpless strain, it was hypothesized that increased exploration in response to novelty, reduced consumption of a rewarding sucrose solution, and enhanced passive avoidance of a harmful stimulus would predict helpless behavior (defined by high escape latencies) in a randomly bred population of rats. To date, no studies have examined the predictive value of all three behavioral dimensions in the development of helplessness.
Individual differences in the responsiveness of the stress pathways including the sympathetic nervous system and hypothalamic-pituitary adrenal (HPA) axis have also been studied in animals and humans [12–18]. For instance, rats that were vulnerable to inescapable stress secreted more corticosterone compared to stress-resistant rats [18]. In addition, infants who displayed greater overt anxiety during maternal separation and exposure to novelty had significantly higher urinary cortisol excretion during the episodes [19], and adult rats that were not handled as neonates had less effective negative feedback regulation of adrenocorticotropic hormone (ACTH), resulting in greater corticosterone secretion during stress [20]. Furthermore, increased levels of glucocorticoid hormones have been shown in a significant percentage of depressed patients [15]. However, a dissociated HPA axis was observed in congenitally helpless rats characterized by a hyperactive paraventricular hypothalamus (PVN) [21] and elevated ACTH [22], and reduced plasma corticosterone both at baseline [23] and in response to stress [22, 24]. Lachman et al. (1993) further observed that, although glucocorticoid receptors are normal in terms of prevalence and binding characteristics, congenitally helpless rats do not alter glucocorticoid receptor mRNA expression in the hippocampus or hypothalamus in response to corticosterone administration [25]. The cause of this dysfunction is unknown, but there appears to be a functional modification that affects some, but not all, glucocorticoid receptors since another glucocorticoid-inducible gene, metallothionein-1 (MT-1), did increase normally in response to corticosterone administration. Thus, an absence of negative feedback via glucocorticoid receptors likely contributes to the hyperactivity of the PVN [21] and pituitary [22], but the mechanism underlying the breakdown in signaling between the pituitary and adrenal glands is unknown.
Reduced heart rate variability has also been observed in depressed patients when compared with non-depressed controls, and the risk of subsequent cardiac events for patients with heart disease has been shown in numerous studies to be related to decreased heart rate variability and/or increased sympathetic tone [17, 26–28]. Furthermore, the presence of major depressive disorder was the best single predictor for the occurrence of cardiac events, and the predictive value was independent of the extent of coronary disease, smoking status, and left ventricular ejection fraction [26]. Temperament or personality is also linked to physiological parameters such as heart rate and cortisol. For example, impulsivity, a personality factor that correlates with novelty seeking [29], has been associated with lower levels of resting heart rate [30], and novelty seeking has been inversely associated with resting stress hormone concentrations [31]. Furthermore, among PTSD combat veterans, increased novelty-seeking was inversely correlated with cortisol levels [11]. The second aim of this study was to identify individual differences in the physiological response to restraint stress, including heart rate, heart rate variability and plasma corticosterone that could predict helpless behavior among Holtzman rats. The hypothesis is that animals with increased susceptibility to helplessness will show higher novelty-seeking scores that will correlate with reduced plasma corticosterone and heart rate measures.
Methods
Subjects
Subjects were 42 male Holtzman rats obtained from Harlan (Madison, WI) at postnatal day 30 (PD 30). The experiments were conducted in juvenile rats to identify behaviors that occur early in development that could be predictive of psychopathology. In addition, adolescent rats perform better and display higher levels of physical activity in the open field, as opposed to adults, which increases the likelihood of identifying behavioral patterns that predict the helpless phenotype. Animals were housed 2–3 per cage and maintained on a 12 h/12 h light/dark photoperiod in a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International. Food and water were available ad libitum. Subjects were handled and weighed for 5 minutes every day throughout 9 consecutive days prior to starting behavioral experiments. Twenty-one subjects were used in a separate control experiment and only underwent learned helplessness training and testing. This was performed to assess whether undergoing prior behavioral screening with the open-field, sucrose and passive avoidance tests would have an effect on helpless behavior. All experiments occurred during the light phase between 0700 h and 1900 h. Experiments were done in accordance with NIH guidelines for the use of experimental animals and were approved by the University of Texas Institutional Animal Care and Use Committee.
Apparati
The open field chamber (43.2 × 43.2 cm) consisted of clear plastic sides 30.5 cm high and a white plexiglass floor. Activity was detected by arrays of infrared light beam motion detectors (16 × 16, 2.5 cm apart) at the sides of each chamber, thus creating a detection grid. Two arrays of detectors were located 1 cm above the floor, and another array was located 13 cm above the floor, to detect rearing. The chambers were controlled by the Activity Monitor program, version 5.10 (Med Associates, St. Albans, VT).
The sucrose chambers consisted of two 30 cm × 25 cm × 20 cm operant chambers (Med Associates, St. Albans, VT). The bottles were designed by Med Associates for use in these operant chambers, and a light beam passing in front of the tip of the bottle registered the time the rat spent drinking. The bottles were located on the right side of the chamber.
The step-down apparatus for passive avoidance testing consisted of one 30 cm × 25 cm × 20 cm operant chamber (Med Associates, St. Albans, VT). Shocks were delivered through metal bars separated by 1.2 cm forming the floor of the chamber, which was wired to shock generators (Med Associates). An acrylic platform (23.4 cm × 14.3 cm × 2.2 cm) was placed on the grid floor.
Two inescapable shock chambers (30 cm × 25 cm × 20 cm) (Med Associates, St. Albans, VT) were enclosed in sound-attenuated boxes and illuminated by a red light. The apparatus had two sides of aluminum, with clear plexiglass for the front, back, and top. A soapy solution was placed in the tray beneath the chambers to provide a distinct olfactory cue for the inescapable context. Shocks were delivered through metal bars separated by 1.2 cm forming the floor of the chamber, which was wired to shock generators (Med Associates). The chamber was controlled by MED-PC, version 4 (Med Associates, St. Albans, VT), using a program written in the MEDSTATE language.
The shuttle box (42 cm × 16 cm × 25 cm) consisted of two compartments of equal size, separated by a door (11 cm × 9 cm) that remained open throughout the session. The chamber was enclosed in a sound-attenuated box and illuminated by a white light. Two sides of the chamber were aluminum, with clear plexiglass for the front, back, and top. Shocks were delivered through metal bars separated by 1.2 cm forming the floor of the chamber, which was wired to shock generators (Med Associates). The subject’s position was detected by eight sets of infrared light beam motion detectors, located 2 cm above the grid floor, spaced 4.4 cm apart from each other, on both sides of the chamber. The chamber was controlled by MED-PC, version 4, using a program written in the MEDSTATE language. This program used beam breaks of the two pairs of beams located at either end of both sides of the chamber as the contingency for terminating shock, to score a complete crossing. An iodine solution was placed in the tray beneath the chamber to provide a distinct olfactory cue.
Heart rate measurements were performed while animals were in restraint tubes (20.2 cm length × 5.08 cm diameter) for 2 to 5 minutes. Using a Mouse Ox™ oximeter (Starr Life Sciences Corp, Oakmont, PA), pulse rates were measured via cyclic changes in absorption of light energy from red and infrared LEDs (light emitting diodes) as it passed through the tissue. These cyclic changes in light absorption are due to the presence of changing quantities of blood that occurred with every heart beat.
Behavioral experiments
Behavioral experiments took place during six consecutive days (Table 1). All animals were tested for open field (OF) activity during the first day of experiments (novel OF) on PD 40 to determine if behavioral characteristics predictive of helplessness were present before learned helplessness training. Each animal was placed in the same corner of the open field chamber and behavior was recorded for 10 minutes. The chambers were washed with a diluted Bio-clean solution between each session.
Table 1.
Experimental design.
| Experiment | Postnatal day (PD) |
|---|---|
| Handling | 31–39 |
| Novel open field | 40 |
| Familiar open field | 41 |
| Sucrose preference training | 41 |
| Sucrose preference testing | 42 |
| Passive avoidance training | 42 |
| Passive avoidance testing | 43 |
| Learned helplessness training | 43 |
| Learned helplessness testing | 44 |
| Heart rate measurement | 46 |
| Trunk blood collection for corticosterone assay | 46 |
Measures included ambulation time in seconds, rearing time in seconds, ambulatory velocity (cm/sec), center time (seconds spent in the 38% center vs. the 62% periphery of the open field as a measure of risk-taking or impulsive behavior), and activity time (seconds spent in either ambulatory or rotational movement, i.e., not resting or rearing). Measures were automatically scored by a computer using MED-PC software. Subjects were re-tested in the open field (familiar OF) the following day. This was done in order to assess whether the behavior was reflective of exploratory activity specific to a novel environment.
Immediately following the familiar open-field session, baseline consumption of a 5% sucrose solution or water was assessed for 30 minutes where rats had free access to two bottles containing the sucrose solution or drinking water. The sucrose test was repeated on the following day and the bottles where counterbalanced with respect to their position the day before. This was performed to account for place preference of the bottles (front or back). A sucrose-to-water drinking ratio was computed for both the baseline and second-day sessions. This ratio is meant to reflect the preference for sucrose solutions over water as an indicator of reward-seeking behavior.
A modified step-down passive avoidance task was performed based on previously described protocols [32, 33]. The procedure consisted of two sessions: an acquisition session and a retention session with an inter-session interval of 24 h. Acquisition session: Immediately after the second sucrose test session, animals were placed on the acrylic platform inside the passive avoidance chamber. As soon as the rat stepped down onto the grid floor with all four paws, a 0.3 mA shock was delivered continuously. Rats could avoid the shock by remaining on the acrylic platform. The acquisition session ended after 180 sec. Retention session: Rats were exposed to the same context with exception of the footshock. They were placed on the acrylic platform, and the latency to step down onto the un-electrified grid floor with all four paws was recorded. The retention session ended after 180 sec.
Immediately following the retention session, subjects were trained in the inescapable shock chamber to induce the helpless phenotype [34–36]. Each session included 60 trials of 10 sec duration 0.7 mA shocks. Pseudorandom inter-trial intervals consisted of durations ranging from 10 to 110 sec.
On the fifth day, subjects were tested with the escapable shock paradigm using a shuttle box to measure escape behavior. First, subjects were tested with fifteen trials of a fixed ratio (FR) 1 schedule consisting of crossing from one side of the box to the other to terminate the shock. The maximum footshock duration was 15 sec, after which the shock was terminated if the subject had not escaped. This was followed by fifteen trials of a FR2 schedule in which animals had to cross twice; in other words, rats had to return to the compartment where the shock was initiated in order to escape the shock. During the FR2 trials, the maximum footshock duration was 30 sec. There was a 1-minute interval in between FR1 and FR2 escape tasks. Based on our previous results using Holtzman rats, a FR1 schedule is sufficient to observe a learned helplessness effect [37]; therefore, we included an equal number of trials for both types of escape responses to obtain comparable results with both schedules. Latency to escape was automatically scored using MED-PC software.
On the last day, subjects were placed inside the restraint tube for heart rate measurements. To increase pulse distention and obtain a stable LED signal, the restraint tube was placed on top of a heating pad. The oximeter sensor was placed on the rat’s tail using a specialized clip (Starr Life Sciences, Oakmont, PA). Animals were covered using a dark cloth to avoid external light from interfering with the LED signal. After 1 – 4 minutes of stabilization time, the heart rate was recorded for 1 minute; therefore the total restraint time was 2–5 minutes. Average heart rates were reported as four 15-sec bins as well as overall mean heart rate. Heart rate variability, a parameter for autonomic control of the heart [38], was determined using the mean standard deviation of measurements taken throughout the 1 minute period [39, 40]. Immediately following the heart rate measurement, animals were decapitated. Trunk blood was collected in BD vacutainer® heparinized 3 ml tubes (BD, Franklin Lakes, NJ) for assessment of plasma corticosterone.
Corticosterone assay
Plasma was separated by centrifuging for 10 minutes at 4°C with a speed of 2500 rpm. Corticosterone was detected in 100 µl samples using the Correlate-EIA™ enzyme immunoassay kit (Assay Designs Inc., Ann Arbor, MI), following the procedure recommended by the manufacturer.
Statistical analyses
A two-step cluster analysis applied Akaike’s Information Criterion to sort subjects into an optimal number of clusters based on their escape latencies. Mean differences between the high-and low-latency clusters in terms of open-field behaviors, sucrose preference, and step-down avoidance latencies were assessed with multivariate analyses of variance (MANOVAs). A principal components analysis with and without varimax rotation was used to decompose open-field activity into orthogonal vectors of general- vs. novelty- vs. familiar-driven activity, and novel- and familiar-adjusted scores were computed from the studentized residuals of a novel × familiar linear regression, as described in detail in the Results. Multiple linear regression was used to evaluate the ability of this effects decomposition to predict escape latency. Pearson pairwise correlations were also computed within the behavioral dimensions as well as between the behavioral dimensions and the physiological parameters of heart rate average, heart rate variability (operationalized as the standard deviation of heart-rate measurements over time for each subject), and corticosterone level. All statistics were computed using SPSS (v. 16.0).
Results
Cluster analysis of shuttle box escape behavior identifies two distinct groups: helpless and not helpless
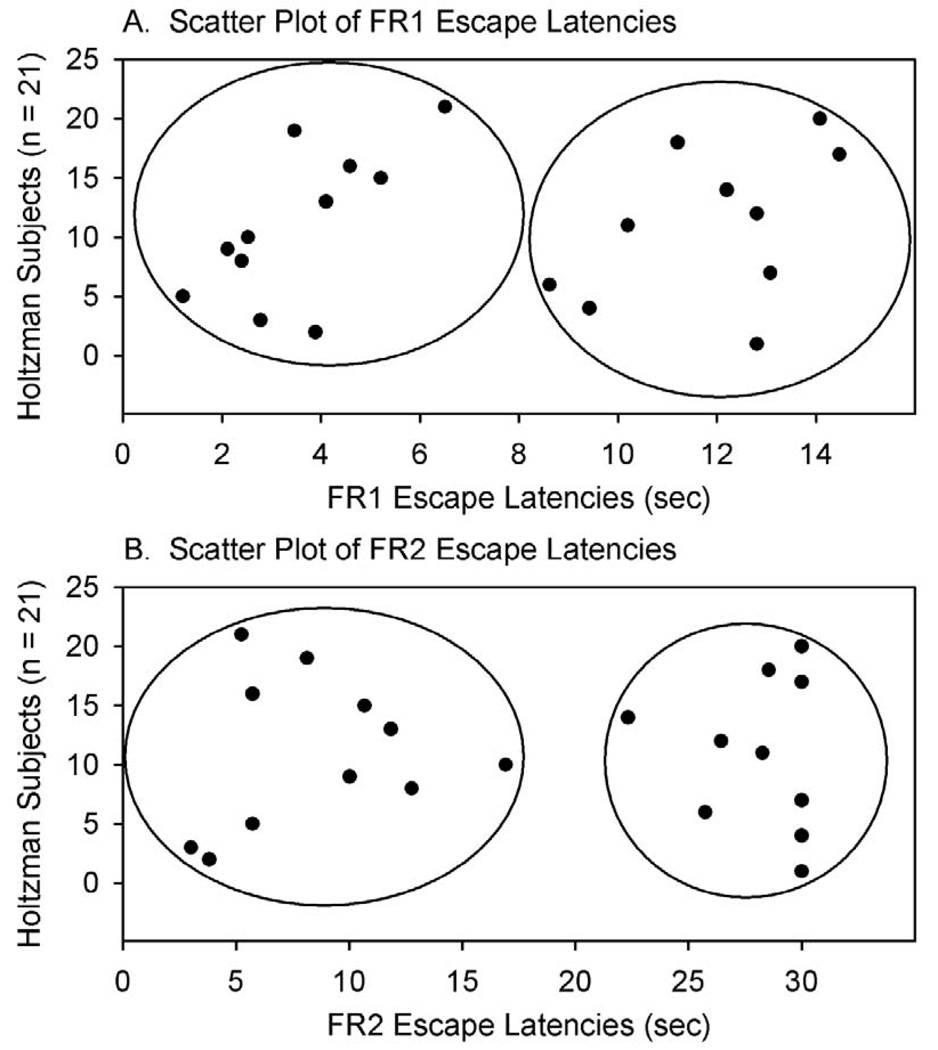
A cluster analysis based on FR1 and a separate analysis using FR2 escape responses divided the same subjects into two distinct clusters with high and low escape latencies, which we termed helpless and non-helpless (Figure 1, Table 2). The helpless group showed three times longer escape latencies as compared to the non-helpless group (p < 0.01).
Figure 1.
Scatter plots of FR1 (top) and FR2 (bottom) escape latencies depicting helpless (n = 10) and non-helpless (n = 11) Holtzman rats. The y-axis corresponds to each individual’s subject number. The same subjects were clustered into the helpless and non-helpless groups using latencies from either response schedule (FR1 or FR2).
Table 2.
Cluster analysis of shuttle box escape responses (mean escape latency in sec ± SEM).
| Rats which received open-field, sucrose-consumption, and passive-avoidance tests: | |||
|---|---|---|---|
| Helpless (N = 10) | Non-helpless (N = 11) | Total sample (N = 21) | |
| FR1 | 11.89 ± 0.62* | 3.53 ± 0.46 | 7.51 ±1.00 |
| FR2 | 28.13 ± 0.82* | 8.54 ±1.30 | 17.87 ± 2.32 |
| Control rats which did not receive behavioral-screening tests: | |||
|---|---|---|---|
| Helpless (N = 12) | Non-helpless (N = 9) | Total sample (N = 21) | |
| FR1 | 12.99 ± 0.39* | 5.83 ± 1.07 | 9.92 ± 0.93 |
| FR2 | 28.23 ± 0.67* | 11.17 ± 1.54 | 20.92 ± 2.03 |
p < 0.01, helpless vs. non-helpless
Prior behavioral screening does not affect learned helpless behavior
In order to evaluate whether the extensive behavioral testing (two days of open field, sucrose consumption, and passive avoidance) altered the subsequent expression of the helpless phenotype, a control group of 21 Holtzman males was subjected to learned helplessness training and testing without any prior behavioral testing. Using the same cluster criteria, 12 out of 21 rats (57%) were classified as helpless (Table 2). This rate is not significantly different from the 10 out of 21 rats (48%) that became helpless following behavioral pretesting, Χ2(1) = 0.76, p = .38, and suggests that the behavioral tests we performed had no substantial impact on the development of the helpless phenotype.
Helpless rats show increased activity in novel environments
A repeated measures MANOVA was used to compare the novel and familiar open field activity for five measures: ambulation, rearing, velocity, center crossing, and total activity. The multivariate main effect of helpless classification was not significant, Pillai’s Trace = .11, F = .365, df = (5,15), p = .86, indicating that there was no general activity difference between helpless and non-helpless rats. However, the multivariate result was significant for the helplessness × novelty interaction, Pillai’s Trace = .55, F = 3.68, df = (5,15), p = .02, indicating that rats classified as helpless showed an overall greater change in behavior between the novel and familiar open fields across all activity measures. Simple tests of univariate effects within each measure and within each open field session showed only three significant differences: helpless rats spent more time ambulating (p = .04), more time in the center of the field (p = .04) and more time generally active (p = .02) in the novel open field, but were not significantly different from non-helpless rats in the familiar open field on these measures (p = .75, .56, and .95, respectively) or any other (Table 3).
Table 3.
Mean novel and familiar open-field activity parameters for helpless and non-helpless rats.
| Open-Field Activity Parameter | Helpless Mean ± SE |
Non-helpless Mean ± SE |
p value |
|---|---|---|---|
| Novel Open Field | |||
| Ambulation time (sec) | 68.9 ±5.2 | 53.4 ± 5.0 | 0.04 |
| Rearing time (sec) | 90.5 ± 9.6 | 82.1 ± 9.1 | 0.54 |
| Average velocity (cm/sec) | 1.2 ± 0.1 | 1.1 ± 0.1 | 0.36 |
| Center time (sec) | 83.2 ±7.8 | 59.4 ± 7.4 | 0.04 |
| Activity time (sec) | 226.2 ±10.6 | 187.6 ±10.1 | 0.02 |
| Familiar Open Field | |||
| Ambulation time (sec) | 41.0 ± 4.5 | 43.0 ± 4.3 | 0.75 |
| Rearing time (sec) | 57.5 ±10.1 | 68.1 ± 9.6 | 0.46 |
| Average velocity (cm/sec) | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.41 |
| Center time (sec) | 40.5 ± 10.3 | 49.1 ± 9.8 | 0.56 |
| Activity time (sec) | 163.3 ± 15.5 | 164.5 ± 14.8 | 0.95 |
A MANOVA of the drinking time data from the sucrose preference test (absolute sucrose drinking time and ratio of drinking time relative to water) showed no significant multivariate effect of helplessness classification, Pillai’s Trace = .19, F = 2.1, df = (2, 18), p = .15. In addition, a univariate ANOVA showed no significant effect of helplessness classification on passive avoidance latencies, F(1,18)= 0.95, p = .34.
Regression residuals associated with novelty reactivity, but not habituation, explain variance in escape learning
The presence of group mean differences in novel, but not familiar, activity measures suggests that a difference in reaction to novel environments, not a difference in habituation learning, is the more relevant predictor of learned helpless behavior. However, for an individual subject, both temperament (novelty reactivity) and learning (habituation) presumably influence the magnitude of the activity difference between novel and familiar environments, and it would be useful to partition the contributions of these two processes so that their relative effects on helpless behavior can be assessed. Because it was the most general measure of activity in the open field and showed the largest mean difference between helpless and non-helpless animals, we focused on total activity time for this analysis.
We operationalized activity in the novel open field as a function of general activity level plus reaction to novel environments, and we operationalized activity in the familiar open field as a function of general activity level minus habituation to familiar environments. As a first step, we decomposed the variance across the novel and open fields into orthogonal dimensions using a principal components analysis. The first component, which explained 83% of the variance, corresponds to general activity level, which is constant across both days of open-field testing and therefore equally correlated with both the novel and familiar open field. The second component, explaining the residual 17% of the variance, corresponds to the novel-familiar contrast in activity and is therefore oppositely correlated with the novel and familiar open field. A linear regression of these two independent components as predictors of escape latency demonstrated that it is the novel-familiar contrast, not general activity level, which predicts helpless behavior (Table 4, Model 1). Only the principal component scores corresponding to the novel-familiar contrast had a significant (p < .01) partial effect in the full model, with a greater shift in activity from novel to familiar environments predicting longer escape latencies.
Table 4.
Regression coefficients predicting escape latency.
| Unstandardized Coefficients |
Standardized Coefficients |
||||
|---|---|---|---|---|---|
| B | Std. Error | Beta | t | P value | |
| Model 1: | |||||
| (Constant) | 12.690 | 1.261 | 10.066 | 0.000 | |
| General activity | 1.856 | 1.292 | 0.250 | 1.437 | 0.168 |
| Novel-familiar contrast | 4.634 | 1.292 | 0.625 | 3.587 | 0.002 |
| Model 2: | |||||
| (Constant) | 12.7 | 1.261 | 10.073 | 0.000 | |
| Novelty reaction | 4.709 | 1.679 | 0.65 | 2.805 | 0.012 |
| Habituation | 0.253 | 1.684 | 0.035 | 0.15 | 0.882 |
| Model 3: | |||||
| (Constant) | 12.690 | 1.261 | 10.066 | 0.000 | |
| Novelty-specific | 4.589 | 1.292 | 0.619 | 3.552 | 0.002 |
| Habituation-specific | 1.964 | 1.292 | 0.265 | 1.521 | 0.146 |
Dependent variable: escape latency (average of FR1 and FR2)
This further demonstrates that it is the change between novel and familiar environments that is associated with helplessness vulnerability. However, this change could be driven by differences in the novel open field (novelty reaction) or by differences in the familiar open field (habituation) or by a combination of both. To partition these effects, we calculated a novelty-reaction index as the positive studentized residual for each subject after regressing novel activity onto familiar activity, and a habituation index as the negative studentized residual for each subject after regressing familiar activity onto novel activity. Thus, the novelty-reaction index is completely uncoupled from familiar open-field activity, and the habituation index is completely uncoupled from novel open-field activity, with the respective correlations rendered zero. A linear regression of these two indices as predictors of escape latency demonstrated that it is reaction to novelty, not habituation, which predicts helpless behavior (Table 4, Model 2). Only the residuals corresponding to novelty reactivity had significant (p < .05) partial effects in the full model, with greater novelty reactivity predicting longer escape latencies.
This suggests that the activity contrast between novel and familiar environments, which was highly associated with helplessness vulnerability, was driven by individual differences in novelty reactivity, not habituation. However, the novelty-reaction and habituation indices, though not highly colinear, are not completely independent either (r = .66). While there is no theoretical reason to expect that these indices should be independent—it may be that novelty-excitable animals tend to be faster habituators—it would be interesting to evaluate orthogonal measures of novel and familiar activity. Such independent measures can be achieved by rotating the principal components. The resulting scores, which we term novelty-specific and habituation-specific activity, have the interesting property of being highly correlated (r = .94) with the novelty-reaction and habituation indices calculated above while also having zero correlation with each other. Again we evaluated a linear regression of escape latency onto these measures, and again the results supported a greater influence of novelty-specific activity (Table 4, Model 3). Only the principal component scores corresponding to novel-specific activity had significant (p < .01) partial effects in the full model, with greater novel-specific activity predicting longer escape latencies.
In summary, the variance in novel and familiar open field activity can be decomposed to elucidate the relationships of general activity, novelty reactivity, and habituation learning with other behavioral and physiological measures. Table 5 provides the correlations among these different scales and an intuitive overview of their construct validity. For example, the general activity scale should be (and is) equally and positively correlated with both novel and familiar open-field measures, and the novel-familiar contrast scale should be (and is) equally and oppositely correlated with the novel and familiar open-field measures. This correlation matrix also aids in interpreting differences between what we have termed the novel-reaction and habituation scales, on the one hand, and the novelty-specific and habituation-specific scales on the other. The scales are highly correlated with each other across novelty and habituation dimensions, respectively. However, the “simple” novelty reaction and habituation scales have the advantage of being selectively correlated with the relevant open-field session (zero correlation with the irrelevant open-field session) and preferentially correlated with the novel-familiar contrast dimension as opposed to the general activity dimension. Novelty reaction and habituation are, however, moderately correlated with each other. The “specific” novelty and habituation scales have the advantage of removing this correlation (thus offering self-specific, linear-independent indices) while retaining a large, preferential correlation with the appropriate open field session. However, they are varimax rotations of the general-activity and novel-familiar-contrast dimensions and are therefore equally correlated to both.
Table 5.
Correlations among decomposed dimensions of activity in the novel and familiar open field.
| Novel activity |
Familiar activity |
General activity |
Novel-familiar contrast |
Novelty reaction |
Habit. | Novel- specific |
|
|---|---|---|---|---|---|---|---|
| Familiar activity | .67 | ||||||
| General activity | .91 | .91 | |||||
| Novel-familiar contrast |
.41 | −.41 | .00 | ||||
| Novelty reaction | .75 | .01 | .41 | .91 | |||
| Habituation | .00 | −.75 | −.41 | .91 | .66 | ||
| Novel-specific | .93 | .36 | .71 | .71 | .94 | .35 | |
| Habit.-specific | −.36 | −.93 | −.71 | .71 | .35 | .94 | .00 |
Bold, correlations over 0.90. See text for explanation of effects decomposition.
Collectively, these scales offer different perspectives onto the different dimensions underlying individual differences in open field behavior. A practical question arises at this point, namely, which is the most useful perspective in terms of predicting susceptibility to learned helplessness? To address this question, we performed a stepwise regression, using all of the variables from Table 5 (Model 3) as potential predictors. The stepwise procedure selected novelty reactivity alone as the best predictor (escape latency = 12.7 + 4.875*novelty reaction), explaining 45% of the variance in escape latencies, F(1,19) = 15.7, p = .001. A rat with a positive novelty reaction was 5.5 times more likely to become helpless than a rat with a negative novelty reaction, as given by the odds ratio (69%/12.5%).
Lower heart rate under stress is associated with higher activity levels in the open field
There were no significant mean differences in corticosterone levels, heart rate average, or heart rate variability between helpless and non-helpless Holtzman rats that were subjected to restraint stress (Table 6). However, heart rate during restraint stress was negatively correlated with general activity, but not the novel-familiar contrast in activity, so further correlations with the decomposed dimensions of the novel-familiar contrast, e.g., novelty reactivity and habituation, were not computed (Table 7). These correlations were computed using all subjects, but the negative correlation between heart rate and general activity was similar for both helpless and non-helpless groups: r(10) = −0.51 for helpless and r(11) = −0.59 for non-helpless. No other physiology-behavior correlations were significant.
Table 6.
Means of physiologic parameters between helpless and non-helpless rats.
| Physiologic parameter | Helpless (n= 10) mean ± SEM |
Non-helpless (n= 11) mean ± SEM |
p value |
|---|---|---|---|
| Corticosterone (pg/ml) | 3234 ± 291.4 | 3023 ± 235.8 | 0.58 |
| Heart rate average (bpm) | 455.81 ± 16.34 | 482.15 ± 12.45 | 0.21 |
| Heart rate variability | 12.83 ± 1.91 | 15.64 ± 2.25 | 0.36 |
Table 7.
Correlations between open-field activity measures and physiologic parameters.
| Open field activity | CORT | HR | HR var |
|---|---|---|---|
| Novel activity | 0.29 | −.63** | −0.2 |
| Familiar activity | 0.15 | −.44* | −0.04 |
| General activity | 0.24 | −.58** | −0.08 |
| Novel-familiar contrast | 0.17 | −0.23 | −0.27 |
| Escape latency | 0.02 | −0.38 | −0.35 |
Significant correlation
p < 0.05,
p < 0.01.
Cort (corticosterone), HR (heart rate), HR var (heart rate variability).
Discussion
Excitability in the novel open field, but neither general hyperactivity nor habituation, predicts heightened susceptibility to helpless behavior
We previously reported a link between susceptibility to learned helplessness and behavioral activation specific to a novel, but not familiar, open-field environment using a selected line of Sprague-Dawley rats bred for helpless behavior [8], a finding replicated by Schulz et al. [41]. In the present study we found the same phenomenon in a randomly bred population of Holtzman rats. The approximately 50% of rats which later developed helpless behavior after inescapable shock showed significantly greater activity in a novel open field when compared to the approximately 50% which did not develop helpless behavior; however, the two groups were equivalent in a familiar open field. This is further reflected in a significant helpless × novelty interaction evident across all open-field measures, indicating a greater novel-to-familiar reduction in activity for those rats that later became helpless. We previously interpreted this interaction in terms of a temperamental difference corresponding to greater excitement by novel environments in helpless-susceptible rats, which has interesting parallels to some human studies linking novelty-seeking temperament to heightened risk for PTSD [8]. However, Schulz et al. [41] interpreted their same finding in terms of a memory difference corresponding to greater habituation learning in helpless-susceptible rats. While it is true that a decrement in activity associated with repeated exposure to a stimulus presupposes a memory of that stimulus, it is a fallacy to conclude that a group difference in activity reduction is caused by a memory difference when there are baseline group differences in activity.
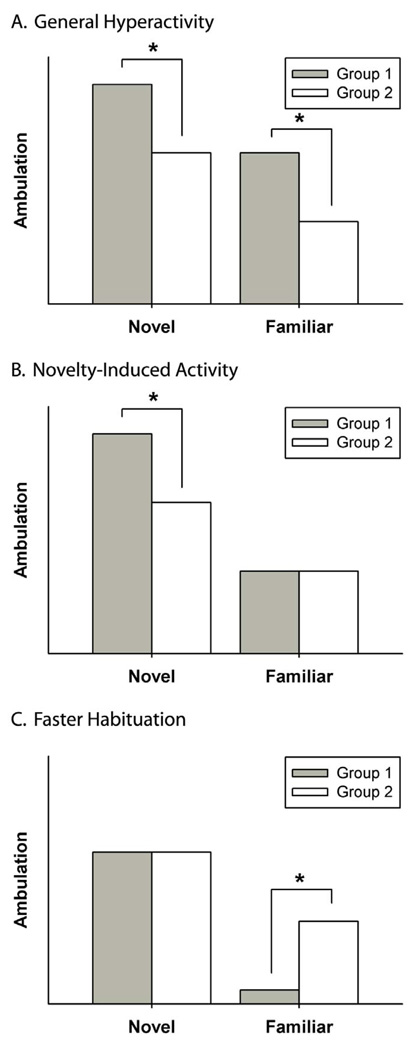
Figure 2 illustrates an idealized depiction of how the concepts of hyperactivity, novelty reactivity, and habituation can be disentangled and what evidence would support each interpretation. If a group difference in activity change is driven entirely by a difference in the novel environment, with no difference in the familiar, then the most plausible explanation is that animals were differentially excited by the novel environment. In this case, the greater reduction in activity across days reflects not superior habituation but a loss of novelty-evoked excitement; the novelty-reactive animals are simply regressing back to their mean activity level after a novelty-evoked departure from it. Differential habituation learning becomes a plausible explanation only when novelty-evoked differences are absent, such that any group difference in activity change could only be driven by a difference in the familiar environment. Thus, our present results fit the pattern of a novelty effect, as opposed to a habituation effect. Moreover, so do the results of Schultz et al. [41]. Their data are consistent with a habituation effect when simply contrasting helpless-susceptible vs. helpless-resistant lines, but when the selected lines are contrasted against wild-type rats, it is evident that resistant rats showed a pattern of hyperactivity (elevated activity relative to wild type in both familiar and novel open fields) and that susceptible rats showed a pattern of novelty reactivity (elevated activity relative to wild type in the novel but not familiar open fields).
Figure 2.
Schematic diagram indicating how to infer general hyperactivity, novelty reactivity, and habituation effects from measurements in the novel and familiar open fields.
While this analysis of group means between novel and familiar open fields provides a simple and intuitive method of interpreting effects in terms of a novelty-excitable temperament vs. superior habituation learning, it would be useful to assign scores of general activity, novelty excitability, and habituation to individual subjects so that relationships between these processes and others (e.g., escape learning) could be correlatively assessed. An innovation introduced in the present study is a statistical method of generating such scores from the novel and familiar open-field data. The first step is to parse the stable activity differences (i.e. general hypoactivity vs. hyperactivity) from the variable activity differences (i.e., novel- vs. familiar-dependent activity). This is accomplished by calculating the unrotated principal components of the two open field sessions, which decomposes the variance into orthogonal vectors: what is common to both sessions (general activity level) and what is opposite (novel-familiar contrast). This decomposition can then be used to generate standardized scores for each subject along these two dimensions. When escape latency was regressed onto these scores, it was evident that a greater novel-familiar shift in activity predicted greater helpless behavior for individual subjects while the impact of general activity level was insignificant.
The next step was to parse the novel-familiar shift into novelty-reaction and habituation effects. Here we developed two alternative methods, one which allows for covariance between the effects and one which forces them to be independent. The first method relies on the same logic employed in the analysis of group means illustrated in Figure 2. Namely, habituation differences can be inferred from differences in the familiar open field when activity in the novel open field is equivalent, and novelty-reaction differences can be inferred from differences in the novel open field when activity in the familiar open field is equivalent. The first method accomplishes this by generating adjusted activity scores for each field which partial out covariance with the other field, enabling the evaluation of individual differences in one field as if there were no individual differences in the other. The second method is simply to rotate the principal components (which by definition are linearly independent) until they are maximally covariant with one open field and minimally covariant with the other. Under the first method, the scores are linearly independent of the opposite open field but somewhat correlated with each other whereas, under the second method, the scores are linearly independent of each other but somewhat correlated with the opposite open field (Table 5). Importantly, both methods yielded the same result when escape latency was regressed onto their scores. Namely, a greater reaction to novelty predicted significantly greater helpless behavior whereas greater habituation did not. Furthermore, a stepwise regression selected novelty reactivity (as calculated by the first method) as the best predictor of helplessness susceptibility.
However, differences in other behavioral measures were not observed between helpless and non-helpless Holtzman rats. For example, the selectively-bred congenitally helpless rat has shown decreased sucrose consumption and increased behavioral inhibition or harm avoidant behavior compared to normal Sprague Dawley controls [8]. One possible explanation for the negative findings is that Holtzman rats may be modeling more of a bipolar than a unipolar depressive phenotype. That is, harm avoidance is more characteristic of unipolar mood disorder, and novelty seeking is more characteristic of bipolar mood disorder, regardless of current mood state [42]. Here, it is interesting to note that lithium suppresses hyperactivity and exploration of novel environments without affecting activity level in familiar environments [43], essentially counteracting the exact behavioral pattern of our novelty-reactive/helpless-vulnerable rats. Thus, a subpopulation of Holtzman rats show a bipolar-like pattern in that they experience excessive behavioral activation in response to novelty followed by excessive behavioral inhibition in response to uncontrollable stress, with normal reward dependence and harm avoidance in the interim.
This finding also has interesting parallels to two studies reporting that high novelty seeking was associated with PTSD symptoms among Vietnam combat veterans [4, 11]. Novelty seeking is also associated with certain comorbidities and sub-types of affective disorders [44, 45]. Specifically, high novelty seeking is associated with multiple substance abuse, including alcohol and nicotine [46–49], and increased suicidality among patients with borderline personality disorder [50]. In addition, high levels of novelty seeking, impulsivity, and sensation seeking were predictive of poor outcome and greater drop-out rates during treatment among patients with substance abuse problems [51]. In a separate study, patients with low levels of novelty seeking, harm avoidance, and reward dependence showed greater symptom improvement after a six week course of anti-depressant treatment [52]. Compared to clinical and demographic variables, temperament was the only predictor which accounted for 50% of the variance in treatment outcome among those severely depressed patients [52].
As discussed previously [8], this associated vulnerability may involve reduced glucocorticoid signaling, which biases animals toward greater activity in the novel open field [53] and greater rates of learned helplessness [54]. Indeed, high novelty seeking and low cortisol levels are reported covariates in veterans with PTSD [11]. However, a correlation between corticosterone and novelty-induced activity was not observed in our sample, and we cannot infer that our rats were novelty seeking since their exploration of the open field was forced and not voluntary. Indeed, one study that allowed rats voluntary access to the open field actually found that greater novelty phobia predicted helplessness susceptibility [55], and reduced exploration of novel objects predicted greater helpless behavior within wild-type, but not selectively bred, rats [41]. Thus, at this point, the finding of greater novelty excitement as a predictor of helpless behavior appears limited to forced exposure to spatial novelty and could reflect anxiogenic agitation or a hedonic response to novelty. However, helpless rats also spent increased time in the center of the novel open field, which is usually interpreted as reduced anxiety [56]. Furthermore, the hypothesis that enhanced memory consolidation, i.e., habituation, accounts for the novel-familiar shift in open field activity that underlies helplessness susceptibility [41] was not supported. Not only was it not supported by our effects-decomposition analysis, but such a difference in general learning mechanisms should manifest across a variety of learning tasks. In particular, enhanced memory formation would predict superior performance in the passive avoidance task for rats predisposed to helplessness, but such an association was not observed in our data. The inclusion of this simple passive avoidance task prior to inescapable shock training provides a useful internal control for the learned helpless paradigm, for the precise reason that it can rule out differences in general learning mechanisms or shock sensitivity as explanations for differences in active escape latency.
Lower heart rate under restraint stress predicts higher activity level
Variations in heart rate and blood pressure reflect activity and responsivity of the autonomic nervous system, providing a measure of the functional state of central neurobiological regulatory mechanisms, and individual differences in the physiological responses to stress that have been linked to depression and anxiety [12, 14, 17]. For example, diminished heart rate variability has been observed among depressed subjects when compared to non-depressed patients, and reduced heart rate variability has been linked to poorer prognosis in patients with coronary artery disease [17]. Negative mood states have also been linked to autonomic nervous system disturbances and cardiac events [57].
Heart rate measurements were not related to differences between helpless and non-helpless rats. However, a lower heart rate did correlate with increased general activity in the open field, especially the novel-open field. This could represent a diminished sympathetic tone or elevated vagal tone present in animals that display greater novelty-induced behavioral activation. For example, novelty seeking was associated with lower heart rate among men and women [16]. Disruptions in the HPA and autonomic nervous system which regulate heart rate and response to stress have been linked to depression and anxiety in humans and animals [12, 14, 15, 17, 22, 58, 59]. Whether the effects on heart rate represent individual differences in response to a stressor or a disruption in these regulatory mechanisms is presently unknown.
Summary
Our finding that novelty-induced exploratory activity predicts helplessness is consistent with literature linking reaction to novelty with a predisposition to PTSD [4, 11], and also with bipolar disorder [42, 60]. Rats with a positive vs. negative reaction to a novel environment (operationalized as enhanced vs. diminished novel-covaried-for-familiar activity) were over five times more likely to become helpless after inescapable stress. In conclusion, increased excitability evoked by novel environments may be a marker of helplessness susceptibility, a finding with interesting practical and theoretical implications for those wishing to model the predisposition to bipolar disorder or PTSD.
Acknowledgements
Supported by NIH grants R01 MH076847 and T32 MH65728 directed by FGL. EP conducted this research in partial fulfillment of her requirements for a Ph.D. degree at the University of Texas at Austin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Cloninger CR, Svrakic DM, Przybeck TR. Can personality assessment predict future depression? A twelve-month follow-up of 631 subjects. J. Affect. Disord. 2006;92:35–44. doi: 10.1016/j.jad.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 2.Enns MW, Cox BJ. Personality dimensions and depression: review and commentary. Can. J. Psychiatry. 1997;42:274–284. doi: 10.1177/070674379704200305. [DOI] [PubMed] [Google Scholar]
- 3.Oquendo MA, Galfalvy H, Russo S, Ellis SP, Grunebaum MF, Burke A, Mann JJ. Prospective study of clinical predictors of suicidal acts after a major depressive episode in patients with major depressive disorder or bipolar disorder. Am. J. Psychiatry. 2004;161:1433–1441. doi: 10.1176/appi.ajp.161.8.1433. [DOI] [PubMed] [Google Scholar]
- 4.Richman H, Frueh BC. Personality and PTSD II: personality assessment of PTSD-diagnosed Vietnam veterans using the cloninger tridimensional personality questionnaire (TPQ) Depress. Anxiety. 1997;6:70–77. doi: 10.1002/(sici)1520-6394(1997)6:2<70::aid-da3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Pucilowski O, Overstreet DH, Rezvani AH, Janowsky DS. Chronic mild stress-induced anhedonia: greater effect in a genetic rat model of depression. Physiol Behav. 1993;54:1215–1220. doi: 10.1016/0031-9384(93)90351-f. [DOI] [PubMed] [Google Scholar]
- 6.Vollmayr B, Bachteler D, Vengeliene V, Gass P, Spanagel R, Henn F. Rats with congenital learned helplessness respond less to sucrose but show no deficits in activity or learning. Behav. Brain Res. 2004;150:217–221. doi: 10.1016/S0166-4328(03)00259-6. [DOI] [PubMed] [Google Scholar]
- 7.Henn FA, Edwards E. Animal models in the study of genetic factors in human psychopathology. In: Papolos DF, Lachman HM, editors. Genetic studies in affective disorders: Overview of basic methods, current directions, and critical research issues. New York: John Wiley and Sons; 1994. pp. 177–192. [Google Scholar]
- 8.Shumake J, Barrett D, Gonzalez-Lima F. Behavioral characteristics of rats predisposed to learned helplessness: reduced reward sensitivity, increased novelty seeking, and persistent fear memories. Behav. Brain Res. 2005;164:222–230. doi: 10.1016/j.bbr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Ballaz SJ, Akil H, Watson SJ. Previous experience affects subsequent anxiety-like responses in rats bred for novelty seeking. Behav. Neurosci. 2007;121:1113–1118. doi: 10.1037/0735-7044.121.5.1113. [DOI] [PubMed] [Google Scholar]
- 10.Stead JD, Clinton S, Neal C, Schneider J, Jama A, Miller S, Vazquez DM, Watson SJ, Akil H. Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviors. Behav. Genet. 2006;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- 11.Wang S, Mason J, Charney D, Yehuda R, Riney S, Southwick S. Relationships between hormonal profile and novelty seeking in combat-related posttraumatic stress disorder. Biol. Psychiatry. 1997;41:145–151. doi: 10.1016/S0006-3223(95)00648-6. [DOI] [PubMed] [Google Scholar]
- 12.Jemerin JM, Boyce WT. Psychobiological differences in childhood stress response. II. Cardiovascular markers of vulnerability. J. Dev. Behav. Pediatr. 1990;11:140–150. [PubMed] [Google Scholar]
- 13.Levine S. Developmental psychobiology. In: Brodie HK, editor. American Handbook of Psychiatry. 2nd ed. vol. 6. New York: Basic Books; 1975. pp. 335–351. [Google Scholar]
- 14.Marchei P, Diverio S, Falocci N, Fatjo J, Ruiz-de-la-Torre JL, Manteca X. Breed differences in behavioural development in kittens. Physiol Behav. 2009;96:522–531. doi: 10.1016/j.physbeh.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Pariante CM. Risk factors for development of depression and psychosis. Glucocorticoid receptors and pituitary implications for treatment with antidepressant and glucocorticoids. Ann. N. Y. Acad. Sci. 2009;1179:144–152. doi: 10.1111/j.1749-6632.2009.04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puttonen S, Elovainio M, Kivimaki M, Koskinen T, Pulkki-Raback L, Viikari JS, Raitakari OT, Keltikangas-Jarvinen L. Temperament, health-related behaviors, and autonomic cardiac regulation: the cardiovascular risk in young Finns study. Biol. Psychol. 2008;78:204–210. doi: 10.1016/j.biopsycho.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Sheps DS, Sheffield D. Depression, anxiety, and the cardiovascular system: the cardiologist's perspective. J. Clin. Psychiatry. 2001;(62) Suppl 8:12–16. [PubMed] [Google Scholar]
- 18.Levay EA, Govic A, Hazi A, Flannery G, Christianson J, Drugan RC, Kent S. Endocrine and immunological correlates of behaviorally identified swim stress resilient and vulnerable rats. Brain Behav. Immun. 2006;20:488–497. doi: 10.1016/j.bbi.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Tennes K, Downey K, Vernadakis A. Urinary cortisol excretion rates and anxiety in normal 1-year-old infants. Psychosom. Med. 1977;39:178–187. doi: 10.1097/00006842-197705000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Meaney MJ, Aitken DH, van Berkel C, Bhatnagar S, Sapolsky RM. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239:766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- 21.Shumake J, Edwards E, Gonzalez-Lima F. Hypermetabolism of paraventricular hypothalamus in the congenitally helpless rat. Neurosci. Lett. 2001;311:45–48. doi: 10.1016/s0304-3940(01)02142-5. [DOI] [PubMed] [Google Scholar]
- 22.King JA, Edwards E. Early stress and genetic influences on hypothalamic-pituitary-adrenal axis functioning in adulthood. Horm. Behav. 1999;36:79–85. doi: 10.1006/hbeh.1999.1525. [DOI] [PubMed] [Google Scholar]
- 23.Edwards E, King JA, Fray JC. Increased basal activity of the HPA axis and renin-angiotensin system in congenital learned helpless rats exposed to stress early in development. Int. J. Dev. Neurosci. 1999;17:805–812. doi: 10.1016/s0736-5748(99)00062-3. [DOI] [PubMed] [Google Scholar]
- 24.Edwards E, King JA, Fray J. Hypertension and insulin resistant models have divergent propensities to learned helpless behavior in rodents. Am. J. Hypertens. 2000;13:659–665. doi: 10.1016/s0895-7061(99)00271-x. [DOI] [PubMed] [Google Scholar]
- 25.Lachman HM, Papolos DF, Boyle A, Sheftel G, Juthani M, Edwards E, Henn FA. Alterations in glucocorticoid inducible RNAs in the limbic system of learned helpless rats. Brain Res. 1993;609:110–116. doi: 10.1016/0006-8993(93)90862-h. [DOI] [PubMed] [Google Scholar]
- 26.Carney RM, Rich MW, Freedland KE, Saini J, teVelde A, Simeone C, Clark K. Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom. Med. 1988;50:627–633. doi: 10.1097/00006842-198811000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Carney RM, Freedland KE, Rich MW, Smith LJ, Jaffe AS. Ventricular tachycardia and psychiatric depression in patients with coronary artery disease. Am. J.Med. 1993;95:23–28. doi: 10.1016/0002-9343(93)90228-h. [DOI] [PubMed] [Google Scholar]
- 28.Frasure-Smith N, Lesperance F, Talajic M. The impact of negative emotions on prognosis following myocardial infarction: is it more than depression? Health Psychol. 1995;14:388–398. doi: 10.1037//0278-6133.14.5.388. [DOI] [PubMed] [Google Scholar]
- 29.Zuckerman M, Cloninger CR. Relationships between Cloninger's, Zuckerman's, and Eysenck's dimensions of personality. Personality and Individual Differences. 1996;21:283–285. doi: 10.1016/0191-8869(96)00042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathias CW, Stanford MS. Impulsiveness and arousal: Heart rate under conditions of rest and challenge in healthy males. Personality and Individual Differences. 2003;35:355–371. [Google Scholar]
- 31.Tyrka AR, Wier LM, Anderson GM, Wilkinson CW, Price LH, Carpenter LL. Temperament and response to the Trier Social Stress Test. Acta Psychiatr. Scand. 2007;115:395–402. doi: 10.1111/j.1600-0447.2006.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cammarota M, Bevilaqua LR, Kohler C, Medina JH, Izquierdo I. Learning twice is different from learning once and from learning more. Neuroscience. 2005;132:273–279. doi: 10.1016/j.neuroscience.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 33.Henningsen K, Andreasen JT, Bouzinova EV, Jayatissa MN, Jensen MS, Redrobe JP, Wiborg O. Cognitive deficits in the rat chronic mild stress model for depression: relation to anhedonic-like responses. Behav. Brain Res. 2009;198:136–141. doi: 10.1016/j.bbr.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 34.Hunziker MH, Dos Santos CV. Learned helplessness: effects of response requirement and interval between treatment and testing. Behav. Processes. 2007;76:183–191. doi: 10.1016/j.beproc.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Maier SF, Albin RW, Testa TJ. Failure to learn to escape in rats previously exposed to inescapable shock depends on nature of escape response. Journal of Comparative and Physiological Psychology. 1973;85:581–592. [Google Scholar]
- 36.Overmier JB, Seligman ME. Effects of inescapable shock upon subsequent escape and avoidance responding. J. Comp Physiol Psychol. 1967;63:28–33. doi: 10.1037/h0024166. [DOI] [PubMed] [Google Scholar]
- 37.Padilla E, Barrett D, Shumake J, Gonzalez-Lima F. Strain, sex, and open-field behavior: factors underlying the genetic susceptibility to helplessness. Behav. Brain Res. 2009;201:257–264. doi: 10.1016/j.bbr.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedman BH, Thayer JF. Anxiety and autonomic flexibility: a cardiovascular approach. Biol. Psychol. 1998;49:303–323. doi: 10.1016/s0301-0511(98)00051-9. [DOI] [PubMed] [Google Scholar]
- 39.Pattij T, Groenink L, Hijzen TH, Oosting RS, Maes RA, van der GJ, Olivier B. Autonomic changes associated with enhanced anxiety in 5-HT(1A) receptor knockout mice. Neuropsychopharmacology. 2002;27:380–390. doi: 10.1016/S0893-133X(02)00317-2. [DOI] [PubMed] [Google Scholar]
- 40.Vinkers CH, Breuer ME, Westphal KG, Korte SM, Oosting RS, Olivier B, Groenink L. Olfactory bulbectomy induces rapid and stable changes in basal and stress-induced locomotor activity, heart rate and body temperature responses in the home cage. Neuroscience. 2009;159:39–46. doi: 10.1016/j.neuroscience.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Schulz D, Mirrione MM, Henn FA. Cognitive aspects of congenital learned helplessness and its reversal by the monoamine oxidase (MAO)-B inhibitor deprenyl. Neurobiol. Learn. Mem. 2010;93:291–301. doi: 10.1016/j.nlm.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 42.Janowsky DS, Morter S, Hong L, Howe L. Myers Briggs Type Indicator and Tridimensional Personality Questionnaire differences between bipolar patients and unipolar depressed patients. Bipolar. Disord. 1999;1:98–108. doi: 10.1034/j.1399-5618.1999.010207.x. [DOI] [PubMed] [Google Scholar]
- 43.O'Donnell KC, Gould TD. The behavioral actions of lithium in rodent models: leads to develop novel therapeutics. Neurosci. Biobehav. Rev. 2007;31:932–962. doi: 10.1016/j.neubiorev.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kashdan TB, Hofmann SG. The high-novelty-seeking, impulsive subtype of generalized social anxiety disorder. Depress. Anxiety. 2008;25:535–541. doi: 10.1002/da.20382. [DOI] [PubMed] [Google Scholar]
- 45.Mulder RT, Joyce PR, Cloninger CR. Temperament and early environment influence comorbidity and personality disorders in major depression. Compr. Psychiatry. 1994;35:225–233. doi: 10.1016/0010-440x(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 46.Batra A, Collins SE, Torchalla I, Schroter M, Buchkremer G. Multidimensional smoker profiles and their prediction of smoking following a pharmacobehavioral intervention. J. Subst. Abuse Treat. 2008;35:41–52. doi: 10.1016/j.jsat.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Gabel S, Stallings MC, Schmitz S, Young SE, Fulker DW. Personality dimensions and substance misuse: relationships in adolescents, mothers and fathers. Am. J. Addict. 1999;8:101–113. doi: 10.1080/105504999305901. [DOI] [PubMed] [Google Scholar]
- 48.Galen LW, Henderson MJ, Whitman RD. The utility of novelty seeking, harm avoidance, and expectancy in the prediction of drinking. Addict. Behav. 1997;22:93–106. doi: 10.1016/s0306-4603(96)00018-4. [DOI] [PubMed] [Google Scholar]
- 49.Wills TA, DuHamel K, Vaccaro D. Activity and mood temperament as predictors of adolescent substance use: test of a self-regulation mediational model. J. Pers. Soc. Psychol. 1995;68:901–916. doi: 10.1037//0022-3514.68.5.901. [DOI] [PubMed] [Google Scholar]
- 50.McGirr A, Paris J, Lesage A, Renaud J, Turecki G. Risk factors for suicide completion in borderline personality disorder: a case-control study of cluster B comorbidity and impulsive aggression. J. Clin. Psychiatry. 2007;68:721–729. doi: 10.4088/jcp.v68n0509. [DOI] [PubMed] [Google Scholar]
- 51.Staiger PK, Kambouropoulos N, Dawe S. Should personality traits be considered when refining substance misuse treatment programs? Drug Alcohol Rev. 2007;26:17–23. doi: 10.1080/09595230601036952. [DOI] [PubMed] [Google Scholar]
- 52.Joyce PR, Mulder RT, Cloninger CR. Temperament predicts clomipramine and desipramine response in major depression. J. Affect. Disord. 1994;30:35–46. doi: 10.1016/0165-0327(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 53.Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J. Neurosci. 2000;20:6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papolos DF, Edwards E, Marmur R, Lachman HM, Henn FA. Effects of the antiglucocorticoid RU 38486 on the induction of learned helpless behavior in Sprague-Dawley rats. Brain Res. 1993;615:304–309. doi: 10.1016/0006-8993(93)90042-l. [DOI] [PubMed] [Google Scholar]
- 55.Minor TR, Dess NK, Ben David E, Chang WC. Individual differences in vulnerability to inescapable shock in rats. J. Exp. Psychol. Anim Behav. Process. 1994;20:402–412. [PubMed] [Google Scholar]
- 56.Levay EA, Govic A, Penman J, Paolini AG, Kent S. Effects of adult-onset calorie restriction on anxiety-like behavior in rats. Physiol Behav. 2007;92:889–896. doi: 10.1016/j.physbeh.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 57.Schwarz AM, Schachinger H, Adler RH, Goetz SM. Hopelessness is associated with decreased heart rate variability during championship chess games. Psychosom. Med. 2003;65:658–661. doi: 10.1097/01.psy.0000075975.90979.2a. [DOI] [PubMed] [Google Scholar]
- 58.Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD., Jr Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am. J. Psychiatry. 1999;156:585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- 59.Shumake J, Gonzalez-Lima F. Brain systems underlying susceptibility to helplessness and depression. Behav. Cogn Neurosci. Rev. 2003;2:198–221. doi: 10.1177/1534582303259057. [DOI] [PubMed] [Google Scholar]
- 60.Goodwin FK, Jamison KR. Manic-Depressive Illness. 2nd ed. Oxford: Oxford University Press; 2007. [Google Scholar]