Abstract
Zebrafish are becoming increasingly popular in behavioral neuroscience as investigators have started to realize the benefits of sophisticated genetic tools specifically developed for this species along with the pharmacological tools already available for other laboratory model organisms. The zebrafish has been proposed as an in vivo tool for the analysis of vertebrate fear responses as well as human psychopathological conditions such as anxiety. We have been developing behavioral tasks for zebrafish that could be utilized for screening mutation or drug induced changes in fear responses. In this paper we present a modified version of a previously developed predator avoidance paradigm that now allows the induction and quantification of avoidance reactions that we previously could not elicit. Most importantly, in the current paradigm zebrafish are now shown to respond to the appearance of a moving image of a sympatric predator, the Indian leaf fish, by increasing their distance from the image, a robust reaction that is easy to quantify in an automated manner. Unexpectedly, however, another fear response, the “diving” response, was seen robustly only at the beginning of the test but not in response to the predator stimulus. We discuss the implications of these results and conclude that although zebrafish fear responses are complex and context dependent, the current paradigm is a significant step towards high throughput screening for alterations in fear responses of zebrafish.
Keywords: anxiety, fear, high throughput behavioral test, zebrafish
INTRODUCTION
The zebrafish is gaining popularity in a number of disciplines that can utilize the sophisticated genetic methods already available for this species, the pharmacological tools originally developed for other laboratory model organisms, and also numerous practical advantages of zebrafish, e.g., its small size and prolific nature. Behavioral brain research is one of these disciplines. However, zebrafish in this research area suffers from a significant bottle neck, the paucity of appropriate behavioral paradigms [20]. Behavioral tests, in principle, should have the ability to detect a broad spectrum of functional alterations induced in the brain by numerous manipulations, including genetic or pharmacological [6, 8] and zebrafish seems to be an ideal subject of large scale screens that could detect a range of such alterations [8, 9]. Therefore, development of high throughput behavioral test paradigms is an important goal. Indeed recently there has been an upsurge of publications that focus on the characterization of the behavior of zebrafish and the development of novel behavioral tasks [8]. A subset of these publications concerns the induction and quantification of fear responses (for review see [9]; also see [5, 15]).
Fear responses are important behavioral reactions that may have a significant fitness component. Properly responding to stimuli that represent or signal the presence of danger may allow the animal (and human) to avoid predation or other forms of danger in nature. Thus analysis of these responses may shed light onto the evolution and ecology of the studied species [18]. In addition, analysis of such fear responses may also have clinical relevance. We, and others, have argued that pathologically exaggerated or misdirected forms of fear are likely the result of dysfunction of neurobiological mechanisms that have evolved to serve adaptive behavioral responses associated with danger avoidance [11, 2]. Thus analysis of the mechanisms of these natural behavioral responses should help us understand the mechanisms of their abnormalities as well.
Human anxiety is a psychopathological condition known to occur without any obvious external threat, i.e. in the absence of aversive or pain-inducing stimuli, a disease that still represents a large unmet medical need [3, 22]. Clearly human anxiety cannot be induced or analyzed in animals, but many believe that animal models will play a pivotal role in the unraveling of its mechanisms [2, 13, 16]. In animal research studies including in the latter cited ones, an operational definition is used that more resembles what we call fear. Fear in this case is defined as a behavioral response to aversive or painful stimuli. Some have argued that fear, anxiety, and phobias are related phenomena at least at the mechanistic level. The former is an evolutionarily adaptive response while the latter two are pathologically distorted, exaggerated, temporally prolonged and/or misdirected responses (or behavioral states) that are due to the dysfunction of biological mechanisms that originally evolved to subserve fear responses.
Due to the numerous biological similarities between zebrafish and humans (e.g. neuroanatomy, neurophysiology, protein function, nucleotide sequence homology, etc.), the zebrafish is regarded as a translationally highly relevant laboratory organism [12, 10, 8, 14]. Appropriate behavioral paradigms capable of the induction and quantification of particular behaviors in zebrafish are believed to represent an important step in this research as such paradigms could aid the discovery of novel genes, or compounds, that affect the behavior in question [5, 15, 8, 6].
Previously, we have developed a behavioral paradigm [11] in which certain fear responses (e.g. erratic movement and jumping) could be induced in zebrafish by presenting a visual cue, an animated (moving) image of a sympatric predator of zebrafish, the Indian leaf fish (Nandus nandus). Although presentation of the image did elicit significant fear related responses, one expected behavioral reaction was conspicuously absent form the repertoire: zebrafish did not move away from the predator image upon its presentation. Furthermore, others have reported that under aversive conditions zebrafish exhibited a typical “diving” reaction ([5, 15, 21, also see [9] for most recent review), i.e. swam to and stayed on the bottom of their tank. This response was also absent in our previous paradigm [11] (also see [1]). The absence of these responses was a potentially crucial drawback of the previous paradigm for two main reasons. One, erratic movement and jumping do not occur frequently even under aversive conditions, and this induces stochasticity (increased error variation) in the data. Two, erratic movement and jumping could thus far only be measured using time consuming observation-based methods [11]. Although we have shown that sophisticated video-tracking parameters such as temporal (within-individual) variability of velocity and temporal variability of absolute turn angle do correlate with these motor patterns [11], these latter video-tracking measures do not precisely quantify erratic movement and jumping as they are influenced by many other motor responses too. The location of the fish (swimming away from the predator stimulus or staying on the bottom) is much simpler to quantify and, if affected by the fear inducing stimulation, should represent a more reliable way of measuring antipredatory responses. The goal of the current paper is to test if a simple modification of the test environment will allow better induction of these easy to quantify location-based avoidance reactions in zebrafish.
It is well documented in the literature that fear may manifest in several ways in fish (for most recent review see [4]). Fish may freeze, hide or, alternatively, choose active escape depending on environmental circumstances. There may be several successful strategies with which the prey may avoid or minimize predation. Thus we proposed [11] that perhaps certain features of the test environment biased zebrafish’s antipredatory reactions toward a strategy that did not include active swimming away from the predator or diving to the bottom of the tank. We proposed that the physical constraints of the small test environment made it impossible for us to elicit such responses in zebrafish. This is the question we are addressing in the current study. In preliminary pilot experiments we have observed (personal observations) that zebrafish tended to stay away from live Indian leaf fish and that the leaf fish’s striking distance (the distance between the leaf fish and the potential zebrafish prey within which the leaf fish responds with a quick ambush reaction) is on average 35–45 cm (also see [1, 21]). We hypothesize that by offering a larger (longer) test tank that allows zebrafish to stay outside of this striking distance, zebrafish will be able to show avoidance reactions including moving away from the stimulus (active avoidance) and spending more time on the bottom of the tank (passive avoidance).
METHODS
Animals and Housing
Experimental subjects (n = 60) were adult (on average 8 months old) sexually mature male and female (50–50%) zebrafish of the AB strain bred in-house in our vivarium. The fish were of the third filial generation from progenitors obtained from the Zebrafish International Resource Center (ZIRC, Eugene, Oregon). The procedural details of our study, including housing, maintenance, and general experimental methods were identical to those described previously [11]. Briefly, the fry were raised in 1.3 liter nursery racks and fed Artemia salina (brine shrimp) nauplii until their age of 3 weeks post fertilization, after which the fish were transferred to a high density multi-stage filtration zebrafish rack (Aquaneering Inc, San Diego California, USA) where they remained in 2.8 liter tanks (15–20 fish per tank) and fed a mixture of Tetramin and spirulina flakes.
Test apparatus and procedure
As opposed to the previously employed [11] 50cm × 25cm × 30cm, (length × width × height) tank, now we used a 100cm × 25cm × 30cm (length × width × height) tank, i.e. one which was twice as long as before. A flat LCD computer monitor (17 inch Samsung SyncMaster 732N) was placed on the left and right side of the tank adjacent and parallel to the short side wall of the tank. Each monitor was connected to a Dell Vostro 1000 laptop running a custom made software application that allowed the presentation of the animated predator, a 15 cm long color photograph (side view) of the Indian leaf fish moving with 0.3 cm/sec speed as described before [11]. The experimental tank was illuminated by a 15 W fluorescent light-tube from above. The back side and the bottom of the test tank were coated with dark green plastic sheets to increase the contrast and reduce glare and reflections for videotracking analysis. The rationale for the elongated tank was that an experimental zebrafish swimming in new experimental tank was, on average, 50 cm away from the stimulus screen placed next to each end of this tank, a distance that is just outside of the previously observed striking distance of the Indian leaf fish.
Each zebrafish was tested singly and only once, and the stimulus (the moving image of the Indian leaf fish) was presented three times (for 1 min each) at the 11th, 15th, and 19th minute of the 19 min behavioral recording session (here our recording session was longer than what we reported on before [11] as we here include the analysis of the habituation period). Some fish were shown the stimulus on only one of the sides of the tank while others received different alternating sequences, and the order of these different stimulus presentation protocols was randomized. The behavioral sessions were recorded and later quantified using EthoVision Color Pro (Noldus Info Tech., Wageningen, The Netherlands) as described before [11]. The tracking system identified the position of the subject and recorded it once every 0.1 sec (10 Hz recording frequency) thus each fish received 600 position coordinate pairs (two dimensional tracking) per minute. Behavioral measures analyzed based upon these coordinates included distance from stimulus screen, distance from bottom, velocity and turn angle, which were expressed as a mean of the values quantified within one minute intervals. In addition, the temporal (within individual) variability of these measures was also calculated and is expressed as the variance of values per individual subject obtained for each one-minute interval.
Statistical analysis
Data were analyzed using repeated measure Variance Analyses (ANOVAs) to investigate whether there were any differences between intervals. Post hoc multiple comparison tests are not appropriate for repeated measure designs and thus to avoid type-1 error, instead of comparing all intervals with each other, we calculated scores (Δ1, Δ2, Δ3 and B) reflecting the numerical differences between certain intervals or between the averages of certain intervals as explained below. The difference scores were compared to the value ‘zero’ using one-sample t-tests as explained below. Because two difference scores were calculated for the ‘distance from stimulus screen’ and the ‘temporal variability of distance from stimulus screen’ and three difference scores were calculated for the ‘temporal variability of distance from bottom’ variables respectively and the ‘distance from bottom’ variable, we employed a Bonferroni correction and set the threshold value for rejecting the null hypothesis at p < 0.05/2 (i.e. p < 0.025) for the former two variables and p < 0.05/3 (i.e. p < 0.017) for the latter two variables.
RESULTS
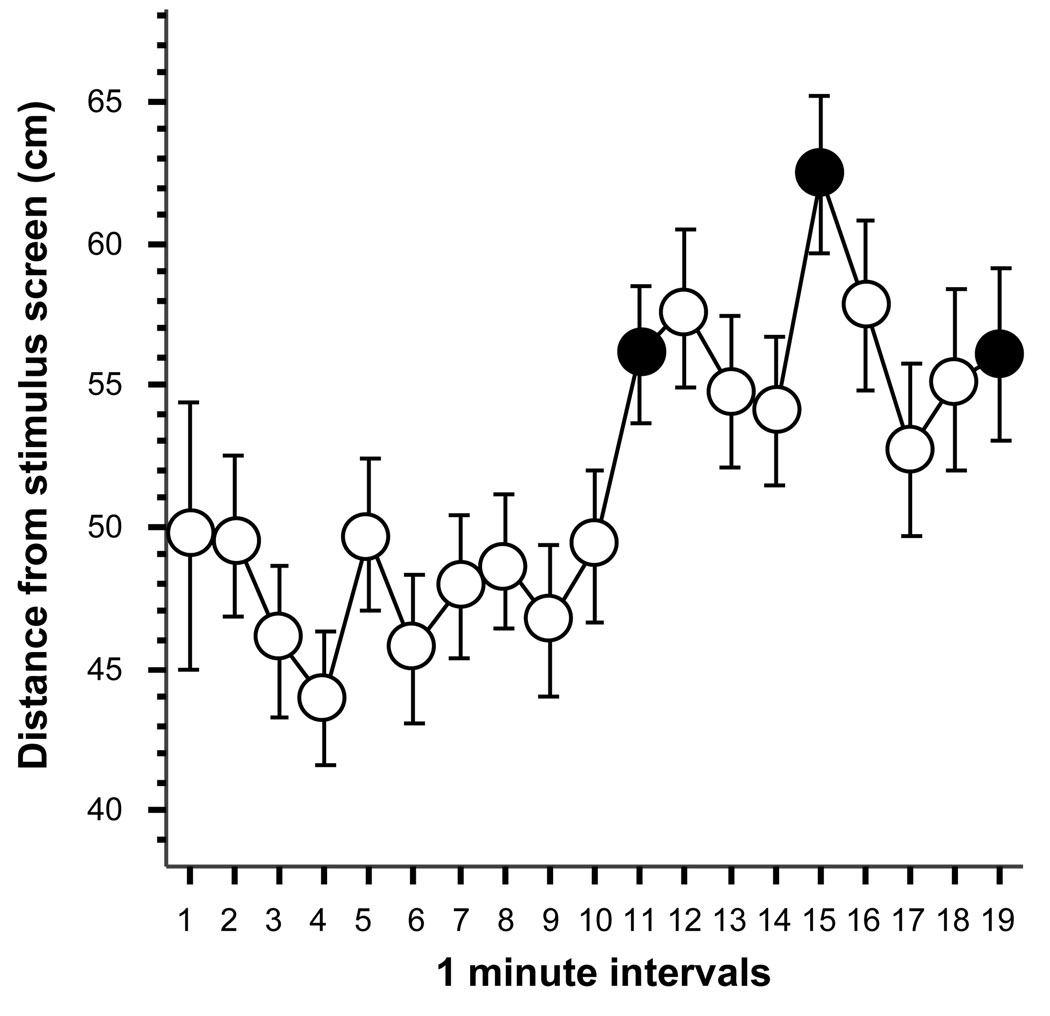
The analyses showed that the side of stimulus presentation or the sex of the fish had no significant effect and thus data were pooled across these factors. The results confirmed what we found before and showed significantly reduced velocity, increased temporal variability of velocity, increased turn angle, and increased temporal variability of turn angle in response to the presentation of the stimulus (data not shown). Importantly, however, our results now also showed significant stimulus effects for distance from the stimulus screen (Figure 1; ANOVA interval effect F(18, 1062) = 3.604, p < 0.0001). We calculated the average difference between the distance from stimulus screen obtained for the one minute interval of stimulus presentation and for the one min interval immediately preceding it: Δ1 = (ΣPi – ΣCi−1)/3 (where P is the interval of predator stimulus presentation, C is the control (no stimulus) interval and i represents the interval number equaling either 11, 15, or 19). The mean and SEM for this difference score was 5.34 ± 2.16, and the one sample t-test showed this score to be significantly above zero (t = 2.47, df = 59, p = 0.016). Thus we conclude that the distance from the stimulus screen significantly increased in response to the predator image presentation as compared to the prior no stimulus interval. From figure 1 it is also apparent that the stimulus presentation increased the distance from the stimulus screen not only while the stimulus was being presented but also it had a carryover effect. That is, during the intervals that followed the stimulus presentation the distance from the stimulus screen appeared higher compared to the distance during the intervals before the first stimulus was shown. We tested the validity of this observation by calculating the average of distance values before the first stimulus presentation interval and subtracted this value from the average of distance values after the first stimulus presentation interval (including only those intervals during which the predator was not shown) as follows: Δ2 = ΣaPi/6 – ΣbPj/10 (where aP represents the 1-min intervals after the first predator stimulus excluding all subsequent predator presentation intervals, i.e. i = 12, 13, 14, 16, 17, 18; and bP represents all 1-min intervals before the first predator stimulus, i.e. j = from 1 to10, the habituation period). One sample t-test comparing the distance increase (7.65±2.40) to zero revealed a significant effect (t = 3.18, df = 59, p < 0.01). This result implies the development of significant short term memory, avoidance of the side of the tank where the stimulus used to be shown even during intervals when the stimulus was not being presented.
Figure 1.
The distance from the stimulus screen significantly increases in zebrafish in response to the presentation of a moving image of the Indian leaf fish. Mean ± SEM are shown. Sample size n = 60. Empty circles represent 1-min intervals during which the predator image was not shown (blank stimulus screen). Black filled circles represent the 1-min intervals during which the predator image was presented.
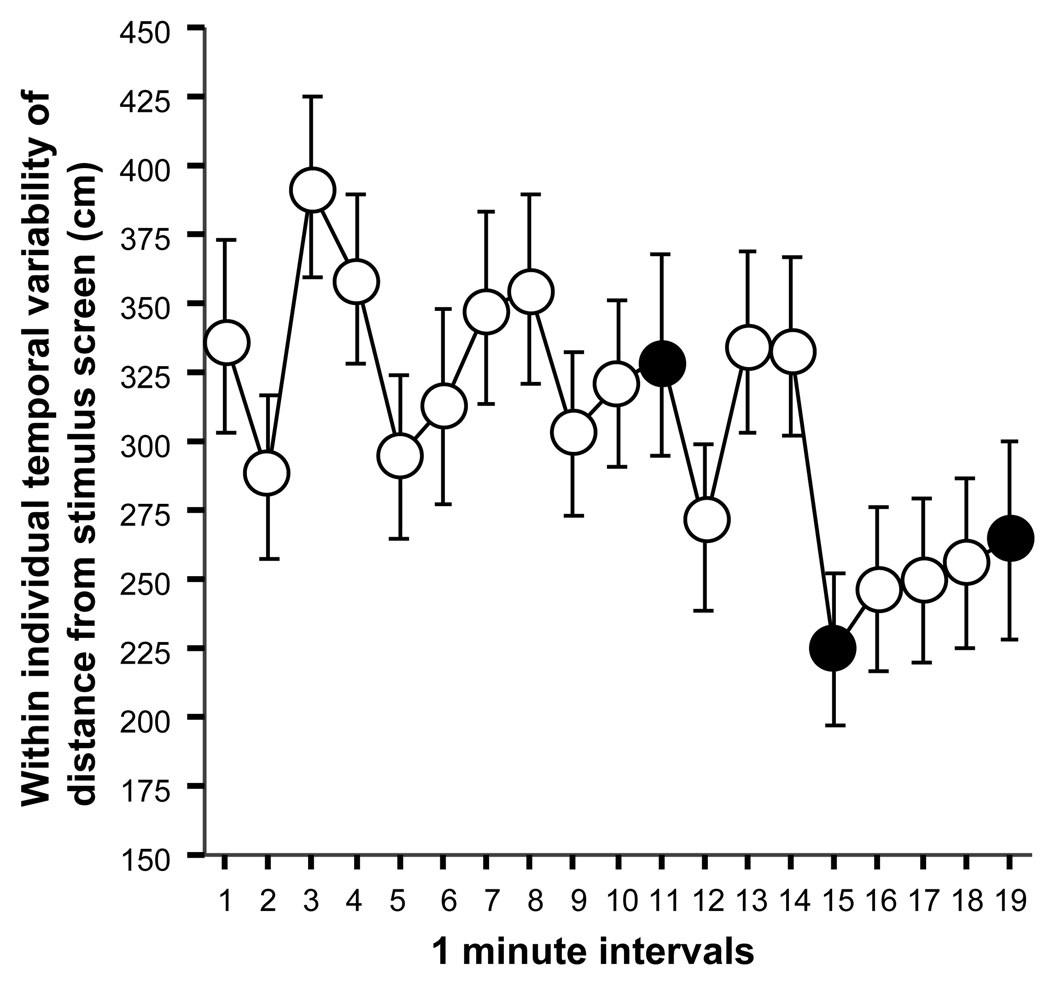
Figure 2 shows the temporal variability of distance from the stimulus screen. Importantly, this variability does not reflect between individual variability but rather refers to within individual variability, i.e. it is the measure of the temporal consistency of the behavioral response with which each individual fish reacted. ANOVA demonstrated a significant interval effect (F(18, 1062) = 2.850, p < 0.0001). It appears that while the actual distance from stimulus screen increased (figure 1), the temporal variability of this response diminished (figure 2). Analysis of the effect of the stimulus presentation compared to the prior interval (the Δ1 difference score, see above) showed that its value, although apparently in the negative range (mean = −30.2, SEM = 23.9) did not significantly differ from zero (t = −1.264, df = 59, p > 0.05). Nevertheless, the difference between the average of the intervals before the first stimulus presentation and after it (the Δ2 difference score, see above) did show a significant decrease (mean = −49.2, SEM = 20.3; t = −2.421, df = 59, p = 0.019) suggesting that zebrafish that has seen the predator image decreased the temporal variability of their distance from the stimulus screen, i.e. stayed consistently farther away from the screen that used to show the predator even when the predator was not being shown.
Figure 2.
The within individual (temporal) variability of the distance from the stimulus screen significantly decreases in zebrafish in response to the presentation of a moving image of the Indian leaf fish. Mean ± SEM are shown. Sample size n = 60. Empty circles represent 1-min intervals during which the predator image was not shown (blank stimulus screen). Black filled circles represent the 1-min intervals during which the predator image was presented.
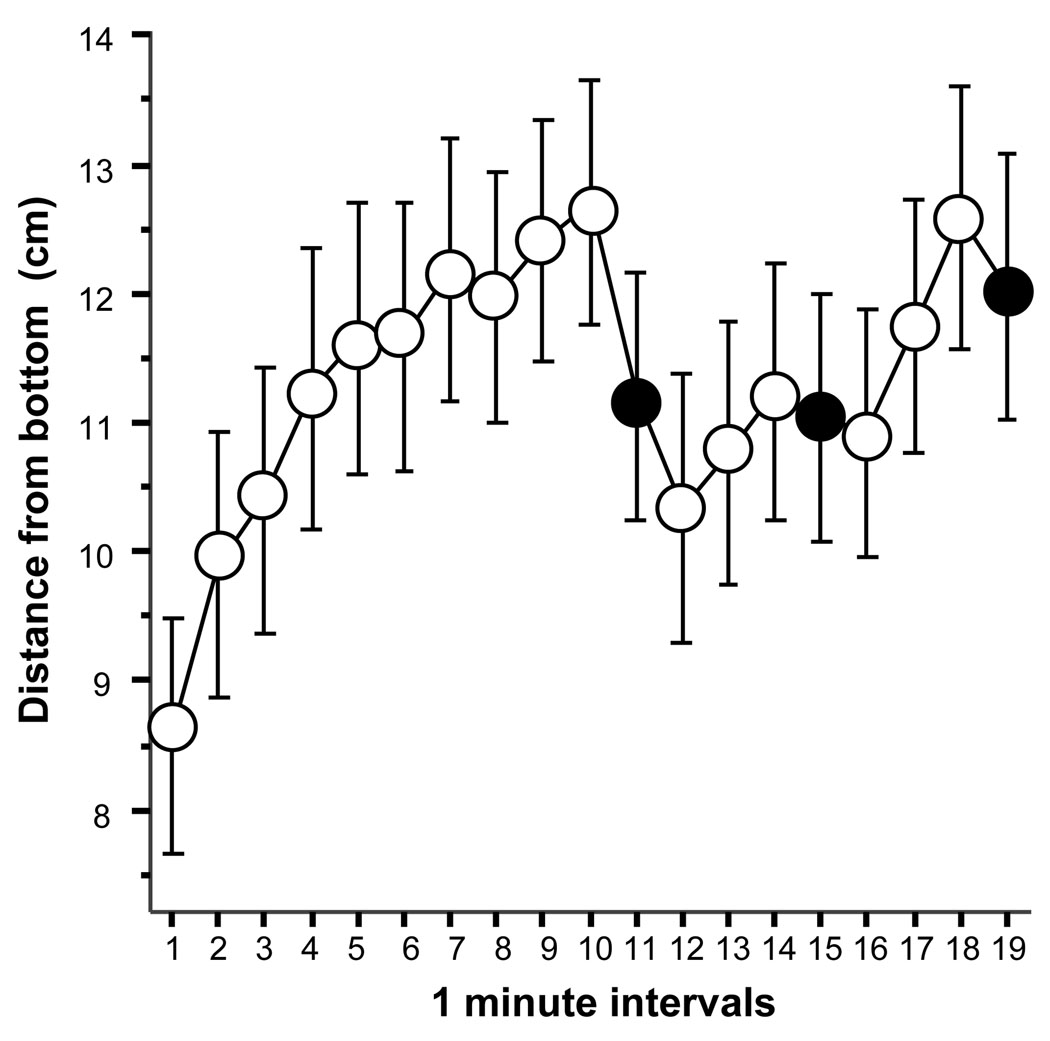
The analysis of distance from bottom also revealed some interesting behavioral changes (figure 3). ANOVA found a significant interval effect (F(18, 1062) = 3.766, p < 0.0001). Figure 3 suggests that there was an initial and gradual increase in the distance from bottom during the habituation period, i.e. before the first stimulus presentation interval, but in response to the stimulus the distance from bottom decreased again. In order to minimize type-1 error, instead of comparing every interval to each other during the habituation period (first 10 intervals), we averaged the first half (1 – 5 min) and the second half (6–10 min) of the habituation period and calculated the difference between them as follows: B = ΣHi+5/5 – ΣHi,/5 where Hi is the distance from bottom value corresponding to the ‘i’ 1 min interval of the habituation period with ‘i’ running from 1 to 5. We then tested whether this difference score is significantly different from zero. The difference score (mean = 9.13, SEM = 3.51) was found to be significantly above zero (one sample t test, t = 2.605, df = 59, p = 0.012) demonstrating that the distance from the bottom increased in the second half of the habituation session compared to the first. This result confirms what others have found (Egan et al., 2009; Levin et al., 2007): novelty itself has fear inducing properties and zebrafish in a novel environment exhibit elevated dwell time on the bottom of their test tank, a response that habituates (the distance increases) with time. Next, we asked the question whether presentation of the predator stimulus could lead to dishabituation, i.e. whether the distance from bottom is decreased in response to the stimulus. We calculated the difference between the value of the second half of the habituation period and the average of intervals 12, 13, 14, 16, 17, 18 (Δ3 = ΣaPi/6 – ΣbPj/5 (where aP represents the 1-min intervals after the first predator stimulus excluding predator presentation intervals, i.e. i = 12, 13, 14, 16, 17, 18; and bP represents the five 1-min intervals before the first predator stimulus, i.e. j = from 6 to10). The mean of the difference score (Δ3) was −0.923 (SEM = 0.619) indicating an apparent reduction of distance from bottom in response to the stimulus presentation, but this reduction turned out to be non-significant (one sample t-test to compare the difference score to zero, i.e. no change, t = 1.49, df = 59, p > 0.05). These results indicate that the stimulus presentation had no carryover effect for intervals that followed the stimulus presentation. We also examined whether the presentation of the predator stimulus changed the distance from bottom as compared to the distance from bottom value obtained for the 1 min intervals immediately preceding the stimulus presentation. We calculated the difference score as described above (Δ1) and compared its value to zero using a one sample t test. The result again showed that the stimulus had no significant effect (t = 0.696, df = 59, p > 0.05).
Figure 3.
The distance from the bottom does not significantly decrease in zebrafish in response to the presentation of a moving image of the Indian leaf fish. Mean ± SEM are shown. Sample size n = 60. Empty circles represent 1-min intervals during which the predator image was not shown (blank stimulus screen). Black filled circles represent the 1-min intervals during which the predator image was presented.
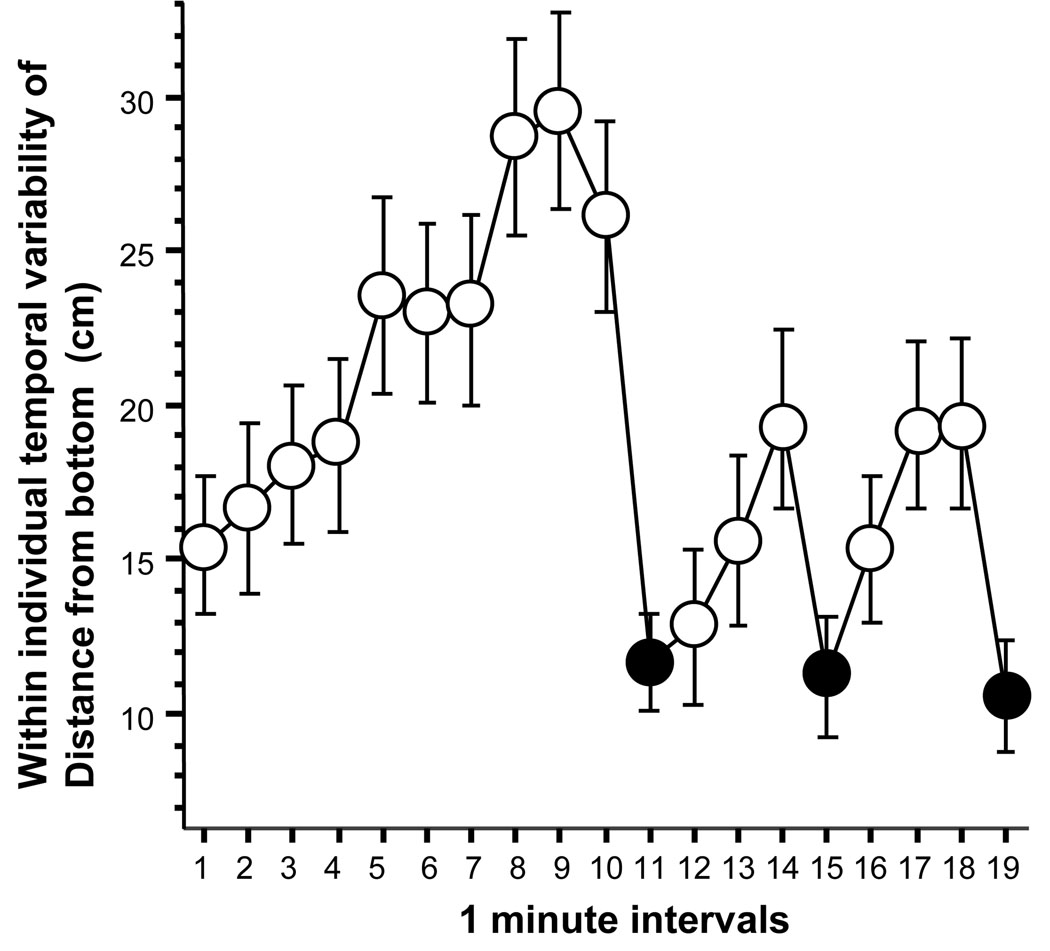
Last, we analyzed the temporal variability of distance from bottom (figure 4). ANOVA indicated a significant interval effect (F(18, 1062) = 7.150, p < 0.0001) and figure 4 again showed an apparent increase of temporal variability during the habituation period. Thus, first we tested whether there was a significant change from the first half to the second half of the habituation period and calculated the difference score (B) and compared it to zero as described above. The results (mean = 37.91, SEM = 10.13) indicated a significant increase of within individual temporal variability (t = 3.74, df = 59, p < 0.0001). Subsequently, we calculated the difference score (Δ3) and compared it to zero. The results (mean = −9.07, SEM = 2.12) showed a significant reduction of within individual temporal variability (t = −4.29, df = 59, p < 0.0001). Similarly, the difference between the stimulus presentation interval and the interval preceding it (difference score Δ1) was also found to be significant (mean = −10.52, SEM = 1.82; t = −581, df = 59, p < 0.0001). These results suggests that although on average zebrafish did not change their vertical position in the water column in response to the predator image, they did reduce the variability of their vertical positioning, i.e. moved less up and down both during predator image presentation and during the intervals that followed the presentation.
Figure 4.
The within individual (temporal) variability of distance from the bottom significantly decreases in zebrafish in response to the presentation of a moving image of the Indian leaf fish. Mean ± SEM are shown. Sample size n = 60. Empty circles represent 1-min intervals during which the predator image was not shown (blank stimulus screen). Black filled circles represent the 1-min intervals during which the predator image was presented.
DISCUSSION
Previously, we have shown that the computer animated image of the Indian leaf fish is sufficient to induce behavioural reactions that are indistinguishable from those elicited by the living predator whether it was presented in a separate tank (visual cues only) or in the same tank (visual, olfactory and auditory and lateral line cues) with the zebrafish [11, 1]. Our results thus showed that this computerized image, which one can precisely control in every respect (including the timing and location of its presentation), is practically equivalent to the live predator. In addition, we also showed that the videotracking quantified parameters including velocity, turn angle and the temporal variability of these parameters correlated well with the manually quantified motor patterns, erratic movement and jumping (leaping), that typify fear in zebrafish. Briefly, in this previous publication [11] we have shown that both automated stimulus delivery and computerized quantification of the behavioural responses are possible, and thus argued that the paradigm we developed is scaleable and will be appropriate for high throughput screening. However, one drawback of our system concerned the relatively sophisticated tracking method needed to quantify the behavioural responses. Tracking systems are widely available of course but these systems are quite sensitive, for instance, to illumination levels (reflections, shades, etc.) as well as small errors including even the slightest change in the camera angle, or air bubbles forming in the water, etc. While many of the tracking systems have numerous ways to compensate for such errors, ultimately, they may not be the ideal way to quantify behaviour in screening applications especially if the behaviour to be quantified is a complex motor pattern such as erratic movement. The second problem we faced was that although we did find significant predator and predator image induced increases in erratic movement and jumping (leaping), these behaviors are exhibited by zebrafish only in a sporadic manner, i.e. quite infrequently even under aversive conditions including aversive visual or olfactory stimuli [1, 11, 17, 21]. The infrequent appearance of these behaviors can potentially lead to stochasticity, and thus increased error variation, in the data and thus these behavioral measures may not be the most reliable way to quantify fear responses and mutation or drug induced alterations in such responses.
Our original goal was to show that simple and easy to measure behavioral parameters may be used for the quantification of antipredatory responses. We were hoping to find zebrafish to move away from the predator presentation screen and/or stay closer to the tank bottom in response to the stimulus. These responses would have been less prone to tracking errors and would have been easier to measure even with simple open field-type photocell arrays employed in rodent research. Our current results suggest that now this is possible. In the longer test tank zebrafish exhibited a robust avoidance reaction and moved significantly farther away from the stimulus screen when it was showing the image of the predator. Why could not we find this reaction previously? We hypothesized that in the previous set up the experimental subject was confined and was too close to the stimulus screen even if it moved to the side of the tank opposite to the stimulus presentation screen. Briefly, we argued that perhaps in this smaller tank zebrafish remained within striking distance from the “predator” and thus simply moving away from the stimulus would not have been an adaptive strategy [11]. In numerous fish species, including the paradise fish (Macropodus opercularis), antipredatory behaviour was found to be context dependent (e.g. [7] and references therein). When paradise fish was in close physical proximity to its sympatric predator, instead of an escape reaction the fish performed elaborate fin erection displays whose function may have been to advertise good health status and make them look bigger for the approaching predator [7]. Furthermore, whether and with what behavioural response the prey fish react to their predator was found to depend upon many factors related to perceived risk and cost of escaping, such as the attack speed and size of the predators, the proximity to refuges, and engagement in other activities (for review see [4]).
A similar argument may be made with regard to finding no decreases in the distance from the bottom in response to the predator image in our current study. Previously others [5, 15] have shown that in novel environments zebrafish tended to stay close to the bottom of their test tank, a response that habituated (diminished) as the fish became acclimatized to the novel environment. We also found that under aversive conditions (due to the delivery of natural but not the chemically synthesized alarm substance) zebrafish increased their bottom dwell time [17, 21]. This response, termed “diving”, was regarded as a measure of fear [5, 15]. Our current study also nicely demonstrated this phenomenon, i.e. the decreased distance from bottom in the novel tank, a response that was prominent at the beginning of the behavioral recording session in the novel tank and one which habituated over time (presumably because the fish became more accustomed to and less afraid of the test tank). However, our results also showed (confirming our previous results) that the predator image presentation did not elicit the diving response. It is possible that the lack of hiding places (empty tank) on the bottom or the physical dimensions of the tank (large and long tank) facilitated active escape and avoidance reactions, and the diving response, which is often followed by immobility (freezing), a passive avoidance reaction, was not the optimal strategy zebrafish would chose in this set up. Briefly, it appears that antipredatory or fear paradigms may need to be “tweaked” in several ways to achieve optimal characteristics, i.e. increase their ability to induce robust and easy to quantify behavioral reactions, and what reaction is induced depends on a number of factors that one will have to systematically analyze. Such systematic analysis will lead to further optimization of fear inducing behavioral paradigms for zebrafish. Nevertheless, we propose that our current paradigm is a significant step towards optimality and may already be appropriate for high throughput screening applications as it provides automated stimulus delivery and automated quantification of a simple and robust behavioral response (distance from stimulus screen). However, an important set of questions must be addressed before the actual screening use of this paradigm: its validity as a fear/anxiety test.
Investigators [2, 16, 19] including us [9, 11] have argued that tasks utilizing natural fear inducing stimuli have face validity. Zebrafish are known to perform certain motor and posture patterns, responses typical under fear inducing or painful stimulation [1, 21, 11, 15]. From this standpoint, the current paradigm also appears to have face validity: it induced a spectrum of behaviors (increased turn angle and speed, increased variability of turn angle and speed, as well as increased distance from the predator stimulus) that has been shown to be associated with fear in zebrafish. However, the relevance of the current paradigm may also have to be determined in terms of construct as well as predictive validity. The former will need to be established by disentangling the mechanisms of zebrafish fear responses and by finding genes and/or biochemical pathways whose homology can be tested against other vertebrate, including human, counterparts. The latter, i.e. predictive validity, will be tested by utilizing classical anxiogenic and anxiolytic drugs, a research that is currently under way in multiple laboratories including ours.
Acknowledgements
This study was supported by NIH/NIAAA grant to RG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bass SLS, Gerlai R. Zebrafish (Danio rerio) responds differentially to stimulus fish: The effects of sympatric and allopatric predators and harmless fish. Behav. Brain Res. 2008;186:107–117. doi: 10.1016/j.bbr.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard DC, Griebel G, Blanchard RJ. Conditioning and residual emotionality effects of predator stimuli: some reflections on stress and emotion. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1177–1185. doi: 10.1016/j.pnpbp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Choy Y, Fyer AJ, Goodwin RD. Specific phobia and comorbid depression: a closer look at the National Comorbidity Survey data. Compr Psychiatry. 2007;48:132–136. doi: 10.1016/j.comppsych.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Domenici P. Context-dependent variability in the components of fish escape response: integrating locomotor performance and behavior. J Exp Zool A Ecol Genet Physiol. 2010;313:59–79. doi: 10.1002/jez.580. 2007. [DOI] [PubMed] [Google Scholar]
- 5.Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, Elkhayat SI, Bartels BK, Tien AK, Tien DH, Mohnot S, Beeson E, Glasgow E, Amri H, Zukowska Z, Kalueff AV. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerlai R. Phenomics: Fiction or the Future? Trends Neurosci. 2002;25:506–509. doi: 10.1016/s0166-2236(02)02250-6. [DOI] [PubMed] [Google Scholar]
- 7.Gerlai R. Can paradise fish (Macropodus opercularis) recognize its natural predator? An ethological analysis. Ethology. 1993;94:127–136. [Google Scholar]
- 8.Gerlai R. High-throughput Behavioral Screens: the First Step towards Finding Genes Involved in Vertebrate Brain Function Using Zebrafish. Molecules. 2010;15:2609–2622. doi: 10.3390/molecules15042609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerlai R. Zebrafish antipredatory responses: A future for translational research? Behav. Brain Res. 2010;207:223–231. doi: 10.1016/j.bbr.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerlai R. Zebra fish: An uncharted behavior genetic model. Behav. Genet. 2003;33:461–468. doi: 10.1023/a:1025762314250. [DOI] [PubMed] [Google Scholar]
- 11.Gerlai R, Fernandes Y, Pereira T. Zebrafish (Danio rerio) responds to the animated image of a predator: Towards the development of an automated aversive task. Behav. Brain Res. 2009;201:318–324. doi: 10.1016/j.bbr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerlai R, Lahav M, Guo S, Rosenthal A. Drinks like a fish: Zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav. 2000;67:773–782. doi: 10.1016/s0091-3057(00)00422-6. [DOI] [PubMed] [Google Scholar]
- 13.Hendrie CA, Weiss SM, Eilam D. Exploration and predation models of anxiety: evidence from laboratory and wild species. Pharmacol Biochem Behav. 1996;54:13–20. doi: 10.1016/0091-3057(95)02176-0. [DOI] [PubMed] [Google Scholar]
- 14.Ingham PW. The power of the zebrafish for disease analysis. Hum Mol Genet. 2009;18(R1):107–112. doi: 10.1093/hmg/ddp091. [DOI] [PubMed] [Google Scholar]
- 15.Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiol Behav. 2007;90:54–58. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Lister RG. Ethologically-based animal models of anxiety disorders. Pharmacol Ther. 1990;46:321–340. doi: 10.1016/0163-7258(90)90021-s. [DOI] [PubMed] [Google Scholar]
- 17.Parra KV, Adrian JC, Jr, Gerlai R. The synthetic substance hypoxanthine 3-N-oxide elicits alarm reactions in zebrafish (Danio rerio) Behav Brain Res. 2009;205:336–341. doi: 10.1016/j.bbr.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reznick DN, Ghalambor CK, Crooks K. Experimental studies of evolution in guppies: a model for understanding the evolutionary consequences of predator removal in natural communities. Mol Ecol. 2008;17:97–107. doi: 10.1111/j.1365-294X.2007.03474.x. [DOI] [PubMed] [Google Scholar]
- 19.Rosen JB, Pagani JH, Rolla KL, Davis C. Analysis of behavioral constraints and the neuroanatomy of fear to the predator odor trimethylthiazoline: a model for animal phobias. Neurosci Biobehav Rev. 2008;32:1267–1276. doi: 10.1016/j.neubiorev.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Sison M, Cawker J, Buske C, Gerlai R. Fishing for genes of vertebrate behavior: Zebra fish as an upcoming model system. Lab Animal. 2006;35:33–39. doi: 10.1038/laban0506-33. [DOI] [PubMed] [Google Scholar]
- 21.Speedie N, Gerlai R. Alarm substance induced behavioral responses in zebrafish (Danio rerio) Behav. Brain Res. 2008;188:168–177. doi: 10.1016/j.bbr.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tyrer P, Baldwin D. Generalised anxiety disorder. Lancet. 2006;16:2156–2166. doi: 10.1016/S0140-6736(06)69865-6. [DOI] [PubMed] [Google Scholar]