Abstract
This paper explicates a theory that oxytocin, a sexually dimorphic neurotransmitter and paracrine hormone, is a plausible mechanism linking early relational trauma with posttraumatic self disorders (e.g., dissociation, somatization, and interpersonal sensitivity), posttraumatic stress disorder per se and pelvicvisceral dysregulationdisorders (e.g., irritable bowel syndrome, chronic pelvic pain, interstitial cystitis, and hyperemesis gravidarum). This Posttraumatic Oxytocin Dysregulation Disorders theory is consistent with historic and contemporary literature. It integrates attention to psychological and physical comorbidities and could account for the increased incidence of these disorders among females. Specific propositions are explored in data from studies of traumatic stress and women's health.
Keywords: affect regulation, attachment theory, chronic pelvic pain, dissociation, hyperemesis gravidarum, interstitial cystitis, irritable bowel syndrome, oxytocin, posttraumatic stress, women's health
Is posttraumatic oxytocin dysregulation a link among posttraumatic self disorders, posttraumatic stress disorder, and pelvicvisceral dysregulation conditions in women?
Overview
Oxytocin is a neuropeptide which, as both a neurotransmitter and paracrine hormone, regulates multiple normative psychological, social, and physical functions: attachment and affiliation, including maternal behavior and pair bonding; stress regulation and memory under stressful conditions; and smooth muscle contractility for digestive, sexual, reproductive, and lactation processes. Less understood is what happens across psychological, social, and physical domains when oxytocin is dysregulated. This theory presentation considers the hypothesis that dysregulated oxytocin is a) a plausible biological mechanism common to psychiatric and physical comorbidity that results from early relational trauma (ERT) and b) a potentiating mechanism for hypothalamic-pituitary-adrenal (HPA) axis dysregulation and posttraumatic stress disorder (PTSD) when childhood maltreatment trauma (CMT) has also occurred. Oxytocin is sexually dimorphic, and the health conditions of interest affect women more than men; therefore this theory explication focuses on women.
Introduction
Although psychological and physical manifestations of early trauma were joined under the concept of “hysteria” at the turn of the last century, contemporary Western health systems tend to divide care of the mind and body into separate clinical domains. There are a handful of “medically unexplained” or “medically idiopathic” functional physical conditions that affect primarily women. These conditions are treated in medical settings,and usually without attention to their potential comorbidity with posttraumatic psychiatric conditions (Roy-Byrne et al., 2007). These conditions map onto the list of somatization disorder symptoms in the taxonomies of the Diagnostic and Statistical Manual of Mental Disorders (DSM, APA, 1994) and the International Classification of Diseases (ICD, WHO, 2007). Associated features of somatization disorder include comorbidity with anxiety and depression and medical treatment-seeking in multiple settings. Functional conditions such as irritable bowel syndrome (IBS) are stated to count as somatic symptoms. IBS and other gastrointestinal (GI) and genitourinary (GU) tract symptoms (i.e., pain or abnormal contractions or peristalsis in pelvic and visceral tissues) often co-occur in the same women. It is widely acknowledged that the diagnosis given depends upon the clinical setting she visits (e.g., chronic pelvic pain (CPP) in gynecology, IBS in gastroenterology, interstitial cystitis (IC) in urology or hyperemesis gravidarum (HG) in obstetric settings) (Wessely, Nimnuan, & Sharpe, 1999; Engel, 2005).
A goal of this theorizing that posttraumatic oxytocin dysregulation (POD) is a mechanism of pelvic visceral dysregulation (PVD) is to return attention to the posited early childhood (sexual) trauma etiology of “hysteria” common to the early work of Freud (Herman, 1992) and the works of Charcot and Janet (van der Hart, Steele, & Nijenhuis, 2006). The DSM somatization disorder diagnostic criteria are written consistent with the manual's aim to be atheoretical and thus non-etiological. However, seen through the lenses of trauma theory and attachment theory, the list of associated features and the sex and gender disparity in prevalence rates suggest somatization disorder could well be a posttraumatic-specific disorder manifesting in the body. The reality that women with traumatic stress histories preferentially seek treatment in medical settings is very understandable given the avoidance characteristic (and diagnostic criterion) of PTSD. The fact that all of the gastrointestinal (GI) and genitourinary (GU) signs and symptoms are non-specific and also occur in people without childhood maltreatment or sexual trauma history muddies the water because medical providers rarely are able to do the psychosocial and psychiatric history-taking needed to obtain disclosure of trauma history and posttraumatic spectrum diagnoses. Nonetheless, the mounting evidence that posttraumatic spectrum disorders may be mediators of physical morbidity makes it imperative that we move toward integrated theories, studies, and eventually therapeutics for this clinical population.
The call for integrating psychological and medical therapies for these functional diagnoses is not new (e.g., Irwin et al, 1996;Milburn, Reiter, & Rhomberg, 1993). The novel contribution of the theory explicated in this paper is to hypothesize a plausible biological mechanism: posttraumatic oxytocin dysregulation. In the simplest possible terms: Oxytocin affects how smooth muscle contracts. Smooth muscle works involuntarily in the GI and GU tracts. Oxytocin in the blood operates on smooth muscle by attaching to receptors. The oxytocin system operates in the brain and body, and it can signal from body to brain and vice-versa via hormonal or vagal neural pathways (Uvnas Moberg, 2003). The number of receptors and the concentration of oxytocin in the blood can vary by individual in response to numerous factors, including stress or distress (Liberzon & Young, 1997). Thus, a posttraumatic trigger, for example, intrusive memory of oral rape, could elicit a wave of upward GI peristalsis (e.g. nausea and gagging). Conversely, an instance of upward GI peristalsis (e.g. nausea from slightly spoiled food) could trigger a posttraumatic intrusive memory of oral rape. In many laboratory studies on how normative phenomena affect oxytocin levels (e.g., effects of warm touch, partner hugs, or breastfeeding; Holt-Lunstad, Birmingham, & Light, 2008, Light, Grewen, & Amico, 2005, White-Traut et al, 2009), higher oxytocin level is associated with the beneficial condition. Data from recent studies of psychopathology, however, suggest that trauma (Pierrehumbert et. al., 2010) and severe PTSD (Seng, Miller,Sperlich, van de Ven, Liberzon & Carter, under review), as well as depression in women (Cyranowski et. al., 2008) are associated with very high or more pulsatile OT levels, and that very low OT levels are associated with schizophrenia (Goldman, Marlow-O'Connor, Torres, & Carter, 2008; Kéri, Kiss, & Kelemen, 2009). This suggests that a feedback mechanism may not be functioning to restrain oxytocin levels. Usually, the OT system response is, in part, balanced or regulated by the release of cortisol, the feedback component of the HPA axis stress-response system (Uvnas Moberg, 2003). However, cortisol function is known to be dysregulated in people with PTSD (de Kloet et al, 2006). Thus, this important feedback to the OT system may be dysregulated. This possibility is why this POD theory posits that dysregulated OT and dysregulated cortisol may potentiate each other. This description in lay terms of how POD could affect the pelvic and visceral tissues is a vastly simplified conceptualization. The hypothesis that OT is associated with GI and GU dysregulations consistent with somatization disorder and with the medically unexplained conditions of IBS, CPP, IC, HG, menstrual pain and dyspareunia is, however, amenable to investigation at this “macro” level. If clinical level associations are affirmed, additional “micro” level physiologic research will be warranted.
Review of the literature
In psychobiological studies, PTSD has been conceptualized primarily as an anxiety disorder, and neuroendocrine and anatomical investigations have focused especially on the hypothalamic-pituitary-adrenal (HPA) axis stress-regulation system.PTSD researchers are starting to examine HPA axis correlates of dissociation and interpersonal sensitivity which are listed in the DSM-IVas PTSD associated features (e.g., Austin, Riniolo &Porges, 2007; Simeon, Yehuda, Knutelska, & Schmeidler, 2008). The consistent association of increased physical morbidity and early mortality related to childhood adversity (Felitti, Anda, Nordenberg, Wiliamson, Spitz,et al., 1998;Shonkoff, Boyce,& McEwen, 2009; childhood sexual abuse (Talbot, Chapman, Conwell, McCollumn, Franus, et al., 2009), and PTSD complicated by depression and self-disorder comorbidity (Seng, Clark, McCarthy, &Ronis, 2006) also is leading to studies of HPA axis dysregulation effects on health outcomes, including physiology (e.g., Altemus, Cloitre, & Dhabhar, 2003),disease(e. g., Dube, Fairweather, Pearson, Felitti, Anda, et. al., 2009; Shonkoff et al., 2009), and childbirth complications (e. g., Seng, Oakley, Sampselle, Killian, Graham-Berman, et. al., 2001). Importantly, traumatic stress genetic studies are recognizing that trauma exposure particularly and contextual factors such as adversity in childhood both are environmental factors that interact with genetic and epigenetic processes to explain variance in models of outcomes (e. g., Koenan, Aiello, Bakshis, Amstadter, Ruggiero, et al., 2009; Norrholm & Ressler, 2009). The past decade's rapprochements of attachment theory, trauma theory, and neurobiology (e. g., Porges, 2001; Schore, 2005) have given credence to the co-factors of early relational trauma and abuse trauma together activating diatheses that manifest in disorder going beyond thePTSD diagnosis itself. What has been missing is an explicit suggestion of what additional biological mechanismsmight activate these diatheses and affect both mind and body.
As described above the oxytotic stress-regulation system is a plausible biological mechanism for somatoform disorders. It is a similarly plausible mechanism of dissociative disorders via effects on the hippocampus and memory (Liberzon, Chalmers, Mansour, Lopez, Watson, et al. 1994). It is a plausible mechanism of interpersonal reactivity by dysregulation of affect and attachment in relation to the primary caregiver in infancy (Schore, 2005). Taken together, we will refer to dissociative, somatoform, and interpersonal reactivity disorders as “posttraumaticself disorders.” If oxytocin is a plausible biological mechanism of these self disorders, it could serve as a biomarker of the psychosocial constructs of attachment and affect dysregulations, and the pelvic visceral pain and functional criteria of the somatization disorder diagnosis that I collectively call “pelvic visceral dysregulation” (PVD) conditions: irritable bowel syndrome, chronic pelvic pain (including dysmenorrhea and dyspareunia), interstitial cystitis, and hyperemesis gravidarum. The oxytotic system is a potentially unifying physiologic mechanism for the psychological and physical manifestations of early relational dysregulation across the lifespan, from infancy through adulthood. It is a manifestation that may be especially important among females, particularly during the reproductive lifespan, because oxytocin is sexually dimorphic and influenced by estrogen levels (Olff, Langeland, Draijer, & Gersons, 2007; Pournajafi-Nazarloo, Perry, Partoo, Papademeteriou, Azizi, et al., 2007).
The focus of this theory explication will be on oxytocin dysregulation as the commonality linking early relational trauma, disorders of the self, and PVD. This theory also attends to the reality that childhood maltreatment trauma often occurs in the same families as early relational trauma. Oxytocin dysregulation that precedes maltreatment trauma could potentiate the HPA axis response to abuse and the onset of PTSD (e.g., Marazziti & Catena Dell'osso, 2008). Interaction of oxytotic and HPA axis dysregulations could synergistically maintain a chronic, complex presentation, increasing allostatic load, and leading to stress-related diseases. I call this the “Posttraumatic Oxytocin Dysregulation Disorders” hypothesis to reflect the idea that oxytocin dysregulation may be a mechanism of the comorbid or combined posttraumatic self disorder and posttraumatic stress disorder manifestationsseen in both mental health and medical settings.
Theoretical Framing
Explication of assumptions in the Posttraumatic Oxytocin Dysregulation Disorders theory
Several assumptions warrant stating. The first assumption is that the stress-regulation systems in the body are numerous and interacting; thus, focusing on any one single system among them is likely to result in a limited perspective on the complexity of human adaptations to traumatic stress. Focus on multiple systems is becoming more feasible and might “tell a better story,” increasing coherence despite also introducing more complexity (Rosenwald & Ochberg, 1992). Second, health and development depend on expanding, overlapping foundations from genetic complement, to prenatal shared physiology, to maternal-infant dyadic physical intimacy, to family, peer, and eventually broad social network interactions; these interactions and adaptations occur at somatic, psychological, and social levels; and dysregulation in infancy and subsequent abuse result in long-term maladaptations and impaired allostasis. Third, early dysregulations can potentiate sequelae of later abuse and then responses to later abuse can reinforce existing dysregulations; so oxytocin dysregulations could affect HPA axis response and vice versa (MacMillan et al., 2009). Fourth, interventions can facilitate physical and mental health and posttraumatic growth, even in the distant aftermath; but lifespan health, quality of life, and contribution to society are optimal when primary prevention (e.g., support of “good enough” parenting) is put in place or, failing that, when secondary prevention (e.g., therapeutic fostering, treatment of ERT and CMT sequelae) occurs as early in the lifespan as feasible. Finally, trauma and dysregulation affect physiologic, somatic, psycho-spiritual, and social domains such that interventions may need to match modalities to the targeted level of repair.
Theoretical Statement
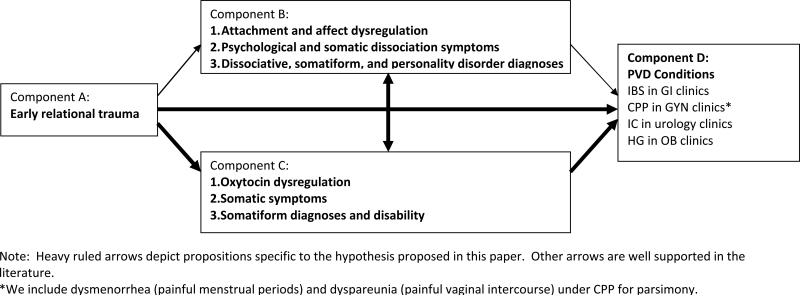
There are four components (A-D) and six propositions that are core to this theory (see Figure 1). The Posttraumatic Oxytocin Dysregulation Disorders theory is stated as a hypothesis to make it readily amenable to empirical testing: Oxytocin dysregulation (C) and self disorders (B) mediate the association of early relational trauma (A) with pelvic visceral dysregulation (D).
Figure 1.
Core theoretical model (without potential childhood maltreatment, posttraumatic stress, or HPA axis influenced depicted).
Explication of core components
There four components can be succinctly defined. Component A: ‘Early relational trauma’ is a term used to mean that an infant's development is compromised by early interactions with the primary caregiver which are characterized by emotional unavailability, misattunements that are unrepaired, and/or which are frightening or betraying of the infant's expectation of care and trust (Erikson, 1950, Schore, 2005). Component B: The consequence is that the infancy developmental outcomes of secure attachment and affect regulation are not achieved. This component is depicted at three levels corresponding to duration across the lifespan and severity. (Level 1) Affect and attachment dysregulation begin as adaptive responses. (Level 2) Symptoms appear as psychological and somatic dissociation, somatization, and/or interpersonal sensitivity and become maladaptive. (Level 3) Self disorder morbidity manifests to an extent corresponding with impairment and/or diagnostic criteria for dissociative, somatoform, and borderline personality disorders. Component C: Oxytocin dysregulation is discernable. This could occur as altered pulsatility, altered secretion (and concentration) of free hormones, altered density of receptor sites, or altered level of enzymes that metabolize oxytocin (e.g., prolyl ethylpeptidase). These alterations could be within the central or peripheral compartments, initiated in an afferent direction, as a paracrine hormone signal from the pelvic viscera or in an efferent direction, as a neurotransmitter signal from the hypothalamus and pituitary. Concomitant vagal tone changes may also be evident (e.g., syncope or decreased heart rate variability). These oxytocin alterations may be (Level 1) adaptive in the short term for the infant, but become (Level 2) maladaptive as they repeatedly facilitate dissociation, dysregulate smooth muscle and vagal tone, and decrease ability to seek affiliation and interactive repair of allostasis. Eventually they manifest as (Level 3) impaired memory processing facilitating dissociation, chronic smooth muscle dysregulation, and the trait of impaired relational functioning, potentially including revictimizing partnerships via impairment in judging trustworthiness (Kéri, Kiss, & Kelemen, 2009). Component D: The outcome of “PVD” manifests as the somatoform symptoms and dysregulated smooth muscle presenting in medical settings. The theory posits that pain will occur in the presence of oxytocin dysregulation of smooth muscles of the bowel, bladder, uterus, oviduct, and possibly the pelvic floor and vaginal introitus.
Propositions linking components in a very parsimonious model
There are six main propositions depicted as arrows in Figure 1 that link ERT with PVD conditions through the mediators of both self disorders and oxytocin dysregulation. These two mediating components also may moderate each other, so there is an arrow proposed between them. ERT also may be related to PVD independently or via another unaddressed mechanism, so there is an unmediated pathway specified as well. Finally, it is possible that the oxytocin dysregulation component should be placed elsewhere in the model, as an antecedent genetic vulnerability expressed as dysregulated oxytocin phenotype (e.g., a colicky infant) that increases risk of occurrence of ERT or risk of non-resilience. As it is now, I place it as a mediator, consistent with the findings from animal studies that there are epigenetic changes in oxytocin expression in the aftermath of ERT (low licking and grooming maternal behavior in rats) such that genetic propensity can be over-ridden (e.g., repaired) by fostering with rat dams characterized by high levels of licking and grooming maternal behavior (Meaney, 2001). Finally, the theory could also be recursive, presuming that the next generation's “starting point” is influenced by the previous generation's endpoint in an intergenerational system where both genetic and contextual substrates affecting the parents contribute to the child's vulnerability. Prenatal physiological interaction, infancy dyadic physical interactions, childhood maltreatment trauma interpersonal interactions and adult trauma exposures are viewed as additive. Measurement of adaptation could thus consider cellular to social levels with epigenetic, neuroendocrine, anatomic, intra-psychic, and social moderators aiding in psychological and physical allostasis.
Evaluationofthe Theoryby Preliminary Testsofthe Hypothesis
Explication of the overall theory has provided a context for empirical testing. The following section provides preliminary empirical testing of the propositions of the theory with data from aprogram of research on posttraumatic stress and women's health and childbearing outcomes. Each test is drawn from our previous publications or with secondary analysis of data from our published or on-going studies. All studies received informed consent from subjects and IRB approval.
Test #1: In women with PTSD diagnoses compared to women with no psychiatric diagnoses, only pregnancy complications that could potentially be mediated by oxytocin would occur at higher rates (Seng, et al., 2001).
In this exploratory epidemiological study of Medicaid claims data, we hypothesized a priori a list of physical complications we expected to occur more with PTSD with the rationale that, “Each of these complications has a behavioral risk factor, a hormonal risk factor related to oxytocin-vasopressin dysregulation, or both” (p. 18). The key findings were that the PTSD-diagnosed women had significantly greater risk for ectopic pregnancies (odds ratio (OR) = 1.7, 95% confidence interval (CI) 1.1, 2.8), miscarriages (OR = 1.9, 95% CI 1.3, 2.9), hyperemesis gravidarum (OR = 3.9, 95% CI 2.0, 7.4), preterm contraction episodes without delivering preterm OR = 1.4, 95% CI 1.1, 1.9);additionally, more ultrasounds were ordered to rule out excessive growth of the fetus OR = 1.5, 95% CI 1.0, 2.2). All five complications are potentially mediated by oxytocin's action on smooth muscle of the oviduct, gut, uterine fundus, and on maternal digestion and fetal growth. No other complications occurred at greater rates, suggesting a specificity of smooth muscle dysregulation. This study provided indirect evidence in support of the proposition linking Component C with Component D: that oxytocin dysregulation is associated specifically with pregnancy complications of smooth muscle, digestion, and growth, including the PVD condition hyperemesis gravidarum.
Test #2: Adult women with complex PTSD would experience the highest risk of PVD conditions (Seng, et al., 2006).
Using a larger sample of Medicaid recipients with a PTSD diagnosis in claims data, we considered odds of having clinician-diagnosed PVD conditions. In this analysis, we were able to also consider the effects of comorbid depression and self disorders. Having a mental health diagnosis other than PTSD, depression, or the self disorder diagnoses was associated with statistically significant increased risk of physical diagnosis across conditions (e.g., odds ratios from 1.6 to2.5), as has been noted previously, consistent with numerous studies showing that people with mental health conditions report more medical complaints (e.g., Costa & McCrae, 1987). There was a dose-response relationship of increasing odds as PTSD became complicated by dissociation and/or borderline personality disorder across all categories of diseases, and it was strongest in relation to the PVDs. However, these ranged from less than 3.0 for disorders not related to somatization disorders (e.g., blood and blood-forming organs 2.0, infectious diseases and parasites 2.7) to twice that level for categories that contain the Category B somatization symptoms: nervous (5.8), digestive (6.4) genitourinary (4.4), and muscular disorders (7.9), as well as signs, symptoms, and ill-defined conditions (10.3). Table 1 presents the re-analysis of these data with rates for IBS, CPP, and urinary pain medical diagnoses shown separately for PTSD-only, depression-only, dissociative, somatoform, and borderline personality diagnostic groups. These findings underscore that clinically-diagnosed PVD conditions are more strongly associated with self disorders (often comorbid with PTSD) than with simple PTSD or depression. The study was not designed to demonstrate the specificity of the association of posttraumatic stress and PVD conditions, but the pattern of odds ratios for the ICD categories with somatization diagnosis B criteria symptoms exceeding those of other categories is consistent with this pattern which has been clinically observed for over 100 years. This study provided indirect evidence in support of the proposition linking Component B with Component D: that self disorder diagnoses are associated with PVD conditions. It also provided indirect evidence that there is a dose response relationship when self disorders are present, over and above the effect of PTSD alone and with some specificity to ICD categories related to the somatization disorder diagnostic criteria.
Table 1.
Test #2 indicates a dose-response relationship between PTSD alone and PTSD complicated by self-disorders in relation to three diagnoses.
| PTSD no depression n=801 (5.8%) | Depression no PTSD n=1,095 (8.0%) | Borderline diagnosis* n=364 (2.6%) | Somatoform diagnosis* n=99 (0.7%) | Dissociative Diagnosis* n=113 (0.8%) | |
|---|---|---|---|---|---|
| Rate (%) Bivariate Odds ratio (95% confidence interval) | |||||
| Irritable bowel syndrome (2.6%) n=353 | 5.1% 2.2 (1.6, 3.1) | 4.4% 1.9 (1.4, 2.5) | 10.4% 4.8 (3.4, 6.9) | 11.1% 4.9 (2.6, 9.2) | 10.6% 4.6 (2.5, 8.5) |
| Chronic pelvic pain (10.5%) n=1,447 | 19.5% 2.2 (1.8, 2.6) | 17.4% 1.9 (1.6, 2.3) | 26.1% 3.1 (2.5, 4.0) | 32.3% 4.1 (2.7, 6.3) | 24.8% 2.8 (1.8, 4.4) |
| Urinary pain (2.6%) n=356 | 3.5% 1.4 (0.9, 2.1) | 4.9% 2.1 (1.6, 2.9) | 9.9% 4.5 (3.1, 6.4) | 6.1% 2.5 (1.1, 5.6) | 8.8% 3.7 (1.9, 7.2) |
NOTE:
79.9% of the women with borderline personality disorder diagnosis also have a PTSD diagnosis. 73.7% of the women with a somatoform, hysteria, or conversion diagnosis also have a PTSD diagnosis. 91.2% of the women with a dissociative disorder diagnosis also have a PTSD diagnosis.
Test #3: Girls with severe PTSD also would have experienced these PVD conditions (Seng, Graham-Bermann, Clark, & Ronis, 2005).
In a parallel study, female adolescent Medicaid recipients, age 9-17, who had been diagnosed with PTSD showed a pattern similar to adult women. The younger girls in this analysis, age 0-8, did not have personality disorder diagnoses, and there were too few cases with the exact IBS diagnosis to analyze. However, those with PTSD carried 40% greater risk for diagnoses in the digestive system category, with an odds ratio of 1.4 (95% CI 1.2-1.7). The adolescent analysis in this study provided indirect evidence in support of the proposition linking Component B with Component D: that self disorder diagnoses are associated with PVD conditions, again in a dose response relationship. It replicated the adult finding in Test #2 and extended it to show that the association may be particularly strong early in the reproductive lifespan. It also indicated that PVD may occur in childhood, but that the adult PVD condition diagnoses are rarely applied to children; other descriptions (e.g., “stomach ache”) are needed for pediatric studies (Alfven, 2004).
Test #4: Early relational trauma, childhood maltreatment, and their co-occurrence would be associated with self disorder symptoms in a dose-response pattern and also related to PTSD and treatment seeking (unpublished secondary analysis).
A community sample of pregnant women in prenatal care(NIH R01 NR008767; methods described in Seng, Kane Low, Sperlich, Ronis & Liberzon, 2009) completed standardized telephone psychiatric diagnostic interviews. Table 2 compares symptom levels and treatment seeking by four childhood trauma groups: neither ERT nor CMT, CMT only, ERT only, and both CMT and ERT. Symptom load follows a dose-response pattern across these groups. We extended this analysis with a stepwise linear regression model (Table 3) which reiterates in Step 1 the independent association of ERT with cumulative self disorder symptoms. Interpretation of the subsequent steps indicates that the significant dose response trauma history pattern of association with self disorder symptoms persists even when controlling for current PTSD and depression symptom levels (Step 2). We also see that cumulative sociodemographic risk factors are not significantly independently associated with self disorder symptom load (Step 3). Pre-pregnancy treatment seeking is associated with higher levels of self disorder symptoms. Sociodemographic disadvantage interacts with treatment seeking. And pre-pregnancy treatment these women received attenuated the strength of association of trauma, PTSD, and depression by uniformly small margins (Step 4). This analysis provided evidence in support of the proposition linking Component A with Component B: that ERT is associated with self disorder symptoms. It also supports the extension of the theory that ERT potentiates the effect of CMT on mental health.
Table 2.
Test #4 shows a dose-response relationship of trauma history with level of self disorder symptoms, probability of depression diagnosis, PTSD symptoms, and treatment seeking.
| Neither ERT nor CMT n=1,120 | CMT only n=145 | ERT only n=150 | Both ERT and CMT n=166 | F | p | |
|---|---|---|---|---|---|---|
| Mean (SD) | ||||||
| SCL-90 Somatization Score | 5.2 (4.5) | 7.8 (5.8) | 8.0 (6.0) | 10.9 (7.6) | 67.3 | <.001 |
| SCL-90 Interpersonal Sensitivity Score | 3.5 (4.2) | 5.2 (5.3) | 8.5 (7.7) | 9.2 (8.0) | 86.3 | <.001 |
| Modified DES-T Score | 0.9 (1.9) | 1.2 (2.2) | 1.6 (3.1) | 2.6 (3.9) | 27.5 | <.001 |
| Major Depression Probability | 0.0 (0.2) | 0.0 (0.2) | 0.1 (0.3) | 0.2 (0.3) | 26.2 | <.001 |
| Current PTSD Symptom Count | 1.4 (2.4) | 2.8 (3.2) | 4.0 (3.9) | 5.5 (4.3) | 121.0 | <.001 |
| Sum of types of treatment sought | 0.8 (1.2) | 1.2 (1.3) | 1.2 (1.4) | 1.8 (1.8) | 36.6 | <.001 |
Table 3.
Stepwise linear regression model for Test #4 shows consistent associations of ERT and CMT with total burden of self disorder symptoms and a strong association with PTSD in pregnancy (n=1,581)
| * β | p | |
|---|---|---|
| Step 1: R2 = .174, p <.001 | ||
| CMT only | .123 | <.001 |
| ERT only | .233 | <.001 |
| Both ERT and CMT | .374 | <.001 |
| Step 2: Adj R2 = .424, change R2 = .251, p < .001 | ||
| CMT only | .056 | <.001 |
| ERT only | .100 | <.001 |
| Both ERT and CMT | .151 | <.001 |
| PTSD current symptoms | .526 | <.001 |
| Depression probability | .088 | <.001 |
| Step 3: Adj R2 = .423, change R2 = .000, p = .461 | ||
| CMT only | .056 | <.001 |
| ERT only | .099 | <.001 |
| Both ERT and CMT | .151 | <.001 |
| PTSD current symptoms | .520 | <.001 |
| Depression probability | .089 | <.001 |
| Cumulative SES risk | .015 | .461 |
| Step 4: Adj R2 = .435, change R2 = .012, p < .001 | ||
| CMT only | .047 | .016 |
| ERT only | .086 | <.001 |
| Both ERT and CMT | .123 | <.001 |
| PTSD current symptoms | .504 | <.001 |
| Depression probability | .078 | <.001 |
| Cumulative SES risk | .060 | .006 |
| Prior treatment used | .122 | <.001 |
The dependent variable is the sum of scores on the measures of dissociation, somatization, and interpersonal sensitivity.
Test #5: The ERT-related symptoms of dissociation, somatization, and interpersonal sensitivity would occur both independently of and as comorbidities with PTSD, a pattern consistent with this theory (Seng, D'Andrea, Liberzon & Ford, in preparation).
In this same study of women in prenatal care, we conducted a cluster analysis using all women with PTSD, depression, and anxiety diagnoses and with dissociation, somatization, and interpersonal sensitivity scores greater than two standard deviations above the mean to examine symptom complexity among women who reported mental health symptomatology in the early pregnancy interview (n= 395). The cluster analysis resulted in a six-group solution. Half of the women in the dissociation cluster met criteria for lifetime PTSD (51.6%, n=32), and 38.7% (n=24) reported CMT. One fourth of women in the somatization cluster met criteria for lifetime PTSD (27.9%, n=12), and 23.3% (n=10) reported CMT. The prevalence rates for these self disorder and posttraumatic clusters were 3.9% (n=62 of the 1,581 women in the entire sample) for dissociation, 2.7% (n=43) for somatization, 4.3% (n=68) for PTSD comorbid with depression, and 4.3% (n=68) for PTSD comorbid with interpersonal sensitivity, depression, and somatization. Combined, these disorders total 15.2% of the prenatal clinic sample, affecting more women than those in the depression-only cluster (6.6%) and anxiety-only cluster (3.1%), which together affect 9.7% of the sample. PTSD alone did not emerge from the cluster analysis as a discrete category. This analysis provided evidence in support of the proposition linking Component A with Component B: that ERT is associated with self-disorder symptoms independent of CMT. It also provides support for the extension of this theory which states that posttraumatic self disorders and posttraumatic stress disorders co-occur when CMT occurs.
Test #6: Smooth muscle dysregulation would be experienced to greater extents by those in the dissociation, somatization, and complex PTSD clusters (unpublished secondary analysis).
Again in the same prenatal clinic sample, women in the three clusters characterized by dissociation, somatization, and complex PTSD reported higher nausea and vomiting scores, (means of 5.2, 6.3, and 5.8 respectively versus 4.7 for those with no mental health diagnosis, F = 7.0, df = 6 p <.001). They also reported more episodes of preterm uterine contractions that worried them (32.1% of women in the dissociation cluster, 66.7% of women in the somatization cluster, 44.0% of those in the complex PTSD cluster, versus 16.5% of those with no mental health disorder, X2 = 58.6, df = 6, p <.001). These findings affirm the finding in Test #1 that relied upon claims for hospital episodes for hyperemesis gravidarum and for obstetric triage visits to rule out preterm labor, extending the support to include subjective reports of nausea and vomiting and preterm uterine contractions. This analysis provided evidence in support of the proposition linking Component B with Component D: self disorders (and PTSD comorbid with self disorders) are associated with PVD in a pregnant sample.
Test #7: Oxytocin levels and dissociation scores would mediate the association of ERT and cumulative PVD conditions, including hyperemesis (Seng, Miller, Sperlich, Brown, van de Ven, & Liberzon, under review).
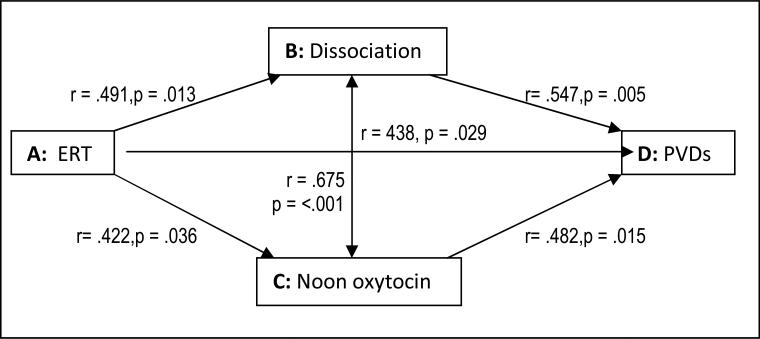
In a case-control pilot study of the severe, unremitting vomiting of pregnancy (hyperemesis gravidarum) we compared noon oxytocin concentrations. We considered hyperemesis to be a severe PVD manifestation that has been historically associated with “hysteria,” and empirically linked with PTSD (Seng et al., 2001) and pre-pregnancy PVD diagnoses (Seng, Schrot, van de Ven, Liberzon, 2007). In this pilot we queried pre-pregnancy history of several PVD conditions, including irritable bowel syndrome, chronic pelvic pain, dysmenorrhea, and dyspareunia. We created a sum score, counting hyperemesis as one additional PVD condition. Data collected in this pilot permit us to test all six propositions in the theory. Figure 2 presents correlations for each relationship, showing that all are significantly and moderately strongly related, with the correlation between modified DES-T (taxonomic Dissociative Experience Scale) score and oxytocin level being the strongest relationship (r = .675, p < .001). Figure 3a shows that the dissociation score mediates the association between ERT and PVDs. Figure 3b shows that oxytocin also mediates this relationship. This analysis provided evidence in support of all six propositions separately and support for the overall hypothesis that Components B and C mediate the association of Component A with Component D: that is, that oxytocin level and dissociative symptoms mediate the relationship between ERT and PVD.
Figure 2.
Test #7 finds significant correlations corresponding to all six propositions.
Figure 3.
Tests of mediation
Test #8: Finally, an influential case with extreme oxytocin levels may represent “the exception that proves the rule.”
Returning our focus to the level of the individual, one participant in the above pilot study had an extreme trauma history, extreme vomiting, an extreme dissociation score, and extreme oxytocin levels. She witnessed the shooting death of her mother when she was a toddler. She was fostered and sexually abused by an older matrilineal relative, suggesting that there may have been an intergenerational pattern of ERT and CMT. Her first pregnancy had ended at term when she was in a motor vehicle accident, after which that infant was stillborn and the woman nearly died due to placental abruption. On the day of the pilot study data collection she vomited or had dry heaves throughout the eight-hour protocol. She reported a history of both IBS and CPP, in addition to having HG. Her oxytocin levels were by far the highest in the study and were consistently above the detectable limit of the assay. This case also points out the complexity of studying oxytocin because these high levels could theoretically reflect either paracrine activity in an afferent pathway signaling distress due to extreme vomiting or efferent signaling of posttraumatic psychological distress. A single case study, a sample size of one, proves nothing. But if the saying is true that “the exception proves the rule,” then this severe case may anchor this theory at one extreme. This case may depict a very powerful synergy of ERT, CMT, and catastrophic adult trauma, of self and posttraumatic psychopathology, of dysregulated oxytocin, and of PVD.
Synthesis of these data
Taken together, these data from several studies within a single program of research on PTSD and women's health and childbearing, lend preliminary support to the “Posttraumatic Oxytocin Dysregulation Disorders” theory. Results of these tests of the six propositions suggest that, in the aftermath of early relational trauma, oxytocin dysregulation may be related to symptoms of self disorders, as well as to pelvic and visceral smooth muscle dysregulation (PVD) conditions. Strengths of this ensemble of eight empirical explorations of the theory include consistency across an array of samples and designs (pregnant and non-pregnant, Medicaid population case-control, prenatal clinics cohorts, and test-of-concept pilot case-control), and measurements (clinician diagnoses, epidemiological diagnoses, symptom counts). Within the studies where PTSD, depression, and the self disorders were measured for research purposes (Tests #4-8), the same measures were used so that effect sizes should be comparable. Important limitations include those inherent in secondary analyses and pilot studies. Taken together these explorations offer initial support for the theory and suggest that further research is warranted.If future studies produce consistent findings, we would have a new biological system to consider in relation to the broader questions of mechanisms of inter-generational transmission of trauma, psychiatric morbidity, and poor health outcomes. Perhaps most important, we would be able to consider theory-generated interventions that target oxytocin regulation for repair of both mental and physical health conditions. We could also target dyadic oxytocin regulation as a means to prevent or decrease intergenerational transmission of ERT, CMT, and vulnerability to their psychological and physical sequelae.
Research on this theory would best combine expertise on dissociation, somatization, interpersonal sensitivity and PTSD in a way that does not usually happen now—advancing the notion of trauma-spectrum disorders that may be discernable by their biological footprints as well as their psychological profiles. Combining studies of HPA axis and PTSD with oxytocin and self disorders would require building bridges across research groups and settings. It will require a lifespan perspective that overrides the separations in academic and clinical settings. This requires attention to infants, girls, and women as they embodydiffering steroid hormone contexts and are players in a cycle of inter-generational patterns. It requires parallel inquiry for boys and men. It also requires imagining scientific links from “crib” to “laboratory bench” to “therapy chair” and “exam table” with investigations analyzing data collected at multiple levels of the phenomena, including epigenetics when that becomes more feasible.
ERT, CMT, trauma spectrum psychological disorders, and trauma-associated physical morbidity and mortality are all around us. They represent a significant burden on individuals and society. Considering oxytocin dysregulation as a potential biological substrate of self disorders and complex PTSD represents an opportunity to align habits of mind that have already formed based on infant development and animal model literatures with our work as clinicians and health scientists so that a new hypothesis might bear valuable fruit as new case conceptualization and empirical efforts.
Acknowledgments
Grant support for empirical data included in this paper comes from the following sources:
National Institutes of Health grant M01-RR00042 to the University of Michigan General Clinical Research Center, grant U013786 to the University of Michigan Office of the Vice President for Research,grant 1P20 NR008367 from the National Institute of Nursing Research's MESA Center for Health Disparities, and grant R01 NR008767 from the National Institute of Nursing Research. This work utilized the Chemistry Core of the Michigan Diabetes Research and Training Center funded by Grant DK020572 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. The Michigan Medicaid data were made available by Health Management Associates, Inc., Lansing, Michigan, with the expert support of Dennis Roberts, PhD. Some of the analyses were supported with a dissertation grant from the Blue Cross and Blue Shield of Michigan Foundation and others were completed while Dr. Julia Seng was a Pfizer Postdoctoral Fellow. The author would especially like to thank Janis Miller, Mickey Sperlich, Rachel Jeltema, and C. Sue Carter.
References
- Alfven G. Plasma oxytocin in children with recurrent abdominal pain. Journal of Pediatric Gastroenterology & Nutrition. 2004;38(5):513–517. doi: 10.1097/00005176-200405000-00010. [DOI] [PubMed] [Google Scholar]
- Altemus M, Cloitre M, Dhabhar FS. Enhanced cellular immune response in women with PTSD related to childhood abuse. American Journal of Psychiatry. 2003;160:1705–1707. doi: 10.1176/appi.ajp.160.9.1705. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Author; Washington (DC): 1994. [Google Scholar]
- Austin MA, Riniolo TC, Porges SW. Borderline personality disorder and emotion regulation: Insights from the polyvagal theory. Brain and Cognition. 2007;65:69–76. doi: 10.1016/j.bandc.2006.05.007. doi:10.1016/j.bandc.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Neuroticism, somatic complaints, anddisease: Is the bark worse than the bite? Journal of Personality. 1987;55:298–316. doi: 10.1111/j.1467-6494.1987.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Hofkins TL, Frank E, Seltman H, Cai HM, Amico JA. Evidence of dysregulated peripheral oxytocin regulation among depressed women. Psychosomatic Medicine. 2008;70:967–975. doi: 10.1097/PSY.0b013e318188ade4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Kavelaars A, Heijen CJ, Westenberg HG. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. Journal of Psychiatric Residency. 2006;40(6):550–567. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosomatic Medicine. 2009;71:243–250. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel C. Somatization and multiple idiopathic physical symptoms: Relationship to traumatic events and posttraumatic stress disorder. In: Schnurr PP, Green BL, editors. Trauma and health: Physical health consequences of exposure to extreme stress. American Psychological Association; Washington DC: 2004. pp. 191–215. [Google Scholar]
- Erikson E. Childhood and Society. W. W. Norton & Co.; New York: 1950. [Google Scholar]
- Felitti V, Anda RF, Nordenberg D, Wiliamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the adverse childhood experiences (ACE) study. American Journal of Preventative Medicine. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Goldman M, Marlow- O'Connor M, Torres I, Carter CS. Diminished plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophrenia Research. 2008;98(1-3):247–255. doi: 10.1016/j.schres.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JL. Trauma and Recovery: The aftermath of violence--from domestic abuse to political terror. BasicBooks; New York: 1992. [Google Scholar]
- Holt-Lunstad J, Birmingham WA, Light KC. Influence of “warm touch” support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosomatic Medicine. 2008;70(9):976–985. doi: 10.1097/PSY.0b013e318187aef7. [DOI] [PubMed] [Google Scholar]
- Irwin C, Falsetti SA, Lydiard RB, Ballenger JC, Brock CD, Brener W. Comorbidity of posttraumatic stress disorder and irritable bowel syndrome. Journal of Clinical Psychiatry. 1996;57:576–8. doi: 10.4088/jcp.v57n1204. [DOI] [PubMed] [Google Scholar]
- Kéri S, Kiss I, Kelemen O. Sharing secrets: Oxytocin and trust in schizophrenia. Social Neuroscience. 2009;4(4):287–293. doi: 10.1080/17470910802319710. [DOI] [PubMed] [Google Scholar]
- Kibler JL. Posttraumatic stress and cardiovascular disease risk. Journal of Trauma & Dissociation. 2009;10(2):135–150. doi: 10.1080/15299730802624577. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Aiello AE, Bakshis E, Amstadter AB, Ruggiero KJ, Acierno R, Kilpatrick DG, Gelernter J, Galea S. Modification of the association between serotonin transporter genotype and risk of posttraumatic stress disorder in adults by county-level social environment. American Journal of Epidemiology. 2009;169(6):704–711. doi: 10.1093/aje/kwn397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Chalmers DT, Mansour A, Lopez JF, Watson SJ, Young EA. Glucocorticoid regulation of hippocampal oxytocin receptor binding. Brain Research. 1994;650:317–22. doi: 10.1016/0006-8993(94)91798-1. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Young EA. Effects of stress and glucocorticoids on CNS oxytocin receptor binding. Psychoneuroendocrinology. 1997;22:411–22. doi: 10.1016/s0306-4530(97)00045-0. [DOI] [PubMed] [Google Scholar]
- Light KC, Grewen KM, Amico JA. More frequent partner hugs and higher oxytocin levels are linked to lower blood pressure and heart rate in premenopausal women. Biological Psychology. 2005;69(1):5–21. doi: 10.1016/j.biopsycho.2004.11.002. [DOI] [PubMed] [Google Scholar]
- MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, Tanaka M, Gensey S, Spree S, Vella E, Walsh CA, De Bellis MD, Van der Meulen J, Boyle MH, Schmidt LA. Cortisol response to stress in female youths exposed to childhood maltreatment: results of the youth mood project. Biological Psychiatry. 2009;66(1):62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D, Catena Dell'osso M. The role of oxytocin in neuropsychiatric disorders. Current Medical Chemistry. 2008;15(7):698–704. doi: 10.2174/092986708783885291. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Milburn A, Reiter RC, Rhomberg AT. Multidisciplinary approach to chronic pelvic pain. Obstetrics & Gynecology Clinics of North America. 1993;20(4):643–661. [PubMed] [Google Scholar]
- Norrholm SD, Ressler KJ. Genetics of anxiety and trauma-related disorders. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.06.036. doi:10.1016/j.neuroscience.2009.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olff M, Langeland W, Draijer N, Gersons BPR. Gender differences in posttraumatic stress disorder. Psychological Bulletin. 2007;133:183–204. doi: 10.1037/0033-2909.133.2.183. [DOI] [PubMed] [Google Scholar]
- Pierrehumbert B, Torrisi R, Laufer D, Halfon O, Ansermet F, Beck Popovic M. Oxytocin response to an experimental psychosocial challenge in adults exposed to traumatic experiences during childhood or adolescence. Neuroscience. Mar 10. 2010;166(1):168–77. doi: 10.1016/j.neuroscience.2009.12.016. Epub 2009 Dec 16. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42(2):123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Pournajafi-Nazarloo H, Perry A, Partoo L, Papademeteriou E, Azizi F, Carter CS, Cushing BS. Neonatal oxytocin treatment modulates oxytocin receptor, atrial natriuretic peptide, nitric oxide synthase, and estrogen receptor mRNAs expression in rat heart. Peptides. 2007;28:1170–1177. doi: 10.1016/j.peptides.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald GC, Ochberg RL. Storied Lives: The cultural politics of self-understanding. Yale University Press; New Haven, CT: 1992. [Google Scholar]
- Roy-Byrne PP, Davidson KW, Kessler RC, Asmundson GJG, Goodwin RD, Kubzansky L, Lydiard RB, Massie MJ, Katon W, Laden SK, Stein MB. Anxiety disorders and comorbid medical illness. General Hospital Psychiatry. 2007;30:208–225. doi: 10.1016/j.genhosppsych.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Samelius L, Wijma B, Wingren G, Wijma K. Somatization in abused women. Journal of Women's Health. 2007;16(6):909–918. doi: 10.1089/jwh.2006.0103. [DOI] [PubMed] [Google Scholar]
- Schore AN. Attachment, affect regulation, and the developing right brain: Linking development neuroscience to pediatrics. Pediatrics in Review. 2005;26(6):204–217. doi: 10.1542/pir.26-6-204. [DOI] [PubMed] [Google Scholar]
- Seng JS, Clark MK, McCarthy AM, Ronis DL. PTSD and physical co-morbidity among women: Results from service use data. Journal of Traumatic Stress. 2006;19:45–56. doi: 10.1002/jts.20097. [DOI] [PubMed] [Google Scholar]
- Seng JS, D'Andrea W, Liberzon I, Ford JD. Trauma history and symptom complexity modeled in a person-centered approach in a sample of women in prenatal care. In preparation.
- Seng JS, Graham-Bermann SA, Clark MK, McCarthy AM, Ronis DL. Posttraumatic stress disorder and physical comorbidity among female children and adolescents: results from service-use data. Pediatrics. 2005;116:e767–76. doi: 10.1542/peds.2005-0608. [DOI] [PubMed] [Google Scholar]
- Seng JS, Kane Low LM, Sperlich M, Ronis D, Liberzon I. Prevalence, trauma history, and risk for posttraumatic stress disorder among nulliparous women in maternity care. Obstetrics & Gynecology. 2009;114(4):839–847. doi: 10.1097/AOG.0b013e3181b8f8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seng JS, Miller JM, Sperlich M, van de Ven CJM, Liberzon I, Carter SC. Mental health sequelae of childhood maltreatment, oxytocin, and hyperemesis gravidarum: a test of concept pilot study. Under revision. [DOI] [PMC free article] [PubMed]
- Seng JS, Oakley DJ, Sampselle CM, Killion C, Graham-Bermann S, Liberzon I. Association of posttraumatic stress disorder with pregnancy complications. Obstetrics & Gynecology. 2001;97:17–22. doi: 10.1016/s0029-7844(00)01097-8. [DOI] [PubMed] [Google Scholar]
- Seng JS, Schrot J, van de Ven CJM, Liberzon I. Service use data analysis of pre-pregnancy psychiatric and somatic diagnoses in women with hyperemesis gravidarum. Journal of Psychosomatic Obstetrics & Gynecology. 2007;28:209–217. doi: 10.1080/01674820701262044. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. Journal of the American Medical Association. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Simeon D, Yehuda R, Knutelska M, Schmeidler J. Dissociation versus posttraumatic stress: Cortisol and physiological correlates in adults highly exposed to the World Trade Center attack on 9/11. Psychiatry Research. 2008;161:325–329. doi: 10.1016/j.psychres.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Talbot NL, Chapman B, Conwell Y, McCollumn K, Franus N, Cotescu S, Duberstein PR. Childhood sexual abuse isassociated with physical illness burden and functioning in psychiatric patients 50 years of age and older. Psychosomatic Medicine. 2009;71:417–422. doi: 10.1097/PSY.0b013e318199d31b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnas Moberg K. The Oxytocin Factor: Tapping the Hormone of Calm, Love, and Healing. Da Capo Press; Cambridge, MA: 2003. [Google Scholar]
- van der Hart O, Nijenhuis ERS, Steele K. The Haunted Self: Structural Dissociation and the Treatment of Chronic Tramatization. W.W. Norton & Co.; New York: 2006. [Google Scholar]
- van der Kolk BA. Developmental trauma disorder. Psychiatric Annals. 2005;35(5):401–408. [Google Scholar]
- Wessely S, Nimnuan C, Sharpe M. Functional somatic syndromes: one or many? The Lancet. 1999;354:936–939. doi: 10.1016/S0140-6736(98)08320-2. [DOI] [PubMed] [Google Scholar]
- White-Traut R, Watanabe K, Pournajafi-Nazarloo H, Schwertz D, Bell A, Carter CS. Detection of salivary oxytocin levels in lactating women. Developmental Psychobiology. 2009;51(4):367–373. doi: 10.1002/dev.20376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . The ICD-10 Classification of Mental and Behavioral Disorders. WHO; Geneva: 2007. version. [Google Scholar]