Abstract
Occlusion of middle cerebral artery (MCAo) is commonly used to generate stroke in experimental animals. Different behavioral assays have been used to evaluate the severity of lesions or recovery after treatment in this model. In this study, we examined the correlation between the size of infarction and locomotor activity after transient MCAo in adult Sprague Dawley rats. The right middle cerebral artery was occluded for 30 to 90 -min by ligation with a 10-O suture. At 2 days after MCAo, animals were individually placed in a 3-D infra-red activity chamber for 1 hour to measure their horizontal, vertical, stereotypic and rotational activity. Body asymmetry was examined using an elevated body swing test. Volume of infarction was measured by triphenyltetrazolium chloride staining. We found that there is a significant correlation between the volume of infarction and vertical movement, such as vertical activity, vertical movement number and vertical movement time. There is also a significant correlation between the infarction volume and body asymmetry. In conclusion, our data suggest that vertical locomotor activity and body asymmetry are useful behavioral indices to estimate the size of lesioning in rodents after MCAo.
Keywords: Stroke, Middle cerebral artery occlusion, behavior
Occlusion of the middle cerebral artery (MCAo) is commonly used to generate focal ischemia in experimental animals. Depending on the loci of occlusion, the methods used for MCAo in rodents can be characterized into two groups: proximal and distal MCAo. Proximal MCAo can be introduced by inserting a monofilament through the carotid artery to reach the junction of MCA, thus blocking the blood flow from the common carotids and the circle of Willis (Chang, Niu et al., 2000). Distal MCAo is often done by occlusion of the MCA at the distal branch, i.e. before the first branch above zygomatic arch, after craniotomy. Distal MCAo can be achieved through laser or cauterization, which generates permanent occlusion, or by ligation with suture for 45 to 90 min, which produces transient ischemia. Depending on the model of MCAo used and the duration of ischemia, animals can develop cerebral infarction in selective ischemic regions.
Several reports have indicated that locomotor activity is suppressed after focal MCAo. Improvement of locomotor function often occurs along with reduction of cerebral infarction after successful neuroprotective treatments (Wang, Chang et al., 2003). These data suggest that locomotor activity can be associated with the change in cerebral infarction. However, the correlation between the size of infarction and locomotor activity after stroke has not been established. Furthermore, locomotor behavior contains many components, such as horizontal or vertical movements and stereotypic activity, as well as head and body orientation. In general, horizontal and vertical locomotor activities are commonly used to evaluate meso-striatal-cortical function (Kalivas, Duffy et al., 1988), stereotypic behaviors are used to study dopaminergic neuronal function (Wallace, Gudelsky et al., 1999), whereas body asymmetry is used to evaluate sensorimotor asymmetry after focal brain ischemia (Borlongan, Hida et al.,1998). It is still not clear which components are more sensitive to the size of the lesion in stroke brain.
In this study, we used the ligation method to generate distal MCAo in adult rats. We found that a minimum of 60-min ischemic time by MCA ligation is required to generate reliable focal infarction. We also report that vertical motor activity and body asymmetry are good behavioral indices to evaluate the size of lesion at 2 days after MCAo, which infarct volume can be readily measured.
Methods
Animals and surgery
Adult male Sprague-Dawley rats (BW =252.3 ± 2.2 g, n=49) were used for this study. Animals were anesthetized with chloral hydrate (400 mg/kg, i.p.) and were subjected to cerebral ischemia. The ligation of the right MCA and bilateral common carotids (CCAs) was performed using methods previously described. The bilateral CCAs were identified and isolated through a ventral midline cervical incision. The CCAs were ligated with non-traumatic arterial clips during MCAo; the clips were removed within 2 minutes after MCAo. A craniotomy of about 4 mm2 was made in the right squamosal bone. The right MCA was ligated with 10-O suture before the first bifurcation above zygomatic arch for 0, 30, 45, 60, or 90 minutes. Control animals received craniotomy with no MCA occlusion. After recovery from the anesthesia, the animals were returned to their home cages for reperfusion of the ischemic brain area. Core body temperature was monitored with a thermistor probe and maintained at 37 °C with a heating pad during anesthesia. After surgery, body temperature was further maintained at 37 °C in a temperature controlled incubator.
Behavioral measurements
Locomotor Behavior
Animals were individually placed in 42×42×31 cm behavioral chambers for 60 minutes (Accuscan activity monitor, Columbus, OH), 2 days after ischemia, for behavioral recording. The monitor contained 16 horizontal and 8 vertical infrared sensors spaced 2.5 cm apart. Locomotor activity was calculated using the number of beams broken by the animals after placement in the chamber. Sixteen parameters from 4 groups of behavior were measured as follows.
-
Vertical movement
Vertical activity (VACTV): The total number of beam interruptions that occurred in the vertical sensor.
Number of vertical movements (VMOVNO): number of time that animal rears up. Vertical time (VTIME): The amount of time, in seconds, the animal rears up.
-
Horizontal movement
Horizontal activity (HACTV): the total number of beam interruptions that occurred in the horizontal sensor.
Total distance travelled (TOTDIST): the distance traveled in centimeters Number of movements (MOVNO): the number of separate horizontal movement for a period greater than 1 second. Individual movements are separated from each other by a rest period of at least 1 second.
Movement time (MOVTIME): The amount of time, in seconds, the animals was in ambulation.
Margin distance (MRGDIST): The distance (in centimeters) an animal travels while at the margin or corners of the cage
Margin time in seconds (MRGTIME): Time spent by the animals in close proximity (within 1 cm) to the walls of the cage
Center distance (CTRDIST): The distance (in centimeters) an animal travels in the center of the cage
Center time (CTRTIME): Time (in seconds) spent by the animals away from the walls of the cage
-
Stereotypic behavior
Stereotype counts (STRCNT): The number of beam breaks during stereotypic activity
Number of stereotypy (STRNO): Number of stereotypic behaviors
Stereotypy time (STRTIME): The total amount of time (in seconds) that stereotypic behavior is exhibited.
Body asymmetry: Stroke behavior was quantitatively analyzed using an elevated body swing test (Borlongan, Hida et al., 1998b). Rats were examined for lateral movements/turning when their bodies were suspended 20 cm above the testing table by lifting their tails. The frequency of initial turning of head or upper body contralateral to the ischemic side was counted in 20 consecutive trials. The maximum impairment in body asymmetry in stroke animals is 20 contralateral turns/20 trials. In normal rats, the average body asymmetry is 10 contralateral turns/20 trials (i.e. the animals turn in each direction with equal frequency).
Measurement of cerebral infarction
Two days after reperfusion, animals were sacrificed and perfused intracardially with saline. The brain tissue was then removed, immersed in cold saline for 5 minutes, and sliced into 2.0-mm thick sections. The brain slices were incubated in 2% triphenyltetrazolium chloride (TTC, Sigma) dissolved in normal saline for 30 minutes at 37 °C, and then transferred into a 4% formaldehyde solution for fixation. The area of infarction in each slice was measured using a digital scanner as previously described (Wang, Lin et al., 1997).
RESULTS
Brain infarction
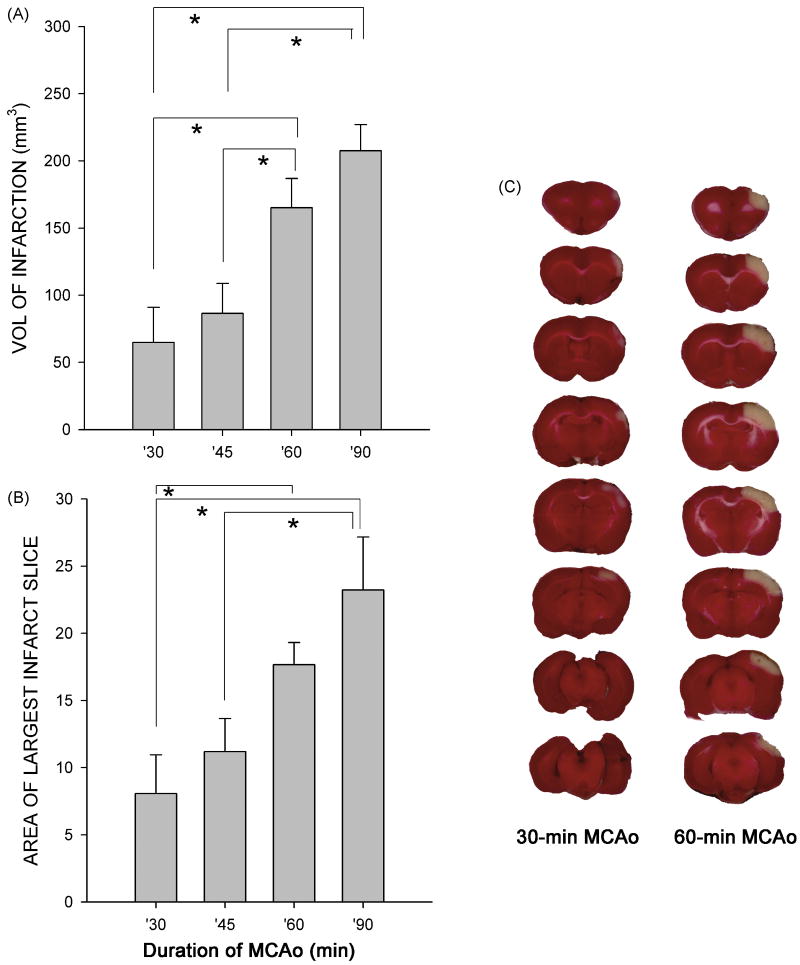
A total of 49 rats were used. Animals were separated into 5 groups based on the duration of MCAo [i.e. a, 90 min MCAo (n=13); b, 60-min MCAo (n=15); c, 45-min MCAo (n=7); d, 30-min MCA (n=8); e: sham surgery (n=6); ]. At 2 days after MCAo, there is a clear-cut infarction in the lesioned side cortex, as examined by TTC staining in all animals (n=28) that receiving 90 or 60 min MCAo (Fig. 1C). The incidence of infarction (number of animals with infarction/number of animals studied) was significantly reduced in animals given 30 or 45 min MCAo (p=0.011, Fisher Exact test). Three of 8 rats that receiving 30-min MCAo and 1 of 7 rats that receiving 45 min MCAo did not demonstrate infarction. These data suggest that a minimal 60 min ligation period is required to generate consistent infarction in adult rats of this strain, age and weight.
Fig 1.
Ischemic time - dependent brain infarction. Rats were subjected to 30 to 90 min MCAo. Brain infarction was examined using TTC staining at 2 days after 30-min or 60-min MCAo (C). The volume of infarction (A) and the area of the largest infarction in a slice (B) were plotted against ischemic time. There is a significant increase in cerebral infarction in animals receiving 60 or 90 min MCAo, compared with those treated with 30 or 45 min MCAo (*p<0.05, One way ANOVA + post-hoc Newman-Keuls test).
The volume of infarction was quantitatively analyzed by two methods: (A) the volume of infarction, i.e. thickness of the slice × sum of the infarction area in all brain slices, (B) the area of the largest infarction in a slice. We found that there is a dose (ischemic-time) dependent increase in volume of infarction and the area of largest infarction slice. Animals receiving more than 60 min ischemia developed larger infarction volumes in cerebral cortex than those given less than 60-min MCAo (Fig 1A, p<0.001, F(3,39)=7.885, One way ANOVA; p<0.05, posthoc Newman-Keuls test). Similarly, there is a significant increase in the area of largest infarction in a slice (Fig 1B, p=0.002, F(3,31)=5.986, One way ANOVA; p<0.05, post-hoc Newman-Keuls test). No infarction was found in animals receiving sham surgery.
Locomotor activity
Four groups of locomotor activity were monitored at 2 days after MCAo. The correlation was made between the changes in behavioral parameters and brain infarction. Because of the inconsistency of infarction in animals receiving 30 min MCAo, animals in this group were excluded from the correlation analysis.
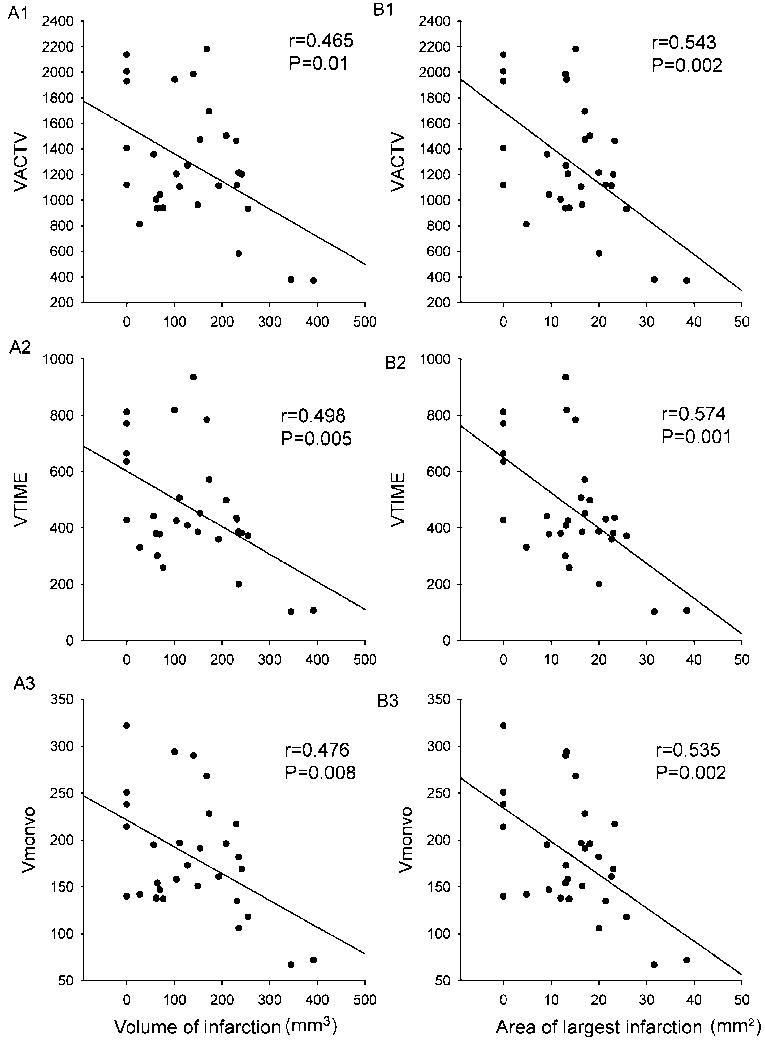
Vertical movement: Three parameters, i.e. VACTV, VMOVNO, and VTIME, were analyzed. There is a significant correlation between the size of infarction, both the volume of infarction and area of the infarction in the largest infarction slice, and all vertical movement parameters (Fig 2, Table 1, p<0.05, linear regression).
Horizontal movement: Of the eight horizontal parameters monitored, we found that there is correlation between reduction of distance travelled in the margin or corners of the cage and the area of the largest infarction in a slice (Table 1, p=0.026) or volume of infarction (p=0.066). There is also marginally significant between the area of the largest infarction in a slice and horizontal activity (p=0.076) or movement time (p=0.061). All other horizontal activities did not show any correlation with the size of infarction (p>0.1).
Stereotypic behavior: There is no correlation (p>0.15) between the size of infarction and all stereotypy parameters (i.e., STRCNT, STRNO, and STRTIME).
Body asymmetry: The incidence of body asymmetry was calculated using the following equation: body asymmetry (%) = (# of contralateral movement in 20 trials 10)/10×100%. There is a significant correlation between body asymmetry and the volume of infarction (p=0.019) or area or the largest infarction in a slice (p=0.012, Table 1).
Fig 2.
Correlation between vertical movement and size of infarction. All animals were placed in the activity chambers to monitor one-hour locomotor activity at 2 days after MCAo. Each dot represents the activity of one animal. Significant correlation was found between the vertical movement and volume (mm3) of infarction (A1-A3) or area (mm2) of largest infarction in a slice (B1-B3).
Table 1.
P and R values of linear correlation between the size of infarction and various locomotor parameters.
| volume of infarction | area of the largest infarction in a slice | |||
|---|---|---|---|---|
| r | p | r | p | |
| A. Vertical movement | ||||
| VACTV | 0.465 | 0.01 | 0.543 | 0.002 |
| VMOVNO | 0.476 | 0.008 | 0.535 | 0.002 |
| VTIME | 0.498 | 0.005 | 0.574 | <0.001 |
| B. Horizontal movement | ||||
| HACTV | 0.258 | 0.168 | 0.328 | 0.076 |
| TOTDIST | 0.245 | 0.193 | 0.305 | 0.102 |
| MOVNO | 0.2 | 0.289 | 0.277 | 0.138 |
| MOVTIME | 0.298 | 0.11 | 0.345 | 0.061 |
| MRGDIST | 0.34 | 0.066 | 0.405 | 0.026 |
| MRGTIME | 0.0743 | 0.696 | 0.0857 | 0.653 |
| CTRDIST | 0.0972 | 0.609 | 0.144 | 0.447 |
| CTRTIME | 0.0743 | 0.696 | 0.0857 | 0.653 |
| C. Stereotyped behavior | ||||
| STRCNT | 0.194 | 0.305 | 0.265 | 0.158 |
| STRNO | 0.221 | 0.241 | 0.279 | 0.135 |
| STRTIME | 0.15 | 0.428 | 0.204 | 0.28 |
| D. Body asymmetry | 0.448 | 0.019 | 0.475 | 0.012 |
Discussion
In this study, we report a dose (ischemic time) dependent increase in focal infarction in brain after transient occlusion of the distal branch of middle cerebral artery by ligation. A significant increase in volume of infarction and area of largest infarction in a slice was found after 60 or 90 min, as compared to 30 or 45 min, MCAo. The incidence of infarction was also affected by the duration of ischemia. All animals receiving 60 or 90 min MCAo developed infarction in brain 2 days after stroke. On the other hand, 3 of 15 rats that receiving 30-min or 45 min MCAo did not demonstrate infarction. These data suggest that duration of ischemic time can alter the size as well as the incidence of infarction. Furthermore, a minimal 60-min ischemic time by MCA ligation is required to generate reliable focal infarction in the strain, age and weight of rats used here.
Previous studies have indicated that locomotor activity can be either increased or decreased in experimental stroke rodents depending on the model of stroke used. For example, increased locomotor activity was found in the global ischemic model where brain ischemia was induced by short term (<15 min) occlusion of common carotids. In these animals, no infarction was present in brain. Microscopic lesions were found in striatum, the damage being mainly located in its dorsolateral part and affecting the medium-sized spiny projection neurons, which may underlie the hyperactivity after global ischemia (Chesselet, Gonzales et al., 1990;Janac, Radenovic et al., 2006). Administration of the D2 receptor antagonists haloperidol, sulpiride, and eticlopride decreased ischemia-induced hyperactivity in Mongolian gerbils, suggesting that the global ischemia – mediated hyperactivity is related to abnormalities in dopaminergic functions (Araki, Hino et al., 1999). In contrast to short term global ischemia, focal ischemia decreases locomotor activity as seen in the present and in previous studies (Hattori, Lee et al., 2000;Tomac, Agulnick et al., 2002;Ji, Chai et al., 2007). Our data indicate that ligation of the distal branch of middle cerebral artery can cause cerebral infarction only in cerebral cortex. The permanent damage to the cell bodies of the corticospinal tract can reduce motor function.
We found a differential correlation between the size of infarction in cortex and reduction in vertical or horizontal movement at 2 days after MCAo. All vertical movement parameters, such as vertical activity, vertical movement number or movement time, significantly correlate with the volume of infarction or the area of the largest infarction in brain. On the other hand, most of horizontal movement parameters, except the distance travelled in the margin or corners of the cage, did not correlate significantly with the size of infarction (Table 1). These data suggest that vertical movement is more sensitive to the size of lesioning in cerebral cortex than horizontal movement after stroke. Similar responses have been found in animal model of Parkinson’s disease. A higher reduction (40 %) in vertical, as compared to horizontal (20%), activity, was found after partial and bilateral 6-hydroxydopamine lesions of substantia nigra in rats (Sakai and Gash, 1994), suggesting that high susceptibility of vertical activity can also be found after nigrostriatal lesioning. Since vertical movement requires weight-supported locomotion by two limbs and axial set of trunk muscles to maintain center of gravity, vertical activity may be less compensated by other limbs and more sensitive to the lesioning after brain injury.
We demonstrated a significant correlation between the reduction of distance travelled in the margin or corners of the cage and infarction. There is no correlation between the infarction and distance travelled in the center of the cage, suggesting that the decrease in locomotor activity is mainly in the peripheral regions of the cages as the size of infarction in brain increases.
We did not see significant correlation in stereotypic behavior with the size of infarction after stroke. Stereotypic behaviors are commonly used to evaluate dopaminergic neuronal activity (Wallace, Gudelsky et al., 1999). For example, increasing DA release by amphetamine facilitates stereotypic activity. Since distal MCAo induces infarction mainly in cortical regions, which have less dopaminergic innervation, these behavioral parameters are thus less likely to be affected by distal MCAo (Fig 1C).
Borlongan et al have previously reported a significant correlation between volume of infarction induced by proximal MCAo and body asymmetry measured by the elevated body swing test (Borlongan, Hida et al., 1998a). In this study, we also noted a significant correlation between body asymmetry and brain infarction after distal MCA ligation. Taken together, these data suggest that the elevated body swing test is a useful tool to estimate the size of the lesion early after stroke in adult rats in both ischemic models.
In addition to the size of infarction, other factors may also affect locomotor activity. For example, it has been reported that post-stroke treatment with bone morphogenetic protein 7 did not alter the size of infarction but enhances locomotor function (Chang, Lin et al., 2003;Chou, Harvey et al., 2006). Similarly, we recently reported that delayed post-treatment with a p53 inhibitor increases locomotor function 2-3 weeks after MCAo. The locomotor recovery in these animals correlated well with new cell proliferation in the subventricular zone (Luo, Kuo et al., 2009). Since these neural reparative processes occur at later stages after MCAo, the changes in locomotor activity at weeks after stroke should be carefully monitored.
In conclusion, our data demonstrate a significant correlation between the size of infarction in stroke brain and vertical movement and body asymmetry. These two locomotor parameters can be used to estimate non-invasively the size of lesioning early after stroke in rodents after focal MCAo.
Acknowledgments
This study is supported by the National Institute on Drug Abuse, IRP
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araki H, Hino N, Karasawa Y, Kawasaki H, Gomita Y. Effect of dopamine blockers on cerebral ischemia-induced hyperactivity in gerbils. Physiol Behav. 1999;66:263–8. doi: 10.1016/s0031-9384(98)00293-5. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Hida H, Nishino H. Early assessment of motor dysfunctions aids in successful occlusion of the middle cerebral artery. Neuroreport. 1998a;9:3615–21. doi: 10.1097/00001756-199811160-00012. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Hida H, Nishino H. Early assessment of motor dysfunctions aids in successful occlusion of the middle cerebral artery. Neuroreport. 1998b;9:3615–21. doi: 10.1097/00001756-199811160-00012. [DOI] [PubMed] [Google Scholar]
- Chang CF, Lin SZ, Chiang YH, Morales M, Chou J, Lein P, Chen HL, Hoffer BJ, Wang Y. Intravenous admininstration of Bone morphogenetic protein-7 after ischemia improves motor function in stroke rats. Stroke. 2003;34:558–64. doi: 10.1161/01.str.0000051507.64423.00. [DOI] [PubMed] [Google Scholar]
- Chang CF, Niu KC, Hoffer BJ, Wang Y, Borlongan CV. Hyperbaric oxygen therapy for treatment of post-ischemic stroke in adult rats. Exp Neurol. 2000;166:298–306. doi: 10.1006/exnr.2000.7506. [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Gonzales C, Lin CS, Polsky K, Jin BK. Ischemic damage in the striatum of adult gerbils: relative sparing of somatostatinergic and cholinergic interneurons contrasts with loss of efferent neurons. Exp Neurol. 1990;110:209–18. doi: 10.1016/0014-4886(90)90032-n. [DOI] [PubMed] [Google Scholar]
- Chou J, Harvey BK, Chang CF, Shen H, Morales M, Wang Y. Neuroregenerative effects of BMP7 after stroke in rats. J Neurol Sci. 2006;240:21–9. doi: 10.1016/j.jns.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Hattori K, Lee H, Hurn PD, Crain BJ, Traystman RJ, DeVries AC. Cognitive deficits after focal cerebral ischemia in mice. Stroke. 2000;31:1939–44. doi: 10.1161/01.str.31.8.1939. [DOI] [PubMed] [Google Scholar]
- Janac B, Radenovic L, Selakovic V, Prolic Z. Time course of motor behavior changes in Mongolian gerbils submitted to different durations of cerebral ischemia. Behav Brain Res. 2006;175:362–73. doi: 10.1016/j.bbr.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Ji HJ, Chai HY, Nahm SS, Lee J, Bae GW, Nho K, Kim YB, Kang JK. Neuroprotective effects of the novel polyethylene glycol-hemoglobin conjugate SB1 on experimental cerebral thromboembolism in rats. Eur J Pharmacol. 2007;566:83–7. doi: 10.1016/j.ejphar.2007.02.061. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, DuMars LA, Skinner C. Behavioral and neurochemical effects of acute and daily cocaine administration in rats. J Pharmacol Exp Ther. 1988;245:485–92. [PubMed] [Google Scholar]
- Luo Y, Kuo CC, Shen H, Chou J, Greig NH, Hoffer BJ, Wang Y. Delayed treatment with a p53 inhibitor enhances recovery in stroke brain. Ann Neurol. 2009;65:520–30. doi: 10.1002/ana.21592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Gash DM. Effect of bilateral 6-OHDA lesions of the substantia nigra on locomotor activity in the rat. Brain Res. 1994;633:144–50. doi: 10.1016/0006-8993(94)91533-4. [DOI] [PubMed] [Google Scholar]
- Tomac AC, Agulnick AD, Haughey N, Chang CF, Zhang Y, Bäckman C, Morales M, Mattson MP, Wang Y, Westphal H, Hoffer BJ. Effects of cerebral ischemia in mice deficient in Persephin. Proc Natl Acad Sci U S A. 2002;99:9521–6. doi: 10.1073/pnas.152535899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TL, Gudelsky GA, Vorhees CV. Methamphetamine-induced neurotoxicity alters locomotor activity, stereotypic behavior, and stimulated dopamine release in the rat. J Neurosci. 1999;19:9141–8. doi: 10.1523/JNEUROSCI.19-20-09141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chang CF, Morales M, Chiang YH, Harvey BK, Su TP, Tsao LI, Chen SY, Thiemermann C. Diadenosine tetraphosphate protects against injuries induced by ischemia and 6-hydroxydopamine in rat brain. J Neurosci. 2003;23:7958–65. doi: 10.1523/JNEUROSCI.23-21-07958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lin SZ, Chiou AL, Williams LR, Hoffer BJ. Glial cell line-derived neurotrophic factor protects against ischemia-induced injury in the cerebral cortex. J Neurosci. 1997;17:4341–8. doi: 10.1523/JNEUROSCI.17-11-04341.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]