The use of UW solution1 has resulted in improved graft function after transplantation2,3 even with long average preservation times.4 However, although the incidence of primary nonfunction (PNF) has decreased, it remains a significant clinical problem, in part because the demand for livers has caused an increased use of previously discarded organs.5 Greig et al6 have reported that prostaglandin E1 (PGE1) can ameliorate, or reverse, severe ischemic injury after liver transplantation, consistent with reports of several prostaglandin agents in experimental hepatic7–11 and nonhepatic12,13 test models of ischemic injury. In addition, we have reported that the combination of PGE1 plus high-dose steroids favorably influences the course of human liver allografts transplanted despite a positive lymphocytotoxic cross match14 and that PGE1 in cross-match negative cases reduces FK 506 nephrotoxicity.15 Consequently, we began in October 1991 to give PGE1 perioperatively to all liver recipients with a subsequent very low (1.1%) incidence of PNF in 174 consecutive negative cross-match cases.
MATERIALS AND METHODS
Case Material
The study was of 174 consecutive adult primary liver recipients between November 1, 1991, and September 30, 1992—excluding cases with cross-match positive donors, multiple organ transplantations, upper abdominal exenterations, and cases in which the liver was split (for two recipients) or surgically reduced in size. Historical controls with the same clinical characteristics and inclusion criteria (Table 1) were treated between July 19, 1990, and October 31, 1991 (n = 304). Patients of both the control (Group I) and study cohort (Group II) were followed until March 1, 1993.
Table 1.
Features in 478 Primary Liver Transplantations
| Group I | Group II | |
|---|---|---|
| Number of patients | 304 | 174 |
| PGE1 usage | no | yes |
| Periods | 7/19/90–10/31/91 | 11/01/91–9/30/92 |
| Age (y) | 49.3 ± 0.7 | 51.0 ± 0.96 |
| Sex | ||
| Male/Female | 183/121 | 107/67 |
| No. excluded for + cross match | 29 (8.4%) | 19 (9.3%) |
| UNOS Score 4 | 108 (35.5%) | 57 (32.8%) |
| CIT (h:min) | 14:18 ± 0:14 | 14:36 ± 0:15 |
| Longer than 20 h of CIT | 25 (8.2%) | 9 (5.2%) |
| More than 20 units of blood transfusion | 104 (34.1%) | 63 (36.0%) |
| Median days follow-up (range) | 749 (497–956) | 322 (157–492) |
CIT = Cold ischemic time: means ± SE for age and CIT.
Definition of Primary Nonfunction
Thirty-day graft loss from all cases was 45/304 (14.8%) in Group I and 19/174 (10.9%) in Group II, defined either as patient death or retransplantation (Table 2). Multiple factors frequently could be identified, but the dominant cause of failure was classified as shown in Table 2 including two examples of irreversible brain injury in patients with fulminant hepatic failure. Technical complications included hepatic artery thrombosis or stricture, portal vein thrombosis, hepatic vein/vena cava stricture or thrombosis, and excessive transfusions (>50 units).
Table 2.
Causes of Graft Failure Within 1 Month
| Group I | Group II (PGE1) |
|
|---|---|---|
| Number of primary grafts | 304 (100%) | 174* (100%) |
| Causes of graft failure in 1 month | ||
| Primary non function (PNF) | 18 (5.9%) | 2 (1.1%) |
| Primary dysfunction | 1 (0.3%) | 0 (0%) |
| HAT, HA stricture, or PVT | 12 (3.9%) | 5 (2.9%) |
| Other technical | 4 (1.3%) | 3 (1.7%) |
| Rejection | 0 (0%) | 0 (0%) |
| Cardiac problems including pulmonary hypertension | 1 (0.3%) | 4 (2.3%) |
| Infection, sepsis | 7 (2.3%) | 5 (2.9%) |
| Encephalopathy, brain death | 2 (0.7%) | 0 (0%) |
| Total no. of graft failures | 45 (14.8%) | 18 (10.9%) |
HAT = hepatic arterial thrombosis; PVT = portal vein thrombosis.
Includes 4 patients who could not tolerate PGE1: They are in the Categories of Cardiac problems (n = 2), Other technical (n = 1), and PNF (n = 1).
A diagnosis of PNF was made when, in the absence of these other complications, a graft had no or nonlife-sustaining function within 2 weeks of the initial transplant. The associated clinical picture included coagulopathy, failure to wake up, renal dysfunction, failure of the liver to initiate or maintain bile production, lactic acidosis, and hemodynamic instability. Histopathologically, the grafts usually showed small infarcts and/or zonal hepatocellular coagulative necrosis (centrilobular or periportal) or severe cholestasis subsequently without evidence of rejection.
Organ Procurement and Preservation
Hepatectomy was performed using standard techniques16 or with a modified rapid-flush technique17 for unstable donors. Grafts were preserved with UW solution (ViaSpan, Du Pont Pharmaceuticals). When there was concern about the quality of a liver, a frozen section biopsy was performed at the time of back-table preparation. The presence of severe macrovesicular steatosis (greater than 50% of hepatocytes), with or without hepatocellular necrosis,18,19 was a discard criterion applied uniformly in both groups. Cold ischemic time (CIT) for Group I ranged 4.1 to 29 hours (14.3 ± 0.29 [SE]) and for Group II it ranged 6.6 to 23.1 hours (14.6 ± 0.30). The CIT exceeded 18 hours in 18.2% of Group I patients and in 15.7% of those in Group II.
Management
Immunosuppression
Intravenous FK 506 was begun in the operating room and continued as a continuous infusion (0.1 to 0.05 mg/kg per day) until oral intake could be resumed. Doses were adjusted to achieve a target plasma level of 1 ng/mL with the method of Tamura20 in the immediate postoperative period, and a similar trough level after oral dosing every 12 hours was started. Although the first-day IV dose was 0.1 mg/kg per day in 88% of the Group I patients and 0.05 mg/kg per day in all patients of Group II, the rapid adjustments based on plasma monitoring made the cumulative doses equivalent.
Patients in both groups received a single 1 g bolus of methylprednisolone intraoperatively and 20 mg d with conversion to the oral route as soon as this was feasible. Rejection episodes that were unresponsive to adjustments of FK 506 maintenance doses were treated with a single 1 g bolus of methylprednisolone. When rejection persisted, an additional 5-day tapering burst of methylprednisolone was given (from 200 mg down to 20 mg), or alternatively a 3- to 5-day course of OKT3 (5 to 10 mg/d).
Prostaglandin E1
Prostaglandin E1 (PGE1, Prostin-VR, Upjohn) was given as a continuous infusion through a central venous catheter, starting at the time of surgery or immediately after its completion. Five hundred micrograms were diluted in 100 mL of 5% dextrose in water, and started at a dose of 0.2 µg/kg per hour, which was gradually increased to 0.6 µg/kg per hour and maintained there for the next 5 to 7 days unless hypotension or cardiovascular instability necessitated an adjustment.
Standard Function Tests
Treatment was guided by the parameters described above, which were correlated with standard liver function tests. Of these, bilirubin, alkaline phosphatase, gamma-glutamyl transpeptidase (gamma-GTP), and transaminases (aspartate aminotransferase [AST], and alanine aminotransferase [ALT]), and prothrombin time were given the greatest weight. Blood ammonia, serum lactic acid, and other special tests were spot-checked. Renal function was monitored with serum creatinine (Cr) and blood urea nitrogen (BUN).
Arterial Ketone Body Ratio (AKBR)
On days 0 through 5, the arterial ketone body ratio (AKBR) was measured within 40 minutes of arterial blood drawing (4 mL) by an enzymatic technique based on the methods of Mellanby and Williamson21,22 using a Ketorex Kit (Sanwa Chemical Company, Ltd., Nagoya, Japan) and a KETO-340 semiautomatic spectrophotometer (Ihara Medics US, Inc, Valencia, Calif). The acetoacetate and β-hydroxybutyrate, from which the AKBR is calculated, are significantly elevated (> 100 µmol/L) with starvation. Consequently, it should be noted that the average patient received 5% dextrose in 0.45% normal saline at a rate of 100 to 120 mL/hour on the day of transplantation and on the first postoperative day. Starting on postoperative day 2, patients usually received parenteral nutrition, which was continued until the enteral route could be used. With this treatment, starvation was observed in 12 patients (2.5%) on the first postoperative day. No hypoglycemia was encountered at the time of AKBR measurement. The AKBR results at 48 hours were placed into three categories:
Class 1, AKBR > 1.0
Class 2, AKBR between 0.7 to 1.0
Class 3, AKBR < 0.7.
Statistical Analysis
Results were expressed as mean ± SEM. The Kaplan-Meier (product limit) method was used to calculate graft survival, and the curves were compared with the generalized Wilcoxon (Breslow) test. One-way analysis of variance (ANOVA) was used to compare means across the three AKBR categories and stratified by PGE1 use or nonuse. The Scheffe F-test was used as a post-hoc multiple comparison technique. Univariate logistic regression analysis was used to evaluate independently the association among graft failure, primary nonfunction, and hepatic arterial thrombosis with PGE1 usage, AKBR on day 2, peak prothrombin time, peak AST and ALT, magnitude of change in AST and ALT levels during the first 24 hours. UNOS score, blood usage, and cold ischemic time. The strength of association of these factors on graft failure, primary nonfunction, and hepatic arterial thrombosis was estimated by the odds ratio. The significance of each odds ratio was tested using the Wald statistic. The one-way analysis of variance and chi-square tests were performed using the statistical package STATVIEW, while logistic regression analyses were performed using SPSS PC+. All tests of significance were two-tailed, with significance at a level of .05.
RESULTS
Patient and Graft Survival
Patient
One-month survival in control Group I was 96.4% vs 95.5% in the PGE1-treated Group II (NS). At the end of the year, the survival rate for the two cohorts was 85.5% and 90.5% respectively (NS) (Fig 1).
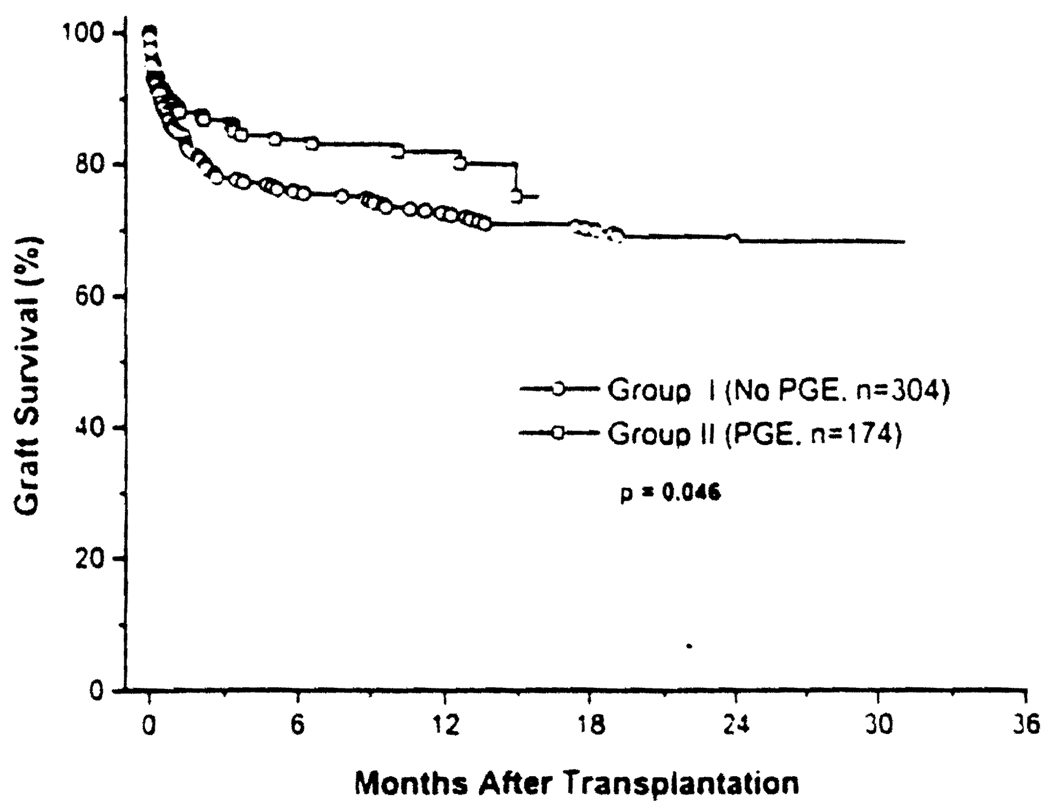
Fig 1.
Patient and graft survival rates after liver transplantation in Group I control and Group II (PGE1). A significantly better graft survival rate was observed in Group II patients with the usage of PGE1 (Breslow, P < .05).
Graft
At one month, 85.2% and 89.1% of the grafts were still in place in Groups I and II, respectively (NS), and at 1 year these survival percentages had fallen to 72.7% vs 82.2% (P < .05) (Fig 1).
Primary Nonfunction and Other Graft Losses
PNF
The incidence of 1.1% in the PGE1 group during the first 2 weeks (Table 2) was significantly lower than 5.9% in the controls (odds ratio = 0.188. P < .05).
Other Causes of Graft Loss (First Month)
The pattern of graft loss from other causes was similar in hath groups including the incidence of vascular and nonvascular technical complications. No grafts were lost to rejection in either cohort.
The four patients who could not tolerate PGE1 were included in the “intent to treat” analysis (Table 2). Three of the four lost their grafts—one by death, and one each to PNF and hepatic artery thrombosis followed by successful retransplantation on postoperative days 2 and 3, respectively. The fourth patient (who survived) had PGE1 stopped because of excessive bleeding perioperatively.
Univariate Logistic Regression Analysis
The PNF rate was not independently affected by UNOS classification, volume of transfusion, and the minor differences in CIT (Table 3). However, PNF was associated with the peak transaminase values in the first 24 hours, prolonged early prothrombin times, and a low 48-hour AKBR (see also below).
Table 3.
Analysis of Risk Factors for Primary Nonfunction and Arterial Thrombosis During First 2 Weeks and the Global Rate of Graft Failure in the First Month*
| Primary nonfunction for the first 2 weeks |
Hepatic arterial thrombosis for the first 2 weeks |
Graft failure for the first month | ||||
|---|---|---|---|---|---|---|
| Univariate analysis | Odds ratio | P-value | Odds ratio | P-value | Odds ratio | P-value |
| Factors | ||||||
| PGE1 Usage | 0.1884 | .0246 | 0.7398 | .5447 | 0.7055 | .232 |
| AKBR at day 2 | 0.0008 | <.00001 | 0.1095 | .0014 | 0.017 | <.00001 |
| Rise in AST in the first 24 h | 1.0006 | .0005 | 1.0001 | .6656 | 1.0004 | <.0001 |
| Rise in ALT in the first 24 h | 1.002 | .0079 | 1.0001 | .5411 | 1.0008 | .0001 |
| Peak AST | 1.0004 | <.00001 | 1.0002 | .0052 | 1.0004 | <.00001 |
| Peak ALT | 1.0005 | .00001 | 1.0002 | .0295 | 1.0004 | <.00001 |
| Prothrombin time | 1.2056 | <.00001 | 1.0788 | .0151 | 1.2069 | <.00001 |
| Cold ischemic time | 4.1886 | .3054 | 0.839 | .906 | 3.261 | .171 |
| UNOS Score 4 | 1.5813 | .3187 | 0.5299 | .2014 | 0.9438 | .8327 |
| Blood transfusion | 0.9877 | .4172 | 0.986 | .437 | 1.012 | .014 |
Univariate logistic regression analysis.
The global loss rate (from all causes) in the first month had the same associations as PNF with peak early transaminases, prothrombin time, and AKBR. In addition, the quantity of intraoperative transfusions emerged by 30 days as a significant risk factor (P = .01) (Table 3).
AKBR and Prognosis
The AKBR determinations up to postoperative day 2 were available in only 83% of Group I and 90% of Group II patients. Because the incidence of PNF in the nonstudied patients of Group I (4/52) and Group II (0/17) was similar to that in the entire respective groups (P = .3), the culled cohorts were considered representative of the whole collection.
Additional patients culled in roughly equal proportion from the control and study groups (Table 4) were 16 requiring insulin for glucose control and 5 more with the clinical diagnosis of moderate or severe pancreatitis. In such cases, the prognostic significance of AKBR is lost.23,24 We also excluded four patients from Group II that did not tolerate PGE1 administration. Finally, 12 patients with the biochemical postoperative diagnosis of starvation could not be studied because the shift from glucose to fatty acid oxidation as the energy substrate in conditions of starvation distorts the meaning of the acetoacetate/B-hydroxybutyrate measures upon which the calculations depend (see Materials and Methods). This left 238 cases in Group I and 134 cases in Group II with which definitive analysis of the prognostic significance of AKBR could be assessed (Table 5).
Table 4.
Exclusion From the AKBR Study
| Group I | Group II (PGE1) | |||||
|---|---|---|---|---|---|---|
| No. of patients (%) |
AKBR in day 2 |
PNF | No. of patients (%) |
AKBR in day 2 |
PNF | |
| Diabetes mellitus | 9 (3.6%) | 0.71 ± 0.12 | 1 | 7 (4.5%) | 0.84 ± 0.03 | 0 |
| Pancreatitis | 2 (0.8%) | 0.94 ± 0.21 | 0 | 3 (1.9%) | 1.09 ± 0.23 | 0 |
| Surgical starvation | 3 (1.2%) | 0.71 ± 0.08 | 0 | 9 (5.7%) | 0.62 ± 0.02 | 0 |
| No PGE1 usage | −(0%) | — | 0 | 4 (2.5%) | 0.76 ± 0.06 | 1 |
| Total no. of patients | 14 (5.6%) | 1 | 23 (14.6%) | 1 | ||
Table 5.
Survival, Causes of Graft Failure, and Liver Function Tests Connected With Results of Arterial Ketone Body Ratio (AKBR) in Patients With or Without PGE1
| Group I | Group II (PGE1) | |||||
|---|---|---|---|---|---|---|
| Range of AKBR | Class 1 AKBR ≥ 1.0 |
Class 2 0.7 ≤ AKBR < 1.0 |
Class 3 AKBR < 0.7 |
Class 1 AKBR ≥ 1.0 |
Class 2 0.7 ≤ AKBR < 1.0 |
Class 3 AKBR < 0.7 |
| Number of primary grafts | 145 | 78 | 15 | 93 | 32 | 9 |
| Graft survival at 1 month | 140 (96.6%) | 63 (80.8%)a | 0 (0%)b | 90 (96.8%) | 31 (96.9%) | 5 (55.6%)c |
| Patient survival at 1 month | 143 (98.6%) | 75 (97.3%) | 12 (80.0%) | 91 (97.8%) | 31 (96.9%) | 9 (100%) |
| Causes of graft failure at 1 month | ||||||
| Primary nonfunction | 0 | 6 | 8 | 0 | 0 | 1 |
| Primary dysfunction | 0 | 2 | 0 | 0 | 0 | 0 |
| HAT, HA stricture. and/or PVT | 1 | 3 | 7 | 0 | 0 | 3 |
| Other technical | 0 | 1 | 0 | 0 | 0 | 0 |
| Rejection | 0 | 0 | 0 | 0 | 0 | 0 |
| Cardiac problems including pulmonary hypertension | 2 | 0 | 0 | 1 | 0 | 0 |
| Infection or sepsis | 2 | 1 | 0 | 2 | 1 | 0 |
| Encephalopathy. brain death | 0 | 2 | 0 | 0 | 0 | 0 |
| Peak AST (IU/L) | 1607 ± 13 | 2852 ± 44d,* | 5836 ± 352#+ | 1800 ± 18 | 2868 ± 94 | 6439 ± 457e,#+ |
| Rise in AST for the first 24 h (IU/L) | 1128 ± 45 | 212 ± 78f,* | 2275 ± 615# | 868 ± 14 | 108 ± 98 | 649 ± 313 |
| Peak PT (sec) | 16.8 ± 0.03 | 20.2 ± 0.1g,* | 22.9 ± 1.1# | 16.7 ± 0.03 | 20.1 ± 0.2h,* | 27.2 ± 1.0#+ |
| CIT (h/min) | 13.49 | 13.46 | 14.52 | 14.20 | 14.09 | 13.53 |
| Range of CIT (h/min) | 4.08–27.21 | 4.43–27.38 | 11.48–23.07 | 6.37–23.02 | 8.53–20.10 | 10.58–19.06 |
Class 1 vs Class 2 in group I P < .005.
Class 2 vs Class 3 in group I P < .0001.
Class 1 vs Class 3 in group II P < .001: Class 2 vs Class 3 in group II P < .01; Class 3 in group I vs Class 3 in group II P < .01.
Peak AST in group I*: Class 1 vs class 2 P < .01. #; Class 1 vs Class 3 P < .01, +; Class 2 vs Class 3 P < .01.
Peak AST in group II #: Class 1 vs Class 3 P < .01, +; Class 2 vs Class 3 P < .01.
Rise in AST in group I*: Class 1 vs Class 2 P < .05, #; Class 1 vs Class 3 P < .01, +; Class 2 vs Class 3 P < .05.
PT (prothrombin time) in group I*: Class 1 vs Class 2 P < .05, #; Class 1 vs Class 3 P < .01.
PT in group II*: Class I vs Class 2 P < .01, #; Class 1 vs Class 3 P < .01, +; Class 2 vs Class 3 P < .01.
HAT, hepatic arteria: thrombosis; PVT, portal vein thrombosis.
Group I
The control patients with good (Class 1) or moderately depressed AKBR (Class 2) had 96.6% and 80.8% 1-month graft survival (P < .01). In contrast, all of the primary grafts were lost within a month in the 15 patients with a 2-day AKBR <0.7 (Class 3). The causes of graft loss in the Class 3 AKBR category were PNF (n = 8) and hepatic artery or portal vein thrombosis (n = 7).
The AKBR classifications correlated well with the results of early AST and prothrombin-time measurements (Table 5), which ranged from the least perturbed in the Class 1 patients to the most grave in the ill-fated Class 3 collection.
Group II
The 1-month graft survival of patients treated with PGE1 was 96.8% for those with Class 1 AKBR and 96.9% with the Class 2 designation (Table 4). The four losses (all with patient death) in these two categories was ascribed to sepsis (n = 3) and a cardiac complication following a graft vena caval anastomosis to the host right atrium. Five (55%) of the nine Class-3 grafts survived. The salvage in this third AKBR category vs the universal failure in the similar control patients who did not receive PGE1 was significant (P < .01). The four organs lost in the Class 3 category failed from PNF in a patient with coincident pulmonary hypertension (n = 1) or from hepatic artery thrombosis (n = 3).
The better course in the PGE1-treated patients was not reflected in the early liver function tests, which were indistinguishable from those in the controls (Table 5).
DISCUSSION
The clinical importance of PNF is underscored by the recent report of the UNOS liver transplant registry of 5658 patients who received liver transplants in the United States from 1988 through the end of 1990.25 Primary nonfunction was the single most frequent cause of graft failure, with a consequent mortality of 5.8%.25,26 The highly variable incidence of this complication in different centers (ranging from 2% to 23%) probably reflects the lack of a generally accepted definition.3,27,28 This is because PNF is a descriptive term encompassing a variety of conditions that represent the common ultimate manifestations of a catastrophic liver injury, which by definition is irreversible.
Etiologic factors ascribed to the donor include preexisting liver disease,19 agonal ischemic episodes.29 the use of vasopressor support.29,30 intraoperativc manipulation,16 faulty procurement technique, and prolonged warm or cold ischemia time.4 Recipient factors could be cardiovascular instability after reperfusion,31 voluminous blood loss,32 the need for pressor agents,33 endotoxemia,32 preformed antigraft antibodies.34–36 and perhaps, above all, an unrecognized hostile immunologic environment that may not be detectable with current screening methodology.37 Such causal ambiguity and heterogenicity plus our previous demonstration of the hepatic as well as renal benefits of prostaglandin-steroid therapy in liver recipients who had a positive lymphocytotoxic cross match prompted our current policy of prophylactic PGE1 therapy for all liver-transplant cases.14
In the present study of cross-match negative patients treated with PGE1, in which an immediately precedent consecutive series of comparable cases was used as a control, the incidence of PNF appeared to have been almost eliminated. The incidence of 1.1% (2/174) was swollen to this figure by the “intent to treat” analysis. The incidence of PNF actually was 1/173 if the patient was excluded who was scheduled for but could not tolerate this therapy.
This low rate of PNF in the PGE1 series, as well as the relatively low incidence of 5.9% in the controls who did not receive PGE1 therapy, reflected in part a strict definition of PNF and the deletion from the PNF list of livers lost to other complications (particularly technical errors). In addition, patients were excluded prospectively from entry into the study who had the positive cytotoxic cross matches that increase the risk of PNF34 unless much higher doses of steroids than in the cases herein reported are given in combination with PGE1.14
The definable operative and early postoperative complications causing liver loss, and that are sometimes passed off as PNF, were not significantly benefitted by the PGE1 treatment. In the culled remaining cases, the patients with an early postoperative course predicted to be favorable by the AKBR test appeared to have no need for prophylactic PGE1 therapy. With this test, pioneered by Ozawa and Pichlmayr and their associates38,39 the redox potential (reduction-oxidation potential) of hepatic mitochondria is assessed by measuring the blood/arterial ketone body ratio (AKBR). Ozawa and Pichlmayr showed that an AKBR below 0.7 at 24 hours after organ recirculation was an early predictor of graft loss. More extensive later studies were provided by Asonuma et al40 who showed a close relationship between ultimate graft prognosis and the 2- to 5-day AKBR.
Patients who appeared to benefit from PGE1 treatment were those with a moderate reduction of energy charge who had an approximately 15% gain in 1-month graft survival and, above all, those with an AKBR below 0.7. In the latter group, five of nine grafts survived compared to none in the control group. Unfortunately, the AKBR is not discriminating in diabetic patients.23,24 recipients with pancreatitis (by inference), and probably patients with biochemical evidence of starvation.41 Under these circumstances, the AKBR tends to underestimate graft quality. Thus, it cannot be wisely used as a routine unless it is interpreted by someone with knowledge of its biochemical basis.
The potential efficacy of PGE1 in liver transplantation could be rationalized in so many ways that it is not tempting to fix upon a single explanation. Members of the prostaglandin family are cytoprotective for hepatocytes.8–11 suppress cell-mediated cytotoxicity.42,43 inhibit cytokine release from activating macrophages,44,45 facilitate hepatic regeneration.45 and inhibit superoxide anion radical (O2−) generation from activated polymorphonuclear leukocytes.13 In addition, various prostaglandins are powerful vasodilators47 and inhibit platelet aggregation,47,48 although at a concentration level that is 1000 times greater than the 100 pg measured in some of our patients receiving the highest dose of 0.6 µg/kg per hour (S. Takaya, unpublished observations).
The sharpest controversy is whether any or all of the foregoing mechanisms are as important in recovery from the insult leading to PNF as the vasodilation caused by the prostaglandins. In our patients with moderately or severely deranged AKBR, there was no evidence from the early liver function tests of ALT or prothrombin time of an ameliorating effect on the acutely damaged hepatocytes. Instead, the therapeutic effect was the inexplicably greater rate of ultimate recovery. Francavilla et al49 recently summarized the evidence that continuing microvascular failure is the reason for the liver’s inability to recover from fulminant hepatic failure, rather than a paucity of growth or other factors subserving repair and regeneration. Thus, protection of the hepatic microvasculature is postulated to be the prime reason for a prostaglandin benefit not limited to its vasodilatory effect. 7,50
Finally, the clinical use of PGE1 as prophylaxis for liver injury has some inherent limitations. It is rapidly inactivated in the lung, making it difficult to deliver a high concentration to the desired target. It may cause hypotension under the already volatile cardiodynamic circumstances of liver transplantation. Other side effects are diarrhea as well as hypoxia due to exacerbation of pulmonary shunting. Nevertheless, the benefits of prophylactic PGE1 for the liver transplant recipient would appear to outweigh its potential morbidity and inevitable inconvenience.
Acknowledgments
This work was supported by Project Grant No. DK 29961 from the National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.Jamieson NV, Sundberg R, Lindell S, et al. Transplantation. 1988;46:517. doi: 10.1097/00007890-198810000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Kalayoglu M, Sollinger HW, Stratta RJ, et al. Lancet. 1988;1:617. doi: 10.1016/s0140-6736(88)91416-x. [DOI] [PubMed] [Google Scholar]
- 3.Todo S, Nery J, Yanaga K, et al. JAMA. 1989;262:711. [PMC free article] [PubMed] [Google Scholar]
- 4.Furukawa H, Todo S, Imventarza O, et al. Transplantation. 1991;51:1000. doi: 10.1097/00007890-199105000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makowka L, Gordon RD, Todo S, et al. Transplant Proc. 1987;19:2378. [PMC free article] [PubMed] [Google Scholar]
- 6.Greig PD, Woolf GM, Sinclair SB, et al. Transplantation. 1989;48:447. doi: 10.1097/00007890-198909000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki S, Nakamura S, Koizumi T, et al. Transplantation. 1991;52:978. doi: 10.1097/00007890-199112000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Stachura J, Tarnawski A, Ivey KJ, et al. Gastroenterology. 1981;81:211. [PubMed] [Google Scholar]
- 9.Sikujara O, Monden M, Toyoshima K, et al. Transplantation. 1983;36:238. doi: 10.1097/00007890-198309000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Araki H, Lefer AM. Am J Physiol. 1980;238:H176. doi: 10.1152/ajpheart.1980.238.2.H176. [DOI] [PubMed] [Google Scholar]
- 11.Mizoguchi Y, Tsutsui H, Miyajima K, et al. Hepatology. 1987;7:1184. doi: 10.1002/hep.1840070603. [DOI] [PubMed] [Google Scholar]
- 12.Tobimatsu M, Konomi K, Saito S, et al. Surgery. 1985;98:45. [PubMed] [Google Scholar]
- 13.Wong K, Freund K. Can J Physiol Pharmacol. 1981;59:915. doi: 10.1139/y81-141. [DOI] [PubMed] [Google Scholar]
- 14.Takaya S, Iwaki Y, Starzl TE. Transplantation. 1992;54:927. doi: 10.1097/00007890-199211000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takaya S, Bronsther O, Abu-Elmagd K, et al. Transplant Proc. 1993;25:2381–2385. [PMC free article] [PubMed] [Google Scholar]
- 16.Starzl TE, Hakala TR, Shaw BW, Jr, et al. Surg Gynecol Obstet. 1984;158:223. [PMC free article] [PubMed] [Google Scholar]
- 17.Starzl TE, Miller C, Broznick B, et al. Surg Gynecol Obstet. 1987;165:343. [PMC free article] [PubMed] [Google Scholar]
- 18.D’Alessandro AM, Kalayoglu M, Sollinger HW, et al. Transplantation. 1991;51:157. doi: 10.1097/00007890-199101000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Todo S, Demetris AJ, Makowka L, et al. Transplantation. 1989;47:903. doi: 10.1097/00007890-198905000-00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamura K, Kobayashi M, Hashimoto K, et al. Transplant Proc. 1987;19 suppl 6:23. [PubMed] [Google Scholar]
- 21.Mellanby J, Williamson DH. In: Methods of Enzymatic Analysis. Bergmeyer HU, editor. New York: Academic Press; 1974. p. 1840. [Google Scholar]
- 22.Williamson DH, Mellanby J. In: Methods of Enzymatic Analysis. Bergmeyer HU, editor. New York: Academic Press; 1974. p. 1836. [Google Scholar]
- 23.Ozawa K, Yamada T, Yamamoto M, et al. Life Sci. 1976;19:1865. doi: 10.1016/0024-3205(76)90118-1. [DOI] [PubMed] [Google Scholar]
- 24.Yamada T, Yamamoto M, Ozawa K, et al. Ann Surg. 1977;185:35. doi: 10.1097/00000658-197701000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belle SH, Beringer KC, Murphy JB, et al. In: Clinical Transplants 1991. Terasaki PI, editor. Los Angeles: UCLA Tissue Typing Laboratory; 1992. p. 13. [Google Scholar]
- 26.Takaya S, Bronsther O, Todo S, et al. Transplant Proc. 1991;23:3026. [PMC free article] [PubMed] [Google Scholar]
- 27.D’Alessandro AM, Kalayoglu M, Sollinger HW, et al. Transplant Proc. 1990;22:474. [PubMed] [Google Scholar]
- 28.Vacanti JP, Lillehei CW, Jenkins RL, et al. Transplant Proc. 1987;19:3261. [PubMed] [Google Scholar]
- 29.Wijnen RMH, Linden CJ. Transpl Int. 1991;4:186. doi: 10.1007/BF00335342. [DOI] [PubMed] [Google Scholar]
- 30.Nagareda T, Kinoshita Y, Tanaka A, et al. Transplantation. 1989;47:792. doi: 10.1097/00007890-198905000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Aggarwal S, Kang Y, Freeman J, et al. Transplant Proc. 1987;19:54. [PubMed] [Google Scholar]
- 32.Yokoyama I, Todo S, Miyata T, et al. Transplant Proc. 1989;21:3833. [PMC free article] [PubMed] [Google Scholar]
- 33.Shaw BW, Jr, Stratta RJ, Donovan JP, et al. Semin Liver Dis. 1989;9:202. doi: 10.1055/s-2008-1040514. [DOI] [PubMed] [Google Scholar]
- 34.Takaya S, Bronsther O, Iwaki Y, et al. Transplantation. 1992;53:400. doi: 10.1097/00007890-199202010-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Starzl TE, Tzakis A, Makowka L, et al. Transplant Proc. 1987;19:4492. [PMC free article] [PubMed] [Google Scholar]
- 36.Demetris AJ, Nakamura K, Yagihashi A, et al. Hepatology. 1992;16:671. doi: 10.1002/hep.1840160310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Starzl TE, Demetris AJ, Todo S, et al. Clin Transpl. 1989;3:37. [Google Scholar]
- 38.Ozawa K. In: Molecular and Cellular Aspects of Shock and Trauma. Lefer AM, Schumer W, editors. New York: Alan R. Liss; 1983. p. 39. [Google Scholar]
- 39.Taki Y, Gubernatis G, Yamaoka Y, et al. Transplantation. 1990;49:535. doi: 10.1097/00007890-199003000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Asonuma K, Takaya S, Selby R, et al. Transplantation. 1991;51:164. doi: 10.1097/00007890-199101000-00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mori K, Ozawa K, Yamamoto Y, et al. Ann Surg. 1990;211:438. doi: 10.1097/00000658-199004000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darrow TL, Tomar RH. Cell Immunol. 1980;56:172. doi: 10.1016/0008-8749(80)90092-1. [DOI] [PubMed] [Google Scholar]
- 43.Pollak R, Dumble LJ, Wiederkehr JC, et al. Transplantation. 1990;50:834. doi: 10.1097/00007890-199011000-00018. [DOI] [PubMed] [Google Scholar]
- 44.Rappaport RS, Dodge GR. J Exp Med. 1982;155:943. doi: 10.1084/jem.155.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker C, Kristensen F, Bettens F, et al. J Immunol. 1983;130:1770. [PubMed] [Google Scholar]
- 46.Miura Y, Fukui T. Cell Mol Biol. 1979;25:179. [PubMed] [Google Scholar]
- 47.Bunting S, Glyglewski RJ, Moncada S, et al. Prostaglandins. 1976;12:817. [Google Scholar]
- 48.Moncada S, Vane JR. N Eng J Med. 1979;300:1142. doi: 10.1056/NEJM197905173002006. [DOI] [PubMed] [Google Scholar]
- 49.Francavilla A, Azzarone A, Carrieri G, et al. Hepatology. 1993;17:429. [PMC free article] [PubMed] [Google Scholar]
- 50.Abecassis M, Falk JA, Makowka L, et al. J Clin Invest. 1987;80:881. doi: 10.1172/JCI113147. [DOI] [PMC free article] [PubMed] [Google Scholar]