CASE
A 3-year-old girl was transferred from an outside hospital to the emergency department after being found shivering and unattended outside of a public shopping area in winter. She presented with decreased mental status and possible hypothermia; she was lethargic and slipped in and out of consciousness. No cause for this alteration in her mental state was obvious; however, a physical examination revealed multiple abrasions and bruises. The patient had a temperature of 37 °C, a blood pressure of 134/74 mmHg, a heart rate of 103 beats/min, and a respiratory rate of 24 breaths/min. Computed tomography scans, a skeletal survey, and an ophthalmologic examination did not reveal additional injuries. An intravenous bolus of the opioid antagonist naloxone (Narcan) was administered shortly after her arrival in the emergency department.
Routine hematology and chemistry tests were performed and revealed decreased serum urea nitrogen, along with increased lactate, aspartate aminotransferase, and alanine aminotransferase (Table 1). Serum acetaminophen and salicylate were not detected, and blood carboxyhemoglobin was not increased. Urine and stool cultures were negative, as was a nasal swab for methicillin-resistant Staphylococcus aureus. Toxicologic screening of a urine sample collected upon the girl’s arrival in the emergency department detected no amphetamines, barbiturates, benzodiazepines, cocaine metabolite, opiates (codeine and morphine), tetrahydrocannabinol (marijuana), or methodone. A gas chromatographic screen for volatile substances in the patient’s serum did not detect ethanol, methanol, or isopropyl alcohol but was positive for acetone. She was admitted to a pediatric floor for further monitoring.
Table 1.
Patient laboratory results (serum).
| Analyte | Result | Reference interval |
|---|---|---|
| Sodium, mmol/L | 142 | 135–148 |
| Potassium, mmol/L | 3.4 | 3.5–5.1a |
| Chloride, mmol/L | 107 | 99–111a |
| CO2, mmol/L | 20 | 21–31 |
| Blood urea nitrogen, mmol/L | 0.7 | 2.5–7.9 |
| Creatinine, μmol/L | 27 | 27–62a |
| Calcium, mmol/L | 2.2 | 2.0–2.6)a |
| Total protein, g/L | 65 | 60–82 |
| Glucose, mmol/L | 4.7 | 3.3–5.5a |
| Alanine aminotransferase, U/L | 77 | 0–31 |
| Aspartate aminotransferase, U/L | 79 | 0–31 |
| Lactic acid, mmol/L | 2.7 | 0.5–2.2 |
| Carboxyhemoglobin, % | 1.2 | 0–2.0 |
| Acetaminophen, μmol/L | <6.614 | 66–132b |
| Salicylate, mmol/L | <0.22 | 0.14–0.72b |
Age-specific reference interval, pediatric.
Therapeutic reference interval.
Early the next day, clinicians requested a second urine toxicology immunoassay screen. This sample was positive for opiates at a cutoff of 300 μg/L. Confirmatory testing by GC-MS was negative for codeine, hydrocodone, oxycodone, morphine, hydromorphone, and oxymorphone (100-μg/L cutoff). A repeat immunoassay in our laboratory substantiated the original positive opiate result, and further investigation was initiated.
DISCUSSION
IMMUNOASSAY CROSS-REACTIVITY
Immunoassays provide a rapid method to screen for the presence of drugs and drug metabolites in urine. Both structurally related and unrelated compounds may cause false-positive assay results by binding non-specifically to the antibodies used in a particular immunoassay. Therefore, a more specific method must be used in forensic testing to confirm a positive immunoassay result. GC-MS is widely accepted as the gold standard method to confirm the presence of drugs of abuse in urine (1).
The opiate cloned enzyme donor immunoassay (CEDIA)3 used by the clinical laboratory in this case is designed to produce a positive result when morphine or codeine is present at 300 μg/L or greater. Studies performed by the manufacturer, however, have determined that 23 additional compounds may also cause a positive response when present at specific concentrations. Of interest to this case is that the opiate antagonist naloxone reportedly cross-reacts with the assay at a concentration of 6000 μg/L.
USE OF NALOXONE IN PEDIATRIC PATIENTS
Naloxone is used in both children and adults for reversal of opioid analgesia and management of opioid overdose. Acting as a competitive antagonist, naloxone binds to and blocks the μ opioid receptor, leading to withdrawal symptoms in frequent users of opioids (2). In contrast with adults, children almost never exhibit opioid tolerance, so precipitation of withdrawal is not a concern. Additionally, minimal side effects from naloxone use have been reported. Thus, naloxone is often administered prophylactically to children who present in emergent situations with depressed respiratory rates and/or mental status before opioid exposure has been confirmed (3).
Naloxone may be administered by an intravenous, intramuscular, intraosseous, subcutaneous, or endo-tracheal route. When administered intravenously, the onset of action is within 1–5 min, with a duration of 20–90 min (4). Intramuscular administration causes a delayed onset but a longer duration of action. Because of the rapid onset of action and the ease of titration, intravenous administration is preferred.
The American Academy of Pediatrics recommends an initial dose of 0.1 mg/kg up to a maximum of 2 mg/dose (5); however, for treatment of a pediatric opioid overdose, a larger naloxone dose may be appropriate. For children presenting to our institution with suspected opioid overdose, an intravenous dose of 2 mg is commonly used, with repeated doses administered until normal ventilation is restored. Exposure to synthetic or semisynthetic opioids may require even higher naloxone doses for reversal.
CONFIRMATION OF NALOXONE CROSS-REACTIVITY
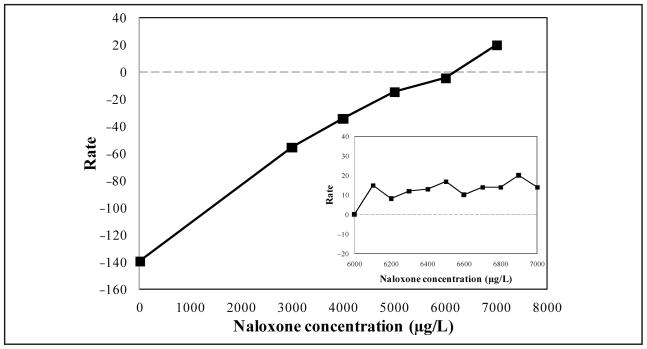
A cross-reactivity study was conducted to confirm whether the opiate the CEDIA used in our clinical laboratory was capable of detecting naloxone in urine samples. The manufacturer of the opiate immunoassay has reported that 6000 μg/L naloxone produced a positive test result at the 300-μg/L cutoff. We added purified naloxone (DuPont) to drug-free urine (UTAK Laboratories) at 3000, 4000, 5000, 6000, and 7000 μg/L to test for cross-reactivity in that range. Supplemented samples and drug-free urine alone were tested with the opiate immunoassay. With the 300-μg/L cutoff, urine samples supplemented with naloxone concentrations between 3000 μg/L and 6000 μg/L were reported as “not detected,” whereas samples containing 7000 μg/L naloxone were reported as “opiate positive” (Fig. 1). Further studies were completed to define more narrowly the threshold at which naloxone may cause a positive opiate result. Urine was supplemented with naloxone concentrations between 6000 μg/L and 7000 μg/L (Fig. 1, inset). Samples supplemented with 6100 μg/L of naloxone or greater were reported as “opiate positive” with the opiate CEDIA.
Fig. 1. Cross-reactivity of naloxone with an opiate CEDIA.
Rate reflects the change in absorbance units per minute subtracted from the rate obtained for the 300-μg/L cutoff calibrator. Dashed line indicates the threshold for positivity. Inset: naloxone concentrations between 6000 μg/L and 7000 μg/L.
The original urine samples from the case study patient were sent to a reference laboratory to confirm whether naloxone was detectable. Naloxone concentrations were quantified with liquid chromatography–tandem mass spectrometry (LC-MS/MS), which detects both conjugated and unconjugated forms of the drug. The earlier urine sample that the immunoassay had reported as “not detected” for opiates, contained 220 μg/L total naloxone. The second urine sample, reported as “opiate positive,” contained 9900 μg/L total naloxone. This naloxone concentration exceeded the 6100-μg/L threshold determined by the cross-reactivity study, indicating that the naloxone concentration in this urine sample was sufficient to produce a positive result in the opiate immunoassay screen.
PATIENT FOLLOW-UP
Further review of the patient’s medical record revealed that the first intravenous naloxone bolus had been administered minutes before the first urine collection. A 24-h naloxone intravenous drip was then initiated approximately 45 min after that collection. Therefore, very little naloxone, if any, would have been detected in the patient’s first urine sample (no opiates detected), and greater amounts would be present in the second urine sample (opiate positive). This drug time course, along with the confirmatory LC-MS/MS test results, implicated naloxone as the agent responsible for the positive opiate results for this patient.
The suspicion of opiate ingestion was not pursued further in this particular patient. The lethargy and decreased mental state of the patient, along with the other biochemical changes observed, are consistent with mild hypothermia. The other positive toxicology result, serum acetone, was also suspected to be due to ketoacidosis caused by poor nutrition or mild hypothermia, given the situation in which the patient was found. After treatment in a favorable environment, the patient regained a typical alert status, including eating and communicating.
CLINICAL IMPLICATIONS
The ability of naloxone to cross-react with an opiate immunoassay is an important consideration when testing for the presence of drugs of abuse. Naloxone cross-reactivity with oxycodone immunoassays has also recently been reported (6). This finding is of particular importance in pediatric patients, because of the frequent use of naloxone in this population and the pharmacokinetic differences between children and adults.
This case highlights the importance of the use of confirmatory tests to validate screening results in certain circumstances, in conjunction with a thorough review of the pertinent medical history of the patient. Immunoassay screening results that do not match the patient’s clinical picture should be confirmed by GC-MS methods. Unexpected screening results should also be confirmed when medications that metabolize into illicit compounds are concurrently prescribed (e.g., acetaminophen with codeine). Confirmatory methods are commonly warranted when managing suspected drug exposure in infants and neonates because of their inability to access or administer these types of substances on their own. Legally sensitive cases, such as those involving child abuse or neglect, require the accurate results that mass spectrometry methods can provide.
For the patient we have presented, this false-positive opiate immunoassay result had no further clinical implications. She received treatment based only on her clinical symptoms, and additional intervention was deemed unnecessary. An opiate-positive result in a pediatric patient, however, may lead to unnecessary hospital admission, additional testing, and further investigation. Child protective services would commonly be contacted and become involved in the management of the case. For these reasons, clinicians should be aware that the commonly used opioid antagonist naloxone can produce false-positive opiate immunoassay results.
QUESTIONS TO CONSIDER.
What clinical situations may produce a positive result in an opiate screen?
How can drug cross-reactivity with clinical immunoassays be confirmed?
What situations may warrant confirmatory testing for drugs of abuse?
What factors should be considered when testing pediatric patients for exposure to drugs of abuse?
POINTS TO REMEMBER.
Immunoassays used to screen for the presence of drugs of abuse may not be specific for the intended drug or class and may cross-react with other prescription and nonprescription drugs.
Confirmatory testing with a second method with greater analytical specificity and lower detection limits is useful when a screening result is questioned or needs to be verified. Confirmatory testing is commonly required in legally sensitive cases.
Both screening and confirmatory tests use cutoff concentrations to distinguish between positive and negative results. The results of drug or drug metabolite tests may be truly negative, or the drugs or their metabolites may be present at concentrations below the cutoff value used and thus be reported as negative or not detected.
Drugs and drug metabolites that share structural similarities with the target compound may cross-react with the antibodies used in screening immunoassays. Cross-reactivity experiments may be useful in confirming conflicting clinical results.
When interpreting toxicology results, clinicians and laboratory scientists should remember that pediatric patients have a different pharmacokinetic profile than adults for many substances.
Footnotes
Nonstandard abbreviations: CEDIA, cloned enzyme donor immunoassay; LC-MS/MS, liquid chromatography–tandem mass spectrometry.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures of Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Potential conflicts of interest:
Employment or Leadership: None declared.
Consultant or Advisory Role: W. Clarke, Thermo Fisher Scientific.
Stock Ownership: None declared.
Honoraria: None declared.
Research Funding: W. Clarke, Thermo Fisher Scientific and the HIV Prevention Trials Network (HPTN) sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), the National Institute on Drug Abuse (NIDA), the National Institute of Mental Health (NIMH), and the Office of AIDS Research, of the NIH, Department of Health and Human Services (U01-AI-068613).
Expert Testimony: None declared.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
References
- 1.Porter WH. Clinical toxicology. In: Burtis CA, Ashwood ER, Bruns DE, editors. Teitz textbook of clinical chemistry and molecular diagnostics. 4. St. Louis: Elsevier Saunders; 2006. pp. 1287–369. [Google Scholar]
- 2.Tornabene VW. Narcotic withdrawal syndrome caused by naltrexone. Ann Intern Med. 1974;81:785–7. doi: 10.7326/0003-4819-81-6-785. [DOI] [PubMed] [Google Scholar]
- 3.Osterhoudt KC, Ewald MB, Shannon M, Henretig FM. Toxicologic emergencies. In: Fleisher GR, Ludwig S, Henretig FM, editors. Textbook of pediatric emergency medicine. 5. Philadelphia: Lippincott Williams & Wilkins; 2006. p. 953. [Google Scholar]
- 4.Howland MA. Opioid antagonists. In: Flomenbaum NE, Goldfrank LR, Hoffman RS, Howland MA, Lewin NA, Nelson LS, editors. Goldfrank’s toxicologic emergencies. 8. New York: McGraw-Hill; 2006. pp. 614–9. [Google Scholar]
- 5.American Academy of Pediatrics Committee on Drugs. Naloxone dosage and route of administration for infants and children: addendum to emergency drug doses for infants and children. Pediatrics. 1990;86:484–5. [PubMed] [Google Scholar]
- 6.Jenkins AJ, Poirier JG, Juhascik MP. Cross-reactivity of naloxone with oxycodone immunoassays: implications for individuals taking Suboxone®. Clin Chem. 2009;55:1434–6. doi: 10.1373/clinchem.2009.125096. [DOI] [PubMed] [Google Scholar]