Abstract
Abdominal multivisceral allotransplantation (MVTX) from Brown Norway donor rats to Lewis recipient rats was performed under a 14-day course of low (0.32 mg/kg) or high-dose (0.64 mg/kg) intramuscular FK 506 to which weekly further injections were added in some of the high-dose animals. With all three regimens, long survival was frequently achieved with good intestinal absorption and weight gain, but histopathologic evidence of intestinal rejection existed in the most lightly treated animals. The liver, stomach, and pancreas had only minor abnormalities. Rejection of isolated intestinal grafts was more difficult to control based on histopathologic criteria, and satisfactory results were obtained only with the most aggressive treatment protocol, suggesting that the liver in the MVTX had provided an advantage to the companion organs of the graft, of which the intestine was most vulnerable. Histopathologically, the lymphoid elements of the intestine, including the Peyer’s patches, appeared to be the most immunogenic component of the intestine. Epithelium near lymphoid areas was secondarily involved with villous atrophy, cryptitis, and abscess formation. Beginning within 12 days in successful MVTX experiments, the lymphoreticular components of the graft intestine, including the Peyer’s patches, lamina propria, and mesenteric nodes, were shown with anti-Ia monoclonal antibodies to be repopulated with recipient cells. This finding in grafts that appeared to be permanently accepted was surprising and contrary to expectations from the literature on intestinal allotransplantation.
The organs included in multivisceral transplantation (MVTX) are the liver, pancreas, stomach, small bowel, and portions of the colon.1 Clinical efforts to transplant this organ composite ultimately have failed for many reasons, including rejection, sepsis, and the development of lymphoproliferative disorders.2–4 To further study these and other problems, we developed a rat model of this operation in nonimmunosuppressed recipients of syngeneic and allogeneic grafts.5 Multivisceral allografts were rejected in 10 days, at about the same time and intensity as isolated intestinal grafts in the same strain combination. In contrast, isolated liver grafts were accepted permanently. It was concluded that the intestinal component of the multivisceral graft was the Achilles heel of this operation, that its vulnerability to rejection was not mitigated in untreated animals by the potential tolerogenic presence of the liver and that the privileged state of the liver was lost when it was part of the organ composite. However, the possibility was left open that a tolerogenic effect of the liver might be unmasked under effective immunosuppression.5
This hypothesis was examined, with the new immunosuppressive agent, FK 506, to treat recipients of multivisceral or isolated intestinal allografts. The intestine proved to be easier to protect from rejection as part of a multi visceral graft than when transplanted alone, and for the first time routine long-term survival could be achieved after MVTX. This was possible without evidence of graft versus host disease (GVHD). In addition, it was shown that the chronically surviving multivisceral or isolated intestinal recipients repopulated the nonepithelial components of their grafts with a range of host cells predominantly of hematopoietic origin. These results have important clinical implications.
MATERIAL AND METHODS
Animals
Inbred male Lewis rats (LEW) weighing 250 to 350 gm and Brown Norway rats (BN) weighing 200 to 300 gm were used as recipients and donors, respectively. With this strain combination, a histocompatibility difference exists for both major histocompatibility complex (MHC) and non-MHC antigens. The animals (Harlan Sprague Dawley Inc., Indianapolis, Ind.) were maintained under standard conditions in conventional facilities.
After surgery, the animals in both experimental groups were housed in individual cages and were given cefamandole nafate (20 mg/day) for 5 days after MVTX and for 3 days after small-bowel transplantation (SBTX). Deaths within 4 days were assumed to have a technical cause, and these animals were excluded from further study. To have 48 complete experiments, a total of 61 experiments were performed. The technical failure rate of 21.3% was almost equally distributed between the MVTX and SBTX experiments.
Operations
MVTX
The operation has been described previously.5 After recipient evisceration, an en bloc graft including the liver, pancreas, stomach, omentum, small intestine, and colon was orthotopically transplanted, with vascular anastomoses between the donor and recipient suprahepatic vena cava, infrahepatic vena cava, and aorta with an interposed small aortic graft. Gastrointestinal continuity was restored proximally by gastrogastrostomy and distally by anastomosis of the donor and recipient colons.
SBTX
The recipient small bowel was resected, including the mesenteric lymph nodes. The donor small bowel from the ligament of the Trietz to the ileocecal valve was isolated on its vascular pedicle.5 The graft superior mesenteric artery was left in continuity with a segment of the donor aorta, which was anastomosed to the recipient infrarenal aorta. The portal vein continuation of the superior mesenteric vein was retained with the graft and anastomosed to the recipient inferior vena cava. Intestinal continuity was restored by proximal and distal intestinal anastomoses.
Treatment and experimental design
All animals were given daily intramuscular injections of FK 506, that had been dissolved in a carrier solvent (HCO-60 D-mannitol; Fujisawa Pharmaceutical Co. Ltd., Osaka, Japan) and diluted in normal saline. Three dose schedules were used (Table I): (1) 0.32 mg/kg for 14 days, (2) 0.64 mg/kg for 14 days, and (3) 0.64 mg/kg for 14 days followed by weekly injections of 0.64 mg/kg to day 200. Treatment was started at the completion of surgery. The FK 506 doses used were modest because in earlier studies in LEW rats the optimal intramuscular dose was 1.28 mg/kg.6
Table I.
Survival after MVTX and isolated SBTX
| Group | Treatment |
n | Survival (days) | ||
|---|---|---|---|---|---|
| Procedure | Dose | Duration | |||
| 1 | MVTX | None | — | 5 | 10, 10, 10, 10, 13 |
| 2 | MVTX | 0.32 mg/kg | 14 Days | 6 | (114), >155, >157, >164, >226, >227 |
| 3 | MVTX | 0.64 mg/kg | 14 Days | 7 | (12*), 18,** (114), (133), (134), >161, >194, |
| 4 | MVTX | 0.64 mg/kg | 14 Days, then weekly | 3 | (203), >218, >224 |
| 5 | SBTX | None | — | 7 | 9, 10, 11, 12, 13, 13, 14 |
| 6 | SBTX | 0.32 mg/kg | 14 Days | 6 | (57*), (88*), (102*), >169, >213, >217 |
| 7 | SBTX | 0.64 mg/kg | 14 Days | 10 | 42,* 105,* (107), 112,* >120, >121, >121, >122, 163,* >163,* >263 |
| 8 | SBTX | 0.64 mg/kg | 14 Days, then weekly | 4 | (109), >212, >212, >242 |
Ileus;
chylothorax.
Note: The animals with survival limes in parentheses were killed for histologic studies. These rats were in good condition at the time of death, except when indicated by * or ** within the parentheses. The other rats with survival times marked * or ** died in the cage or were killed because they were dying and could not be used for the special pathologic studies.
Nutrition and intestinal absorption
Body weight and activity were recorded daily for the first 2 weeks and twice a week thereafter.
Maltose absorption was determined by the measure of change in blood glucose after an enteral maltose challenge. The intestinal absorption of maltose requires the enzyme disaccharidase in the mucosal brush border for the breakdown of maltose in glucose and active glucose absorption.7 Before challenge, the animals were fasted for 9 hours but were allowed free access to water. The maltose was dissolved in 0.45% saline at a concentration of 1 gm/ml and administered at a dose of 3 gm/kg body weight by a gastric gavage tube. Blood glucose levels were measured with a glucose analyzer (Glucometer 2; Miles Laboratories Inc., Elkart, Ind.) before and 15, 30, 45, 60, 90, and 120 minutes after maltose loading. Each sample required one drop of capillary blood from the tail.
Pathologic studies
For routine histologic studies, the tissues were fixed in neutral buffered formalin, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin.
Tissues for special immunohistochemical studies were immediately frozen in optimal cold temperature compound (Tissue-Tek; Miles Laboratories Inc.). To determine the origin of the lymphoid tissues in the graft, immunohistochemical studies were done with immunoperoxidase techniques on a variety of allograft and recipient tissues. A mouse monoclonal anti-rat class II MHC antibody (L-21-6)8 was used, which reacts with an Ia determinant present on the tissues of several strains of rats including the recipient strain (LEW) but not the donor strain (BN).8 Six microns cryostat sections were cut, fixed in cold acetone for 5 minutes, and air dried overnight. Staining was performed with a three-step avidin-biotin complex procedure. Before incubation with the primary antibody, endogenous peroxidase activity was quenched by reacting the tissue with 0.6% hydrogen peroxidase in methanol for 5 minutes. Then, the slides were washed in buffer and reacted with the primary antibody for 45 minutes, followed by a buffer wash. Next, the tissues were incubated with a biotinylated rat anti-mouse F(ab)2 fragment and washed again. Finally, the tissues were reacted with an avidin-biotin complex, and color development was obtained with aminoethylcarbazole. Three sets of controls were used: (1) the primary antibody was omitted, to rule out endogenous peroxidase activity; (2) the complete procedure was applied to BN specimens from control rats to confirm our previous observations8 that the primary antibody did not bind to tissues of this strain (negative control); and (3) the distribution of staining in normal Lewis rat tissues was confirmed to be typical of class II MHC antigens (positive control).9
The complete pathologic studies were performed in seven long-term survivors, which were killed between 100 and 200 days after transplantation (Table I). In the MVTX experiments, samples were obtained from the graft stomach, small bowel, cecum, colon, mesenteric lymph nodes, pancreas, and liver. Recipient tissue samples were of thymus, thoracic lymph nodes, lung, heart, kidney, and skin. In the SBTX experiments, the same recipient tissues were studied, and graft samples were taken from jejunum, ileum, and mesenteric lymph nodes. In both kinds of allografts, special attention was paid to the Peyer’s lymphoid patches.
RESULTS
Survival
MVTX and SBTX recipients that did not receive immunosuppressive therapy died from acute rejection and consequent small-bowel perforation and/or sepsis after a median survival of 10 and 12 days, respectively. These operations (Table I; groups 1 and 5), which have been reported previously,5 were performed contemporaneously with those in the FK 506 treatment groups of this study. Extended survival of MVTX recipients (groups 2 to 4) and SBTX recipients (groups 6 to 8) was accomplished frequently with FK 506 treatment. This survival was not significantly different with variable drug doses or with one operation versus the other (Table I). However, subtle differences were noted in clinical behavior and in the pathologic observations.
MVTX recipients
With two exceptions (both in group 3), all of the FK 506-treated MVTX rats that were alive at the end of 4 days survived for at least 155 days or until they were killed for tissue studies (Table I). The two early deaths in group 3 were from an ileus and chylothorax. All of the dose schedules were effective (groups 2 to 4; Table I). The five animals that were killed 114 to 203 days after surgery for tissue studies were healthy, and their grafts were grossly normal (Table I). Their deaths for pathologic studies distorted the aforementioned survival comparisons.
SBTX recipients
In contrast to the MVTX experiments, the results were markedly affected by FK 506 dose schedules. Three (50%) of the six rats given 0.32 mg/kg/day for 2 weeks (group 6) and four (40%) of 10 rats given double this dose for 2 weeks (group 7) died or were killed because they were dying after 42 to 163 days. The gross causes of death or terminal illness were intestinal obstruction, abscess formation, and adhesions that were highly concentrated at or near the intestinal anastomoses. Keys for the diagnosis in individual animals are indicated in the footnotes of Table I. The graft intestinal walls were thickened, and Peyer’s patches could not be found. The graft mesentery was thickened with lymphatic congestion and white fibrotic lymph nodes. An apparently healthy rat from the higher dose group 7 also had swollen bowel and thickened mesentery when killed after 107 days, whereas the graft appeared normal at 109 days in a rat from group 8 that was treated with further weekly FK 506 after an initial 14-day high-dose course.
Body weight
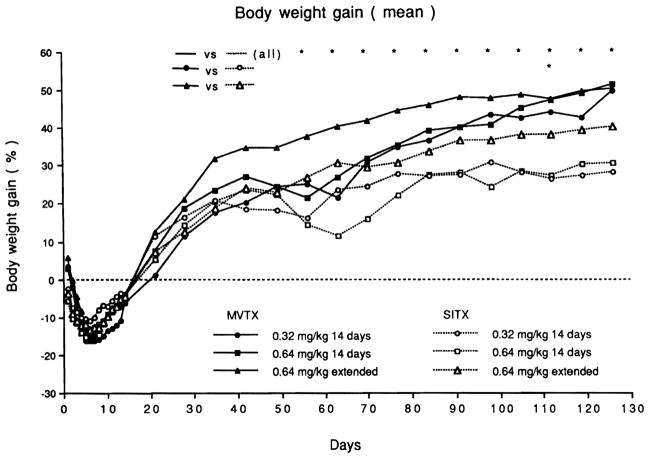
In contrast to the SBTX recipients that usually resumed a regular diet in 1 or 2 days, the MVTX rats required food withdrawal for 4 days. The pooled MVTX rats lost weight to a significantly greater extent than the SBTX rats for each of the first 9 days, but after 8 weeks their weight was significantly higher than the pooled SBTX rats (Fig. 1).
Fig. 1.
Body weight (mean) after transplantation for each treatment group. Weight gain was significantly better (p < 0.05 Student t test) in the pooled MVTX animals versus pooled SBTX animals at the times indicted (*) and also in the low dose experiments at the single time noted.
MVTX
These animals gained weight (Fig. 1) and were active. The animals given extended FK 506 therapy (group 4) had a better weight gain than those with short-term treatment (groups 2 and 3).
SBTX
Weight gain in the SBTX rats was less satisfactory than in the MVTX rats. The best SBTX weight gain was in rats given extended high-dose FK 506 treatment (group 8; Fig. 1). Group 6 and 7 SBTX rats had diarrhea.
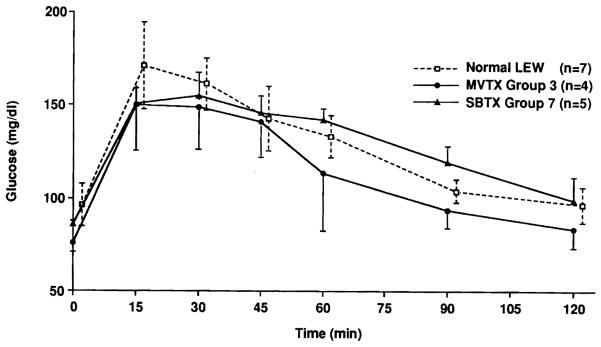
Maltose absorption test
Sixteen animals were studied, including seven unoperated LEW, four MVTX recipients from group 3, and five SBTX recipients from group 7, between 107 and 203 days after transplantation (Fig. 2). Fifteen minutes after maltose loading, peak blood glucose levels were higher in the normal LEW than in those of the experimental groups, but the differences were not statistically significant (p > 0.05; Student t test). Blood glucose levels returned to normal within 120 minutes. Minor differences between the experimental and control animals were observed at various time points (Fig. 2).
Fig. 2.
Maltose absorption test (mean ± SD) of MVTX and SBTX recipients with short term high dose FK506 treatment.
Histopathologic studies
Histopathologic studies were performed only if fresh tissues could be obtained at death. The animals appeared healthy at the time of death, except for those with ileus and chylothorax (Table I). The data from animals used for standard histopathologic studies are shown in Table II.
Table II.
Summary of findings in the small bowel of rats killed 57 to 203 days after surgery for detailed histopathologic studies
| Villi |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose of FK 506 | Time sample in days | Necrosis | Blunting | Cryptitis | Depleted lamina propria | Arteriopathy | Thickened muscularis | Peyer’s patches | Mesenteric lymph nodes |
| 0.32 mg/kg/day × 14 days | |||||||||
| MVTX (n = 1) (group 2) | 114 | No | Focal | Focal | No | No | No | Normal | Depleted and scarred |
| SBTX (n = 3) (group 6) | 57,* 88,* 102* | Focal | Diffuse | Diffuse | Depleted | Yes | Yes | Absent | Depleted and scarred |
| 0.64 mg/kg/day × 14 days | |||||||||
| MVTX (n = 3) (group 3) | 114, 133, 134 | No | Focal (Fig. 4) | Rare | No | No | No | Normal (Fig. 4) | Normal (2 of 3)** (Fig. 4) |
| SBTX (n = 1) (group 7) | 107 | No | Diffuse (Fig. 5) | Common (Fig. 5) | Depleted (Fig. 5) | No | Yes (Fig. 5) | Absent (Fig. 5) | Depleted and scarred (Fig. 6) |
| 0.64 mg/kg/day × 14 days and weekly | |||||||||
| MVTX (n = 1) (group 4) | 203 | No | None (Fig. 3) | None | No | No | No | Normal (Fig. 3) | Normal |
| SBTX (n = 1) (group 8) | 109 | No | Focal | None | No | No | No | Normal | Intact but distorted |
Moribund at time of sacrifice (see Table 1);
Other node depleted and scarred.
Depleted, Lymphoid depletion.
Standard histopathology
MVTX
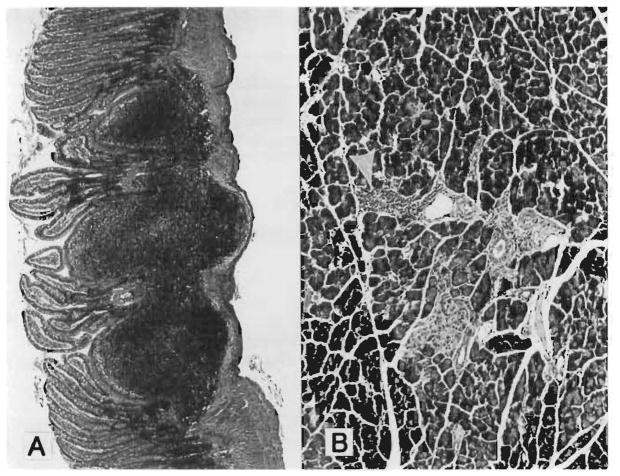
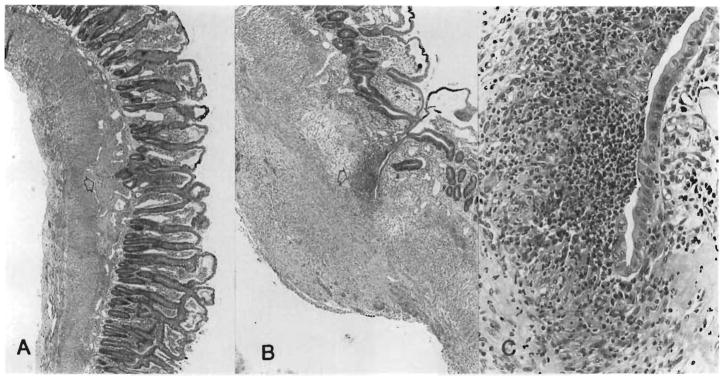
The intestine was essentially normal in the group 4 MVTX rats killed 203 days after surgery after a 14-day course and subsequent weekly high-dose treatment with FK 506 (Fig. 3, A). The villi were long and slender with normal lamina propria, except for mild focal lymphatic dilatation. Peyer’s patches were normal (Table II). The stomach was normal, and the colon could not be distinguished above the colocolostomy from below the recipient’s colon. A mild periductular lymphocytic infiltrate was found in the pancreas (Fig. 3, B) and in the portal tracts of the liver, but the parenchyma was undisturbed.
Fig. 3.
At 203 days after MVTX under high dose continuous FK 506 (group 4). A, Essentially normal small intestine and intact Peyer’s patches. (Hematoxylin-eosin; original magnification ×48. ) B, Pancreas with mild periductular mononuclear infiltrate (arrow). (Hematoxylin-eosin; original magnification ×125.)
The findings were almost as favorable, including good preservation of Peyer’s patches and mesenteric lymph nodes (Fig. 4), in animals given a 14-day high dose course of FK 506 (group 3) and killed 114 to 134 days after surgery. However, in an animal killed 114 days after surgery after receiving one half this dose for 14 days, abnormalities were detected including depletion and scarring of the mesenteric lymph nodes, villous blunting, and focal cryptitis (Table II). In this animal, the grafted pancreas and liver had focal lymphocyte infiltration, but the parenchymal architecture was not disturbed.
Fig. 4.
At 134 days after MVTX under high dose FK 506 for 14 days only (group 3). A, Normal intestine and Peyer’s patches. (Hematoxylin-eosin; original magnification ×48.) B, Intact mesenteric lymph node with full complement of lymphoid cells. (Hematoxylin-eosin; original magnification ×48.)
SBTX
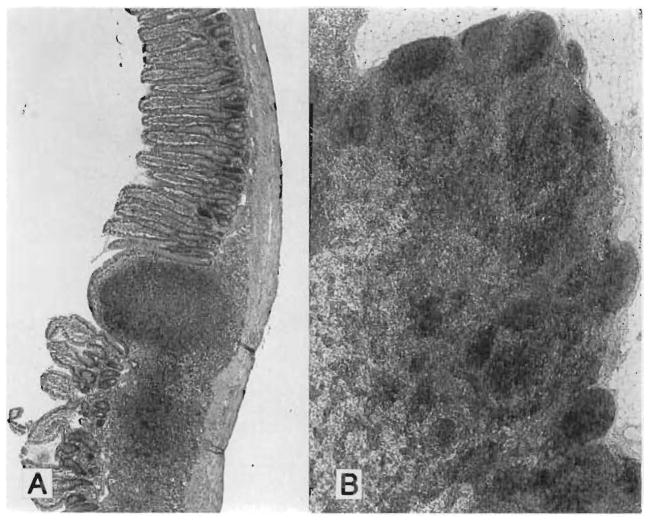
The only recipients with relatively satisfactory results were those in group 8 treated with a 14-day course of high-dose FK 506 followed by weekly injections (Tables I and II). However, with all dose schedules, the changes in the isolated intestinal grafts were more common and severe than in the intestine of the MVTX rats, which had received comparable treatment (Table II). This was exemplified by villous blunting, cryptitis, and lymphoid depletion of the lamina propria (Fig. 5) in an animal of group 7 at 107 days that had been given a 14-day course of high-dose FK 506, which in MVTX experiments completely protected the intestine (Table II). The Peyer’s patches and mesenteric lymph nodes were particularly affected even though the epithelial intestinal components were still relatively intact (Table II); the lymphoid components were completely replaced by scar tissue (Figs. 5 and 6).
Fig. 5.
Intestine 107 days after SBTX under high dose FK 506 for 14 days only (group 7). The conditions of treatment were the same as for the MVTX group 3 (compare with Fig. 4). A, Villous blunting, scarring at Peyer’s patch remnant (arrow) and thickening of muscularis. (Hematoxylin-eosin; original magnification ×48.) B, Mucosal breakdown and intramural abscess (arrow) at site of Peyer’s patch disappearance. (Hematoxylin-eosin; original magnification magnificatoin ×48.) C, High power or abscess. (Hematoxylin-eosin; original magnification ×300.)
Fig. 6.
Host (A) and graft (B) mesenteric lymph nodes from the same rat as in Fig. 5, 107 days after SBTX. Note the total lymphoid depletion and extensive scarring of the allograft node in which remnants of the subcapsular sinus can be seen (arrow). (Hematoxylin-eosin; (hematoxylin-eosin; original magnification ×48.)
The intestinal grafts from the animals treated for 14 days with low-dose FK 506 (group 6) had even more severe and diffuse abnormalities 57 to 102 days after surgery. These specimens were obtained earlier because the rats had become ill as a result of the graft damage and were killed because of their terminal condition (Table I).
Recipient tissues
Host tissues after MVTX or SBTX did not have consistent abnormalities of thoracic lymph nodes, ear skin, tongue, esophagus, heart, lung, and kidneys. The liver, pancreas, stomach, and colon after SBTX were essentially normal. Thymic medullary depletion was common after both MVTX and SBTX. This is a known consequence of FK 506 therapy.10
Immunohistochemical studies
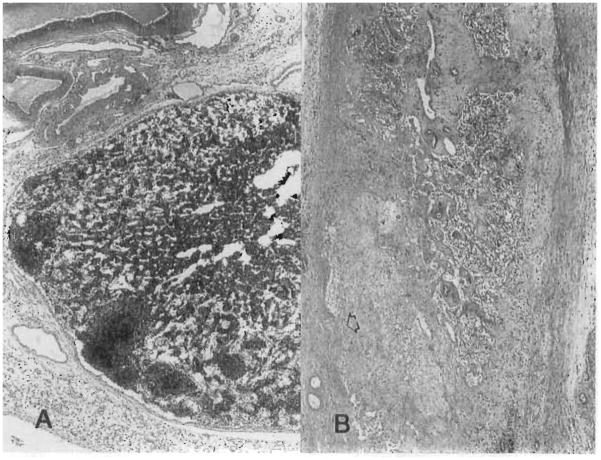
Immunohistochemical staining for recipient cells was performed in four MVTX recipients after 12 to 203 days; three rats were from group 3, and one rat was from group 4. The findings were identical. In the liver, spindle-shaped cells, which were intensely positive for L-21-6, were noted in the portal triads (Fig. 7) immediately subjacent to the terminal hepatic venules and in the capsule of the liver. Similar cells were seen in the pancreatic interstitium and capsule. A few of the mononuclear inflammatory cells in the pancreas and liver were also L-21-6. Practically all of the cells in the lymphoid tissue of the intestine were L-21-6 and thus of recipient origin (Fig. 8). Most cells in the dome region of the Peyer’s patches were also positive, as were dendritic-shaped cells within the follicles. Lymphoid cells in the interfollicular T-cell zones did not stain for L-21-6 (Fig. 8B). These repopulation changes were seen as early as 12 days after MVTX (Fig. 8A).
Fig. 7.
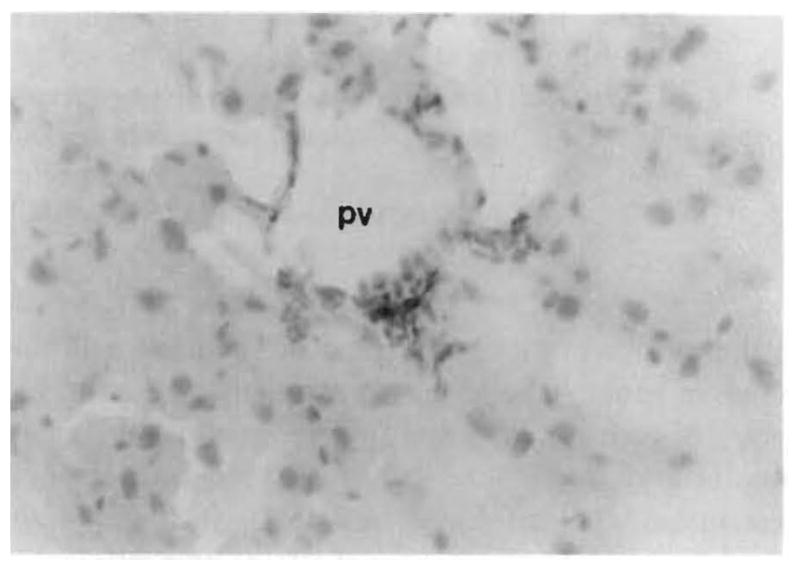
Replacement of intensely L-21-6+ spindle-shaped cells was noted in the liver of a MVTX recipient killed for histologic study 114 days after transplantation under transplantation under high dose FK 506 for 14 days only on (group 3). These cells are probably bone marrow–derived dendritic cells. (L-21-6, red with hematoxylin counterstain; original magnification ×300.); pv, Portal vein.
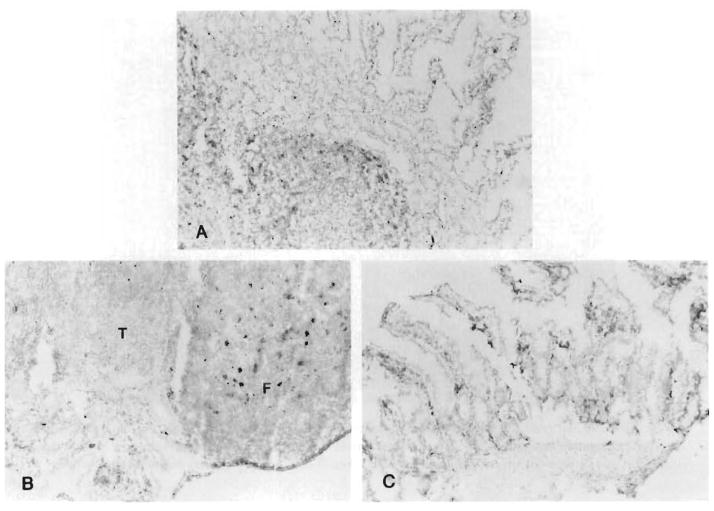
Fig. 8.
Replacement of the donor lymphoid tissue with recipient cells. A, Twelve days after MVTX under high-dose FK 506 for the 12 days of survival (group 3) about one half of the intestinal lymphoid cells were replaced (L-21-6, red; hematoxylin hematoxyling counterstain). B, At 114 days (group 3), most of the cells in the follicles of the Peyer’s patches displayed recipient Ia determinants. C, Same replacement in the lamina propria of the villi (group 3) at 134 days. (L-21-6, red; hematoxylin counterstain; original counterstain (original magnification X300.) T, T-cell interfollicular zone; F, B-cell follicle.
DISCUSSION
These experiments showed that with FK 506 long-term survival can be repeatedly achieved in rats after MVTX across a strong histocompatibility barrier. Systematic or even sporadic success with this difficult operation has not been possible in the past with any species. FK 506, which has been shown in heterotopic11 and orthotopic12 intestinal transplantation models to be superior to cyclosporine for prevention of both rejection and GVHD, was given daily for 2 weeks in low and high-dose schedules and was continued weekly in a subgroup of high-dose animals. Most MVTX rats with all of these therapeutic variations gained weight, were active, and (in those tested) had nearly normal maltose absorption. The parenchyma in all of the constituent organs was well preserved. However, with the less aggressive treatment regimens, the graft mesenteric lymph nodes were sometimes depleted of lymphoid cells; focal cryptitis at the base of the villi was detectable in the small intestine; and periductular lymphocytic infiltration was more prominent in the graft liver and pancreas than when heavier treatment was used. None of the animals had any clinical or histopathologic evidence of GVHD. It is possible that the BN to LEW combination may not be conducive to this complication. The potent ability of FK 506 to prevent GVHD has been shown in other strain combinations.11, 12
Isolated small-bowel transplantation also was successfully done, but control of rejection seemed more difficult to achieve with all of the treatment schedules than with MVTX. Long-term survival comparisons were meaningless because so many healthy MVTX rats were killed for histopathologic studies. Therefore, reliance was placed on the pathologic studies for comparisons of rejection. Differences between MVTX and SBTX were particularly evident at the lowest FK 506 doses, where rejection of the isolated small bowel was associated with severe morbidity, including diarrhea and poor weight gain, even though good maltose absorption was retained. The intestine and mesentery in these animals were grossly swollen. Histopathologically, the intestinal villi had focal necrosis and blunting, many areas of cryptitis (often with abscess formation), and depletion of the lymphocytes in the lamina propria. The lymphoid content of Peyer’s patches and mesenteric lymph nodes was also depleted and was replaced with scar tissue to an extent never seen with comparable treatment in the intestine of the multivisceral grafts. In spite of higher doses of FK 506, many of these same morphologic changes were found, although less advanced, in the isolated intestinal grafts although the animals were clinically normal at the time of their death. The protection of the isolated intestinal grafts was complete only when FK 506 was used in high doses initially and continued weekly afterwards.
These observations are compatible with, but do not prove, previous claims that the liver is protective of or tolerogeneic for concomitantly transplanted organs.13, 14 In the past, the reason for this “sanctuary” influence of the liver has been explained in several ways14, 15 of which one unlikely possibility that has not been ruled out is that the other organs of a multivisceral graft (pancreas, stomach, duodenum, and colon) have an ameliorating influence.15 An attractive recent hypothesis is that excretion of soluble HLA antigens by the liver grafts may be responsible.16 Whatever the explanation, the clinical implication is that intestinal transplantations in the kind of organ composites that have been described for patients with hepato-intestinal disorders2,3,17 may have a distinct immunologic advantage, as has been suspected since the first description of MVTX in dogs.1
The design of our experiments was inappropriate for determination of the reasons for the seeming advantage of the intestine in multivisceral grafts because the venous drainage in the MVTX rats was through the natural transhepatic route, whereas the venous outflow from our isolated intestinal grafts was into the recipient inferior vena cava. Because splanchnic venous blood,18, 19 including that from the intestine,20 is hepatotrophic, the systemic drainage used in our SBTX rats could result in liver damage,20 as suggested by Schraut et al.21 They showed moderate hepatic atrophy, hyperammonemia, and lipid and other metabolic abnormalities in host rat livers after SBTX experiments similar to ours. Although these same metabolic changes were seen by Koltun and Kirkman,22 none of these findings, including hepatic atrophy, could be documented by Shaffer et al.23 when intestinal grafts were drained systemically.
A further question that could not be resolved by our experiments because of the unbalanced intestinal venous drainage in the MVTX and SBTX procedures is whether the liver modifies allograft antigens or immunoreactive cells in ways that mitigate rejection24, 25 or GVHD.23, 26 Numerous claims have been made25 that whole organ or cell grafts drained or implanted in the portal vein are rejected less vigorously than those drained systemically. Schraut et al.26 reported such a mitigating effect on intestinal grafts drained transhepatically, but this could not be confirmed by Shaffer et al.23 Resolution of these important controversies will require further experiments with equivalent intestinal venous drainage after MVTX and SBTX.
Whatever the results of these experiments, they should not invalidate the observations reported herein about the events of intestinal rejection, which were the same in MVTX and SBTX experiments although more pronounced in the latter. The hallmarks of chronic rejection have been reasonably well characterized for the kidney, liver, heart, and lung, but not for the small bowel. The findings in the present experiments and in those previously reported in the absence of immunosuppression5 allow a profile of both acute and chronic intestinal rejection to be drawn after either MVTX or SBTX. With or without immunosuppression, the first insult apparently is to the most immunogenic portions of the intestines, namely the Peyer’s patches and regional lymph nodes. This is seen by day 6 or 7 in untreated animals5 and at variable times under immunosuppression. Villous atrophy and cryptitis near the Peyer’s patches occur as the lymphoid tissue, including that of the lamina propria, is depleted. When necrosis and discontinuity of the epithelium occurs, a loss of the intestinal barrier occurs, which has been illustrated by permeability studies in the landmark human case of Grant et al.17 Bacterial translocation through the damaged and porous intestine would account for the mysterious and usually lethal septic complications that have terminated most clinical efforts at intestinal transplantation. The distortion and destruction of lymph nodes with consequent disruption and stasis in the lymph drainage probably contribute to the swelling of the chronically rejected intestine. The epithelial component evidently is preserved until relatively late because good maltose absorption was maintained in our animals in spite of gross anatomic distortion. All the while, there is a progressive obliteration of the lumens of mesenteric arteries by intimal hyperplasia, characteristic of “chronic” rejection of all organ allografts.
If our observations are verified, revision will be required about some of the assumptions on the central events of both rejection and (in successful transplantation) of graft acceptance. It has been assumed in most descriptions of intestinal rejection, including our own,2,4,27 that the appearance of alloreactive cells in the graft is strongly associated with or equivalent to rejection.
The findings in the studies herein reported suggest that at some stages the opposite may be the truth in that repopulation with recipient cells is associated with graft acceptance. As early as 12 days after transplantation of multivisceral grafts, a major infiltration of recipient lymphoreticular cells had entered the lymphoid components of the graft, including the lamina propria of the villi, the Peyer’s patches, and the regional lymph nodes. Most of the lymphoreticular population in perfectly accepted grafts as late as 203 days were of recipient origin, including the spindle-shaped stromal cells, which were thought to be the dendritic cells so important in antigen presentation. These findings were consistent with those in earlier studies of accepted rat liver grafts in which lymphoid elements that had normal alloreactivity against the original donor strain could be found in the recipient, in spite of the fact that no evidence of graft dysfunction was found.28 It is highly probable that most of the cells of hematopoietic origin in the accepted MVTX and SBTX grafts had undergone this change, although the only ones specifically studied were those with Ia receptors.
Because the demonstration of lymphoreticular repopulation in our experiments was dependent on the specificity of a monoclonal antibody technique, the possibility had to be considered that the results were due to a staining artifact. This was ruled out with both the negative and positive controls. It was also questioned if the change in staining characteristics of the graft could have been due to the increased expression of Ia antigens of donor tissue, which is known to occur during rejection or nonrejection related damage,29–31 rather than reflecting repopulation by recipient cells. However, other experiments in our laboratory on the traffic of dendritic cells in rejecting DA livers transplanted to BN recipients have not revealed any change in the staining of the BN cells in the grafts or in the recipient spleen, thymus, or lymph nodes.32 Aside from these observations, repopulation of the lymphoreticular cells has been shown in three successfully transplanted human intestinal grafts in which Class I HLA phenotypes of the intestinal lymphoreticular tissues were shown with HLA-specific monoclonal antibodies to become those of the recipient (unpublished observations). In both the rat and human studies, the epithelial components of the grafts were thought to have retained donor specificity.
The congruence of these observations in rats and humans with different phenotype detection techniques is reassuring. That lymphoreticular repopulation could occur in humans has received little attention in spite of the fact that replacement of the Kupffer cell population31, 33 or cells in the hepatic hilar lymph nodes34 has been reported under immunosuppression both with azathioprine or cyclosporine-based regimens. Thus the repopulation probably is not specific to FK 506, but merely more easily achieved with FK 506 because of the drug’s superior immunosuppressive qualities during the critical induction phase when cell movement is occurring.
ADDENDUM
Documentation of the same kind of cell repopulation in humans that was seen in these animal experiments is in Iwaki Y, et al.35
Acknowledgments
Supported by research grants from the Veterans Administration and project grant DK 29961 from the National Institutes of Health, Bethesda, Md.
References
- 1.Starzl TE, Kaupp HA, Jr, Brock DR, Butz GW, JR, Linman JW. Homotransplantation of multiple visceral organs. Am J Surg. 1962;103:219–29. doi: 10.1016/0002-9610(62)90491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Rowe MI, Todo S, et al. Transplantation of multiple abdominal viscera. JAMA. 1989;261:1449–57. [PMC free article] [PubMed] [Google Scholar]
- 3.Williams JW, Sankary HN, Foster P, Lowe J, Goldman GM. Splanchnic transplantation: an approach to the infant dependent on parenteral nutrition who develops irreversible liver disease. JAMA. 1989;261:1458–62. doi: 10.1001/jama.261.10.1458. [DOI] [PubMed] [Google Scholar]
- 4.Jaffe R, Trager JDK, Zeevi A, et al. Multivisceral intestinal transplantation: surgical pathology. Pediatr Pathol. 1989;9:633–54. doi: 10.3109/15513818909022372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murase N, Demetris AJ, Kim DG, Todo S, Fung JJ, Starzl TE. Rejection of multivisceral allografts in rats: a sequential analysis with comparison to isolated orthotopic small-bowel and liver grafts. Surgery. 1990;108:880–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Murase N, Todo S, Lee PH, et al. Heterotopic heart transplantation in the rat under FK 506 alone or with cyclosporine. Transplant Proc. 1987;19(Suppl 6):71–5. [PMC free article] [PubMed] [Google Scholar]
- 7.Billiar TR, Garberoglio C, Schraut WH. Maltose absorption as an indicator of small intestinal allograft rejection. J Surg Res. 1984;37:75–82. doi: 10.1016/0022-4804(84)90164-1. [DOI] [PubMed] [Google Scholar]
- 8.Yagihashi A, Iwaki Y, Murase N, Demetris AJ, Starzl TE. L-21-6 a monoclonal antibody recognizing Ia antigens in the rat. Characterization, tissue and strain distribution. Transplantation. (in press) [Google Scholar]
- 9.Hart DNJ, Fabre JW. Demonstration and characterization of Ia-positive dendritic cells in the interstitial connective tissues of rat heart and other tissues, but not brain. J Exp Med. 1981;154:347–61. doi: 10.1084/jem.154.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nalesnik MA, Todo S, Murase N, et al. Toxicology of FK 506 in the Lewis rat. Transplant Proc. 1987;19(Suppl 6):82–92. [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman AL, Makowka L, Banner B, et al. The use of FK 506 for small intestine allotransplantation: inhibition of acute rejection and prevention of fatal graft-versus-host disease. Transplantation. 1990;49:483–90. doi: 10.1097/00007890-199003000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee K, Stangl MJ, Todo S, Langrehr JM, Starzl TE, Schraut WH. Successful orthotopic small bowel transplantation with short term FK 506 immunosuppressive therapy. Transplant Proc. 1990;22:78–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Calne RY, Sells RA, Pena JR, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–6. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 14.Kamada N. Experimental liver transplantatin. Boca Raton, Fla: CRC Press, Inc; 1988. pp. 67–74.pp. 85–97. [Google Scholar]
- 15.Starzl TE. Experience in hepatic transplantation. Philadelphia: WE Saunders Company; 1969. pp. 85–8. (with the assistance of C.W. Putnam) [Google Scholar]
- 16.Davies HS, Pollard SG, Calne RY. Soluble HLA antigens in the circulation of liver graft recipients. Transplantation. 1989;47:524–7. doi: 10.1097/00007890-198903000-00025. [DOI] [PubMed] [Google Scholar]
- 17.Grant D, Wall W, Mimeault R, et al. Successful small-bowel/liver transplantation. Lancet. 1990;335:181–4. doi: 10.1016/0140-6736(90)90275-a. [DOI] [PubMed] [Google Scholar]
- 18.Starzl TE, Watanabe K, Porter KA, Putnam CW. Effects of insulin, glucagon, and insulin/glucagon infusions on liver morphology and cell division after complete portacaval shunt in dogs. Lancet. 1976;1:821–5. doi: 10.1016/s0140-6736(76)90477-3. [DOI] [PubMed] [Google Scholar]
- 19.Starzl TE, Porter KA, Francavilla A. The Eck fistula in animals and humans. Curr Probl Surg. 1983;20:687–752. doi: 10.1016/s0011-3840(83)80010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Starzl TE, Lee IY, Porter KA, Putnam CW. The influence of portal blood upon lipid metabolism in normal and diabetic dogs and baboons. Surg Gynecol Obstet. 1975;140:381–96. [PMC free article] [PubMed] [Google Scholar]
- 21.Schraut WH, Abraham VS, Lee KW. Portal versus caval venous drainage of small bowel allografts: technical and metabolic consequences. Surgery. 1986;99:193–8. [PubMed] [Google Scholar]
- 22.Koltun WA, Kirkman RL. Nutritional and metabolic aspects of total small bowel transplantation in inbred rats. Transplant Proc. 1987;19:1120–2. [PubMed] [Google Scholar]
- 23.Shaffer D, Diflo T, Love W, Clowes GHA, Maki T, Monaco AP. Immunologic and metabolic effects of caval versus portal venous drainage in small-bowel transplantation. Surgery. 1988;104:518–24. [PubMed] [Google Scholar]
- 24.Mazzoni G, DiMartino C, Demofonti A, et al. A comparison of portal and systemic venous drainage in porcine renal allografts. Br J Surg. 1972;59:541–4. doi: 10.1002/bjs.1800590710. [DOI] [PubMed] [Google Scholar]
- 25.Mazzoni G, Benichou J, Porter KA, Starzl TE. Renal homotransplantation with venous outflow or infusion of antigen into the portal vein of dogs or pigs. Transplantation. 1977;24:268–73. doi: 10.1097/00007890-197710000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schraut WH, Rosemurgy AS, Riddell RM. Prolongation of intestinal allograft survival without immunosuppressive drug therapy. J Surg Res. 1983;34:597–607. doi: 10.1016/0022-4804(83)90116-6. [DOI] [PubMed] [Google Scholar]
- 27.Trager JDK, Zeevi A, Jaffe R, Rowe MI, Starzl TE, Duquesnoy RJ. Functional characteristics of lymphocytes infiltrating a human multivisceral allograft. Transplantation. (in press) [PMC free article] [PubMed] [Google Scholar]
- 28.Murase N, Kim DG, Todo S, Cramer DV, Fung JJ, Starzl TE. FK 506 suppression of heart and liver allograft rejection. II. The induction of graft acceptance in the rat. Transplantation. 1990;50:739–44. doi: 10.1097/00007890-199011000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takacs L, Szende B, Monostroi E, et al. Expression of HLA-DR antigens on bile duct cells of rejected liver transplant. [Letter] Lancet. 1983;2:1500. doi: 10.1016/s0140-6736(83)90845-0. [DOI] [PubMed] [Google Scholar]
- 30.Demetris AJ, Lasky S, Van Thiel D, Slarzl TE, Whiteside T. Induction of DR/IA antigens in human liver allografts. Transplantation. 1985;40:504–9. doi: 10.1097/00007890-198511000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gouw AH, Houthoff HJ, Huitema S, Beelen JM, Gips CH, Poppema S. Expression of major histocompatibility complex antigens and replacement of donor cells by recipient ones in human liver grafts. Transplantation. 1987;43:291–6. doi: 10.1097/00007890-198702000-00025. [DOI] [PubMed] [Google Scholar]
- 32.Demetris AJ, Qian S, Sun H, et al. Early events in liver allograft rejection: delineation of sites of simultaneous infragraft and recipient lymphoid tissue sensitization. Am J Pathol. (in press) [PMC free article] [PubMed] [Google Scholar]
- 33.Porter KA. In: Pathology of the orthotopic homograft and heterograft. Starzl TE, editor. Philadelphia: WE Saunders Company; 1969. [Google Scholar]
- 34.Fung JJ, Zeevi A, Demetris A, et al. Origin of lymph node-derived lymphocytes in human hepatic allografts. Clin Transplant. 1989;3:316–24. [PMC free article] [PubMed] [Google Scholar]
- 35.Iwaki Y, Starzl T, Yagihashi A, et al. Replacement of donor lymphoid tissue in human small bowel transplants under FK 506 immunosuppression. Lancet. doi: 10.1016/0140-6736(91)92517-6. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]