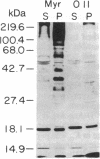
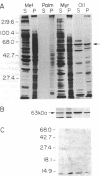
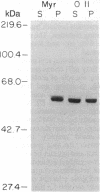
Abstract
A number of viral and cellular proteins contain covalently bound lipid. In a subset of these acyl proteins, the 14-carbon saturated fatty acid myristic acid is attached through an amide linkage to an NH2-terminal glycine residue. Myristoyl-CoA:protein N-myristoyltransferase (NMT) transfers the myristoyl moiety from myristoyl-CoA to these nascent proteins and is highly selective for fatty acid chain length. We have found that 10-(propoxy)decanoyl-CoA (11-oxymyristoyl-CoA), an analog of myristic acid with reduced hydrophobicity, acts as a substrate for NMT both in vitro and in vivo. Comparison of the in vitro kinetic properties of a number of synthetic octapeptide substrates of NMT using myristoyl-CoA or 11-oxymyristoyl-CoA indicated that there is an interaction between the acyl-CoA and peptide binding sites of this acyltransferase. Peptide catalytic efficiency with 11-oxymyristoyl-CoA was reduced relative to that with myristoyl-CoA, but the extent of the reduction varied widely among the octapeptides tested. These in vitro data accurately predicted that only a subset of myristoyl proteins synthesized in Saccharomyces cerevisiae and a murine myocyte-like cell line (BC3H1) would incorporate 11-oxy[3H]myristate. Substitution of the myristoyl moiety by the 11-oxymyristoyl moiety does not significantly affect the membrane association of most N-myristoyl proteins. However, for the tyrosine kinase p60v-src and a 63-kDa N-myristoyl protein in BC3H1 cells, analog incorporation results in marked redistribution from the membrane to the cytosolic fraction. These studies demonstrate the utility of heteroatom-containing analogs for analysis of the role of myristate in acyl protein targeting. The sequence-specific nature of analog incorporation and the protein-specific effects on membrane association suggests that these compounds may represent a useful class of antiviral and antitumor agents.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Gould K., Sefton B. M. The absence of myristic acid decreases membrane binding of p60src but does not affect tyrosine protein kinase activity. J Virol. 1986 May;58(2):468–474. doi: 10.1128/jvi.58.2.468-474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Solski P. A., Schaeffer J. P., MacDonald M. J., Der C. J. Activation of the cellular proto-oncogene product p21Ras by addition of a myristylation signal. Science. 1989 Mar 24;243(4898):1600–1603. doi: 10.1126/science.2648572. [DOI] [PubMed] [Google Scholar]
- Cham B. E., Knowles B. R. A solvent system for delipidation of plasma or serum without protein precipitation. J Lipid Res. 1976 Mar;17(2):176–181. [PubMed] [Google Scholar]
- Chow M., Newman J. F., Filman D., Hogle J. M., Rowlands D. J., Brown F. Myristylation of picornavirus capsid protein VP4 and its structural significance. Nature. 1987 Jun 11;327(6122):482–486. doi: 10.1038/327482a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Pellman D., Hanafusa H. A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol Cell Biol. 1984 Sep;4(9):1834–1842. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross G. A. Eukaryotic protein modification and membrane attachment via phosphatidylinositol. Cell. 1987 Jan 30;48(2):179–181. doi: 10.1016/0092-8674(87)90419-3. [DOI] [PubMed] [Google Scholar]
- Duronio R. J., Towler D. A., Heuckeroth R. O., Gordon J. I. Disruption of the yeast N-myristoyl transferase gene causes recessive lethality. Science. 1989 Feb 10;243(4892):796–800. doi: 10.1126/science.2644694. [DOI] [PubMed] [Google Scholar]
- Garber E. A., Cross F. R., Hanafusa H. Processing of p60v-src to its myristylated membrane-bound form. Mol Cell Biol. 1985 Oct;5(10):2781–2788. doi: 10.1128/mcb.5.10.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings B. A., Zubenko G. S., Hasilik A., Jones E. W. Mutant defective in processing of an enzyme located in the lysosome-like vacuole of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Jan;78(1):435–439. doi: 10.1073/pnas.78.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuckeroth R. O., Glaser L., Gordon J. I. Heteroatom-substituted fatty acid analogs as substrates for N-myristoyltransferase: an approach for studying both the enzymology and function of protein acylation. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8795–8799. doi: 10.1073/pnas.85.23.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuckeroth R. O., Towler D. A., Adams S. P., Glaser L., Gordon J. I. 11-(Ethylthio)undecanoic acid. A myristic acid analogue of altered hydrophobicity which is functional for peptide N-myristoylation with wheat germ and yeast acyltransferase. J Biol Chem. 1988 Feb 15;263(5):2127–2133. [PubMed] [Google Scholar]
- Kamps M. P., Buss J. E., Sefton B. M. Mutation of NH2-terminal glycine of p60src prevents both myristoylation and morphological transformation. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4625–4628. doi: 10.1073/pnas.82.14.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Saltiel A. R. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988 Jan 15;239(4837):268–275. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- Olson E. N., Caldwell K. L., Gordon J. I., Glaser L. Regulation of creatine phosphokinase expression during differentiation of BC3H1 cells. J Biol Chem. 1983 Feb 25;258(4):2644–2652. [PubMed] [Google Scholar]
- Olson E. N., Towler D. A., Glaser L. Specificity of fatty acid acylation of cellular proteins. J Biol Chem. 1985 Mar 25;260(6):3784–3790. [PubMed] [Google Scholar]
- Omura S. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriol Rev. 1976 Sep;40(3):681–697. doi: 10.1128/br.40.3.681-697.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal R. A., Jr, Mannarelli S. J., Ziering D. L. 10-Thiastearic acid inhibits both dihydrosterculic acid biosynthesis and growth of the protozoan Crithidia fasciculata. J Biol Chem. 1986 Sep 25;261(27):12441–12443. [PubMed] [Google Scholar]
- Pellman D., Garber E. A., Cross F. R., Hanafusa H. Fine structural mapping of a critical NH2-terminal region of p60src. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1623–1627. doi: 10.1073/pnas.82.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Poirier F., Calothy G., Karess R. E., Erikson E., Hanafusa H. Role of p60src kinase activity in the induction of neuroretinal cell proliferation by rous sarcoma virus. J Virol. 1982 Jun;42(3):780–789. doi: 10.1128/jvi.42.3.780-789.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., McClure M. R., Rice N. R., Luftig R. B., Schultz A. M. Myristylation site in Pr65gag is essential for virus particle formation by Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7246–7250. doi: 10.1073/pnas.83.19.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. Myristylation is required for intracellular transport but not for assembly of D-type retrovirus capsids. J Virol. 1987 Apr;61(4):1045–1053. doi: 10.1128/jvi.61.4.1045-1053.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S. Fatty acylation of proteins. Annu Rev Cell Biol. 1988;4:611–647. doi: 10.1146/annurev.cb.04.110188.003143. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Oroszlan S. In vivo modification of retroviral gag gene-encoded polyproteins by myristic acid. J Virol. 1983 May;46(2):355–361. doi: 10.1128/jvi.46.2.355-361.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Buss J. E. The covalent modification of eukaryotic proteins with lipid. J Cell Biol. 1987 Jun;104(6):1449–1453. doi: 10.1083/jcb.104.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler D. A., Adams S. P., Eubanks S. R., Towery D. S., Jackson-Machelski E., Glaser L., Gordon J. I. Myristoyl CoA:protein N-myristoyltransferase activities from rat liver and yeast possess overlapping yet distinct peptide substrate specificities. J Biol Chem. 1988 Feb 5;263(4):1784–1790. [PubMed] [Google Scholar]
- Towler D. A., Adams S. P., Eubanks S. R., Towery D. S., Jackson-Machelski E., Glaser L., Gordon J. I. Purification and characterization of yeast myristoyl CoA:protein N-myristoyltransferase. Proc Natl Acad Sci U S A. 1987 May;84(9):2708–2712. doi: 10.1073/pnas.84.9.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler D. A., Eubanks S. R., Towery D. S., Adams S. P., Glaser L. Amino-terminal processing of proteins by N-myristoylation. Substrate specificity of N-myristoyl transferase. J Biol Chem. 1987 Jan 25;262(3):1030–1036. [PubMed] [Google Scholar]
- Towler D. A., Gordon J. I., Adams S. P., Glaser L. The biology and enzymology of eukaryotic protein acylation. Annu Rev Biochem. 1988;57:69–99. doi: 10.1146/annurev.bi.57.070188.000441. [DOI] [PubMed] [Google Scholar]
- Towler D., Glaser L. Acylation of cellular proteins with endogenously synthesized fatty acids. Biochemistry. 1986 Feb 25;25(4):878–884. doi: 10.1021/bi00352a021. [DOI] [PubMed] [Google Scholar]
- Towler D., Glaser L. Protein fatty acid acylation: enzymatic synthesis of an N-myristoylglycyl peptide. Proc Natl Acad Sci U S A. 1986 May;83(9):2812–2816. doi: 10.1073/pnas.83.9.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]