Abstract
Two-pore-domain potassium (K2P) channels are responsible for background leak currents which regulate the membrane potential and excitability of many cell types. Their activity is modulated by a variety of chemical and physical stimuli which act to increase or decrease the open probability of individual K2P channels. Crystallographic data and homology modelling suggest that all K+ channels possess a highly conserved structure for ion selectivity and gating mechanisms. Like other K+ channels, K2P channels are thought to have two primary conserved gating mechanisms: an inactivation (or C-type) gate at the selectivity filter close to the extracellular side of the channel and an activation gate at the intracellular entrance to the channel involving key, identified, hinge glycine residues. Zinc and hydrogen ions regulate Drosophila KCNK0 and mammalian TASK channels, respectively, by interacting with the inactivation gate of these channels. In contrast, the voltage dependence of TASK3 channels is mediated through its activation gate. For KCNK0 it has been shown that the gates display positive cooperativity. It is of much interest to determine whether other K2P regulatory compounds interact with either the activation gate or the inactivation gate to alter channel activity or, indeed, whether additional regulatory gating pathways exist.
Alistair Mathie (left), Emma Veale (centre) and Ehab Al Moubarak (right) are based in the Medway School of Pharmacy, University of Kent. Their research backgrounds are in electrophysiology, molecular biology and bioinformatics, respectively. They use a combination of these experimental approaches to study the structural and functional properties and the regulation of two-pore domain potassium channels, the channels which underlie leak currents in many cells. A.M. is currently a Royal Society Industry Fellow with a particular interest in developing research and training links between the pharmaceutical industry and academia.

Two-pore-domain potassium channels
Background, or leak, K+ currents regulate the resting membrane potential and excitability of many mammalian cells. The two-pore-domain potassium (K2P) channel family are open across the physiological voltage range and are therefore believed to underlie many of these leak currents (Goldstein et al. 2005; Lotshaw, 2007; Bayliss & Barrett, 2008; Enyedi & Czirjak, 2010). Each K2P channel subunit comprises two pore regions (P1 and P2; hence their name) and four transmembrane domains (M1–M4). Functional channels form as dimers with a single potassium selective conductance pore. There are 15 members of this K2P channel family, which can be divided into six distinct subfamilies on the basis of both their structural and their functional properties (Goldstein et al. 2005; Lotshaw, 2007), namely the TWIK, TASK, TREK, THIK, TALK and TRESK subfamilies (see Table 1 and, for example, Enyedi & Czirjak, 2010). In addition to many pharmacological regulators (Mathie & Veale, 2007), K2P channels are regulated by a number of physiological mediators (Table 1) which alter channel open probability and underpin the role of these channels in many diverse physiological processes, including neuroprotection, cerebrovascular vasodilatation, regulation of aldosterone production and secretion, depression, chemoreception and pulmonary vasoconstriction (see Enyedi & Czirjak, 2010).
Table 1.
Physiological regulators of K2P channel function
| Family | Family members | Physiological activators | Physiological inhibitors |
|---|---|---|---|
| TWIK | TWIK1 (K2P1.1) | Acid pHi | |
| TWIK2 (K2P6.1) | PKC | ||
| KCNK7 (K2P7.1) | |||
| TREK | TREK1 (K2P2.1) | Acid pHi | Acid pHo (TREK2) |
| TREK2 (K2P10.1) | Acid pHo (TREK1) | Gαq | |
| TRAAK (K2P4.1) | Arachidonic acid | Gαs | |
| Depolarizing voltage | PKA (not TRAAK) | ||
| Gαi | PKC (not TRAAK) | ||
| Heat | |||
| Lysophospholipids | |||
| Nitric oxide (TREK1) | |||
| Polyunsaturated fatty acids | |||
| Stretch | |||
| TASK | TASK1 (K2P3.1) | Alkaline pHo (TASK1) | Acid pHo |
| TASK3 (K2P9.1) | Depolarizing voltage (TASK3) | Gαq | |
| TASK5 (K2P15.1) | Zinc (TASK3) | ||
| TALK | TASK2 (K2P5.1) | Alkaline pHo | |
| TALK1 (K2P16.1) | |||
| TASK4 (K2P17.1) | |||
| THIK | THIK1 (K2P13.1) | Arachidonic acid | |
| THIK2 (K2P12.1) | |||
| TRESK | TRESK (K2P18.1) | Calcium | Arachidonic acid |
| Gαq |
A number of physiologically important molecules regulate the gating of K2P channels, either increasing or decreasing channel open probability (see text and Enyedi & Czirjak, 2010 for further details). Note that functional currents have not been recorded for KCNK7 (K2P7.1), THIK2 (K2P12.1) or TASK5 (K2P15.1).
Currently most information concerning the regulation and gating of K2P channels is available from TASK and TREK subfamilies and additionally, particularly in terms of channel gating, from the Drosophila homologue KCNK0.
The TASK subfamily comprises TASK1 (K2P3.1), TASK3 (K2P9.1) and the, to date, non-functional channel TASK5 (K2P15.1) (Duprat et al. 1997; Leonoudakis et al. 1998; Kim et al. 2000; Rajan et al. 2000; Ashmole et al. 2001). TASK1 and TASK3 (and TASK1/TASK3 heterodimer) K2P channels are regulated by a wide variety of chemical stimuli including general anaesthetic agents, pH, zinc, ruthenium red, certain G-protein coupled receptors (GPCRs) and methanandamide (Talley & Bayliss, 2002; Czirjak & Enyedi, 2003; Mathie, 2007; Veale et al. 2007b; Clarke et al. 2008). TASK channels are responsible for leak K+ currents in many neurons including the standing outward current, IKSO, in cerebellar granule neurons (e.g. Millar et al. 2000; Kang et al. 2004; Aller et al. 2005; Brickley et al. 2007).
The TREK subfamily comprises TREK1 (K2P2.1), TREK2 (K2P10.1) and TRAAK (K2P4.1). Gating of these channels can be regulated by physical stimuli such as mechanical stimulation and temperature and pH changes (e.g. Fink et al. 1996; Maingret et al. 2000; Honoréet al. 2002; Honore, 2007; Sandoz et al. 2009) as well as chemical stimuli such as lipids and anaesthetics (e.g. Patel et al. 1999; Honore, 2007) and by GPCRs (see Mathie, 2007).
Gating of potassium channels
Crystallographic data from the bacterial channels KcsA, MthK and KvAP and the mammalian channel Kv1.2 suggest a highly conserved structure for ion selectivity and gating mechanisms for all K+ channels (Doyle et al. 1998; Jiang et al. 2002a,b; Long et al. 2005). K+ channels are thought to have two primary conserved gating mechanisms: an activation gate at the intracellular entrance to the channel and a distant slow inactivation gate at the selectivity filter close to the extracellular side of the channel (Yellen, 2002). A third mechanism exists, termed N-type inactivation. This is unique to certain KV channels and is due to the binding of an auto-inhibitory peptide that is part of the N terminus of the channel protein, which inhibits current flow leading to fast inactivating A-type currents (see Yellen, 2002).
The first primary mechanism for closing the pore occurs when amino acids at the narrow selectivity filter itself pinch shut. This occurs more slowly than N-type inactivation and is often termed C-type inactivation or C-type gating (Choi et al. 1991; Hoshi et al. 1991). This gating is sensitive to mutations at residues near the external entrance of the pore and has been visualised as an alteration in the structure of the selectivity filter in KcsA channels under certain conditions (see Yellen, 2002). For KcsA channels, Cordero-Morales et al. (2007) have shown that hydrogen bonds between amino acid residues in the selectivity filter and its adjacent pore helix determine the degree of C-type inactivation and, although the precise residues involved may vary, these authors suggest that this may be a general mechanism for C-type inactivation in other K+ channels.
In the second mechanism the channel opens or closes through movement of amino acids at the intracellular entrance to the pore region. For voltage-gated (KV) channels this occurs in response to depolarisation and movement of the S4 transmembrane segment. This region was identified when the crystal structures of the bacterial channels KcsA in its closed conformation and MthK in its open conformation were compared (Jiang et al. 2002a). Comparison of these two structures revealed hinge glycine residues located in the inner helices which bend to create a pathway for ions to pass when the channel is open. These hinge glycine residues are conserved in most potassium channels and, as such, are also found in K2P channels (but see below).
Whilst K2P channels are not voltage sensitive they are gated and are regulated by a variety of physiological and pharmacological mediators. Gating can be seen from recordings of individual ion channel activity, which reveal both the opening and closing of individual channels at rest (e.g. Kang et al. 2004), and the effects of modulatory compounds which may increase or decrease channel activity by influencing gating regions of the channel (e.g. Zilberberg et al. 2001) can also be observed. In contrast to voltage-gated K+ channels, until recently relatively little was known about the regions of mammalian K2P channels that underlie these gating processes.
The inactivation gate of K2P channels
Evidence is beginning to accumulate that C-type gating is a mechanism utilised by many mammalian K2P channels to regulate channel activity. For example, hydrogen ions reduce current through TASK1 and TASK3 channels, primarily by binding to a histidine residue (H98) next to the GFG selectivity filter (Kim et al. 2000; Lopes et al. 2000; Rajan et al. 2000), which alters channel gating (Yuill et al. 2007; Stansfeld et al. 2008). Yuill et al. (2007) have shown that residues in the outer pore mouth of TASK1 channels contribute to ion selectivity and that protonation of H98 initiates a ‘C-type’ gating response that involves a conformational change in the selectivity filter of TASK1.
Stansfeld et al. (2008) have modelled the inactivation gate region of the channel for TASK1 (and hERG) and have shown that there is a region of structural instability in the selectivity filter. A water molecule stabilises the backbone of the selectivity filter by bridging two residues previously associated with pH sensitivity in TASK1 (H98 and T89) and inactivation in hERG (N629 and S620). This water molecule is more readily retained within the unprotonated TASK1 channels and non-inactivating hERG mutants compared to protonated TASK1 and WT hERG channels.
Similarly, a study on the related TALK family of K2P channels has suggested that pH gating of these channels, which involves a key arginine residue (R224 for TASK2) near the pore, may interact with the selectivity filter and influence C-type gating of TALK channels in a similar manner (Niemeyer et al. 2007).
A conserved glutamate residue, located at the end of the first transmembrane domain of the channel at the extracellular side of the membrane, has been shown to be important for gating in Drosophila KCNK0 K2P channels (E28; Zilberberg et al. 2001) and for C-type inactivation of Shaker voltage-gated K+ channels (E418; Larsson & Elinder, 2000). The homologous amino acid in TASK3 channels is amino acid E30 while in TREK1 channels it is E84 (Fig. 1A).
Figure 1. A key glutamic acid residue regulates the inactivation gate of K2P channels.
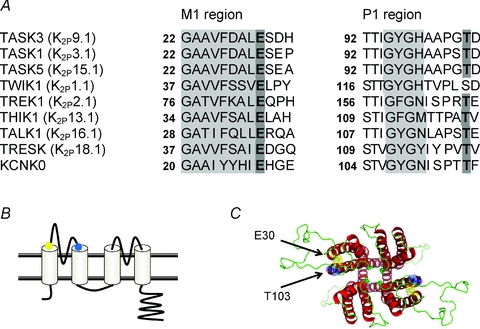
A, sequence alignment showing the end of the first transmembrane domain (M1 region in grey) and the end of the first pore domain (with selectivity filter in grey) for Drosophila KCNK0, human TASK1, TASK3 and TASK5, and a representative member of each of the other 5 human K2P channel subfamilies. Note the conserved glutamate residue (E in bold, position E30 for TASK3) immediately following the end of each M1 region and the threonine residue (T in bold) present 5 amino acids after the selectivity filter in KCNK0 and all mammalian K2P channels except TWIK1 and KCNK7. B, schematic representation of K2P channel structure with the position of the relevant E (yellow dot) and T (blue dot) residues highlighted. C, homology model of the TASK3 K2P channel illustrating the proximity of the two E30 (yellow) and two T103 (blue) residues (view looking from the top down). The model was created using Modeller 9v7 (Sali & Blundell, 1993) based on the KcsA structure as template (originally solved by Doyle et al. 1998).
The glutamate residue at position 28 (E28) in Drosophila KCNK0 channels is critical for channel gating, since mutation of this amino acid to a cysteine (C) residue leads to a reduction in KCNK0 current density (Zilberberg et al. 2001). Thus E28 normally stabilises the open conformation of the channel, perhaps by forming hydrogen bonds with amino acids in (or adjacent to) the pore region of the channel. Following mutation E28C, C28 is no longer able to stabilise the open state of the channel and the channel, therefore, spends more time in its closed state, reducing whole-cell current amplitude. For TASK3 channels, mutation of the equivalent amino acid E30 to C also reduces whole-cell current amplitude (Veale et al. 2005).
Furthermore, Zilberberg et al. (2001) showed that zinc was a more effective blocker of KCNK0 channels following mutation of the same conserved E residue. Zilberberg et al. (2001) have suggested that zinc binds preferentially to the closed form of KCNK0 channels, thus it is a more effective blocker when the 28C mutation causes the channel to spend more time in its closed conformation.
For Drosophila KCNK0 channels, it has been shown that E28 interacts with a threonine residue (T115) close to the pore region of the channel (Zilberberg et al. 2001) and can form disulphide bonds when both residues are mutated to C. This T residue is conserved in 13 out of the 15 mammalian K2P channels and is T103 in TASK3 channels (see Fig. 1A and B). The close spatial relationship between E30 and T103 in TASK3 is highlighted in our homology model of the channel in Fig. 1C.
More direct evidence of the critical role of the conserved E residue in C-type gating of mammalian K2P channels has come from recent work on the pH sensitivity of human TREK1 channels (Cohen et al. 2008), which showed a facilitation of C type gating by protonation of extracellular histidine residues and a change in ion selectivity following protonation. Cohen et al. (2008) suggested that this protonation of histidine residues adds positive charge to pore-adjacent residues, which draws the conserved E84 residue away from its natural interactions and causes the selectivity filter to collapse. Thus E84 is critical for C-type gating in TREK1 channels.
The activation gate of K2P channels
A homology model of KCNK0 based on the crystal structure of Kv1.2 and the identification of many pairs of interacting sites by systematic introduction of charged and counter-charged amino acids at putative interacting sites has been developed by Kollewe et al. (2009). This model provided strong evidence to support the hitherto widely held assumption that K2P channels form functional dimers with each subunit contributing two P regions to the pore. In addition, the model showed that the conserved glycine hinge residues implicated in channel opening and present in almost all K+ channels are present in the M2 regions of K2P channels but not in the M4 regions (although there is another glycine residue shifted by two positions in M4; Kollewe et al. 2009) reflecting asymmetry of the domains. Whilst K2P channels are fourfold symmetrical in the selectivity filter region (although individual amino acids can differ in P1 compared to P2 in this region), below this they are only bilaterally symmetrical (Kollewe et al. 2009) reflecting the low amino acid identity between M2 and M4.
Ben-Abu et al. (2009) took advantage of a mutated, reduced form of KCNK0, lacking most of its C terminus, to show the presence of the activation gate and that, for this mutated channel, it was mostly held open, giving the channel a high single channel open probability (Po) of 0.8. This very high single channel Po implies that K2P channels are preferentially in their open state and the authors suggested that the putative activation gate lined by glycine residues in K2P channels (Ben-Abu et al. 2009; Cohen et al. 2009) promotes the gate being open. This is in contrast to related KV channel sequences which promote the activation gate being closed (in the absence of depolarisation driven activation). In agreement with this suggestion, mutation of these glycine residues with hydrophobic residues in both KCNK0 and indeed in TASK3 (Ashmole et al. 2009) led to a reduction in channel open probability. However, for full-length mammalian K2Ps the resting Po is not nearly so high (e.g. Ashmole et al. 2009). Indeed, mutations in amino acids close to the activation gate can increase Po severalfold (A237T, Ashmole et al. 2009, see Fig. 2A and B). It was suggested that this introduced threonine residue in M4 stabilises the open state of the channel through altered side-chain interactions between residues, possibly with N133 in M2. Furthermore, it was suggested that the natural alanine residue stabilises the shut state of the channel through an interaction with residue L128 in M2 (Ashmole et al. 2009). As such, channel activity may either increase or decrease through the action of regulators which influence this gate. In hERG channels an equivalent alanine residue (A653) close to the glycine hinge forms tight contacts with residues in neighbouring subunits and supports channel closure at membrane potentials below the activation threshold (Stepanovic et al. 2009).
Figure 2. A237 in TASK3 regulated the activation gate.

A, homology model of part of the TASK3 channel in its open state. Note the hinge glycine residues G117 and G231 and the amino acid A237 altered to T237 in the M4 segment. From Ashmole et al. (2009) Fig. 5C. B, relationship between membrane potential and Po for WT TASK3 channels and TASK3_A237T. From Ashmole et al. (2009) Fig. 7A.
Anaesthetic activation, methanandamide inhibition and GPCR mediated inhibition of TASK1 and TASK3 channels all require a region of six amino acids; VLRFLT in the case of TASK3; at the interface between the final transmembrane domain (M4) and the cytoplasmic C terminus to be present, suggesting a shared molecular site of action (Talley & Bayliss, 2002; Andres-Enguix et al. 2007; Veale et al. 2007a). It is probable, however, that this ‘VLRFLT region’ is important in transducing the signal to the gate following binding of the regulatory molecule, rather than being part of either the gate or the binding site(s) themselves (Talley & Bayliss, 2002; Andres-Enguix et al. 2007; Veale et al. 2007a). Since this region is located close to the alanine residue (A237) in M4 it is tempting to suggest that regulation by these compounds may interact with the activation gate. Indeed, TASK channel activity is moderately sensitive to membrane potential (Lopes et al. 2000; Yuill et al. 2007; Ashmole et al. 2009) and Ashmole et al. (2009) demonstrated that this voltage dependence was altered – there was a shift in the relationship between Po and voltage to less positive voltages – in the A237T mutated TASK3 channel (Fig. 2B). Furthermore, Ashmole et al. (2009) suggest parallels between the response of TASK channels to voltage and their regulation by anaesthetics and GPCRs. Thus it is important to determine whether these latter responses are indeed altered in A237T mutated TASK3 channels.
TREK1 channels are highly regulated by a wide range of physiological and pharmacological mediators (see above and Honore, 2007). Like the VLRFLT region in TASK3 channels, the cytosolic C terminus of the TREK channel immediately following M4 plays a key structural role in these regulatory mechanisms. The actions of many of these regulatory compounds converge on a single intracellular glutamic acid residue, E306 (Honore et al. 2002; Chemin et al. 2005), which is critical in the gating pathway and, again, it is tempting to suggest that this regulatory pathway interacts with the activation gate (see also Sandoz et al. 2009).
Conclusion
There is accumulating evidence that K2P channel activity is regulated through at least two separate gates and that regulatory compounds, in addition to directly plugging the channel to occlude ion flow, can modulate activity through one or other of these gates. It is not clear at this stage, however, whether these gates interact. For KV channels it has been shown that there is negative co-operativity between the activation and inactivation gate (e.g. Panyi & Deutsch, 2006); however, Ben-Abu et al. (2009) have provided strong evidence in favour of the opposite (positive co-operativity) between the gates in KCNK0 channels. On the other hand, Ashmole et al. (2009) have shown that pH regulation of TASK channels (which occurs through the inactivation gate) is unaltered by mutations that affect the activation gate. Similarly, separate, independent gating mechanisms control the regulation of both TREK channels and TASK2 by changes in either intracellular or extracellular pH (Sandoz et al. 2009; Niemeyer et al. 2010). It will be of much interest to determine whether the majority of regulatory molecules that interact with mammalian K2P channels produce their effects by interacting with one or other of these gates and whether the gates operate co-operatively or independently of each other.
Acknowledgments
A.M. is a Royal Society Industry Fellow and E.A.-M. is supported by a BBSRC CASE studentship. This symposium was a Festschrift for Professor Peter Stanfield and A.M. would like to take this opportunity to thank Peter for invaluable career advice when a PhD student at Leicester, acknowledge the contribution of Peter and his colleagues to this area of research in recent years and wish Peter well in his retirement.
Glossary
Abbreviations
- GPCR
G-protein coupled receptor
- K2P
two pore domain potassium channel
- PKA
protein kinase A
- PKC
protein kinase C
Author contributions
A.M. drafted the article and all authors contributed to revising it critically. All authors approve of the final version to be published.
References
- Aller MI, Veale EL, Linden AM, Sandu C, Schwaninger M, Evans LJ, Korpi ER, Mathie A, Wisden W, Brickley SG. Modifying the subunit composition of TASK channels alters the modulation of a leak conductance in cerebellar granule neurons. J Neurosci. 2005;25:11455–11467. doi: 10.1523/JNEUROSCI.3153-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres-Enguix I, Caley A, Yustos R, Schumacher MA, Spanu PD, Dickinson R, Maze M, Franks NP. Determinants of the anesthetic sensitivity of TASK channels: molecular cloning of an anesthetic-activated potassium channel from Lymnaea stagnalis. J Biol Chem. 2007;282:20977–20990. doi: 10.1074/jbc.M610692200. [DOI] [PubMed] [Google Scholar]
- Ashmole I, Goodwin PA, Stanfield PR. TASK-5, a novel member of the tandem pore K+ channel family. Pflugers Arch. 2001;442:828–833. doi: 10.1007/s004240100620. [DOI] [PubMed] [Google Scholar]
- Ashmole I, Vavoulis DV, Stansfeld PJ, Mehta PR, Feng JF, Sutcliffe MJ, Stanfield PR. The response of the tandem pore potassium channel TASK-3 (K2P9.1) to voltage: gating at the cytoplasmic mouth. J Physiol. 2009;587:4769–4783. doi: 10.1113/jphysiol.2009.175430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss DA, Barrett PQ. Emerging roles for two-pore-domain potassium channels and their therapeutic impact. Trends Pharmacol Sci. 2008;29:566–575. doi: 10.1016/j.tips.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Abu Y, Zhou Y, Zilberberg M, Yifrach O. Inverse coupling in leak and voltage-activated K channel gates underlies distinct roles in electrical signaling. Nat Struct Mol Biol. 2009;16:71–79. doi: 10.1038/nsmb.1525. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Aller MI, Sandu C, Veale EL, Alder FG, Sambi H, Mathie A, Wisden W. TASK-3 two-pore domain potassium channels enable sustained high-frequency firing in cerebellar granule neurons. J Neurosci. 2007;27:9329–9340. doi: 10.1523/JNEUROSCI.1427-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin J, Patel AJ, Duprat F, Lauritzen I, Lazdunski M, Honore E. A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO J. 2005;24:44–53. doi: 10.1038/sj.emboj.7600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KL, Aldrich RW, Yellen G. Tetraethylammonium blockade distinguishes two inactivation mechanisms in voltage-activated K+ channels. Proc Natl Acad Sci U S A. 1991;88:5092–5095. doi: 10.1073/pnas.88.12.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CE, Veale EL, Wyse K, Vandenberg JI, Mathie A. The M1P1 loop of TASK3 K2P channels apposes the selectivity filter and influences channel function. J Biol Chem. 2008;283:16985–16992. doi: 10.1074/jbc.M801368200. [DOI] [PubMed] [Google Scholar]
- Cohen A, Ben-Abu Y, Hen S, Zilberberg N. A novel mechanism for human K2P2.1 channel gating. Facilitation of C-type gating by protonation of extracellular histidine residues. J Biol Chem. 2008;283:19448–19455. doi: 10.1074/jbc.M801273200. [DOI] [PubMed] [Google Scholar]
- Cohen A, Ben-Abu Y, Zilberberg N. Gating the pore of potassium leak channels. Eur Biophys J. 2009;39:61–73. doi: 10.1007/s00249-009-0457-6. [DOI] [PubMed] [Google Scholar]
- Cordero-Morales JF, Jogini V, Lewis A, Vasquez V, Cortes DM, Roux B, Perozo E. Molecular driving forces determine potassium channel slow inactivation. Nat Struct Mol Biol. 2007;14:1062–1069. doi: 10.1038/nsmb1309. [DOI] [PubMed] [Google Scholar]
- Czirjak G, Enyedi P. Ruthenium red inhibits TASK-3 potassium channel by interconnecting glutamate 70 of the two subunits. Mol Pharmacol. 2003;63:646–652. doi: 10.1124/mol.63.3.646. [DOI] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. A human background K channel to sense external pH variations near physiological pH. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M. Cloning, functional expression and brain localisation of a novel unconventional outward-rectifier K+ channel. EMBO J. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- Goldstein SAN, Bayliss DA, Kim D, Lesage F, Plant LD, Rajan S. International Union of Pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacol Rev. 2005;57:527–540. doi: 10.1124/pr.57.4.12. [DOI] [PubMed] [Google Scholar]
- Honore E. The neuronal background K2P channels: focus on TREK1. Nature Rev Neurosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- Honore E, Maingret F, Lazdunski M, Patel AJ. An intracellular proton sensor commands lipid- and mechano-gating of the K+ channel TREK-1. EMBO J. 2002;21:2968–2976. doi: 10.1093/emboj/cdf288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T, Zagotta WN, Aldrich RH. Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxy-terminal region. Neuron. 1991;7:547–556. doi: 10.1016/0896-6273(91)90367-9. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R. The open pore conformation of potassium channels. Nature. 2002a;417:523–526. doi: 10.1038/417523a. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2002b;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- Kang D, Han J, Talley EM, Bayliss DA, Kim D. Functional expression of TASK-1/TASK-3 heteromers in cerebellar granule cells. J Physiol. 2004;554:64–77. doi: 10.1113/jphysiol.2003.054387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Bang H, Kim D. TASK-3, a new member of the tandem pore K channel family. J Biol Chem. 2000;275:9340–9347. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- Kollewe A, Lau AY, Sullivan A, Roux B, Goldstein SAN. A structural model for K2P potassium channels based on 23 pairs of interacting sites and continuum electrostatics. J Gen Physiol. 2009;134:53–68. doi: 10.1085/jgp.200910235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson HP, Elinder F. A conserved glutamate is important for slow inactivation in K+ channels. Neuron. 2000;27:573–583. doi: 10.1016/s0896-6273(00)00067-2. [DOI] [PubMed] [Google Scholar]
- Leonoudakis D, Gray AT, Winegar BD, Kindler CH, Harada M, Taylor DM, Chavez RA, Forsayeth JR, Yost CS. An open rectifier potassium channel with two pore domains in tandem cloned from rat cerebellum. J Neurosci. 1998;18:868–877. doi: 10.1523/JNEUROSCI.18-03-00868.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SB, Campbell EB, MacKinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- Lopes CM, Gallagher PG, Buck ME, Butler MH, Goldstein SA. Proton block and voltage gating are potassium-dependent in the cardiac leak channel Kcnk3. J Biol Chem. 2000;275:16969–16978. doi: 10.1074/jbc.M001948200. [DOI] [PubMed] [Google Scholar]
- Lotshaw D. Biophysical, pharmacological and functional characteristics of cloned and native mammalian two-pore domain potassium channels. Cell Biochem Biophys. 2007;47:209–256. doi: 10.1007/s12013-007-0007-8. [DOI] [PubMed] [Google Scholar]
- Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, Lazdunski M, Honore E. TREK-1 is a heat-activated background K+ channel. EMBO J. 2000;19:2483–2491. doi: 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A. Mammalian K2P channels and their regulation by G protein coupled receptors. J Physiol. 2007;578:377–385. doi: 10.1113/jphysiol.2006.121582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A, Veale EL. Therapeutic potential of neuronal two-pore domain potassium-channel modulators. Curr Opin Invest Drugs. 2007;8:555–562. [PubMed] [Google Scholar]
- Millar JA, Barratt L, Southan AP, Page KM, Fyffe RE, Robertson B, Mathie A. A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proc Natl Acad Sci U S A. 2000;97:3614–3618. doi: 10.1073/pnas.050012597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer MI, Gonzalez-Nio FD, Zuniga L, Gonzalez W, Cid LP, Sepulveda FV. Neutralization of a single arginine residue gates open a two-pore domain alkali-activated K channel. Proc Natl Acad Sci U S A. 2007;104:666–671. doi: 10.1073/pnas.0606173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer MI, Cid LP, Pena-Munzenmayer G, Sepulveda FV. Separate gating mechanisms mediate the regulation of K2P potassium channel TASK-2 by intra- and extracellular pH. J Biol Chem. 2010;285:16467–16475. doi: 10.1074/jbc.M110.107060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyi G, Deutsch C. Cross talk between activation and slow inactivation gates of Shaker potassium channels. J Gen Physiol. 2006;128:547–559. doi: 10.1085/jgp.200609644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- Rajan S, Wischmeyer E, Liu GX, Preisig-Muller R, Daut J, Karschin A, Derst C. TASK-3 a novel tandem pore domain acid sensitive potassium channel. An extracellular histidine as pH sensor. J Biol Chem. 2000;275:16650–16657. doi: 10.1074/jbc.M000030200. [DOI] [PubMed] [Google Scholar]
- Sali A, Blundell TL. Comparative protein modeling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- Sandoz G, Douguet D, Chatelain F, Lazdunski M, Lesage F. Extracellular acidification exerts opposite actions on TREK1 and TREK2 potassium channels via a single conserved histidine residue. Proc Natl Acad Sci U S A. 2009;106:14628–14633. doi: 10.1073/pnas.0906267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfeld PJ, Grotessi A, Sands ZA, Sansom MSP, Gedeck P, Gosling M, Cox B, Stanfield PR, Mitcheson JS, Sutcliffe MJ. Insight into the mechanism of inactivation and pH sensitivity in potassium channels from molecular dynamic simulations. Biochemistry. 2008;47:7414–7422. doi: 10.1021/bi800475j. [DOI] [PubMed] [Google Scholar]
- Stepanovic SV, Potet F, Petersen CI, Smith JA, Meiler J, Balser JR, Kupershmidt S. The evolutionarily conserved residue A653 plays a key role in HERG channel closing. J Physiol. 2009;587:2555–2566. doi: 10.1113/jphysiol.2008.166694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Bayliss DA. Modulation of TASK-1 (Kcnk3) and TASK-3 (Kcnk9) potassium channels: volatile anesthetics and neurotransmitters share a molecular site of action. J Biol Chem. 2002;277:17733–17742. doi: 10.1074/jbc.M200502200. [DOI] [PubMed] [Google Scholar]
- Veale EL, Buswell R, Clarke CE, Mathie A. Identification of a region in the TASK3 two pore domain potassium channel that is critical for its blockade by methanandamide. Br J Pharmacol. 2007a;152:778–786. doi: 10.1038/sj.bjp.0707436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veale EL, Clarke CE, Mathie A. A key glutamate residue (E30), conserved in many K channels, regulates the gating of TASK3 two pore domain potassium channels. J Physiol. 2005;567.P:PC196. abstract. [Google Scholar]
- Veale EL, Kennard LE, Sutton GL, MacKenzie G, Sandu C, Mathie A. Gαq mediated regulation of TASK3 two pore domain potassium channels: the role of protein kinase C. Mol Pharmacol. 2007b;71:1666–1675. doi: 10.1124/mol.106.033241. [DOI] [PubMed] [Google Scholar]
- Yellen G. The voltage-gated potassium channels and their relatives. Nature. 2002;419:35–42. doi: 10.1038/nature00978. [DOI] [PubMed] [Google Scholar]
- Yuill KH, Stansfeld PJ, Ashmole I, Sutcliffe MJ, Stanfield PR. The selectivity, voltage-dependence and acid sensitivity of the tandem pore potassium channel TASK-1, contributions of the pore domains. Pflugers Arch. 2007;455:333–348. doi: 10.1007/s00424-007-0282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberberg N, Ilan N, Goldstein SAN. KCNKO: Opening and closing the 2-P-domain potassium leak channel entails “C-type” gating of the outer pore. Neuron. 2001;32:635–648. doi: 10.1016/s0896-6273(01)00503-7. [DOI] [PubMed] [Google Scholar]


