Abstract
Many types of ion channel localize to cholesterol and sphingolipid-enriched regions of the plasma membrane known as lipid microdomains or ‘rafts’. The precise physiological role of these unique lipid microenvironments remains elusive due largely to difficulties associated with studying these potentially extremely small and dynamic domains. Nevertheless, increasing evidence suggests that membrane rafts regulate channel function in a number of different ways. Raft-enriched lipids such as cholesterol and sphingolipids exert effects on channel activity either through direct protein–lipid interactions or by influencing the physical properties of the bilayer. Rafts also appear to selectively recruit interacting signalling molecules to generate subcellular compartments that may be important for efficient and selective signal transduction. Direct interaction with raft-associated scaffold proteins such as caveolin can also influence channel function by altering gating kinetics or by affecting trafficking and surface expression. Selective association of ion channels with specific lipid microenvironments within the membrane is thus likely to be an important and fundamental regulatory aspect of channel physiology. This brief review highlights some of the existing evidence for raft modulation of channel function.
Caroline Dart completed her doctorate at the University of Oxford before moving to the then Ion Channel Group in the Department of Cell Physiology and Pharmacology at the University of Leicester. There, she worked with Profs Nick Standen and Peter Stanfield on projects investigating the structure/function and regulation of potassium channels, particularly those of the inwardly rectifying family. Following the award of a Royal Society University Fellowship, she continued her research at Leicester investigating the organisation of signalling complexes made up of potassium channels and proteins that modulate their activity. She currently works in the School of Biological Sciences at the University of Liverpool where her interests include the role of caveolae in the compartmentation of vascular ATP-sensitive potassium channel signalling.

Introduction
The plasma membrane can consist of in excess of 2000 different species of lipid (Barenholz, 2000). These separate into distinct populations within the bilayer forming a patchwork of different lipid environments at the cell surface. Cholesterol, sphingolipids and phospholipids with saturated acyl tails coalesce to form tightly packed aggregates known as lipid ‘rafts’ (Simons & Ikonen, 1997; Simons & Toomre, 2000). These represent a relatively rigid, ordered phase of the membrane in contrast to the more fluid bulk of the bilayer where the kinked hydrocarbon chains of the largely unsaturated phospholipids prevent close packing. Multiple types of raft are likely to exist based on differences in lipid and protein composition (Pike, 2004), but despite many years of study rafts in native membranes remain relatively controversial in terms of size, stability and physiological importance (Edidin, 2003; Munro, 2003). The only morphologically identifiable raft-like domain is a caveola (Fig. 1). Here, association with the protein caveolin causes the cholesterol- and sphingolipid-enriched regions of the membrane to bulge into the cell forming small (50–100 nm) flask-shaped pits that are clearly visible in scanning or transmission electron microscopy (Razani et al. 2002; Cohen et al. 2004; Parton & Simons, 2007).
Figure 1. Lipid microdomains potentially influence ion channel activity by a variety of different mechanisms.
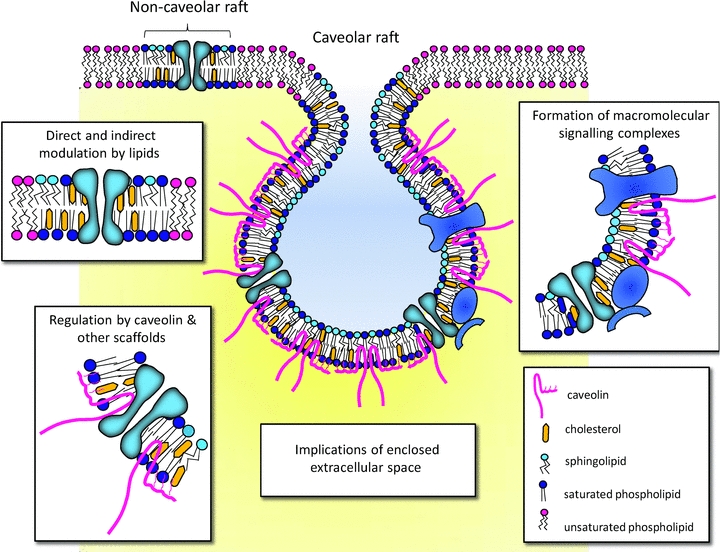
Cartoon illustrating the segregation of different types of lipids in raft and non-raft regions of the plasma membrane. Cholesterol, sphingolipids and saturated phospholipids aggregate in tightly packed microdomains known as rafts. The lateral association of these lipids in the membrane is driven by tight hydrophilic and hydrophobic van der Waal interactions between sphingolipids and cholesterol (Simons & Ikonen, 1997). Cholesterol tends to increase the packing density in these regions by filling in the spaces between bulky sphingolipids. Sphingomyelin, the most prevalent sphingolipid, localizes predominantly to the outer leaflet of the membrane. In caveolar-type rafts, association with the cholesterol-binding protein caveolin causes the formation of flask-shaped invaginations of the surface membrane. Lipid microdomains may regulate channel function in a number of different ways. This regulation can occur by direct and indirect modulation by lipids; recruitment of interacting molecules to facilitate localized signalling; and/or modulation by caveolae-associated scaffold proteins, such as caveolin. This final form of modulation may be through direct alterations in channel kinetics or through changes in channel trafficking and surface expression. Additionally, the restricted opening at the mouth of the caveola would allow the build up of ions within the caveolar ‘pit’ to concentrations far in excess of that found in the bulk extracellular fluid. This would additionally influence the activity of proteins localized to these compartments.
Despite the controversy, membrane rafts have attracted considerable attention in the ion channel field over recent years. This is chiefly because of their apparent ability to selectively aggregate interacting signalling molecules and the implication that they may be involved in the spatial organization of cell signalling pathways (Simons & Toomre, 2000; Patel et al. 2008). In addition, a considerable number of channel proteins that associate with caveolar rafts interact directly with the cholesterol-binding protein, caveolin. This interaction appears to regulate channel function either directly by altering channel kinetics or indirectly by affecting trafficking and surface expression (Alioua et al. 2008; Jiao et al. 2008; Garg et al. 2009b; Lee et al. 2009). Raft-enriched lipids such as cholesterol and sphingolipids can also exert effects on channel activity either through direct protein-lipid interactions (Epshtein et al. 2009; Fantini & Barrantes, 2009) or by influencing the physical characteristics of the bilayer (Andersen & Koeppe, 2007). The ability of ion channels to associate with specific lipid domains is thus likely to be an important regulatory aspect of channel physiology.
Ion channels and membrane rafts: methods of study
Channel proteins from virtually every class have been reported to associate with lipid microdomains. These include certain voltage-gated (Kv) and inwardly rectifying (Kir) K+ channels; voltage-gated (Nav) and epithelial (ENaC) Na+ channels; L-type Ca2+ channels; hyperpolarization-activated cyclic nucleotide-gated (HCN) channels; transient receptor potential (TRP) channels; various connexins; chloride channels; and P2X receptors (Table 1). Evidence for the association of ion channels with membrane rafts comes from three broad approaches: biochemical techniques that exploit the unusual properties of these domains to isolate them and their associated proteins from the bulk of the bilayer; microscopic approaches designed to directly visualize rafts; and functional approaches that destroy these domains and assess the effects on channel activity.
Table 1.
Examples ion channel association with lipid microdomains
| Channel | Tissue | Biochemical isolation | Electron microscopy | Association caveolin | Functional effects/Comments | References |
|---|---|---|---|---|---|---|
| Kv1.3 | Jurkat T-lymphocytes | yes | — | — | Constituitively present in rafts. Hydrolysis of sphingomyelin causes rafts to merge into large ceramide-enriched domains. Association of Kv1.3 with ceramide-enriched domains inhibits channel activity | (Bock et al. 2003) |
| Kv1.4 | Brain HEK293T cells | yes | — | — | Requires PSD-95 for raft targeting | (Wong & Schlichter, 2004) |
| Kv1.5 | Heart | yes | yes | ? | Cholesterol depletion modulates gating | |
| Cell lines | yes | — | yes | Interaction with SAP-97 and caveolin-3 involved in raft targeting. Coexpression with caveolin or addition of exogenous cholesterol causes a depolarizing shift in steady-state activation and inactivation | (Reviewed by Balijepalli & Kamp, 2008) | |
| Kv2.1 | L cell fibroblasts | yes | — | no | Cholesterol depletion causes hyperpolarizing shift in the inactivation curve | (Martens et al. 2000) |
| Pancreatic β cells | yes | — | — | Cholesterol depletion reduces current amplitude and causes hyperpolarizing shift in inactivation curve | (Xia et al. 2004) | |
| Oocytes | Regulation of gating by sphingomyelin | (Ramu et al. 2006) | ||||
| Kv4.2 | Brain | yes | — | — | (Wong & Schlichter, 2004) | |
| Kv7.1 (KvLQT1) | Heart HEK293 cells | yes | — | — | (Balijepalli et al. 2007) | |
| Kv11.1 (hERG1) | Heart HEK293 cells | yes | — | no | Cholesterol depletion causes positive shift in voltage dependence of activation and accelerates deactivation kinetics | (Balijepalli et al. 2007) |
| BK, hSLO | Aorta | yes | — | yes | Caveolin-regulated surface expression | (Alioua et al. 2008) |
| Myometrium | yes | — | yes | Knockdown of caveolin with siRNA suppresses total BK current | (Brainard et al. 2009) | |
| Aortic endothelial | yes | — | yes | BK channel inactive under control conditions but activated by cholesterol depletion or knockdown of caveolin | (Wang et al. 2005) | |
| Kir2.1 | Aortic endothelial | yes | — | no | Cholesterol depletion increases current density. Single channel properties unaffected – cholesterol may modulate number of active channels | (Romanenko et al. 2002) |
| Kir3.1/3.2 | Neurones CHO cells | yes | — | — | Rafts may be involved in surface delivery | (Delling et al. 2002) |
| Kir4.1 | Astrocytes HEK293 cell line | yes | — | — | Associate with non-caveolar lipid rafts. Cholesterol depletion results in loss of channel activity | (Hibino & Kurachi, 2007) |
| Kir6.1 | Aorta | yes | yes | yes | Cholesterol depletion abolishes cAMP/PKA regulation of channel | (Sampson et al. 2004, 2007) |
| Vascular smooth muscle HEK293 cells | PKC-mediated caveolin-dependent internalization | (Jiao et al. 2008) | ||||
| Kir6.2 | Heart | yes | — | yes | Currents suppressed by association with caveolin-3, but not caveolin-1 | (Garg et al. 2009a,b;) |
| Nav1.5 | Heart | yes | — | yes | Gαs-mediated recruitment of Nav1.5-containing caveolae to surface membrane – increase in current density | (Reviewed by Balijepalli & Kamp, 2008) |
| ENaC | Cell lines | yes | — | yes | Caveolin-dependent ubiquitination and subsequent internalization | (Hill et al. 2007; Lee et al. 2009) |
| Cav1.2 | Pancreatic β cells | yes | — | — | (Xia et al. 2004) | |
| Smooth muscle | yes | — | — | |||
| Heart | yes | yes | yes | Destruction of caveolae causes loss in β2AR regulation (neonatal mice myocytes) or enhanced β2AR regulation (adult rat myocytes) | (Balijepalli et al. 2006) (Calaghan & White, 2006) | |
| HCN4 | Sinus node | yes | — | yes | Cholesterol depletion causes positive shift in activation; reduces deactivation; loss of β2AR regulation | (Barbuti et al. 2004, 2007) |
| Disruption of caveolae by expression of dominant negative caveolin mutants shifts voltage dependence of activation to more negative potentials and increases time constant of activation | (Ye et al. 2008) | |||||
| Connexin-43 | Cell lines | yes | — | yes | Sucrose density gradients also suggest that Cx32, Cx36, and Cx46 are targeted to lipid rafts, while Cx26 and Cx50 are specifically excluded | (Schubert et al. 2002) |
| CFTR | Epithelial cells | yes | — | — | Raft association required for CFTR-dependent bacterial internalization and activation of innate immune response | (Kowalski & Pier, 2004) |
| CLIC4 | HEK293 cell line | yes | — | — | (Suginta et al. 2001) | |
| TRPC | Submandibular gland cells Smooth muscle Platelets | yes | — | yes | Cholesterol depletion inhibits TRPC1-store operated Ca2+ signals | (Kwiatek et al. 2006; Pani et al. 2009; Sundivakkam et al. 2009) |
| P2X1 | Platelets Vascular smooth muscle | yes | — | — | Cholesterol depletion inhibits P2X1-mediated currents and artery contraction | (Reviewed by Garcia-Marcos et al. 2009) |
| P2X3 | Neurones | yes | — | — | ||
| P2X4 | Epithelial cells | yes | — | — | ||
| P2X7 | Submandibular gland cells | yes | — | yes | Cholesterol depletion inhibits P2X7-mediated lipid signalling |
Biochemical isolation of rafts
This is by far the most widely exploited method of identifying proteins that associate with lipid rafts. The tight packing of the lipid acyl chains in these domains results in resistance to solubilization by cold non-ionic detergents (Brown & Rose, 1992), and the high lipid content of these complexes enables them to float to a low density during sucrose gradient centrifugation. Appropriate fractions can then be isolated from the density gradient and proteins associated with these fractions characterized by Western blot analysis. The most widely used detergent-based method consists of solubilization of membranes with 1% Triton X-100 at 4°C, although other methods have been described using lower detergent concentrations or other non-ionic detergents such as Chaps, Lubrol and Brij-98 (Pike, 2004).
Different detergents, and indeed different concentrations of the same detergent, produce rafts of very different protein and lipid composition (Pike, 2004; Babiychuk & Draeger, 2006). This may reflect the inherent heterogeneity of membrane rafts and the ability of some detergents to produce more ‘pure’ raft fractions by efficiently removing contaminating non-raft proteins and lipids. Equally, however, it may mean that some detergents selectively extract subsets of proteins and lipids from rafts, leaving behind a domain that bears little resemblance to its in vivo form. Problems such as these led to the development of non-detergent based methods for the isolation of rafts (Smart et al. 1995; Song et al. 1996). These essentially use sonication to disrupt the membrane followed by sucrose density centrifugation to separate the buoyant low-density raft component. While this method suffers from none of the potential selective extraction that plagues detergent-based methods, there is evidence that contaminating non-raft proteins may stay associated at the periphery of rafts and float with them to the low density layers (Foster et al. 2003; Pike, 2004). In general, it seems that biochemical isolation should be seen as a starting point for assessing the association of specific proteins with rafts and that, where possible, additional techniques should be employed to confirm the interaction.
Direct visualization: microscopic methods
Much of the controversy surrounding the existence of non-caveolar rafts in native cell membranes stems from the lack of suitable, non-invasive imaging techniques that allow direct visualization of these potentially extremely small (estimates range between 5–200 nm) and dynamic domains. Evidence for this form of microdomain is based largely on analysis of non-random clustering of fluorescently labelled raft-associated proteins in the membrane and the segregation of these clusters away from non-raft markers (Varma & Mayor, 1998; Kenworthy et al. 1999; Sharma et al. 2004). Additional evidence for the existence of distinct lipid microenvironments comes from monitoring changes in the diffusion behaviour of proteins in the bilayer (Pralle et al. 2000; Meder et al. 2006) or from the use of fluorescent probes such as 6-acyl-dimethylaminonapthalene (Laurdan) that are sensitive to changes in membrane fluidity (Gaus et al. 2003).
Morphologically, caveolae, which represent a subset of membrane raft, are much easier to study as they resemble small, stable pits on the membrane surface that are easily recognized in electron micrographs. These pits label heavily for caveolae marker proteins such as caveolin, and the direct visualization of proteins within these distinctive membrane compartments through immunogold electron microscopy provides perhaps the most convincing evidence for channel association with lipid microdomains. A subpopulation of Cav1.2 L-type Ca2+ channels localize with a muscle-specific isoform of caveolin in sarcolemmal caveolae in ventricular cardiomyocytes for example (Balijepalli et al. 2006). A particularly useful method here is the immunogold labelling of sheets of plasma membrane ripped from the surface of cells directly onto electron microscopy grids (Prior et al. 2003). In this plane, caveolae appear as circular, donut-shaped structures that label for caveolin. This method not only allows visualization of the plasma membrane in two dimensions over relatively large areas, but also permits quantitative analysis of clustering through the distribution of gold particles. This has been used to assess the association of the pore-forming subunit of the vascular ATP-sensitive K+ (KATP) channel, Kir6.1, with aortic caveolae (Sampson et al. 2007).
Functional approaches
These approaches usually involve the destruction of cholesterol-enriched microdomains either through the depletion of cellular cholesterol by agents such as methyl-β-cyclodextrin, or, in the case of caveolae, by use of small interfering RNA (siRNA) to knock down caveolin isoforms. This has been a useful technique to assess potential roles of rafts and/or cholesterol and caveolins in channel modulation, although methyl-β-cyclodextrin has been reported to have potentially important general effects on membrane and cell function (see, for example, Rodal et al. 1999; Kwik et al. 2003).
The hydrolysis of sphingomyelin, another important component of membrane rafts, has also been used to alter functional raft properties. Hydrolysis of sphingomyelin by the action of sphingomyelinase generates ceramide, which self-associates to form small ceramide-enriched microdomains that then merge into large ceramide-enriched platforms (Bollinger et al. 2005). This change in lipid (and probably protein) organization within the membrane upon addition of exogenous sphingomyelinase can be used to assess the functional role of sphingolipids/ceramides (Bock et al. 2003). Sphingomyelinase is also naturally activated by a number of pro-apoptotic stimuli suggesting this membrane reorganization may represent a general mechanism in signal transduction.
Regulation of channel function by lipid microdomains
Modulation by lipids
Ion channels are both directly and indirectly sensitive to the lipid composition of the membrane in which they are embedded. Caveolar and non-caveolar rafts form tightly packed aggregates of cholesterol and sphingolipids, chiefly sphingomyelin and glycosphingolipids, and this lipid composition influences physical characteristics of the membrane such as thickness, curvature and the ability to bend and compress. Every time a channel protein undergoes a conformational change it causes a local disturbance in the surrounding bilayer. The overall energetic cost of a channel transition will thus include not only the intrinsic channel activation energy, but also energy associated with membrane deformation (reviewed by Andersen & Koeppe, 2007). The energetics and kinetics of channel gating, for example, will therefore be modulated by the local lipid environment and this may account for some of the changes in kinetic behaviour seen upon altering cholesterol levels in the membrane (see Table 1).
In some cases, there is evidence for direct modulation of channel activity through interaction with surrounding lipids. The optical isomer of cholesterol, epicholesterol, which essentially mimics the effects of cholesterol on general membrane properties, is unable to substitute for cholesterol in its modulatory effects on inwardly rectifying K+ channels (Romanenko et al. 2002). This suggests that cholesterol exerts direct effects on K+ channel activity and indeed recent studies have identified specific residues in the cytosolic C-terminal domain of Kir2.1 that confer cholesterol sensitivity (Epshtein et al. 2009). Evidence also suggests that specific interaction between channel proteins and sphingolipids may regulate voltage sensing and/or channel gating. Treatment of cells with sphingomyelinase, an enzyme that cleaves the positively charged choline group from sphingomyelin, results in a pronounced negative shift in Kv2.1 activation and has led to the suggestion that key channel residues may interact with lipid head groups to modify channel activity (Ramu et al. 2006).
As discussed above, removal of the choline headgroup from sphingomyelin by the action of sphingomyelinase generates ceramide, which tends to aggregate into large ceramide-enriched membrane domains (Bollinger et al. 2005). Association of Kv1.3 with ceramide-enriched domains in lymphocytes inhibits whole-cell current amplitude, although whether this effect is due to changes in the lipid environment per se is unclear (Bock et al. 2003).
A discussion of lipid modulation of channel function would not be complete without mention of the best known lipid modulator, phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) (Suh & Hille, 2008). The compartmentalization of PI(4,5)P2 in membrane rafts is controversial, although recent data using a freeze fracture electron microscopic method suggest it is highly concentrated in the rim of caveolae in cultured fibroblasts and mouse smooth muscle (Fujita et al. 2009).
Modulation by association: macromolecular signalling complexes
The compartmentation of ion channels with proteins that regulate channel function will clearly have an impact upon channel behaviour. A wealth of signalling proteins aside from ion channels have been shown to accumulate in rafts, including a number of G-protein coupled receptors, various classes of G protein, adenylyl cyclase, protein kinase C, nitric oxide synthase, tyrosine kinases, H-ras and mitogen-activated protein kinase (reviewed by Patel et al. 2008). Protein recruitment to lipid microdomains may result from specific targeting signals, such as palmitoylation and myristoylation (Zacharias et al. 2002), or from specific transmembrane domain residues (Munro, 1995). Additionally, targeting may be through association with caveolae-associated scaffolding proteins, like caveolin or membrane-associated guanylate kinase (MAGUK) proteins such as postsynaptic density protein-95 (PSD-95) and synapse-associated protein-97 (SAP97), which have been suggested to be important in the localization of KV1.4 and KV1.5, respectively, to membrane rafts (Folco et al. 2004; Wong & Schlichter, 2004).
In the heart, activation of L-type Ca2+ channels by the stimulation of β-adrenergic receptors and activation of the cAMP/PKA signalling pathway is a well described response to sympathetic activation. Immunoprecipitation studies in mouse ventricular myocytes have identified a macromolecular signalling complex comprising caveolin-3, Cav1.2 L-type channels and a number of signalling molecules in the β2-adrenergic pathway (Balijepalli et al. 2006). Destruction of caveolar compartments by methyl-β-cyclodextrin-induced cholesterol depletion or siRNA for caveolin-3 perturbs the response of Cav2.1 to β2-adrenergic stimulation (Balijepalli et al. 2006; Calaghan & White, 2006). A similar form of cAMP signalling compartmentation is seen in vascular smooth muscle where the PKA-dependent regulation of vascular KATP channels depends upon the integrity of smooth muscle caveolae containing KATP channel subunits and adenylyl cyclase (Sampson et al. 2004).
There is also good evidence in endothelial and smooth muscle cells that caveolae act as integration sites for Ca2+ signalling due to their ability to aggregate proteins involved in Ca2+ regulation and excitation–contraction coupling (Bergdahl & Sward, 2004). In smooth muscle cells, caveolae are in particularly close association with the peripheral sarcoplasmic reticulum (SR) and appear to be the major site of calcium entry following store depletion (Shaw et al. 2006). Depletion of cholesterol has been shown to inhibit TRPC1-related store operated Ca2+ entry in smooth muscle and submandibular gland cells. Recent studies suggest a critical role for caveolin-1 in not only retaining TRPC1 channels at distinct junction sites between the plasma membrane and the peripheral SR where it can mediate store-operated calcium entry, but also in regulating TRPC1 activity (Kwiatek et al. 2006; Pani et al. 2009; Sundivakkam et al. 2009).
Aside from roles in caveolae formation and stability, caveolins have been shown to interact with many caveolae-localized signalling molecules (Razani et al. 2002; Patel et al. 2008). Each caveolin molecule has an unusual hairpin-like topology with a central hydrophobic core embedded in the membrane and the hydrophilic N and C termini free in the cytosol (Fig. 1). The N terminus contains an oligomerization domain that allows interaction with other caveolin molecules and an overlapping region known as the caveolin scaffolding domain (CSD) that is responsible for association with other signalling molecules. The CSD is known to bind to specific sequences on target proteins (ϕXϕXXXXϕ, ϕXXXXϕXXϕ and ϕXϕXXXXϕXXϕ where ϕ is an aromatic amino acid (tryptophan, phenylalanine or tyrosine) and X is any amino acid; Couet et al. 1997). Interaction with caveolins has been shown to directly modulate the activity of a number of ion channels. Interestingly, the activity of the cardiac KATP channel (Kir6.2/SUR2A) is suppressed by interaction with caveolin-3, but not caveolin-1, suggesting some specificity in regulation (Garg et al. 2009a,b;). Caveolin-3 has also recently been shown to regulate the current density and activation/inactivation kinetics of the HCN4 channel (Ye et al. 2008).
The suppressive effects of interaction with caveolin can in some cases be explained by caveolin-regulated changes in surface expression. Jiao et al. demonstrated that activation of protein kinase C facilitates caveolin-1-dependent internalization of vascular KATP channels (Jiao et al. 2008). Association with caveolin-1 has also been shown to negatively regulate ENaC activity by promoting ubiquitination of the channel and subsequent internalization (Lee et al. 2009). In cell lines, caveolin-1 has been suggested to play a dual role in regulating surface targeting of the large conductance Ca2+-activated (BK) channel α subunit, Slo1, by maintaining channels in intracellular compartments and/or anchoring channels in the membrane (Alioua et al. 2008). In the heart, β-adrenergic stimulation of Nav1.5 activity, which is responsible for the initial upstroke of the cardiac action potential, occurs by both PKA-dependent phosphorylation of channel subunits and by direct PKA-independent Gαs modulation of current density. Recent studies indicate that the increase in Nav1.5 current density results from direct interaction between Gsα and caveolin-3, which promotes recruitment of Nav1.5-containing caveolae to the surface membrane (reviewed by Balijepalli & Kamp, 2008).
Considerations of an enclosed extracellular compartment
Finally it is worth noting, particularly in the context of ion channel function that the flask-shaped pit formed by a caveola often has a small, restricted opening. This would potentially allow for the build up of ions within the caveolar ‘pit’ (caveolae have a diameter of 50–100 nm) to concentrations far in excess of what would be experienced in the bulk extracellular fluid. Interestingly, a very recent study by Alday et al. (2010) suggests that a subpopulation of cardiac Kv channels may avoid this problem by localizing predominantly to the rim of caveolae. Implications linked to the geometry of caveolae have not been fully investigated but may add an additional layer of complexity when considering the activity of channels localized to these specialized membrane compartments.
Concluding remarks
The idea that the lipid bilayer acts only as an inert solvent for membrane proteins is now superseded by the idea that membrane lipids play an integral part in the regulation of channel function. Regulation of channel activity by PI(4,5)P2 is well characterized, but evidence suggests that raft-associated lipids such as cholesterol and sphingolipids and the lipid domains themselves also directly and/or indirectly regulate channel activity. Much of the information we have so far represents only static snapshots of channel interaction with microdomains, where in vivo it is likely that these associations are dynamic and regulated. It may take the development of additional non-invasive imaging technologies before the full dynamic complexity of these regions and their regulatory interactions are fully appreciated.
Glossary
Abbreviations
- CLIC
chloride intracellular channel
- CSD
caveolin scaffolding domain
- ENaC
epithelial Na+ channel
- HCN
hyperpolarization-activated cyclic nucleotide-gated
- hERG1
human ether-à-go-go related gene
- KATP
ATP-sensitive K+ channel
- Kir
inwardly rectifying K+ channel
- Kv
voltage-gated K+ channel
- MAGUK
membrane-associated guanylate kinase
- Nav
voltage-gated Na+ channel
- PI(4,5)P2
phosphatidylinositol 4,5-bisphosphate
- PSD-95
postsynaptic density protein-95
- SAP97
synapse-associated protein-97
- siRNA
small interfering RNA
- SR
sarcoplasmic reticulum
- TRP
transient receptor potential
References
- Alday A, Urrutia J, Gallego M, Casis O. α1-Adrenoreceptors regulate only the caveolae-located subpopulation of cardiac Kv4 channels. Channels. 2010 doi: 10.4161/chan.4.3.11479. in press DOI 10.4161/chan.4.3.11479. [DOI] [PubMed] [Google Scholar]
- Alioua A, Lu R, Kumar Y, Eghbali M, Kundu P, Toro L, Stefani E. Slo1 caveolin-binding motif, a mechanism of caveolin-1-Slo1 interaction regulating Slo1 surface expression. J Biol Chem. 2008;283:4808–4817. doi: 10.1074/jbc.M709802200. [DOI] [PubMed] [Google Scholar]
- Andersen OS, Koeppe RE. Bilayer thickness and membrane protein function: An energetic perspective. Annu Rev Biophys Biomol Struct. 2007;36:107–130. doi: 10.1146/annurev.biophys.36.040306.132643. [DOI] [PubMed] [Google Scholar]
- Babiychuk EB, Draeger A. Biochemical characterization of detergent-resistant membranes: a systematic approach. Biochem J. 2006;397:407–416. doi: 10.1042/BJ20060056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balijepalli RC, Delisle BP, Balijepalli SY, Foell JD, Slind JK, Kamp TJ, January CT. Kv11.1 (ERG1) K+ channels localize in cholesterol and sphingolipid enriched membranes and are modulated by membrane cholesterol. Channels. 2007;1:263–272. doi: 10.4161/chan.4946. [DOI] [PubMed] [Google Scholar]
- Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac L-type Ca2+ channels to a caveolar macromolecular signalling complex is required for β2-adrenergic regulation. Proc Natl Acad Sci U S A. 2006;103:7500–7505. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balijepalli RC, Kamp TJ. Caveolae, ion channels and cardiac arrhythmias. Prog Biophys Mol Biol. 2008;98:149–160. doi: 10.1016/j.pbiomolbio.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbuti A, Gravante B, Riolfo M, Milanesi R, Terragni B, DiFrancesco D. Localization of pacemaker channels in lipid rafts regulates channel kinetics. Circ Res. 2004;94:1325–1331. doi: 10.1161/01.RES.0000127621.54132.AE. [DOI] [PubMed] [Google Scholar]
- Barbuti A, Terragni B, Brioschi C, DiFrancesco D. Localization of f-channels to caveolae mediates specific β2-adrenergic receptor modulation of rate in sinoatrial myocytes. J Mol Cell Cardiol. 2007;42:71–78. doi: 10.1016/j.yjmcc.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Barenholz Y. Liposomology. Progr Lipid Res. 2000;39:1–2. doi: 10.1016/s0163-7827(99)00015-6. [DOI] [PubMed] [Google Scholar]
- Bergdahl A, Sward K. Caveolae-associated signalling in smooth muscle. Can J Physiol Pharmacol. 2004;82:289–299. doi: 10.1139/y04-033. [DOI] [PubMed] [Google Scholar]
- Bock J, Szabo I, Gamper N, Adams C, Gulbins E. Ceramide inhibits the potassium channel Kv1.3 by the formation of membrane platforms. Biochem Biophys Res Commun. 2003;305:890–897. doi: 10.1016/s0006-291x(03)00763-0. [DOI] [PubMed] [Google Scholar]
- Bollinger CR, Teichgraber V, Gulbins E. Ceramide-enriched membrane domains. Biochim Biophys Acta. 2005;1746:284–294. doi: 10.1016/j.bbamcr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Brainard AM, Korovkina VP, England SK. Disruption of the maxi-K-caveolin-1 interaction alters current expression in human myometrial cells. Reprod Biol Endocrinol. 2009;7:131. doi: 10.1186/1477-7827-7-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of Gpi-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell-surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Calaghan S, White E. Caveolae modulate excitation-contraction coupling and β2-adrenergic signalling in adult rat ventricular myocytes. Cardiovasc Res. 2006;69:816–824. doi: 10.1016/j.cardiores.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- Couet J, Li SW, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain: Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997;272:6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- Delling M, Wischmeyer E, Dityatev A, Sytnyk V, Veh RW, Karschin A, Schachner M. The neural cell adhesion molecule regulates cell-surface delivery of G-protein-activated inwardly rectifying potassium channels via lipid rafts. J Neurosci. 2002;22:7154–7164. doi: 10.1523/JNEUROSCI.22-16-07154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M. The state of lipid rafts: From model membranes to cells. Annu Rev Biophys Biomol Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- Epshtein Y, Chopra AP, Rosenhouse-Dantsker A, Kowalsky GB, Logothetis DE, Levitan I. Identification of a C-terminus domain critical for the sensitivity of Kir2.1 to cholesterol. Proc Natl Acad Sci U S A. 2009;106:8055–8060. doi: 10.1073/pnas.0809847106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini J, Barrantes FJ. Sphingolipid/cholesterol regulation of neurotransmitter receptor conformation and function. Biochim Biophys Acta. 2009;1788:2345–2361. doi: 10.1016/j.bbamem.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Folco EJ, Liu GX, Koren G. Caveolin-3 and SAP97 form a scaffolding protein complex that regulates the voltage-gated potassium channel Kv1.5. Am J Physiol Heart Circ Physiol. 2004;287:H681–H690. doi: 10.1152/ajpheart.00152.2004. [DOI] [PubMed] [Google Scholar]
- Foster LJ, de Hoog CL, Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci U S A. 2003;100:5813–5818. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita A, Cheng JL, Tauchi-Sato K, Takenawa T, Fujimoto T. A distinct pool of phosphatidylinositol 4,5-bisphosphate in caveolae revealed by a nanoscale labeling technique. Proc Natl Acad Sci U S A. 2009;106:9256–9261. doi: 10.1073/pnas.0900216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Marcos M, Dehaye JP, Marino A. Membrane compartments and purinergic signalling: the role of plasma membrane microdomains in the modulation of P2XR-mediated signalling. FEBS J. 2009;276:330–340. doi: 10.1111/j.1742-4658.2008.06794.x. [DOI] [PubMed] [Google Scholar]
- Garg V, Jiao JD, Hu KL. Regulation of ATP-sensitive K+ channels by caveolin-enriched microdomains in cardiac myocytes. Cardiovasc Res. 2009a;82:51–58. doi: 10.1093/cvr/cvp039. [DOI] [PubMed] [Google Scholar]
- Garg V, Sun W, Hu KL. Caveolin-3 negatively regulates recombinant cardiac K-ATP channels. Biochem Biophys Res Commun. 2009b;385:472–477. doi: 10.1016/j.bbrc.2009.05.100. [DOI] [PubMed] [Google Scholar]
- Gaus K, Gratton E, Kable EPW, Jones AS, Gelissen I, Kritharides L, Jessup W. Visualizing lipid structure and raft domains in living cells with two-photon microscopy. Proc Natl Acad Sci U S A. 2003;100:15554–15559. doi: 10.1073/pnas.2534386100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H, Kurachi Y. Distinct detergent-resistant membrane microdomains (lipid rafts) respectively harvest K+ and water transport systems in brain astroglia. Eur J Neurosci. 2007;26:2539–2555. doi: 10.1111/j.1460-9568.2007.05876.x. [DOI] [PubMed] [Google Scholar]
- Hill WG, Butterworth MB, Wang H, Edinger RS, Lebowitz J, Peters KW, Frizzell RA, Johnson JP. The epithelial sodium channel (ENaC) traffics to apical membrane in lipid rafts in mouse cortical collecting duct cells. J Biol Chem. 2007;282:37402–37411. doi: 10.1074/jbc.M704084200. [DOI] [PubMed] [Google Scholar]
- Jiao JD, Garg V, Yang BF, Elton TS, Hu KL. Protein kinase C-epsilon induces caveolin-dependent internalization of vascular adenosine 5′-triphosphate sensitive K+ channels. Hypertension. 2008;52:499–506. doi: 10.1161/HYPERTENSIONAHA.108.110817. [DOI] [PubMed] [Google Scholar]
- Kenworthy AK, Petranova N, Hubbard AL, Edidin M. Membrane organization of GPI-anchored proteins in hepatocyte plasma membranes probed by imaging FRET. Mol Biol Cell. 1999;10:1758. [Google Scholar]
- Kowalski MP, Pier GB. Localization of cystic fibrosis transmembrane conductance regulator to lipid rafts of epithelial cells is required for Pseudomonas aeruginosa-induced cellular activation. J Immunol. 2004;172:418–425. doi: 10.4049/jimmunol.172.1.418. [DOI] [PubMed] [Google Scholar]
- Kwiatek AM, Minshall RD, Cool DR, Skidgel RA, Malik AB, Tiruppathi C. Caveolin-1 regulates store-operated Ca2+ influx by binding of its scaffolding domain to transient receptor potential channel-1 in endothelial cells. Mol Pharmacol. 2006;70:1174–1183. doi: 10.1124/mol.105.021741. [DOI] [PubMed] [Google Scholar]
- Kwik J, Boyle S, Fooksman D, Margolis L, Sheetz MP, Edidin M. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc Natl Acad Sci U S A. 2003;100:13964–13969. doi: 10.1073/pnas.2336102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IH, Campbell CR, Song SH, Day ML, Kumar S, Cook DI, Dinudom A. The activity of the epithelial sodium channels is regulated by caveolin-1 via a Nedd4-2-dependent mechanism. J Biol Chem. 2009;284:12663–12669. doi: 10.1074/jbc.M809737200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens JR, Navarro-Polanco R, Coppock EA, Nishiyama A, Parshley L, Grobaski TD, Tamkun MM. Differential targeting of shaker-like potassium channels to lipid rafts. J Biol Chem. 2000;275:7443–7446. doi: 10.1074/jbc.275.11.7443. [DOI] [PubMed] [Google Scholar]
- Meder D, Moreno MJ, Verkade P, Vaz WLC, Simons K. Phase coexistence and connectivity in the apical membrane of polarized epithelial cells. Proc Natl Acad Sci U S A. 2006;103:329–334. doi: 10.1073/pnas.0509885103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. A comparison of the transmembrane domains of Golgi and plasma memebrane proteins. Biochem Soc Trans. 1995;23:527–530. doi: 10.1042/bst0230527. [DOI] [PubMed] [Google Scholar]
- Munro S. Lipid rafts: Elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- Pani B, Ong HL, Brazer SCW, Liu XB, Rauser K, Singh BB, Ambudkar IS. Activation of TRPC1 by STIM1 in ER-PM microdomains involves release of the channel from its scaffold caveolin-1. Proc Natl Acad Sci U S A. 2009;106:20087–20092. doi: 10.1073/pnas.0905002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–391. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike LJ. Lipid rafts: heterogeneity on the high seas. Biochem J. 2004;378:281–292. doi: 10.1042/BJ20031672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pralle A, Keller P, Florin EL, Simons K, Horber JKH. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J Cell Biol. 2000;148:997–1007. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior IA, Parton RG, Hancock JF. Observing cell surface signalling domains using electron microscopy. Science STKE. 2003;2003:PL9. doi: 10.1126/stke.2003.177.pl9. [DOI] [PubMed] [Google Scholar]
- Ramu Y, Xu YP, Lu Z. Enzymatic activation of voltage-gated potassium channels. Nature. 2006;442:696–699. doi: 10.1038/nature04880. [DOI] [PubMed] [Google Scholar]
- Razani B, Woodman SE, Lisanti MP. Caveolae: From cell biology to animal physiology. Pharmacol Rev. 2002;54:431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- Rodal SK, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K. Extraction of cholesterol with methyl-β-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell. 1999;10:961–974. doi: 10.1091/mbc.10.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanenko VG, Rothblat GH, Levitan I. Modulation of endothelial inward-rectifier K+ current by optical isomers of cholesterol. Biophys J. 2002;83:3211–3222. doi: 10.1016/S0006-3495(02)75323-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson LJ, Davies LM, Barrett-Jolley R, Standen NB, Dart C. Angiotensin II-activated protein kinase C targets caveolae to inhibit aortic ATP-sensitive potassium channels. Cardiovasc Res. 2007;76:61–70. doi: 10.1016/j.cardiores.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Sampson LJ, Hayabuchi Y, Standen NB, Dart C. Caveolae localize protein kinase A signalling to arterial ATP-sensitive potassium channels. Circ Res. 2004;95:1012–1018. doi: 10.1161/01.RES.0000148634.47095.ab. [DOI] [PubMed] [Google Scholar]
- Schubert AL, Schubert W, Spray DC, Lisanti MP. Connexin family members target to lipid raft domains and interact with caveolin-1. Biochemistry. 2002;41:5754–5764. doi: 10.1021/bi0121656. [DOI] [PubMed] [Google Scholar]
- Sharma P, Varma R, Sarasij RC, Ira, Gousset K, Krishnamoorthy G, Rao M, Mayor S. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell. 2004;116:577–589. doi: 10.1016/s0092-8674(04)00167-9. [DOI] [PubMed] [Google Scholar]
- Shaw L, Sweeney MA, O’Neill SC, Jones CJP, Austin C, Taggart MJ. Caveolae and sarcoplasmic reticular coupling in smooth muscle cells of pressurised arteries: The relevance for Ca2+ oscillations and tone. Cardiovasc Res. 2006;69:825–835. doi: 10.1016/j.cardiores.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Smart EJ, Ying YS, Mineo C, Anderson RGW. A detergent-free method for purifying caveolae membrane from tissue-culture cells. Proc Natl Acad Sci U S A. 1995;92:10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KS, Li SW, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains: Detergent-free purification of caveolae membranes. J Biol Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- Suginta W, Karoulias N, Aitken A, Ashley RH. Chloride intracellular channel protein CLIC4 (p64H1) binds directly to brain dynamin I in a complex containing actin, tubulin and 14-3-3 isoforms. Biochem J. 2001;359:55–64. doi: 10.1042/0264-6021:3590055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: How and why? Annu Rev Biophys. 2008;37:175–195. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundivakkam PC, Kwiatek AM, Sharma TT, Minshall RD, Malik AB, Tiruppathi C. Caveolin-1 scaffold domain interacts with TRPC1 and IP3R3 to regulate Ca2+ store release-induced Ca2+ entry in endothelial cells. Am J Physiol Cell Physiol. 2009;296:C403–C413. doi: 10.1152/ajpcell.00470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- Wang XL, Ye D, Peterson TE, Cao S, Shah VH, Katusic ZS, Sieck GC, Lee HC. Caveolae targeting and regulation of large conductance Ca2+-activated K+ channels in vascular endothelial cells. J Biol Chem. 2005;280:11656–11664. doi: 10.1074/jbc.M410987200. [DOI] [PubMed] [Google Scholar]
- Wong W, Schlichter LC. Differential recruitment of Kv1.4 and Kv4.2 to lipid rafts by PSD-95. J Biol Chem. 2004;279:444–452. doi: 10.1074/jbc.M304675200. [DOI] [PubMed] [Google Scholar]
- Xia FZ, Gao XD, Kwan E, Lam PPL, Chan LL, Sy KY, Sheu L, Wheeler MB, Gaisano HY, Tsushima RG. Disruption of pancreatic beta-cell lipid rafts modifies Kv2.1 channel gating and insulin exocytosis. J Biol Chem. 2004;279:24685–24691. doi: 10.1074/jbc.M314314200. [DOI] [PubMed] [Google Scholar]
- Ye B, Balijepalli RC, Foell JD, Kroboth S, Ye Q, Luo YH, Shi NQ. Caveolin-3 associates with and affects the function of hyperpolarization-activated cyclic nucleotide-gated channel 4. Biochemistry. 2008;47:12312–12318. [PMC free article] [PubMed] [Google Scholar]
- Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]


