Abstract
In this review we take a physiological perspective on the role of voltage-gated potassium channels in an identified neuron in the auditory brainstem. The large number of KCN genes for potassium channel subunits and the heterogeneity of the subunit combination into K+ channels make identification of native conductances especially difficult. We provide a general pharmacological and biophysical profile to help identify the common voltage-gated K+ channel families in a neuron. Then we consider the physiological role of each of these conductances from the perspective of the principal neuron in the medial nucleus of the trapezoid body (MNTB). The MNTB is an inverting relay, converting excitation generated by sound from one cochlea into inhibition of brainstem nuclei on the opposite side of the brain; this information is crucial for binaural comparisons and sound localization. The important features of MNTB action potential (AP) firing are inferred from its inhibitory projections to four key target nuclei involved in sound localization (which is the foundation of auditory scene analysis in higher brain centres). These are: the medial superior olive (MSO), the lateral superior olive (LSO), the superior paraolivary nucleus (SPN) and the nuclei of the lateral lemniscus (NLL). The Kv families represented in the MNTB each have a distinct role: Kv1 raises AP firing threshold; Kv2 influences AP repolarization and hyperpolarizes the inter-AP membrane potential during high frequency firing; and Kv3 accelerates AP repolarization. These actions are considered in terms of fidelity of transmission, AP duration, firing rates and temporal jitter. An emerging theme is activity-dependent phosphorylation of Kv channel activity and suggests that intracellular signalling has a dynamic role in refining neuronal excitability and homeostasis.
Jamie Johnston did his PhD with Ian Forsythe and a postdoctoral fellowship with Kerry Delaney in British Columbia before returning to Leon Lagnardo's laboratory in the MRC Laboratory of Molecular Biology. Ian Forsythe started in Leicester as a postdoc in Peter Stanfield's Ion Channel Group in 1988; following a Wellcome Trust Senior Research Fellowship, he became Professor of Neuroscience in the Department of Cell Physiology and Pharmacology in 2000 and in 2005 moved from the University of Leicester to the MRC Toxicology Unit. Conny Kopp-Scheinpflug did her PhD in Leipzig with Rudolf Rübsamen and a postdoctoral fellowship with Bruce Tempel in Seattle, thereafter returning to Leipzig for her Habilitation; she joined Ian Forsythe's group in 2009. We share a common interest in understanding synaptic transmission and integration in the central auditory pathway and in the control of neuronal excitability by voltage-gated potassium channels. Our research methods span from in vivo single-unit recording and multi-photon imaging to in vitro whole-cell patch recording, voltage-clamp and immunohistochemistry.

Introduction: defining potassium currents in native neurons
When delayed rectifying potassium currents were shown to be the basis of action potential (AP) repolarization (Hodgkin & Huxley, 1952), it was never envisaged that there would be such a huge number of genes dedicated to this function: there are around 40 subunit genes divided into around 12 families (Coetzee et al. 1999; Gutman et al. 2003). Generally a functional channel requires association of four α subunits, usually from within the same family and may include β subunits or other accessory proteins. K+ channels share a highly conserved pore structure, providing high selectivity for K+ ion permeation (Doyle et al. 1998; Yellen, 2002; MacKinnon, 2003). Elaboration of this structure forms the basis for the rich diversity of potassium channel properties and functions, but these subtle differences make it difficult to confidently assign specific roles to identified subunits in most neurons.
Knowledge of K+ channels is dominated by studies of recombinant homomeric channels in cell lines, but this gives limited understanding of heteromeric channels in native neurons. First one must obtain good quality voltage-clamp data, but the extensive dendritic trees of many neurons make space-clamp imperfect, while the magnitude of outward currents means that the voltage-clamp error (generated by the current passing across the pipette series resistance in whole-cell recording) is very large, even with series resistance compensation circuitry. These and other issues have been well considered elsewhere (Williams & Mitchell, 2008; Clay, 2009).
Resting membrane potentials are generally set by expression of inward rectifiers mediated by the Kir channel family and by the leak channels, ‘outward rectifiers’ mediated by the tandem-pore (K2p) K+ channels (see review from A. Mathie in this issue). These channels are not gated, but show rectification either through pore block (Kir) or arising from the highly asymmetric internal and external [K+]. Kv channels activating around resting potentials, such as Kv1 and hyperpolarization-activated non-specific cation channels, IH (HCN are structurally related to Kv) will also influence the resting potential and synaptic integration (Garden et al. 2008; Oertel et al. 2008; Hassfurth et al. 2009).
Basic gating/kinetic properties of the K+ channel families
Within the limitations of voltage-clamp in the whole-cell patch configuration, we can obtain biophysical parameters of the currents which are characteristic of particular families (Gutman et al. 2003). The dominant neuronal delayed rectifiers in mammals are from the core voltage-gated channel families: Kv1–Kv4. Each family contains multiple genes specifying subunits which are homologous to the single prototypical genes expressed in Drosophila: shaker, shab, shaw and shal, respectively. The biophysical properties of native ion channels differ in rather subtle ways and given their heterogeneous composition, precise identification is possible only under special circumstances. Family traits are recognizable, so it seems reasonable to propose that Kv families are specialized for particular functions, but there is necessarily considerable overlap and interaction. Kv1, Kv4 and Kv7 channels are low-voltage-activated (LVA; Fig. 1) opening on small depolarizations from around resting potentials, and so are a powerful means of regulating AP number on depolarization or during an EPSP (Brew et al. 2003, 2007; Coetzee et al. 1999; Brown & Passmore, 2009). Kv4 channels generate the classic A-current, a transient K+ current, which requires prior hyperpolarization to remove steady-state inactivation before it can activate (Maffie & Rudy, 2008). Kv3 currents are high voltage activated (HVA; Fig. 1) requiring voltages achieved only during APs, and their fast kinetics mean that they contribute to AP duration and fast firing. Kv2 are also HVA, but have slower kinetics, and their association with accessory proteins (gene families: Kv5, Kv6, Kv8 and Kv9) makes their properties difficult to match with recombinant counterparts.
Figure 1. Functional classification of voltage-gated potassium currents.
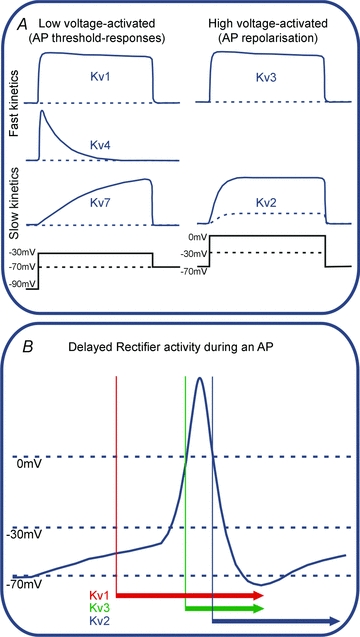
A, voltage clamp permits the biophysical properties of the native channel types to be distinguished in terms of voltage-dependent activation kinetics. Generalization of these properties is expressed here as a matrix of low-voltage-activated (LVA) channels such as Kv1, Kv4 and Kv7, and high-voltage-activated channels (HVA) Kv3 and Kv2. Kv4 channels (mediating A-type currents) are inactivated at rest and so require prior hyperpolarization before they will activate. The spectrum of their channel kinetics further divides these groups into those that activate rapidly (Kv1, Kv4 and Kv3) and more slowly (Kv7 and Kv2). B, under conventional voltage recording (current clamp) the Kv channel kinetics mean that each channel activates over a characteristic part of the overall AP waveform as represented by the coloured bars/arrows: LVA channels open on depolarization from resting potentials (−70 mV) to around −40 to −30 mV, so influencing the threshold for AP firing. HVA channels require further depolarization, approaching 0 mV, which is only achieved during APs, so these channels contribute to repolarization. Kv1 channels would turn on with small depolarizations, while Kv3 would be delayed until further depolarization. Kv2 would be even later due to its slow kinetics, but its activity extended to longer time points as it is slow to turn off. Although this diagram is a vast oversimplification, it gives a framework in which to identify native potassium currents and their different roles in determining neuronal excitability. Note that little emphasis is placed on inactivation, since this is so dependent on the precise subunit composition of the channel, but it is nonetheless important; Kv4 channels invariably inactivate, with a time course which is highly dependent on subunit and accessory proteins. Inactivation (for reviews see Robertson, 1997; Aldrich, 2001) plays an important role in reducing the Kv contribution during sustained activation, for instance increasing AP duration during repetitive firing.
The IUPHAR web site (Gutman et al. 2003) provides broad introductory information on ion channel properties and their pharmacology. Many pharmacological agents are not so specific that they can be used as the sole means of identification for potassium channels. Tetraethylammonium (TEA) will block many Kv channels at high concentrations (Armstrong & Binstock, 1965; Stanfield, 1983) but at low concentrations it is relatively effective at blocking Kv3 channels (Fig. 2). The approach we have taken is to build a series of simple pharmacological tests, which when combined with biophysical data can indicate the Kv family of a native neuronal conductance, with additional immuno-histochemical, PCR and/or transgenic data providing further confirmation. The basic pharmacological protocol is presented in Fig. 2. At present it has only been applied to auditory brainstem neurons, but it should be applicable to other brain areas. Certain antagonists such as the dendrotoxins are highly specific and provide clear evidence for the channel family (Harvey & Robertson, 2004); in a few cases, subunit-specific toxins can be employed to give even more precise data (e.g. Kv1.1 block by dendrotoxin-K; see Dodson et al. 2002).
Figure 2. Pharmacological profile for native delayed rectifier Kv families.
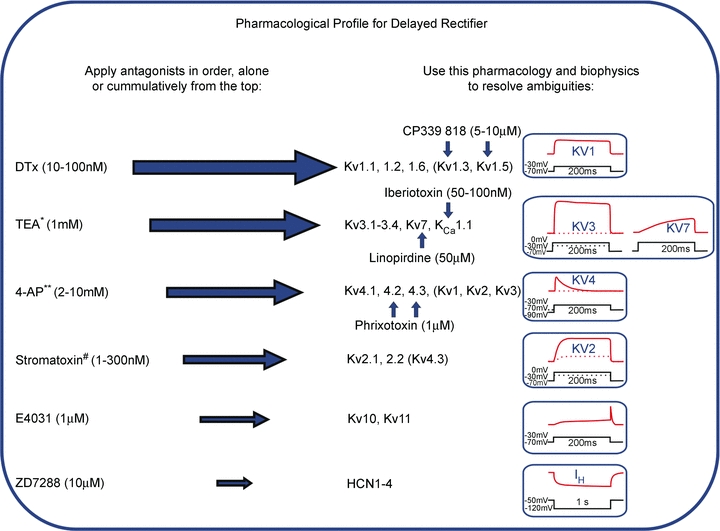
Since native channels are often heteromers, precise biophysical and pharmacological matching to recombinant homomeric channels is difficult. Measurement of ion channel current defines the functional plasma membrane channel pool; this is a crucial advantage over biochemical approaches (Western blot or PCR). This pharmacological profile method should be applied with caution. The antagonists (or gating modifier no.) listed on the left are applied cumulatively in order to inhibit the conductances indicated in the middle. Resolution of ambiguous pharmacology is achieved by secondary experiments using the additional pharmacology indicated by the vertical blue arrows. Additional constraints and confirmation of Kv identity is obtained from the time course and current–voltage relations of the current measured under voltage clamp, as summarized on the right. *Low concentrations of TEA will also block Kv1.1 homomeric channels. ** 4-aminopyridine will block many Kv1 and Kv3 subunits at micromolar concentrations and most Kvs at millimolar concentrations; If Kv4 (A-current) is present it may be effectively removed by induction of inactivation, without need of pharmacological agents. #Stromatoxin is a gating modifer, shifting the activation voltage to more positive potentials, rather than an antagonist.
The crucial physiological insights to understanding the biophysical roles of different potassium channel families may be realized by focusing studies on a specific identified neuron, which participates in a particular computational process. Our interest in the control of excitability in the medial nucleus of the trapezoid body (MNTB) and its giant synapse, the calyx of Held (Dodson & Forsythe, 2004; Schneggenburger & Forsythe, 2006), provides an ideal preparation to explore native K+ channels in the context of auditory processing. This nucleus has the advantages of simple morphology (the calyx of Held synapse forms on the soma of a neuron with few dendrites) and predictable neuronal properties. The MNTB is an ideal site at which to address these integrative questions; but first let us briefly consider the function of the MNTB in auditory processing: what is it doing, where does it project, how is it adapted to these postulated computational roles?
What is the MNTB doing?
Three elementary properties of sound are encoded into AP trains of the 8th nerve by the cochleae: frequency (tone), intensity (volume) and time (onset, phase). The frequency of sound is transformed into a place code (tonotopy) by the resonance of the basilar membrane, so that inner hair cells close to the oval window (at the base of the cochlea) respond to high frequencies and as the resonance evolves with distance along the basilar membrane, increasingly low frequencies are represented toward the apex. This tonotopic map is preserved and adapted in the central auditory projection (Kandler et al. 2009) with each neuron having a characteristic frequency (CF) to which it fires with the lowest threshold. For a given frequency (and within neuronal populations) sound intensity is encoded by AP firing rate; usually with higher rates representing louder sounds. Finally, timing is important for localization of a sound source, which requires integration of AP encoded sounds from two cochleae for computation of interaural timing and intensity differences (Oertel, 1999; Trussell, 1999). This computation first occurs in two distinct nuclei of the brainstem superior olivary complex, each of which receives inhibitory inputs from the MNTB.
The MNTB is an inverting relay, providing an ipsilateral glycinergic inhibition from contralateral excitation (i.e. from the sound received on the opposite side of the head). This input arrives from the bushy cells of the ventral cochlear nucleus (VCN) via the calyx of Held (Fig. 3, inset). Each MNTB projects to four auditory nuclei: the medial superior olive (MSO; Smith et al. 2000; for review see Grothe, 2003; Fig. 3B), the lateral superior olive (LSO; for review see Tollin, 2003; Kandler et al. 2009; Fig. 3C), the superior paraolivary nucleus (SPN; Banks & Smith, 1992; Kulesza et al. 2007; Fig. 3D) and the nuclei of the lateral lemniscus (NLL; Glendenning et al. 1981; Yavuzoglu et al. 2010; Fig. 3E). We will briefly review the function of each nucleus, highlighting the role of the MNTB at each stage.
Figure 3. Contribution of MNTB-inhibition to auditory brainstem processing.
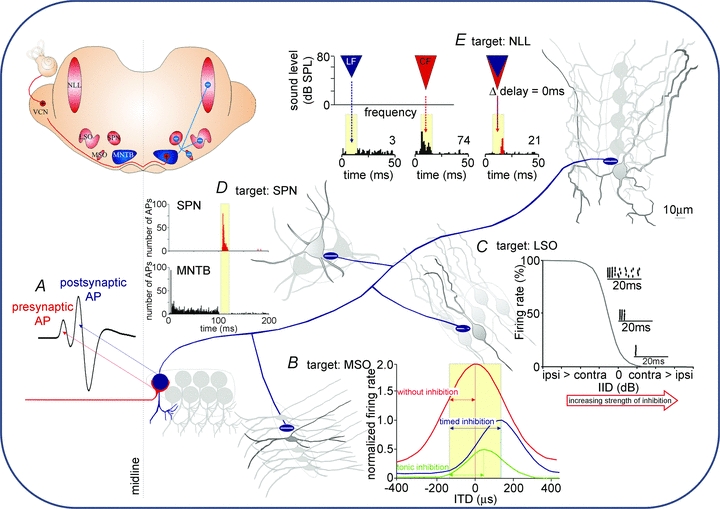
Inset, a sketch of a coronal brain section at the level of the superior olivary complex. Sound-evoked activity from the contralateral ear arrives as AP trains in the ventral cochlear nucleus (VCN) which sends excitatory projections to the MNTB (red lines, +). MNTB neurons give inhibitory projections (blue lines, −) to the medial superior olive (MSO), the lateral superior olive (LSO), the superior paraolivary nucleus (SPN) and the nuclei of the lateral lemniscus (NLL). All four target nuclei receive direct excitatory inputs from the VCN (not shown). A, excitation to each MNTB neuron is mediated via a calyx of Held synapse (red line) whose activity can be detected in extracellular recordings as a presynaptic AP preceding the postsynaptic AP (modified from Kopp-Scheinpflug et al. 2008b with permission from Elsevier). B, MSO cells receive glycinergic IPSPs directly onto their somata. Without inhibition, the peak of the ITD function lies within the physiologically relevant range of ITDs (yellow shaded area) and ITDs are encoded by a topographic map. Well-timed inhibition reduces the firing rate but also shifts the peak ITD function out of the physiological range. In this scenario the slope of the ITD function can be used to encode ITDs via a rate code. Tonic (non-phase-locked) inhibition causes a reduction in firing rate but without the respective shift of the ITD function (figure modified from Pecka et al. 2008 with permission from the Society for Neuroscience). C, LSO cells respond to ipsilateral sound stimulation with increased firing rates. Increasing the strength of the inhibitory input leads to successive reductions in firing rate. Dot raster plots resemble the activity at about 20, 50 and 80% reduction of firing rate. The inhibition needs to be rather strong to suppress the onset component (figure modified from Park et al. 1997, with permission from the American Physiological Society). D, SPN cells receive a sustained inhibitory input which effectively suppresses AP firing during sound stimulation. The termination of a sound is then highlighted by an AP offset response (unpublished data; see also Kadner et al. 2006). E, NLL neurons receive low-frequency inhibition (blue area and histogram below), in addition to a higher-frequency excitatory CF-stimulation (red area and histogram below). When both stimuli are presented at 0 ms delay, the excitatory CF response is suppressed (overlaying blue and red areas) and a post-inhibitory rebound is observed (red part of the histogram; figure modified from Peterson et al. 2009 with permission from the American Physiological Society).
(1) The MSO projection
Low-frequency sounds (<2 kHz) with a wavelength long enough to give two distinct phases at opposite ears within one sound cycle are localized by interaural time differences (ITDs) of the sound stimulus. There are two theories for ITD processing based on coincidence detection: first, via ‘hard-wired delay lines’ (Jeffress, 1948; for discussion see Joris & Yin, 2007) and second via ‘virtual delay lines’ introduced by either tonic inhibition (Zhou et al. 2005) or well-timed inhibition from the MNTB (Pecka et al. 2008). Both theories require temporal and spatial summation of phase-locked inputs, in which summation of EPSPs from left and right ears are refined by voltage-gated conductances, such as Kv1 (Mathews et al. 2010). MNTB-mediated inhibition shifts the slope (rather than the peak) of the ITD functions into the physiologically relevant range, so that near linear changes in firing rate equate to azimuth sound location (McAlpine et al. 2001; Grothe, 2003). IPSPs from the MNTB need to be as precisely timed as the EPSPs, i.e. for phase-locked firing beyond 1 kHz during the sound (Smith et al. 1998; Paolini et al. 2001; Kopp-Scheinpflug et al. 2003; Tollin & Yin, 2005) and ensure that inhibition is linearly represented so that firing is restricted to only one AP per cycle of the sound wave.
(2) The LSO projection
High-frequency sounds (≥2 kHz) are located by comparison of interaural differences in sound intensity (IID) caused by reflection from and refraction around the head. The LSO integrates EPSPs originating from the ipsilateral VCN and IPSPs from the MNTB via the contralateral VCN at a matching sound frequency (Tsuchitani, 1997). Firing evoked by the ipsilateral EPSP is suppressed by increased sound intensity at the contralateral (inhibitory) ear, with the firing rate being described by the IID function (Fig. 3C). The dot raster plots in Fig. 3C show that complete suppression of the onset APs is achieved by intense inhibition. Changes in either timing (of the IPSP or EPSP) or intensity, can substitute for the other parameter (Pollak, 1988). IIDs are generally integrated over short time periods (Tollin, 2003) which makes onset precision essential. Over these periods the MNTB inhibitory input must have a large firing range so as to encode inherently small intensity differences.
(3) The SPN projection
The mammalian auditory brainstem also contains circuits adapted for gap and sound duration detection which define the end of a sound (Kadner et al. 2006; Kadner & Berrebi, 2008). This contributes to vocal communication and speech in humans (see review Walton, 2010). The SPN receives predominantly contralateral excitatory input from the cochlear nucleus (Schofield, 1995) and a strong, tonotopic inhibitory input from the MNTB (Banks & Smith, 1992). AP firing is suppressed during the sound by MNTB IPSPs and when the sound ceases, the SPN is released from the inhibition and generates rebound APs as an ‘offset response’ (Fig. 3D). This offset response is blocked by glycinergic antagonists (Kulesza et al. 2007). The MNTB must convey sustained inhibition for the whole duration of the stimulus and have a rapid termination, so as not to suppress the offset firing in the SPN.
(4) The NLL projection
The NLL analyzes complex sounds by temporal segregation of spectrally integrated inputs and is suggested to be involved in echo suppression (Pecka et al. 2007). Inhibition has two roles. First, the excitatory response to a CF stimulus is suppressed by simultaneous delivery of a low-frequency (LF) inhibitory stimulus, while a change in timing between the two sounds abolishes the suppressive effect (Peterson et al. 2009; Fig. 3E). Second, a powerful onset inhibition in the NLL delays the CF excitatory response (Nayagam et al. 2005) and undermines the precise mutual timing between LF and CF tones. This onset inhibition has broad frequency tuning, so it may arise from convergence of MNTB neurons with a range of CFs. The glycinergic inhibition may also act directly at the excitatory input formed by another calyx synapse which originates from broadly tuned octopus cells in the VCN (Vater & Feng, 1990). In the MNTB, glycine can be excitatory at the CF, while maintaining its classical inhibitory role at spectrally distant frequencies (Kopp-Scheinpflug et al. 2008a); this paradox may involve presynaptic modulation as reported for the calyceal synapse (Turecek & Trussell, 2001). Similar mechanisms underlying glycinergic transmission have been proposed in the NLL (Kutscher & Covey, 2009). Thus, a precisely timed strong inhibition from the MNTB would be required by the NLL.
Understanding the requirement of the target nuclei allows us to frame ideas concerning the properties to which the output of MNTB neurons must conform. Precise timing with low jitter and no aberrant firing is required for ITD discrimination in the MSO. For IID processing in the LSO, the MNTB requires a large firing range to encode the range of sound intensities. Offset encoding is probably the least demanding in terms of precision but requires sustained firing for long time periods. The NLL projection must also have a precisely timed onset, although our understanding of its overall role here is still to be elucidated. For each of these discrete circuits the MNTB is providing IPSPs that must integrate with EPSPs arising from a shorter synaptic chain, and therefore the transmission delay to the MNTB must be brief, and the APs well timed and able to sustain high frequencies with minimal aberrant firing.
Physiology of the MNTB, a key role for K+ channels
To fulfil its role in the circuitry of the auditory brainstem, the MNTB needs to have the following intrinsic properties: fidelity in terms of input vs. output, ability to generate high and sustained firing rates, and AP timing accuracy. A further refinement is rapid synaptic transmission from its presynaptic input to minimize latency jitter. A physical constraint on fast transmission imposed by all neuronal membranes is the exponential (capacitive) rather than instantaneous charging of the membrane. Each MNTB neuron receives a large excitatory synapse called the calyx of Held (Held, 1893; Morest, 1968), which originates from the globular bushy cells of the contralateral cochlear nucleus (Spirou et al. 1990; Kuwabara et al. 1991; Smith et al. 1991). In response to a single presynaptic stimulus, this giant synapse generates an excitatory postsynaptic conductance (EPSG) of between 100 and 300 nS (Johnston et al. 2009), which effectively supercharges the MNTB membrane, rapidly bringing it to firing threshold. Although the exuberant size of the presynaptic input confers minimal time delays between the presynaptic and postsynaptic APs, the initial current magnitude and short term depression during repetitive stimulation generate other more subtle problems for information transmission.
Fidelity: preservation of the timing and pattern of AP trains across the MNTB relay
The large calyceal input generates an EPSC which can be 30-fold larger than that required to trigger one AP and so should trigger multiple APs in response to a single presynaptic AP. This would undermine processing in the LSO, where the inhibition should scale linearly with intensity, and the MSO, where aberrant APs would be mis-timed and poorly synchronized with the phase. However, the MNTB never fires more than a single AP for each presynaptic input. This remarkable one-to-one fidelity is due to low voltage-activated channels formed from Kv1.1 and Kv1.2 subunits (Brew & Forsythe, 1995; Dodson et al. 2002). In the MNTB Kv1 channels begin to activate from around –67 mV and are half-activated by ∼ −40 mV (Fig. 4Ac and d; Brew & Forsythe, 1995). Their crucial role was demonstrated by application of DTx-I, which results in multiple postsynaptic APs being generated in response to a single EPSC (Fig. 4Aa). Kv1 channels also contribute to AP firing threshold and AP amplitude (Dodson et al. 2002; Klug & Trussell, 2006).
Figure 4. K+ channel physiology of the MNTB.
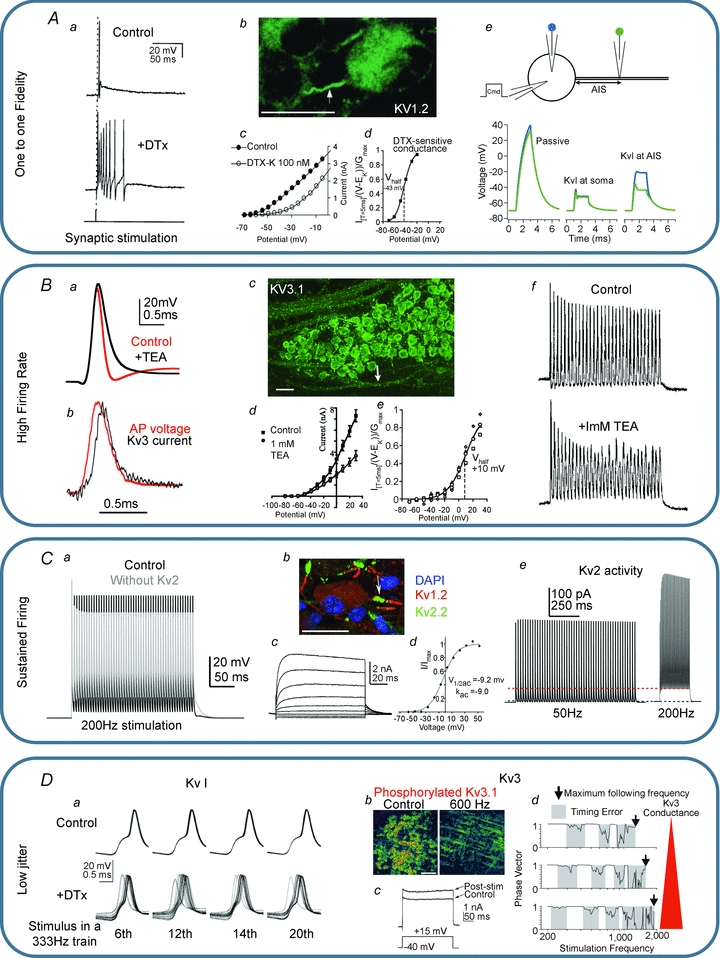
A, Kv1 channels ensure one-to-one fidelity. Aa, application of DTx results in multiple action potentials on the tail of the EPSP generated in response to each presynaptic stimulus (Brew & Forsythe, 1995). Ab, Kv1 immunohistochemistry shows that Kv1 channels are located in the axon initial segment (Dodson et al. 2002); scale bar is 20 μm. Ac and d, DTx blocks a LVA K+ current with a V0.5 of −43 mV (Brew & Forsythe, 1995; Dodson et al. 2002 with permission, SfN.). Ae, a simple NEURON model (Hines & Carnevale, 2001) of the MNTB with a 1.8 nA current injection at the soma. The voltage at the soma and at the end of the AIS is plotted in blue and green, respectively, and shown assuming passive properties (left) Kv1 channels located at the soma (middle) and with Kv1 channels in the AIS (right). Note that with Kv1 channels in the AIS the voltage in the soma differs from the AIS, which explains the apparent lowering of action potential threshold seen with DTx application (Dodson et al. 2002). B, Kv3 channels ensure high firing rates. Ba, blocking Kv3 channels with TEA slows action potential repolarization. Bb, Kv3 current activates with the action potential peaking at the start of repolarization (reproduced from Klug & Trussell, 2006 with permission from the American Physiological Society). Bc, Kv3 immunohistochemistry shows these channels in the MNTB cell body and in the axon (arrow indicates nodes of Ranvier); scale bar is 40 μm. Bd and e, 1 mm TEA blocks a high voltage-activated K+ current with a V0.5 of ∼10 mV. Bf, the Kv3 mediated rapid repolarization is essential for high firing rates (Wang et al. 1998). C, Kv2 channels enable sustained firing. Ca, in a single compartment NEURON model, removing Kv2 results in a depolarized inter-spike potential and reduced availability of Nav channels causing shorter APs. Cb, Kv2 channels are located in the AIS along with Kv1 channels; scale bar is 20 μm. Cc and d, Kv2 channels mediate a slow activating high voltage-activated K+ current. Ce, Kv2 current is activated in a frequency-dependent manner and remains active during the inter-spike potential (Johnston et al. 2008a). D, decreased jitter with Kv1 channels and regulation of Kv3 channels. Da, Kv1 channels decrease the membrane time constant and improve the timing of MNTB neurons during repetitive stimulation (reproduced from Gittelman & Tempel, 2006 with permission from the American Phsyiological Society). Db, Kv3 phosphorylation state is regulated by activity; scale bar is 100 μm. Dc, dephosphorylation occurs in loud auditory environments or with high frequency stimulation and increases Kv3 current magnitude. Dd, large Kv3 magnitudes prolong firing but introduce jitter (Song et al. 2005 reproduced with permission, Nature Neuroscience); under quiet conditions Kv3 is phosphorylated and timing errors are minimized.
The powerful control on AP firing exerted by a relatively small Kv1 conductance is made possible by their special subcellular location in the axon initial segment (AIS) of the MNTB axon (Fig. 4Ab) (Dodson et al. 2002; Johnston et al. 2008a; Clark et al. 2009) along with voltage-gated sodium channels (Kuba et al. 2006; Kuba & Ohmori, 2009). A simple NEURON model of the MNTB (shown in Fig. 4Ae) reinforces this, showing that passive properties alone permit spread of large and sustained depolarization into the axon. With somatic Kv1 channels, depolarization is largely shunted; however, when placed in the AIS, the Kv1 shunt is even more effective. Kv1 channels located in the AIS will act as a high-pass filter permitting only large fast rising voltage signals to trigger APs.
Although they are not present in the MNTB (Johnston et al. 2008a), Kv7 channels (M-current) are present in initial segments of some neurons (Brown & Passmore, 2009), where they serve analogous roles in suppressing firing. In mice, ether-à-go-go-related gene (ERG) channels serve a complementary role with Kv1 (Hardman & Forsythe, 2009). Since ERG channels show increased current when the extracellular [K+] is raised, ERG channels may exert most influence during periods of high-frequency firing when extracellular K+ accumulates and EK is depolarized.
High firing rate
Brief APs allow a neuron/axon to sustain higher firing rates. The MNTB has extremely brief APs with half-widths of ∼400 μs due to the rapid kinetics of Kv3 channels (Fig. 4Bd and e) (Brew & Forsythe, 1995; Wang et al. 1998a; Rudy & McBain, 2001). The function of Kv3 channels is conceptually simple: the positive activation voltage of Kv3 channels limits their activation to voltages only achieved during the AP (Fig. 4Bb and e), yet their rapid kinetics ensure they are deactivating during the falling phase of the AP (Fig. 4Bb), but can contribute to a fast after-hyperpolarization. The contribution of Kv3 channels to brief APs is demonstrated by the AP broadening following application of 1–3 mm TEA (Fig. 4Ba) (Brew & Forsythe, 1995; Wang et al. 1998a). Short APs will minimize Nav inactivation and thus assist maintained firing (Fig. 4Bf) while the fast Kv3 deactivation will avoid extending refractory periods. However, all active Kv conductances (Fig. 1B) will contribute to the AP amplitude and time course, including Kv1 (see above) and Kv2. Indeed the large synaptic conductance will influence AP waveform (Johnston et al. 2009) and during the peak of an overshooting AP (while Vm is positive to EEPSC) could assist AP repolarization.
Kv3 channels are located in the somatic membrane and axons, including nodes of Ranvier (Devaux et al. 2003) in the trapezoid body (Fig. 4Bc); this will ensure rapid discharging of the postsynaptic membrane after each EPSP. Kv3 channels are also present at the calyx of Held terminal, but are located on the non-release face of the terminal and postsynaptically between the fingers of the calyx (Elezgarai et al. 2003). This exclusion from the synaptic cleft will minimize K+ efflux into the small volume of the cleft which would otherwise cause local depolarization or depletion of intracellular [K+] from fine structures (Wang et al. 1998b).
Maintained firing
Although the large AMPA receptor (AMPAR)-mediated EPSC decays extremely rapidly, a slow component remains (Barnes-Davies & Forsythe, 1995; Taschenberger & von Gersdorff, 2000; Johnston et al. 2009) and summates with repetitive stimulation (>50 Hz), resulting in APs being triggered during sustained depolarization (Taschenberger & von Gersdorff, 2000). Depolarization during the inter-spike period reduces the pool of available sodium channels and can lead to AP failure (Jung et al. 1997; Johnston et al. 2008a). In the MNTB Kv2.2 causes inter-spike potential to be more negative and consequently sodium channels recover more quickly from inactivation (Fig. 4Ca). Kv2.2 is a delayed rectifier with relatively slow kinetics and a positive activation range (Fig. 4Cc and d), so intuitively it will be less activated during single short APs. However, their slow deactivation rate allows maintained activation during the inter-spike interval and also results in cumulative activation during high-frequency firing. Like Kv1 channels, the Kv2.2 channels are localized at the AIS (Fig. 4Cb, the location of sodium channels) where they provide a hyperpolarizing drive whose gain is determined by firing frequency (Fig. 4Ce). Similar AIS targeting of Kv2.1 and Kv2.2 is observed in hippocampal and cortical neurones (Hwang et al. 1993; Sarmiere et al. 2008).
Accurate timing and low jitter
Preservation of timing information requires precision and minimal jitter (variance) in the response of the postsynaptic cell to the presynaptic input. As already mentioned the large synaptic input ensures a rapid response, but the MNTB also implements other mechanisms to further reduce jitter. Kv1 channels have been shown to lower the time constant of the MNTB, promoting more precise firing (Fig. 4Da); blocking Kv1 channels in vitro resulted in increased jitter with repetitive stimulation (Gittelman & Tempel, 2006; Klug & Trussell, 2006). Increased levels of jitter have also been observed in in vivo recordings from Kv1.1 KO mice (Kopp-Scheinpflug et al. 2003), though this was largely achieved upstream of the MNTB, probably in the presynaptic axons of the calyx.
The large Kv3 conductance facilitates high frequency firing; however, it also introduces timing errors at moderate frequencies (Fig. 4Dd). This could be due to increases in the relative refractory period (Macica et al. 2003; Song et al. 2005), which may introduce a shunt at the cell body, preventing the AIS from charging rapidly (see Fig. 4Ae). The magnitude of the Kv3 current in the MNTB is dynamically regulated by its phosphorylation state (Fig. 4Db and c). Under basal conditions Kv3 is partially inactive, but with high frequency synaptic stimulation (e.g. 600 Hz for 20 s) or moderate sound stimulation, Kv3 becomes dephosphorylated and active (Song et al. 2005), which promotes higher frequency firing.
Discussion
The auditory brainstem is an example of a system in which ion channel function can be addressed at the molecular, cellular and physiological level. Whole-cell patch recordings permit identification of voltage-gated potassium currents from MNTB neurons. A common theme in ion channel regulation of excitability is precise localization in specific neuronal compartments; many Kv channels are located in the axon initial segment (Kv1, Kv2, Kv3) while Kv3, but neither Kv1 nor Kv2, channels were observed in excised somatic membrane patches (Johnston et al. 2008a). A generalized overview of Kv channel localization is provided in Fig. 5. Kv1 channels limit multiple firing following activation of the giant synapse and Kv3 channels repolarize the action potential. Recent evidence shows that Kv2.2 complements Kv3 and is of increasing relevance during inter-spike intervals in stimulus trains. A similar role has been proposed for sodium-activated K+ (IKNa) channels (Yang et al. 2007) although IKNa may not contribute under all experimental conditions (Johnston et al. 2008a). Other potassium conductances mediated by HCN, ERG and Kv4 channels (Johnston et al. 2008b) are present, but with relatively small conductances and further work is required to understand their various specific and complementary roles in MNTB function.
Figure 5. Subcellular localization of voltage-gated K+ channels.
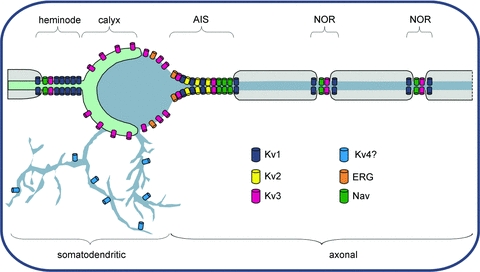
Somatic Kv channels include Kv3 and ERG, but the major part of the conductance arises from channels in the axon initial segment (AIS) which are dominated by Kv1, Kv2 and Kv3 channels (as well as voltage-gated sodium channels, Nav). The location of Kv4 on dendrites is implied from their absence in MNTB somatic membrane and evidence from other cell types. Nodes of Ranvier (NOR) contain Nav and Kv3, with Kv1 channels in the juxtaparanodal region, under the myelin sheath (Wang et al. 1993). The last NOR of the axon known as the heminode is particularly large for the Calyx (Leão et al. 2005) and contains Kv1 and Kv3 channels, with the majority of Kv3 being on the non-release face of the synaptic terminal.
Potassium currents are crucial for MNTB neurons to maintain high firing rates, avoiding aberrant firing, and to permit sustained longer periods of firing. The physiological roles of ion channels in AP firing are often probed by current injection through the recording pipette rather than synaptic stimulation. Step current injection generates only a single initial AP in an MNTB neuron and does not produce physiological AP waveforms (Johnston et al. 2009). The physiological input is a train of EPSPs, each generating a single postsynaptic AP at its peak, but as input frequencies approach refactory period intervals, so latency fluctuations and AP failures become inevitable as the synaptic conductance depresses (Hermann et al. 2007; Hennig et al. 2008). MNTB cells fire at much higher rates for stimulus onset than for later times in the train, as seen in the phasic–tonic (primary-like post-stimulus time histogram) responses in vivo (Smith et al. 1998). In brain slices, MNTB cells can attain ‘instantaneous’ high firing rates up to 800 Hz (Taschenberger & von Gersdorff, 2000), which requires the high safety factor of the calyx for postsynaptic AP generation especially at stimulus onsets (Kopp-Scheinpflug et al. 2008a). However, during maintained (>30 s to minutes) stimuli or background spontaneous activity (Hermann et al. 2007), failures in synaptic transmission start to occur, EPSPs drop below threshold and the MNTB firing rates decline. The calyx of Held–MNTB synapse has often been described as maintaining a ‘one-to-one’ input/output fidelity; this is true at low stimulus frequencies, where Kv1 channels suppress multiple firing to the giant calyceal input (Brew & Forsythe, 1995). But in terms of auditory physiology, the MNTB relay is best considered as a ‘one-to-no-more-than-one’ transmission; i.e. failures in terms of skipped cycles during high firing rates are tolerable (Macica et al. 2003; Kaczmarek et al. 2005; Song et al. 2005) from the computational viewpoint, since the APs which do propagate are still well timed with respect to the sound wave (albeit at a lower overall firing rate). Failures of the EPSP to trigger APs in the target neuron have been described in some studies (Guinan & Li, 1990; Kopp-Scheinpflug et al. 2002; Steinert et al. 2008; Lorteije et al. 2009) although not all (McLaughlin et al. 2008). One issue is that the incoming AP train to the MNTB will already have been conditioned and AP firing limited by transmission through the VCN (Englitz et al. 2009) and the cochlea. However, attainment of high transmission rates must require similar adaptations at all levels of the pathway. Clearly activity-dependent modulation during spontaneous firing (Hermann et al. 2007) and activation of nitrergic signalling induces transmission failures under physiological conditions (Steinert et al. 2008).
Ligand-activated or inhibited K+ conductances are familiar concepts in terms of Kv7 (KCNQ), KIR3 (KATP) and KCa1.1 (BK), but there is increasing evidence for activity-dependent modulation of voltage-gated potassium channels (e.g. Kv2.1, Chen et al. 2006; Park et al. 2006) across many areas of the nervous system. Reduced Kv1 currents (Leao et al. 2004) and Kv3 channel activity (von Hehn et al. 2004) are seen in hearing impaired or deaf animals and during incubation with elevated [K+]o (Liu & Kaczmarek, 1998; Tong et al. 2010). Future work will focus on the signalling and homeostatic mechanisms by which synaptic input modulates Kv channel function and the extent to which changes in Kv current are mediated by ion channel phosphorylation or trafficking. These approaches will provide important insights into the mechanisms influencing ion channel activity during auditory insult, deafness and disease. Recent evidence suggests multiple levels of activity-dependent control in the MNTB. Synaptic stimulation, lasting seconds and involving PKC (Macica et al. 2003; Desai et al. 2008) and the protein phosphatases PP1/PP2A, mediates a short-term facilitation of Kv3 conductances through net dephosphorylation in response to moderate sound levels (Song et al. 2005; Strumbos et al. 2010). Stimulation of the calyceal input over tens of minutes causes activation of nitric oxide signalling and suppression of Kv3 currents (Steinert et al. 2008) and potentiation of other delayed rectifiers by cGMP/PKG-dependent mechanisms. These observations highlight the concept that the excitatory synaptic input can modulate the excitability of the target neuron by direct changes in voltage-gated potassium channels. This supports the notion that the calyx of Held–MNTB synapse is not an inert secure relay, but requires active feedback and feedforward control in order to tune neuronal excitability to recent synaptic activity.
Acknowledgments
Thanks to Martine Hamann and Joern Steinert for comments on the manuscript. We gratefully acknowledge funding from the Medical Research Council, UK.
Glossary
Abbreviations
- AIS
axon initial segment
- AP
action potential
- CF
characteristic frequency
- DTx-I
Dendrotoxin-I
- ERG
ether-a-go-go related gene K+ channel family
- HVA
high-voltage activated
- HCN
hyperpolarization-activated, non-specific cation channels mediating IH.
- IID
interaural intensity difference
- ITD
interaural time difference
- Kv1.1 etc
Voltage-gated potassium channel family 1, member 1
- LSO
lateral superior olive
- LVA
low-voltage activated
- MNTB
medial nucleus of the trapezoid body
- MSO
medial superior olive
- NLL
nuclei of the lateral lemniscus
- SPN
superior paraolivary nucleus
- TEA
tetraethylammonium
- VCN
ventral cochlear nucleus
References
- Aldrich RW. Fifty years of inactivation. Nature. 2001;411:643–644. doi: 10.1038/35079705. [DOI] [PubMed] [Google Scholar]
- Armstrong CM, Binstock L. Anomalous rectification in the squid giant axon injected with tetraethylammonium chloride. J Gen Physiol. 1965;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks MI, Smith PH. Intracellular recordings from neurobiotin-labelled cells in brain slices of the rat medial nucleus of the trapezoid body. J Neurosci. 1992;12:2819–2837. doi: 10.1523/JNEUROSCI.12-07-02819.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes-Davies M, Forsythe ID. Pre- and postsynaptic glutamate receptors at a giant excitatory synapse in rat auditory brainstem slices. J Physiol. 1995;488:387–406. doi: 10.1113/jphysiol.1995.sp020974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew HM, Forsythe ID. Two voltage-dependent K+ conductances with complementary functions in postsynaptic integration at a central auditory synapse. J Neurosci. 1995;15:8011–8022. doi: 10.1523/JNEUROSCI.15-12-08011.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew HM, Gittelman JX, Silverstein RS, Hanks TD, Demas VP, Robinson LC, Robbins CA, McKee-Johnson J, Chiu SY, Messing A, Tempel BL. Seizures and reduced life span in mice lacking the potassium channel subunit Kv1.2, but hypoexcitability and enlarged Kv1 currents in auditory neurons. J Neurophysiol. 2007;98:1501–1525. doi: 10.1152/jn.00640.2006. [DOI] [PubMed] [Google Scholar]
- Brew HM, Hallows JL, Tempel BL. Hyperexcitability and reduced low threshold potassium currents in auditory neurons of mice lacking the channel subunit Kv1.1. J Physiol. 2003;548:1–20. doi: 10.1113/jphysiol.2002.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol. 2009;156:1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, Schwarz TL, Sweatt JD, Johnston D. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 2006;26:12143–12151. doi: 10.1523/JNEUROSCI.2667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BD, Goldberg EM, Rudy B. Electrogenic tuning of the axon initial segment. Neuroscientist. 2009;15:651–668. doi: 10.1177/1073858409341973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay JR. Determining K+ channel activation curves from K+ channel currents often requires the Goldman-Hodgkin-Katz equation. Front Cell Neurosci. 2009;3:20. doi: 10.3389/neuro.03.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Desai R, Kronengold J, Mei J, Forman SA, Kaczmarek LK. Protein kinase C modulates inactivation of Kv3.3 channels. J Biol Chem. 2008;283:22283–22294. doi: 10.1074/jbc.M801663200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux J, Alcaraz G, Grinspan J, Bennett V, Joho R, Crest M, Scherer SS. Kv3.1b is a novel component of CNS nodes. J Neurosci. 2003;23:4509–4518. doi: 10.1523/JNEUROSCI.23-11-04509.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson PD, Barker MC, Forsythe ID. Two heteromeric Kv1 potassium channels differentially regulate action potential firing. J Neurosci. 2002;22:6953–6961. doi: 10.1523/JNEUROSCI.22-16-06953.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson PD, Forsythe ID. Presynaptic K+ channels: electrifying regulators of synaptic terminal excitability. Trends Neurosci. 2004;27:210–217. doi: 10.1016/j.tins.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Elezgarai I, Díez J, Puente N, Azkue JJ, Benítez R, Bilbao A, Knöpfel T, Doñate-Oliver F, Grandes P. Subcellular localization of the voltage-dependent potassium channel Kv3.1b in postnatal and adult rat medial nucleus of the trapezoid body. Neuroscience. 2003;118:889–898. doi: 10.1016/s0306-4522(03)00068-x. [DOI] [PubMed] [Google Scholar]
- Englitz B, Tolnai S, Typlt M, Jost J, Rubsamen R. Reliability of synaptic transmission at the synapses of Held in vivo under acoustic stimulation. PLoS One. 2009;4:e7014. doi: 10.1371/journal.pone.0007014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden DL, Dodson PD, O’Donnell C, White MD, Nolan MF. Tuning of synaptic integration in the medial entorhinal cortex to the organization of grid cell firing fields. Neuron. 2008;60:875–889. doi: 10.1016/j.neuron.2008.10.044. [DOI] [PubMed] [Google Scholar]
- Gittelman JX, Tempel BL. Kv1.1 containing channels are critical for temporal precision during spike initiation. J Neurophysiol. 2006;96:1203–1214. doi: 10.1152/jn.00092.2005. [DOI] [PubMed] [Google Scholar]
- Glendenning KK, Brunso-Bechthold JK, Thompson GC, Masterton RB. Ascending auditory afferents to the nuclei of the lateral lemniscus. J Comp Neurol. 1981;197:673–703. doi: 10.1002/cne.901970409. [DOI] [PubMed] [Google Scholar]
- Grothe B. New roles for synaptic inhibition in sound localization. Nat Rev Neurosci. 2003;4:540–550. doi: 10.1038/nrn1136. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Jr, Li RY. Signal processing in brainstem auditory neurons which receive giant endings (calyces of Held) in the medial nucleus of the trapezoid body of the cat. Hear Res. 1990;49:321–334. doi: 10.1016/0378-5955(90)90111-2. [DOI] [PubMed] [Google Scholar]
- Gutman GA, Chandy KG, Adelman JP, Aiyar J, Bayliss DA, Clapham DE, Covarriubias M, Desir GV, Furuichi K, Ganetzky B, Garcia ML, Grissmer S, Jan LY, Karschin A, Kim D, Kuperschmidt S, Kurachi Y, Lazdunski M, Lesage F, Lester HA, McKinnon D, Nichols CG, O’Kelly I, Robbins J, Robertson GA, Rudy B, Sanguinetti M, Seino S, Stuehmer W, Tamkun MM, Vandenberg CA, Wei A, Wulff H, Wymore RS. International Union of Pharmacology. XLI. Compendium of voltage-gated ion channels: potassium channels. Pharmacol Rev. 2003;55:583–586. doi: 10.1124/pr.55.4.9. [DOI] [PubMed] [Google Scholar]
- Hardman RM, Forsythe ID. Ether-à-go-go-related gene K+ channels contribute to threshold excitability of mouse auditory brainstem neurons. J Physiol. 2009;587:2487–2497. doi: 10.1113/jphysiol.2009.170548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AL, Robertson B. Dendrotoxins: structure-activity relationships and effects on potassium ion channels. Curr Med Chem. 2004;11:3065–3072. doi: 10.2174/0929867043363820. [DOI] [PubMed] [Google Scholar]
- Hassfurth B, Magnusson AK, Grothe B, Koch U. Sensory deprivation regulates the development of the hyperpolarization-activated current in auditory brainstem neurons. Eur J Neurosci. 2009;30:1227–1238. doi: 10.1111/j.1460-9568.2009.06925.x. [DOI] [PubMed] [Google Scholar]
- Held H. Die zentrale Gehörleitung. In: His W, Du Bois-Reymond E, editors. Archiv fur Anatomie und Physiologie. Leipzig: Leipzig Verlag von Veit und Comp; 1893. pp. 201–380. [Google Scholar]
- Hennig MH, Postlethwaite M, Forsythe ID, Graham BP. Interactions between multiple sources of short-term plasticity during evoked and spontaneous activity at the rat calyx of Held. J Physiol. 2008;586:3129–3146. doi: 10.1113/jphysiol.2008.152124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann J, Pecka M, von Gersdorff H, Grothe B, Klug A. Synaptic transmission at the calyx of Held under in vivo like activity levels. J Neurophysiol. 2007;98:807–820. doi: 10.1152/jn.00355.2007. [DOI] [PubMed] [Google Scholar]
- Hines ML, Carnevale NT. NEURON: a tool for neuroscientists. Neuroscientist. 2001;7:123–135. doi: 10.1177/107385840100700207. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang PM, Fotuhi M, Bredt DS, Cunningham AM, Snyder SH. Contrasting immunohistochemical localizations in rat brain of two novel K+ channels of the Shab subfamily. J Neurosci. 1993;13:1569–1576. doi: 10.1523/JNEUROSCI.13-04-01569.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffress LA. A place theory of sound localization. J Comp Psychol. 1948;41:35–39. doi: 10.1037/h0061495. [DOI] [PubMed] [Google Scholar]
- Johnston J, Griffin SJ, Baker C, Forsythe ID. Kv4 (A-type) potassium currents in the mouse medial nucleus of the trapezoid body. Eur J Neurosci. 2008b;27:1391–1399. doi: 10.1111/j.1460-9568.2008.06116.x. [DOI] [PubMed] [Google Scholar]
- Johnston J, Griffin SJ, Baker C, Skrzypiec A, Chernova T, Forsythe ID. Initial segment Kv2.2 channels mediate a slow delayed rectifier and maintain high frequency action potential firing in medial nucleus of the trapezoid body neurons. J Physiol. 2008a;586:3493–3509. doi: 10.1113/jphysiol.2008.153734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J, Postlethwaite M, Forsythe ID. The impact of synaptic conductance on action potential waveform: evoking realistic action potentials with a simulated synaptic conductance. J Neurosci Methods. 2009;183:158–164. doi: 10.1016/j.jneumeth.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Joris P, Yin TC. A matter of time: internal delays in binaural processing. Trends Neurosci. 2007;30:70–78. doi: 10.1016/j.tins.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Jung HY, Mickus T, Spruston N. Prolonged sodium channel inactivation contributes to dendritic action potential attenuation in hippocampal pyramidal neurons. J Neurosci. 1997;17:6639–6646. doi: 10.1523/JNEUROSCI.17-17-06639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek LK, Bhattacharjee A, Desai R, Gan L, Song P, von Hehn CA, Whim MD, Yang B. Regulation of the timing of MNTB neurons by short-term and long-term modulation of potassium channels. Hear Res. 2005;206:133–145. doi: 10.1016/j.heares.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Kadner A, Berrebi AS. Encoding of temporal features of auditory stimuli in the medial nucleus of the trapezoid body and superior paraolivary nucleus of the rat. Neuroscience. 2008;151:868–887. doi: 10.1016/j.neuroscience.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner A, Kulesza RJ, Jr, Berrebi AS. Neurons in the medial nucleus of the trapezoid body and superior paraolivary nucleus of the rat may play a role in sound duration coding. J Neurophysiol. 2006;95:1499–1508. doi: 10.1152/jn.00902.2005. [DOI] [PubMed] [Google Scholar]
- Kandler K, Clause A, Noh J. Tonotopic reorganization of developing auditory brainstem circuits. Nat Neurosci. 2009;12:711–717. doi: 10.1038/nn.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug A, Trussell LO. Activation and deactivation of voltage-dependent K+ channels during synaptically driven action potentials in the MNTB. J Neurophysiol. 2006;96:1547–1555. doi: 10.1152/jn.01381.2005. [DOI] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Lippe WR, Dorrscheidt GJ, Rubsamen R. The medial nucleus of the trapezoid body in the gerbil is more than a relay: comparison of pre- and postsynaptic activity. J Assoc Res Otolaryngol. 2002;4:1–23. doi: 10.1007/s10162-002-2010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Fuchs K, Lippe WR, Tempel BL, Rübsamen R. Decreased temporal precision of auditory signalling in Kcna1-null mice: an electrophysiological study in vivo. J Neurosci. 2003;23:9199–9207. doi: 10.1523/JNEUROSCI.23-27-09199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Dehmel S, Tolani S, Dietz B, Milenkovic I, Rübsamen R. Glycine mediated changes of onset reliability at a mammalian central synapse. Neuroscience. 2008a;157:432–445. doi: 10.1016/j.neuroscience.2008.08.068. [DOI] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Tolnai S, Malmierca MS, Rubsamen R. The medial nucleus of the trapezoid body: Comparative physiology. Neuroscience. 2008b;154:160–170. doi: 10.1016/j.neuroscience.2008.01.088. [DOI] [PubMed] [Google Scholar]
- Kuba H, Ishii TM, Ohmori H. Axonal site of spike initiation enhances auditory coincidence detection. Nature. 2006;444:1069–1072. doi: 10.1038/nature05347. [DOI] [PubMed] [Google Scholar]
- Kuba H, Ohmori H. Roles of axonal sodium channels in precise auditory time coding at nucleus magnocellularis of the chick. J Physiol. 2009;587:87–100. doi: 10.1113/jphysiol.2008.162651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesza RJ, Jr, Kadner A, Berrebi AS. Distinct roles for glycine and GABA in shaping the response properties of neurons in the superior paraolivary nucleus of the rat. J Neurophysiol. 2007;97:1610–1620. doi: 10.1152/jn.00613.2006. [DOI] [PubMed] [Google Scholar]
- Kutscher A, Covey E. Functional role of GABAergic and glycinergic inhibition in the intermediate nucleus of the lateral lemniscus of the big brown bat. J Neurophysiol. 2009;101:3135–3146. doi: 10.1152/jn.00766.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara N, DiCaprio RA, Zook JM. Afferents to the medial nucleus of the trapezoid body and their collateral projections. J Comp Neurol. 1991;314:684–706. doi: 10.1002/cne.903140405. [DOI] [PubMed] [Google Scholar]
- Leao RN, Berntson A, Forsythe ID, Walmsley B. Reduced low-voltage activated K+ conductances and enhanced central excitability in a congenitally deaf (dn/dn) mouse. J Physiol. 2004;559:25–33. doi: 10.1113/jphysiol.2004.067421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leão RM, Kushmerick C, Pinaud R, Renden R, Li GL, Taschenberger H, Spirou G, Levinson SR, von Gersdorff H. Presynaptic Na+ channels: locus, development, and recovery from inactivation at a high-fidelity synapse. J Neurosci. 2005;25:3724–3738. doi: 10.1523/JNEUROSCI.3983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SQ, Kaczmarek LK. Depolarization selectively increases the expression of the Kv3.1 potassium channel in developing inferior colliculus neurons. J Neurosci. 1998;18:8758–8769. doi: 10.1523/JNEUROSCI.18-21-08758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorteije JA, Rusu SI, Kushmerick C, Borst JG. Reliability and precision of the mouse calyx of Held synapse. J Neurosci. 2009;29:13770–13784. doi: 10.1523/JNEUROSCI.3285-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macica CM, von Hehn CA, Wang LY, Ho CS, Yokoyama S, Joho RH, Kaczmarek LK. Modulation of the Kv3.1b potassium channel isoform adjusts the fidelity of the firing pattern of auditory neurons. J Neurosci. 2003;23:1133–1141. doi: 10.1523/JNEUROSCI.23-04-01133.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon R. Potassium channels. FEBS Lett. 2003;555:62–65. doi: 10.1016/s0014-5793(03)01104-9. [DOI] [PubMed] [Google Scholar]
- Maffie J, Rudy B. Weighing the evidence for a ternary protein complex mediating A-type K+ currents in neurons. J Physiol. 2008;586:5609–5623. doi: 10.1113/jphysiol.2008.161620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews PJ, Jercog PE, Rinzel J, Scott LL, Golding NL. Control of submillisecond synaptic timing in binaural coincidence detectors by Kv1 channels. Nat Neurosci. 2010;13:601–609. doi: 10.1038/nn.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin M, Van Der Heijden M, Joris PX. How secure is in vivo synaptic transmission at the calyx of Held? J Neurosci. 2008;28:10206–10219. doi: 10.1523/JNEUROSCI.2735-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlpine D, Jiang D, Palmer AR. A neural code for low-frequency sound localization in mammals. Nat Neurosci. 2001;4:396–401. doi: 10.1038/86049. [DOI] [PubMed] [Google Scholar]
- Morest DK. The growth of synaptic endings in the mammalian brain: a study of the calyces of the trapezoid body. Z Anat Entwicklungsgesch. 1968;127:201–220. doi: 10.1007/BF00526129. [DOI] [PubMed] [Google Scholar]
- Nayagam DA, Clarey JC, Paolini AG. Powerful onset inhibition in the ventral nucleus of the lateral lemniscus. J Neurophysiol. 2005;94:1651–1654. doi: 10.1152/jn.00167.2005. [DOI] [PubMed] [Google Scholar]
- Oertel D. The role of timing in the brain stem auditory nuclei of vertebrates. Annu Rev Physiol. 1999;61:497–519. doi: 10.1146/annurev.physiol.61.1.497. [DOI] [PubMed] [Google Scholar]
- Oertel D, Shatadal S, Cao XJ. In the ventral cochlear nucleus Kv1.1 and subunits of HCN1 are colocalized at surfaces of neurons that have low-voltage-activated and hyperpolarization-activated conductances. Neuroscience. 2008;154:77–86. doi: 10.1016/j.neuroscience.2008.01.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini AG, FitzGerald JV, Burkitt AN, Clark GM. Temporal processing from the auditory nerve to the medial nucleus of the trapezoid body in the rat. Hear Res. 2001;159:101–116. doi: 10.1016/s0378-5955(01)00327-6. [DOI] [PubMed] [Google Scholar]
- Park KS, Mohapatra DP, Misonou H, Trimmer JS. Graded regulation of the Kv2.1 potassium channel by variable phosphorylation. Science. 2006;313:976–979. doi: 10.1126/science.1124254. [DOI] [PubMed] [Google Scholar]
- Park TJ, Monsivais P, Pollak GD. Processing of interaural intensity differences in the LSO: role of interaural threshold differences. J Neurophysiol. 1997;77:2863–2878. doi: 10.1152/jn.1997.77.6.2863. [DOI] [PubMed] [Google Scholar]
- Pecka M, Brand A, Behrend O, Grothe B. Interaural time difference processing in the mammalian medial superior olive: the role of glycinergic inhibition. J Neurosci. 2008;28:6914–6925. doi: 10.1523/JNEUROSCI.1660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecka M, Zahn TP, Saunier-Rebori B, Siveke I, Felmy F, Wiegrebe L, Klug A, Pollak GD, Grothe B. Inhibiting the inhibition: a neuronal network for sound localization in reverberant environments. J Neurosci. 2007;27:1782–1790. doi: 10.1523/JNEUROSCI.5335-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DC, Nataraj K, Wenstrup J. Glycinergic inhibition creates a form of auditory spectral integration in nuclei of the lateral lemniscus. J Neurophysiol. 2009;102:1004–1016. doi: 10.1152/jn.00040.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak GD. Time is traded for intensity in the bat's auditory system. Hear Res. 1988;36:107–124. doi: 10.1016/0378-5955(88)90054-8. [DOI] [PubMed] [Google Scholar]
- Robertson B. The real life of voltage-gated K+ channels: more than model behaviour. Trends Pharmacol Sci. 1997;18:474–483. doi: 10.1016/s0165-6147(97)01140-1. [DOI] [PubMed] [Google Scholar]
- Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24:517–526. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- Sarmiere PD, Weigle CM, Tamkun MM. The Kv2.1 K+ channel targets to the axon initial segment of hippocampal and cortical neurons in culture and in situ. BMC Neurosci. 2008;9:112–127. doi: 10.1186/1471-2202-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Forsythe ID. The calyx of Held. Cell Tissue Res. 2006;326:311–337. doi: 10.1007/s00441-006-0272-7. [DOI] [PubMed] [Google Scholar]
- Schofield BR. Projections from the cochlear nucleus to the superior paraolivary nucleus in guinea pigs. J Comp Neurol. 1995;360:135–149. doi: 10.1002/cne.903600110. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Owens S, Forsythe ID. Characterisation of inhibitory and excitatory postsynaptic currents of the rat medial superior olive. J Physiol. 2000;529:681–698. doi: 10.1111/j.1469-7793.2000.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Joris PX, Carney LH, Yin TC. Projections of physiologically characterized globular bushy cell axons from the cochlear nucleus of the cat. J Comp Neurol. 1991;304:387–407. doi: 10.1002/cne.903040305. [DOI] [PubMed] [Google Scholar]
- Smith PH, Joris PX, Yin TC. Anatomy and physiology of principal cells of the medial nucleus of the trapezoid body (MNTB) of the cat. J Neurophysiol. 1998;79:3127–3142. doi: 10.1152/jn.1998.79.6.3127. [DOI] [PubMed] [Google Scholar]
- Song P, Yang Y, Barnes-Davies M, Bhattacharjee A, Hamann M, Forsythe ID, Oliver DL, Kaczmarek LK. Acoustic environment determines phosphorylation state of the Kv3.1 potassium channel in auditory neurons. Nat Neurosci. 2005;8:1335–1342. doi: 10.1038/nn1533. [DOI] [PubMed] [Google Scholar]
- Spirou GA, Brownell WE, Zidanic M. Recordings from cat trapezoid body and HRP labelling of globular bushy cell axons. J Neurophysiol. 1990;63:1169–1190. doi: 10.1152/jn.1990.63.5.1169. [DOI] [PubMed] [Google Scholar]
- Stanfield PR. Tetraethylammonium ions and the potassium permeability of excitable cells. Rev Physiol Biochem Pharmacol. 1983;97:1–67. doi: 10.1007/BFb0035345. [DOI] [PubMed] [Google Scholar]
- Steinert JR, Kopp-Scheinpflug C, Baker C, Challiss RA, Mistry R, Haustein MD, Griffin SJ, Tong H, Graham BP, Forsythe ID. Nitric oxide is a volume transmitter regulating postsynaptic excitability at a glutamatergic synapse. Neuron. 2008;60:642–656. doi: 10.1016/j.neuron.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Strumbos JG, Polley DB, Kaczmarek LK. Specific and rapid effects of acoustic stimulation on the tonotopic distribution of Kv3.1b potassium channels in the adult rat. Neuroscience. 2010;167:567–572. doi: 10.1016/j.neuroscience.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H, von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity. J Neurosci. 2000;20:9162–9173. doi: 10.1523/JNEUROSCI.20-24-09162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollin DJ. The lateral superior olive: a functional role in sound source localization. Neuroscientist. 2003;9:127–143. doi: 10.1177/1073858403252228. [DOI] [PubMed] [Google Scholar]
- Tollin DJ, Yin TC. Interaural phase and level difference sensitivity in low-frequency neurons in the lateral superior olive. J Neurosci. 2005;25:10648–10657. doi: 10.1523/JNEUROSCI.1609-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Steinert JR, Robinson SW, Chernova T, Read DJ, Oliver DL, Forsythe ID. Regulation of Kv channel expression and neuronal excitability in rat medial nucleus of the trapezoid body maintained in organotypic culture. J Physiol. 2010;588:1451–1468. doi: 10.1113/jphysiol.2009.186676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell LO. Synaptic mechanisms for coding timing in auditory neurons. Annu Rev Physiol. 1999;61:477–496. doi: 10.1146/annurev.physiol.61.1.477. [DOI] [PubMed] [Google Scholar]
- Tsuchitani C. Input from the medial nucleus of trapezoid body to an interaural level detector. Hear Res. 1997;105:211–224. doi: 10.1016/s0378-5955(96)00212-2. [DOI] [PubMed] [Google Scholar]
- Turecek R, Trussell LO. Presynaptic glycine receptors enhance transmitter release at a mammalian central synapse. Nature. 2001;411:587–590. doi: 10.1038/35079084. [DOI] [PubMed] [Google Scholar]
- Vater M, Feng AS. Functional organization of ascending and descending connections of the cochlear nucleus of horseshoe bats. J Comp Neurol. 1990;292:373–395. doi: 10.1002/cne.902920305. [DOI] [PubMed] [Google Scholar]
- von Hehn CA, Bhattacharjee A, Kaczmarek LK. Loss of Kv3.1 tonotopicity and alterations in cAMP response element-binding protein signaling in central auditory neurons of hearing impaired mice. J Neurosci. 2004;24:1936–1940. doi: 10.1523/JNEUROSCI.4554-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993;365:75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- Wang L, Gan L, Forsythe ID, Kaczmarek LK. Contribution of the Kv3.1 potassium channel to high-frequency firing in mouse auditory neurones. J Physiol. 1998a;509:183–194. doi: 10.1111/j.1469-7793.1998.183bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LY, Gan L, Perney TM, Schwartz I, Kaczmarek LK. Activation of Kv3.1 channels in neuronal spine-like structures may induce local potassium ion depletion. Proc Natl Acad Sci U S A. 1998b;95:1882–1887. doi: 10.1073/pnas.95.4.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JP. Timing is everything: Temporal processing deficits in the aged auditory brainstem. Hear Res. 2010;264:63–69. doi: 10.1016/j.heares.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Mitchell SJ. Direct measurement of somatic voltage clamp errors in central neurons. Nat Neurosci. 2008;11:790–798. doi: 10.1038/nn.2137. [DOI] [PubMed] [Google Scholar]
- Yang B, Desai R, Kaczmarek LK. Slack and Slick KNa channels regulate the accuracy of timing of auditory neurons. J Neurosci. 2007;27:2617–2627. doi: 10.1523/JNEUROSCI.5308-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavuzoglu A, Schofield BR, Wenstrup JJ. Substrates of auditory frequency integration in a nucleus of the lateral lemniscus. Neuroscience. 2010 doi: 10.1016/j.neuroscience.2010.04.073. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. The voltage-gated potassium channels and their relatives. Nature. 2002;419:35–42. doi: 10.1038/nature00978. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Carney LH, Colburn HS. A model for interaural time difference sensitivity in the medial superior olive: interaction of excitatory and inhibitory synaptic inputs, channel dynamics, and cellular morphology. J Neurosci. 2005;25:3046–3058. doi: 10.1523/JNEUROSCI.3064-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


