Abstract
Recent studies have shown that antibodies targeting Lewis × (Lex) antigen are a valuable tool in the isolation and identification of glycoproteins in plasma. A focus of this study was to determine whether sialylated Lewis × (sLex) antigen carrying glycoproteins occur in human plasma and whether an antibody targeting this antigen could be used to isolate and identify glycoproteins bearing this antigen. An additional objective was to determine the degree to which proteins conjugated to Lex and sLex antigens are similar in structure. A specific anti-sLex antibody (anti-sLexAb), CHO-131, immobilized in an immunoaffinity column was used to select a set of specific sLex bearing proteins from human plasma, after which they were identified by either of two analytical strategies. One approach was to further resolve the affinity selected proteins by reversed phase chromatography (RPC), tryptic digest the RPC fractions, and identify peptide fragments by MALDI-MS/MS. The second was to tryptic digest the affinity selected protein fraction, further resolve the tryptic fragments by RPC, and identify peptides from RPC fractions by MALDI-MS/MS. Histidine-rich glycoprotein, plasminogen, apolipoprotein A-I, vitronectin, proteoglycan-4, clusterin, Ig gamma-2 chain C region, Ig mu chain C region, and inter-alpha-trypsin inhibitor heavy chain H4 were found to change three folds or more in association with breast cancer. Fifty percent of the glycoproteins carrying either sLex antigen from CHO-131 selection, Lex antigen from selection with TG-1 antibody, or both were found to be changed three folds or more in concentration in breast cancer plasma relative to controls.
Keywords: sialyl Lewis × antigen; CHO-131 antibody; immunoaffinity chromatography; targeted proteomics; sialylation; glycosylation; GlcNAc-β-(1,6)-branching; breast cancer; posttranslational modification; quantification; plasma
Introduction
Aberrations of cellular glycosylation involving a small number of biosynthetic pathways are a common phenotype of cancer.1 The associated structural changes that accompany these aberrations can alter the antigenic and adhesive properties of tumor cells, their potential to invade peripheral tissues, and their capacity to metastasize.2–3 Combinations of Lewis × (Lex), sialyl Lewis × (sLex), sulfosialyl Lewis × (SsLex), and sialyl Lewis a (sLea) antigens are often elevated in the glycoproteins of cancer patients along with increased β-(1,6)-branching, sialylation, and fucosylation of glycans.4–5 These changes are commonly observed during cancer progression in N- and O-linked glycoproteins on the surface of malignant cells. Tumor-associated glycans have also been connected to tumor grade, metastasis, and poor prognosis.6
Subsequent to initiation and proliferation of a tumor at a primary site, metastasis occurs in a series of steps involving i) dissociation of malignant cells from the tumor, ii) their invasion of the basal membrane to which the tumor is attached, iii) migration through tissue into the circulatory or lymphatic system, and iv) transport to remote sites.7 A critical element of metastasis is that malignant cells find a favorable remote site to bind and proliferate. Without this, migrating tumor cells die. Attachment at distant sites is greatly facilitated by the selectin family of lectins.8–10 Expression of sLex and sLea on cell-surface glycoproteins gives malignant cells the ability to adhere to L-selectin on leukocytes, E-selectin and P-selectin on the vascular endothelium, and P-selectin on platelets,11–17 all of which are parts of metastasis. Sialylated Lewis structures such as sLex and sLea are highly expressed in colon, gastric, pancreatic, squamous cell, and breast tumors and melanomas. Elevation of Lewis antigens on tumors correlates with tumorigenesis and poor patient prognosis.18–20 Moreover, sLex is a selectin targeting ligand capable of mediating the binding of tumor cells to endothelia, platelets, and neutrophils.21
Much of what we know about the role of Lewis antigens in cancer is based on glycan recognition with antibodies on cell surfaces.22–23 The number of proteins to which the sLex antigens are appended, their identity, and the role they play in metastasis are either poorly understood or unknown in most cases. If these glycoproteins are shed from tumors, an antibody such as CHO-131, known to target sLex might be useful in the recovery of these sLex bearing proteins from plasma for identification. Whether sLex bearing proteins associated with tumorigenesis are shed into plasma is not clear. The ease with which tumor associated sLex bearing glycoproteins can be detected in plasma will depend on the number of sLex containing glycoproteins that are already in plasma and their relative concentration. These questions are the focus of this paper.
Immunoaffinity chromatography (IAC) based on immobilized CHO-131 monoclonal antibody was used to capture sLex bearing glycoproteins. CHO-131 antibody has been shown to select sLex antigen(s) coupled to the glycan matrix of glycoproteins at points of branching in which the GlcNAc residue of sLex is coupled in a β-(1,6)- branching.24 It is well documented that antigen capture by this antibody requires that sLex on N-linked glycosylation sites of glycoproteins is conjugated to the glycan matrix through a GlcNAc-β-(1,6)-Man branching while at O-linked glycosylation sites the antigen is conjugated to a core 2 β-(1,6)-O-glycan through a GlcNAc-β-(1,6)-GalNAc branching. Excessive fucosylation, sialylation, and GlcNAc-β-(1,6)-branching25 are all properties associated with aberrant glycosylation in cancer and are part of sLex antigen.26 Subsequent to capture by the immunosorbent, proteins were desorbed with an acidic mobile phase, then trypsin digested, and the peptides were further fractionated by reversed phase chromatography (RPC) before identification by tandem mass spectrometry. This particular analytical strategy identifies glycoproteins based on non-glycosylated peptides instead of glycopeptides27 as has been done previously.28 This protocol was chosen because it is possible to capture and identify both O- and N-glycosylated proteins carrying sLex antigen without deglycosylation of peptides. Through derivatization with the stable isotope labeled iTRAQ coding agent, peptides in tryptic digests of affinity captured samples were isotope coded according to sample origin and their relative concentrations compared by isotope ratio measurements during mass spectral analysis. Based on the isotope ratio of peptide isotopomers it was observed that in a small number of cases CHO-131 captured specific glycoproteins from cancer patient blood samples that were more than three fold higher than in controls. Although the number of cancer patient samples examined was too small to allow definitive statistical analyses, sLex bearing glycoproteins were found in plasma, their concentrations were elevated in cancer patients, and those which were elevated in cancer patients had previously been reported to be associated with cancer. In multiple cases the Lex glycotype of sLex bearing glycoproteins has been found to be elevated in breast cancer patients as well.29
Experimental section
Materials and Chemicals
Agarose-conjugated anti-sialyl Lewis × IgM affinity chromatography sorbent was purchased from Santa Cruz Biotech (Santa Cruz, CA). iTRAQ™ and the ABI 4700 Proteomics Analyzer Calibration Mixture (4700 Cal Mix, bradykinin, angiotensin I, glu-fibrinopeptide B, ACTH fragment 1–17, ACTH fragment 18–39, and ACTH fragment 7–38) were purchased from Applied Biosystems (ABI, Foster City, CA). Normal pooled human plasma from 100 subjects was generously supplied by the National Institute of Standards and Technology (NIST, Gaithersburg, MD). Four human breast cancer plasma samples from ductal carcinoma patients were purchased from Asterand, Inc. (Detroit, MI). Another set of cancer plasma samples was supplied from M. D. Anderson Cancer Center (Houston, TX).
Monobasic potassium phosphate, acetic acid, trifluoroacetic acid (TFA), and HPLC grade acetonitrile were purchased from Mallinckrodt Chemicals (Phillipsburg, NJ). Glycine, α-Cyano-4-hydroxy-cinnamic acid (CHCA), proteomics grade N-p-tosyl-phenylalanine chloromethyl ketone (TPCK)-treated trypsin, 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES), iodoacetic acid (IAA), and L-cysteine were obtained from Sigma-Aldrich (St. Louis, MO). Sodium dodecyl sulfate (SDS) was purchased from Fluka Biochemika (Buchs, Switzerland). Dithiothreitol (DTT) and urea were provided by Bio-Rad Laboratories (Hercules, CA). The DI water system was purchased from Millipore (Boston, MA).
IAC of human plasma
An agarose-conjugated anti-sialyl Lewis × IgM (clone name: CHO-131) was self-packed in a 4.6 × 50 mm PEEK column at a flow rate of 0.3 mL/min. Protein concentrations of human plasma from the NIST and ductal carcinoma patients were estimated using the Bradford assay. This information was used to ensure that the total amount of proteins injected into the IAC column was the same with each sample. Human plasma was applied directly to this soft-gel immunosorbent column with loading buffer A (0.15 M phosphate buffered saline, pH 7.4) at a flow rate of 0.3 mL/min. Following extensive washing with loading buffer A, affinity selected proteins were eluted from the IAC column with solution B (0.1M glycine/2 % acetic acid-HCl solution, pH 2.5). Elution curves were obtained with an absorbance detector operating at 280 nm using a ProteomeLab™ PF 2D liquid chromatography from Beckman Coulter, Inc. (Fullerton, CA).
RPC of selected proteins
Chromatographic fractionation of proteins was achieved with the ProteomeLab™ PF 2D. Affinity-selected proteins from the first separation dimension were fractionated in the second dimension using a 4.6 × 33 mm column packed with non-porous silica of 1.5 μm particle diameter with a C18 coating (Beckman Coulter, Inc.). Proteins were eluted from the RPC column with 34 min linear gradient ranging from solvent A (DI water with 0.1% TFA) to 100% solvent B (acetonitrile with 0.09% TFA). Protein elution was monitored at 214 nm.
Trypsin digestion
Proteolysis for protein identification was achieved with a previously described method.28 In brief, vacuum dried affinity selected proteins were dissolved in HEPES buffer, pH 7.4 and incubated overnight with trypsin at 37 °C after denaturation, reduction, and alkylation. The digest was stored at −20 °C until needed.
MALDI-MS/MS based protein identification
Glycoproteins carrying the sLex antigen were identified with a 4800 Proteomics Analyzer mass spectrometer (ABI). Individual peaks were collected from the antibody column or the RPC column and following trypsin digestion, proteins were identified based on the presence of peptide fragments from tandem mass spectra.
Experimental details of the chromatographic and MALDI mass spectral analysis are described in previous publications.29–30 Proteins identified are listed in Tables according to their Swiss-Prot entry names and accession numbers.
Quantitative comparison of protein abundance with iTRAQ
Tryptic digested proteins from either IAC fractions or RPC fractions were labeled with iTRAQ™ reagent to compare the control plasma and cancer patient plasma samples. Trypsin digestion and labeling with iTRAQ™ reagent were performed according to the supplier’s guidelines (ABI). The NIST control sample was labeled with the 114-dalton iTRAQ labeling agent while cancer samples were labeled with the 116-dalton iTRAQ labeling agent. The 115-dalton and 117-dalton iTRAQ reagents were also used in these analyses.
Again the Pepmap C18 trap column and a nano-RPC column were used for desalting and RPC of peptides from affinity-selected proteins as described above in the “MALDI-MS/MS based protein identification” section. Peptides were analyzed on the ABI 4800 Proteomics Analyzer mass spectrometer. Automated acquisition of MS and MS/MS data was controlled by 4000 Series Explorer software. Automated MS/MS data analysis was performed utilizing Protein Pilot software 2.0 with the Pro Group™ algorithm for protein identification and quantification of iTRAQ reporter ions. Only peptides that were completely labeled with iTRAQ reagent at their N-terminus and lysine residues and had a non-zero relative isotope ratio were considered in comparative proteomics measurements.
Results
Analytical strategy
Identification of glycoproteins by affinity selection with lectins or antibodies has been widely reported.31–33 In many cases identification is based on affinity selected glycopeptides from tryptic digests of samples. Following deglycosylation, the affinity captured peptide fragments are identified by tandem mass spectrometry. The ease with which N-linked glycans are removed from glycopeptides by PNGase F accounts for the fact that much more work has been done on N-linked glycoproteins. O-linked glycans in contrast cannot be released from polypeptides by a single enzyme, or even a small set of enzymes. Because the goal of this work was to identify O- and N-linked glycoproteins carrying the sLex antigen, glycoproteins were selected instead of glycopeptides. This allows glycoproteins to be identified following affinity selection and tryptic digest through identification of multiple peptides derived from proteins, not just glycopeptides. It also circumvents the need for deglycosylation and as a consequence, simplifies the identification process along with providing more peptide candidates for identification. The limitation of this approach is that glycosylation sites are not being identified.
CHO-131 is a mouse IgM monoclonal antibody that has been widely reported to target glycoproteins bearing the sLex antigen coupled to glycan matrixes through GlcNAc-β-(1,6)-branching on either mannose with N-linked glycoproteins or N-acetylgalactosamine with O-linked glycoproteins.1, 34 The specificity of CHO-131 was further tested in CHO-131 affinity chromatography experiments with haptoglobin (HAP) in which sLex is coupled in a β-(1,4)-linkage. HAP carries both N- and O-glycans. The O-glycans in HAP are of the core 1 O-glycan type. Core 1 glycans do not have the GlcNAc-β-(1,6)-branching structure reportedly required by CHO 131 for binding. The N-linked glycosylation sites in which sLex is coupled to HAP are through a β-(1,4)-linkage.35–37 As expected CHO-131 did not bind to HAP. [Data not shown.] Removal of fucose or sialic acid from sLex results in loss of CHO-131 binding affinity as well.
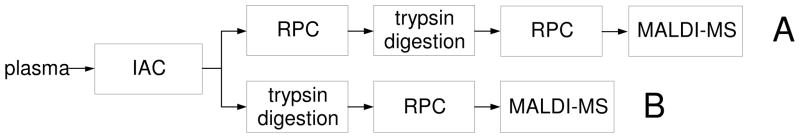
IAC columns in which this antibody was immobilized on agarose were used to select sLex glycotypes of proteins from plasma samples. Glycoproteins thus selected were eluted from the IAC column as a single fraction and identified in two ways as illustrated in Routes A and B of Figure 1. In Route A, glycoproteins selected by CHO-131 IAC were further fractionated by RPC. Following trypsin digestion of fractions collected from RPC peaks, peptides were further resolved by RPC and identified by MALDI-MS/MS. Protein identification in Route B was achieved by digesting the IAC selected fraction with trypsin and RPC of the peptide fragments before identification with MALDI-MS/MS.
Figure 1.
Analytical protocol.
Quantification of differences in glycoprotein concentrations between subjects was achieved by isotope coding of peptides in tryptic digests of protein fractions from the RPC column in Route A and in tryptic digests of protein fractions from the affinity column in Route B. Individual tryptic digests were isotope coded differentially according to sample origin with iTRAQ reagents,38 samples thus coded were then mixed, and the inter-sample isotope ratio of peptides was determined by MALDI-MS/MS. The control in all cases was the NIST pooled human plasma sample.
Immunoaffinity selection
IAC was carried out with a CHO-131 immunoaffinity sorbent packed at a flow rate of 0.3 mL/min. This low flow rate was required to prevent compaction of the agarose matrix. Plasma samples ranging up to 200 μL in volume were loaded on the column directly, without abundant protein removal. Subsequent to sample loading, a 15 column volume wash with the loading buffer A was carried out to elute proteins bound to the column with low affinity. High volume washing of IAC columns has been reported to be efficacious in removing non-specifically bound proteins.30 The extent to which low binding affinity proteins had been eluted was assessed by how nearly detector absorbance returned to the preloading level at 0.0 to 0.02 full scale absorbance.
Affinity selected proteins were eluted from the IAC column with solution B in a single step going from the loading to the eluting mobile phase. Column effluent was monitored by absorbance at 280 nm to minimize base-line perturbations during elution. Based on absorbance of the column effluent there was no significant quantitative difference between the normal control and cancer patient samples at the IAC level. [Data not shown.] Assuming both the non-retained and retained peaks were composed of proteins with equivalent molar extinction coefficients, approximately 0.1% of the total plasma protein was captured by the CHO-131 column.
After antigen desorption the IAC column was recycled with a 20 column volume wash of loading buffer. A single IAC column was used in all the studies reported here. During the course of at least 30 capture-release cycles there was no apparent loss in binding capacity.
RPC of selected proteins
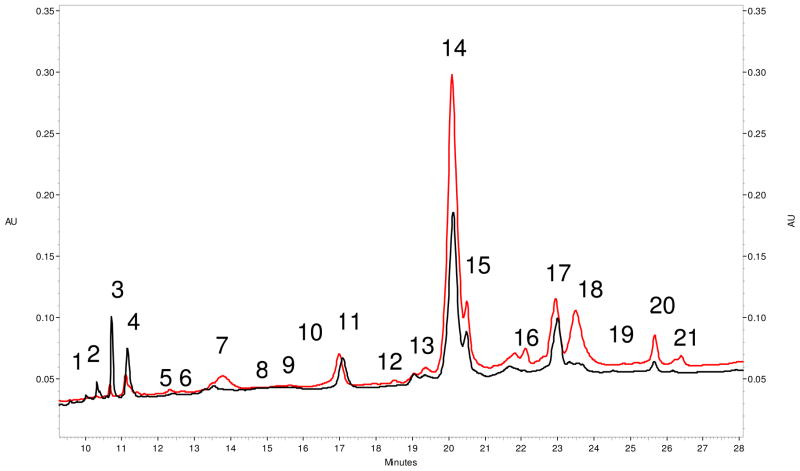
RPC of CHO-131 IAC selected glycoproteins was carried out on a non-porous silica with a C18 coating. Twenty one distinct protein fractions from both the pooled control and cancer patient samples were collected from the RPC column (Figure 2). Considering that plasma contains a complex array of proteins, the RPC chromatograms show that the IAC column provides a substantial degree of sample simplification.
Figure 2.
Reversed phase chromatograms of CHO-131 selected proteins. The red chromatogram is that of IAC selected protein from a plasma sample obtained from a stage-2 ductal carcinoma patient. The black chromatogram is that of IAC selected proteins from the NIST normal pooled plasma sample. Proteins were eluted from the RPC column in a linear 34 min gradient ranging from solvent A (DI water with 0.1% TFA) to 100% solvent B (acetonitrile with 0.09% TFA). Protein elution was monitored at 214 nm.
Quantitative differences between the pooled control and breast cancer patient samples were assessed chromatographically using peak area measurements at 280 nm (Figure 2). Although UV absorbance measurements were not used in the quantification of individual proteins, major differences between the control and a cancer patient are seen in peaks 1, 2, 3, 4, 7, 16, 18, 20, and 21. Peaks 1, 2, and 3 were 2 fold lower in cancer patients while peaks 7, 16, 18, 20, and 21 were elevated more than 2 fold.
Protein identification and quantification by Route A
Proteins fractionated by RPC (Figure 2) and identified according to Route A in Figure 1 are listed in Table 1. A total of 24 proteins were identified. The criterion for positive identification was that two or more peptides from a protein had to be identified at greater than 99% confidence level. Among the glycoproteins identified the concentrations of histidine-rich glycoprotein, plasminogen, apolipoprotein A-I, vitronectin, proteoglycan-4, and clusterin changed three fold or more in breast cancer patients based on iTRAQ quantification.
Table 1.
Identification and quantification of proteins selected from plasma by CHO-131 IAC and further resolved by RPC.
| Peak # | Swiss-Prot Accession # and entry name | Protein name | a peptides with 99 % confidence level | b iTRAQ ratio |
|---|---|---|---|---|
| Peak 1 | P01877|IGHA2_HUMAN | Ig alpha-2 chain C region | c1 | N/A |
| Peak 2 | P01024|CO3_HUMAN | Complement C3 | 3 | 0.1 |
| P02654|APOC1_HUMAN | Apolipoprotein C-I | 2 | 2.7 | |
| Peak 3 | P04264| K2C1_HUMAN | Keratin 1 | 14 | 0.3 |
| P13645|K1C10_HUMAN | Keratin 10 | 7 | 0.3 | |
| P35527|K1C9_HUMAN | Keratin 9 | 2 | 0.3 | |
| Peak 7 | P02776|PLF4_HUMAN | Platelet factor 4 | 6 | 6.1 |
| P02675|FIBB_HUMAN | Fibrinogen beta chain | 3 | 1.1 | |
| Peak 10 & 11 | P08603|CFAH_HUMAN | Complement factor H | 24 | 0.8 |
| Q9BXR6|FHR5_HUMAN | Complement factor H-related protein 5 | 10 | 2 | |
| P04264| K2C1_HUMAN | Keratin 1 | 8 | 1 | |
| P13645|K1C10_HUMAN | Keratin 10 | 5 | 1 | |
| P02735|SAA_HUMAN | Serum amyloid A protein | 2 | 3.4 | |
| Peak 14 & 15 | P04196|HRG_HUMAN | Histidine-rich glycoprotein | 34 | 4.3 |
| P00747|PLMN_HUMAN | Plasminogen | 16 | 4.3 | |
| P02654|APOC1_HUMAN | Apolipoprotein C-I | 9 | 3.3 | |
| P04004|VTNC_HUMAN | Vitronectin | 4 | 4.8 | |
| Q92954|PRG4_HUMAN | Proteoglycan-4 | 2 | 4.3 | |
| Peak 17 & 18 | P02671|FIBA_HUMAN | Fibrinogen alpha chain | 19 | 1.9 |
| P02768|ALBU_HUMAN | Serum albumin | 17 | 0.8 | |
| P02675|FIBB_HUMAN | Fibrinogen beta chain | 16 | 2 | |
| P02654|APOC1_HUMAN | Apolipoprotein C-I | 15 | 0.9 | |
| P02679|FIBG_HUMAN | Fibrinogen gamma chain | 12 | 1.6 | |
| P04264| K2C1_HUMAN | Keratin 1 | 11 | 1.3 | |
| P13645|K1C10_HUMAN | Keratin 10 | 8 | 1.2 | |
| P04196|HRG_HUMAN | Histidine-rich glycoprotein | 6 | 0.7 | |
| P04004|VTNC_HUMAN | Vitronectin | 4 | 0.9 | |
| P10909|CLUS_HUMAN | Clusterin | 3 | 0.9 | |
| P02647|APOA1_HUMAN | Apolipoprotein A-I | 3 | 0.2 | |
| P48668lK2C6C_HUMAN | Cytokeratin 6E | 2 | 0.9 | |
| P02760|AMBP_HUMAN | AMBP protein | 2 | 1.1 | |
| P05090|APOD_HUMAN | Apolipoprotein D | 2 | 1 | |
| P02656|APOC3_HUMAN | Apolipoprotein C-III | 2 | 0.5 | |
| Peak 20 | P10909|CLUS_HUMAN | Clusterin | 12 | 4 |
| P04264| K2C1_HUMAN | Keratin 1 | 3 | 2.2 |
Protein identity is based on the identification of at least two peptide fragments at a confidence level of 99% or greater.
iTRAQ ratio signifies the relative concentration of a protein in cancer patient samples versus a disease free control sample based on the iTRAQ method of quantification.21
The protein was identified through one peptide with 99% confidence level multiple times.
It is significant to note that clusterin, vitronectin, histidine-rich glycoprotein, fibrinogen beta chain, apolipoprotein C-I, keratin-1, and keratin-10 appeared in two or more non-adjacent chromatographic peaks (Table 1). The most likely explanation is that these proteins exist in multiple structural isoforms, probably as a result of posttranslational modification(s). Whether these isoforms exist in-vivo or are generated in-vitro remains to be determined. One of the strengths of the Route A identification protocol (Figure 1) is that isoforms of proteins arising from such mechanisms can often be detected. Shotgun proteomics methods similar to Route B will not detect isoforms.
Although RPC analysis of CHO-131 selected proteins clearly distinguished breast cancer patient samples from the normal pooled control, some proteins may co-migrate and not be distinguishable. The degree to which this is true can be seen by comparing results in Figure 2 and Table 1. Mass spectral analysis detects multiple proteins in most of the peaks seen in Figure 2. Peak 18 is a good example. This peak was always elevated three fold or more in chromatograms from the eight breast cancer patient samples examined. Moreover, this was not the case in ovarian and colon cancer. [Data not shown.] Elevation of this peak is a hallmark of breast cancer patient samples examined with this chromatographic method, yet it is seen to be composed of multiple proteins by MS/MS [Table 1]. It is important to note that with iTRAQ based quantification by MS/MS this peak shows no protein that increased in concentration to the extent seen by chromatographic analysis. This discrepancy is clearly due to the presence of protein(s) seen by UV absorbance detection but not mass spectrometry. Moreover, the UV absorbance detector is seeing a mixture of proteins while the mass spectrometer is quantifying a single protein. Peaks 14 and 15 showed the reverse; iTRAQ based quantification showed more than four-fold elevation of proteins in these peaks with cancer patient samples while a two fold increase was noted by chromatographic peak area analysis. Again, it is likely due to differences in what these two detectors are seeing. One sees mixtures while the other is looking at a single analyte in the mixture. It is to be expected there will be poor correlation between chromatographic quantification by UV absorbance in HPLC and isotope ratio based quantification by MS/MS.
It is important to note that keratin 1, keratin 9, keratin 10, cytokeratin 6E, platelet factor 4, serum amyloid A protein, apolipoprotein C-I, and human serum albumin appearing in Table 1 have not been reported to be glycoproteins. For this reason, they are not included in Tables 2, 3, and 4. Selection of these proteins by the affinity column is most likely due to a capture mechanism other than antigen:antibody association.30 This issue will be discussed more extensively below.
Table 2.
Glycoproteins identified by Route A and Route B.
| N | Swiss-Prot Accession # and entry name | Name | Rt. A | Rt. B | a iTRAQ ratio |
|---|---|---|---|---|---|
| 1 | P00747|PLMN_HUMAN | Plasminogen | + | ||
| 2 | P01024|CO3_HUMAN | Complement C3 | + | + | 0.4 |
| 3 | P01859|IGHG2_HUMAN | Ig gamma-2 chain C region | + | 3.9 | |
| 4 | P01871|MUC_HUMAN | Ig mu chain C region | + | 3.5 | |
| 5 | P01877|IGHA2_HUMAN | Ig alpha-2 chain C region | + | ||
| 6 | P02647|APOA1_HUMAN | Apolipoprotein A-I | + | ||
| 7 | P02656|APOC3_HUMAN | Apolipoprotein C-III | + | ||
| 8 | P02671|FIBA_HUMAN | Fibrinogen alpha chain | + | ||
| 9 | P02675|FIBB_HUMAN | Fibrinogen beta chain | + | ||
| 10 | P02679|FIBG_HUMAN | Fibrinogen gamma chain | + | ||
| 11 | P02760|AMBP_HUMAN | AMBP protein | + | ||
| 12 | P04004|VTNC_HUMAN | Vitronectin | + | + | 3 |
| 13 | P04196|HRG_HUMAN | Histidine-rich glycoprotein | + | + | 3.2 |
| 14 | P05090|APOD_HUMAN | Apolipoprotein D | + | ||
| 15 | P08603|CFAH_HUMAN | Complement factor H | + | ||
| 16 | P10909|CLUS_HUMAN | Clusterin | + | ||
| 17 | Q14624|ITIH4_HUMAN | Inter-alpha-trypsin inhibitor heavy chain H4 | + | 4.9 | |
| 18 | Q92954|PRG4_HUMAN | Proteoglycan-4 | + | + | 3.5 |
| 19 | Q9BXR6|FHR5_HUMAN | Complement factor H-related protein 5 | + |
Quantification of Route B selected glycoproteins only
Table 3.
Glycoproteins identified by Lex and sLex IAC that were altered in concentration 3-fold or more in breast cancer patient plasma.
| N | Swiss-Prot Accession # and entry name | Name | Lex | sLex |
|---|---|---|---|---|
| 1 | P00747|PLMN_HUMAN | Plasminogen | + | (+) |
| 2 | P00748|FA12_HUMAN | Coagulation factor XII | + | |
| 3 | P01024|CO3_HUMAN | Complement C3 | + | |
| 4 | P01042|KNG1_HUMAN | Kininogen-1 | (+) | |
| 5 | P01859|IGHG2_HUMAN | Ig gamma-2 chain C region | + | (+) |
| 6 | P01860|IGHG3_HUMAN | Ig gamma-3 chain C | + | |
| 7 | P01871|MUC_HUMAN | Ig mu chain C region | (+) | (+) |
| 8 | P01877|IGHA2_HUMAN | Ig alpha-2 chain C | + | + |
| 9 | P02647|APOA1_HUMAN | Apolipoprotein A-I | (+) | |
| 10 | P02649|APOE_HUMAN | Apolipoprotein E (Apo-E) | + | |
| 11 | P02656|APOC3_HUMAN | Apolipoprotein C-III | + | |
| 12 | P02671|FIBA_HUMAN | Fibrinogen alpha chain | (+) | + |
| 13 | P02675|FIBB_HUMAN | Fibrinogen beta chain | (+) | + |
| 14 | P02679|FIBG_HUMAN | Fibrinogen gamma chain | + | + |
| 15 | P02747|C1QC_HUMAN | Complement C1q subcomponent subunit C | + | |
| 16 | P02751|FINC_HUMAN | Fibronectin | + | |
| 17 | P02760|AMBP_HUMAN | AMBP protein | + | |
| 18 | P04004|VTNC_HUMAN | Vitronectin | (+) | (+) |
| 19 | P04196|HRG_HUMAN | Histidine-rich glycoprotein | (+) | |
| 20 | P05090|APOD_HUMAN | Apolipoprotein D | + | |
| 21 | P08603|CFAH_HUMAN | Complement factor H | + | |
| 22 | P10720|PF4V_HUMAN | Platelet factor 4 variant | (+) | |
| 23 | P10909|CLUS_HUMAN | Clusterin | (+) | (+) |
| 24 | Q08380|LG3BP_HUMAN | Galectin-3-binding protein | + | |
| 25 | Q14624|ITIH4_HUMAN | Inter-alpha-trypsin inhibitor heavy chain H4 | (+) | |
| 26 | Q92954|PRG4_HUMAN | Proteoglycan-4 | + | (+) |
| 27 | Q9BXR6|FHR5_HUMAN | Complement factor H-related protein 5 | + |
+: identified glycoprotein
(+): glycoprotein which was identified and altered its concentration 3-fold or more on the breast cancer plasma compared to the control
Table 4.
Glycoproteins identified by CHO-131 immunoaffinity selection. These proteins showed 3 fold or more change in cancer compared to the control.
| N | Swiss-Prot Accession # & entry name | Name | Glycosylation | Function |
|---|---|---|---|---|
| 1 | P00747|PLMN_HUMAN | Plasminogen | N-linked, O-linked | tissue remodeling, tumor invasion53 |
| 2 | P02647|APOA1_HUMAN | Apolipoprotein A-I | N-linked | androgen regulated mammary gland homeostasis in breast cancer45 |
| 3 | P04004|VTNC_HUMAN | Vitronectin | N-inked, O-linked | cell adhesion and spreading factor54 |
| 4 | P04196|HRG_HUMAN | Histidine-rich glycoprotein | N-linked O-linked |
modulates antiangiogenic activity55 |
| 5 | P10909|CLUS_HUMAN | Clusterin | N-linked | Tumor cell survival56 |
| 6 | Q92954|PRG4_HUMAN | Proteoglycan-4 | N-linked, O-linked | Tumor cell proliferation57 |
| 7 | P01859|IGHG2_HUMAN | Ig gamma-2 chain C region | N-linked | immune response |
| 8 | P01871|MUC_HUMAN | Ig mu chain C region | N-linked O-linked |
immune response |
| 9 | Q14624|ITIH4_HUMAN | Inter-alpha-trypsin inhibitor heavy chain H4 | N-linked, O-linked | signaling, acute phase reactions58 |
Protein identification and quantification by Route B
The second route to identification of the proteins selected by the CHO-131 IAC column was by conventional shotgun proteomics, i.e. Route B in Figure 1. In this approach proteins selected by the IAC column were tryptic digested, iTRAQ labeled, and after RPC fractionation, peptide fractions were identified by MALDI-MS/MS. It was seen in comparing proteins identified by Route A versus Route B (Table 2), that more proteins were identified by Route A. Again the criterion for positive identification was that two or more peptides from a protein had to be identified at greater than 99% confidence level. A total of 19 glycoproteins were identified using Routes A and B together. Of the total, 12 were identified by Route A exclusively, 3 by Route B alone, and 4 by both routes. The concentration of all glycoproteins identified in the Route B analysis were elevated 3 folds or more in cancer patients except complement C3.
Comparative proteomics of Lex and sLex proteins
Comparative studies were done using Lex data from the literature29 and sLex quantification data from these studies obtained by UV absorbance and LC-MS/MS analyses. Samples from 8 breast cancer patients were used to derive sLex experimental data for RPC comparisons. Quantification of sLex bearing proteins by LC-MS/MS was achieved through iTRAQ isotope labeling using normal and stage 2 samples. Samples were analyzed twice and the values averaged.
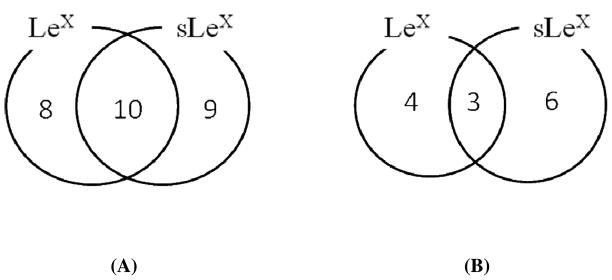
Based on antibody targeting Lewis antigens, Table 3 shows a total of 27 glycoproteins from plasma that carry either Lex or sLex antigens. The Lex data in Table 3 were taken from the previous study showing Lex bearing glycoproteins that were elevated in the plasma of breast cancer patients.29 Lex antigen was conjugated to 18 glycoproteins while 19 of the 27 carried the sLex antigen. Eight bore the Lex antigen but not the sLex antigen while the converse was true with 9 glycoproteins (Figure 3A). Ten of the 27 glycoproteins were found to carry both the Lex and sLex glycoforms. Whether both antigens were present in the same protein molecule was not determined. Of the 10 glycoproteins with both antigens, vitronectin, clusterin, and Ig mu chain C region were cancer associated (Tables 3 and literature29). The concentration of plasminogen, vitronectin, proteoglycan-4, clusterin, histidine-rich glycoprotein, inter-alpha-trypsin inhibitor heavy chain H4, apolipoprotein A-I, Ig gamma-2 chain C region, and Ig mu chain C region glycoforms carrying sLex antigen was elevated 3 folds or more in the breast cancer patients (Table 4). Six of these 9 glycoproteins did not vary similarly in concentration with respect to the Lex antigen (Figure 3B and Table 3). Of the 7 Lex containing glycoproteins showing similar concentration changes in cancer patients,29 5 were also found to carry the sLex antigen and 3 (vitronectin, clusterin, and Ig mu chain C region) of the 5 were elevated in cancer patients (Table 3).
Figure 3.
Number of glycoproteins identified from Lex and/or sLex IAC selection from Table 3. (A) Total number of glycoproteins identified by Lex and/or sLex IAC selection. (B) The number of glycoproteins which altered 3 folds or more in concentrations in the cancer plasma relative to the pooled normal plasma from Lex and/or sLex IAC selection. The Lex data were taken from the previous study showing Lex bearing glycoproteins which were elevated in the plasma of breast cancer patients.29
Discussion
An objective of this work was to determine whether glycoproteins bearing the sLex antigen are shed from cell surfaces into blood. Clearly they are in both control subjects and women diagnosed to have stage 2 breast cancer. A total of 19 sLex bearing glycoproteins were selected by the monoclonal antibody CHO-131 and identified in human blood plasma during the course of this work. Given that the mass spectrometry based methods used are capable of identifying a thousand or more proteins from a single sample and 50% of the proteins in plasma have been estimated to be glycosylated,39 this is a surprisingly small number.
A second objective in this work was to determine whether sLex bearing glycoproteins found in plasma would be associated with tumorigenesis. In this context it is important that 1) the monoclonal antibody CHO-131 has been widely used in histochemical studies to identify a specific sLex antigen on the outer surface of breast cancer cells and 2) among the CHO-131 selected glycoproteins, histidine-rich glycoprotein, plasminogen, apolipoprotein A-I, vitronectin, proteoglycan-4, clusterin, Ig gamma-2 chain C region, Ig mu chain C region, and inter-alpha-trypsin inhibitor heavy chain H4 were all elevated 3 folds or more in cancer patients. Moreover, all of these proteins have been associated with breast cancer in the literature (Table 4). These results make it highly likely these proteins arose from being shed into the circulatory system by the expanding number of tumor cells in the cancer patients. The roles of these proteins in cancer are reported to range from tumor invasion,40 maintaining homeostasis,41 and tumor cell adhesion,42 to anti-angiogenesis,43 tumor survival,44 immune response,45 and cell signaling.46
Although histidine-rich glycoprotein, plasminogen, apolipoprotein A-I, vitronectin, proteoglycan-4, clusterin, Ig gamma-2 chain C region, Ig mu chain C region, and inter-alpha-trypsin inhibitor heavy chain H4 were elevated in breast cancer patients, they were still found in normal subjects. The presence of sLex antigen in these glycoproteins appears not to be totally cancer specific.47 Indeed, sLex antigen plays a signaling role in a small number of other cell types that use the selectin family of lectins in intercellular recognition. Tumor cells may even be mimicking normal cell signaling to facilitate metastasis.
Interesting facts emerged from the analytical component of these studies as well. Between the Route A and B glycoprotein identification methods, fractionation of the CHO-131 selected glycoproteins by RPC before proteolysis and mass spectral analysis (Route A) was the superior method. Four times more proteins were identified in this approach. The fact that some glycoproteins were found in multiple chromatographic peaks in the Route A top-down approach is also important. This suggests that these proteins either exist in isoforms of different primary, tertiary, or quaternary structure or differ in some post-translational modification that impacts their partition coefficient in RPC. Elucidation of the nature of these differences was beyond the scope of these studies and must be undertaken in a future study.
CHO-131 selectivity is another important issue. This antibody captured both N- and O-linked glycoproteins bearing the sLex antigen (Table 4), but not all proteins carrying this antigen. An essential element of CHO-131 selection of glycoproteins appears to be that the requisite sLex antigen is coupled through a GlcNAc-β-(1,6)-branching which is well-known in cancer subjects. CHO-131 has been widely used in recognizing O-linked glycosylation in which GlcNAc of sLex antigen is conjugated through a β-(1,6)-branching on core 2 glycan structures (C2-O-sLex). Some examples of C2-O-sLex bearing glycoproteins identified in these studies are vitronectin, proteoglycan-4, and plasminogen. The β-(1,6)-branching is also required with N-linked glycoproteins. Apolipoprotein A-I, clusterin, and the gamma-2 chain C region of Ig are known N-linked glycoproteins found in these studies through CHO-131 selection. Based on preliminary experiments with HAP it was expected that glycoproteins in which sLex antigen is coupled to the glycan matrix through a β-(1,4)-linkage would not be selected. Indeed, HAP, α-1-acid glycoprotein (AGP), and α-1-antichymotrypsin (ACT), all of which carry sLex antigen attached in a β-(1,4)-linkage48–49 were not selected by CHO-131 IAC. CHO-131 is more fastidious in its stereochemical selectivity than other anti-sLexAbs such as CSLEX1.
It is also important to note in the context of CHO-131 selectivity that keratin 1, keratin 9, keratin 10, cytokeratin 6E, platelet factor 4, serum amyloid A protein, apolipoprotein C-I, and human serum albumin appearing in Table 1 are not glycoproteins. It is probable they were selected by the immunosorbent because they were part of a protein:protein complex,50 of either native or artificial origin. When the glycoprotein component of a protein complex is affinity selected, non-glycosylated members of the complex will be isolated as well. This phenomenon is widely exploited in the tandem affinity purification method when studying protein complexes.30 Some of these proteins could also have been captured by non-specific binding. Both types of binding have been reported in abundant protein removal. Immunosorbent columns that remove albumin and other abundant proteins from plasma have been shown to bind more than a hundred other proteins, some of which are glycosylated.51 It is known for example that platelet factor-4 non-covalently binds to proteoglycans (www.expasy.org) such as proteoglycan-4, both of which were selected by CHO-131. Since proteoglycan-4 concentration was elevated in cancer samples it is expected that platelet factor-4 would appear to be elevated as well because it accompanied platelet factor-4.
A further objective was to determine the extent to which a structural analogue of sLex antigen differing in a single sialic acid residue from Lex would be found on the same glycoproteins. It was determined that the sLex and Lex antigens were conjugated to the same proteins in 10 of the 19 sLex bearing glycoproteins observed in this study. In three cases both were observed to be cancer associated, i.e. their concentrations were elevated more than three fold above that of the controls. Although these two antigens differ by a single monosaccharide residue, they appear to be synthesized and regulated differently. This is because both the Lex and sLex antigens are fucosylated and fucosylation is the final step in their synthesis.49, 52
Conclusions
An objective of the work described here was to determine whether sLex bearing glycoproteins occur in plasma along with those carrying the Lex antigen and if so to determine their identity. Based on the results of these studies it can be concluded that indeed a small number of glycoproteins carrying the sLex antigen are shed from tumors and are present in plasma. Their relationship to breast cancer in general and metastasis in specific is still unknown. What can be concluded is that like glycoproteins with conjugated Lex, this small set of sLex bearing glycoproteins 1) has been non-mechanistically associated with cancer in previous studies,32 2) their concentrations were found to be elevated three folds or more in breast cancer patients, and 3) the same glycoproteins were observed to be conjugated to the Lex antigen in multiple cases. There is potential that Lex and sLex bearing glycoproteins could be biomarkers but it will be necessary to examine a much larger, more diverse patient population to determine whether these glycoproteins are in fact cancer markers.
Acknowledgments
The authors gratefully acknowledge support of this work through US National Institutes of Health grant U24-CA-126480. This grant is part of the larger National Cancer Institute Clinical Proteomic Technology Assessment for Cancer (CPTAC) consortium involving the Broad Institute of MIT and Harvard; Memorial Sloan-Kettering Cancer Center; Purdue University; the University of California, San Francisco; and Vanderbilt University School of Medicine.
The pooled plasma control sample used in this work was kindly provided by the National Institute of Standards and Technology. Mass spectral analyses were carried out in the Purdue Proteomics Facility (PPF) supported by the Purdue Cancer Center and the Bindley Bioscience Center. The authors also wish to acknowledge the technical assistance and operational management at the PPF provided by Dr. Dorota Inerowitz and Dr. Charles Buck, respectively.
References
- 1.St Hill CA, Bullard KM, Walcheck B. Expression of the high-affinity selectin glycan ligand C2-O-sLeX by colon carcinoma cells. Cancer Lett (Amsterdam, Neth) 2005;217(1):105–113. doi: 10.1016/j.canlet.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi H, Boelte KC, Lin PC. Endothelial cell adhesion molecules and cancer progression. Curr Med Chem. 2007;14(4):377–386. doi: 10.2174/092986707779941032. [DOI] [PubMed] [Google Scholar]
- 3.Hakomori S. Glycosylation defining cancer malignancy: New wine in an old bottle. Proc Natl Acad Sci U S A. 2002;99(16):10231–10233. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukuda M. Possible roles of tumor-associated carbohydrate antigens. Cancer Res. 1996;56(10):2237–44. [PubMed] [Google Scholar]
- 5.Lattova E, Tomanek B, Bartusik D, Perreault H. N-Glycomic Changes in Human Breast Carcinoma MCF-7 and T-Lymphoblastoid Cells After Treatment with Herceptin and Herceptin/Lipoplex. J Proteome Res. 2010;9(3):1533–1540. doi: 10.1021/pr9010266. [DOI] [PubMed] [Google Scholar]
- 6.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56(23):5309–18. [PubMed] [Google Scholar]
- 7.Fuster MM, Esko JD. The sweet and sour of cancer: Glycans as novel therapeutic targets. Nat Rev Cancer. 2005;5(7):526–542. doi: 10.1038/nrc1649. [DOI] [PubMed] [Google Scholar]
- 8.Sass PM. The involvement of selectins in cell adhesion, tumor progression, and metastasis. Cancer Invest. 1998;16(5):322–8. doi: 10.3109/07357909809084652. [DOI] [PubMed] [Google Scholar]
- 9.Krause T, Turner GA. Are selectins involved in metastasis? Clin Exp Metastasis. 1999;17(3):183–192. doi: 10.1023/a:1006626500852. [DOI] [PubMed] [Google Scholar]
- 10.Haier J, Nicolson GL. Cell biology and clinical implications of adhesion molecules in colorectal diseases: Colorectal cancers, infections and inflammatory bowel diseases. Clin Exp Metastasis. 2001;18(8):623–638. doi: 10.1023/a:1013114200750. [DOI] [PubMed] [Google Scholar]
- 11.Dejana E, Martin-Padura I, Lauri D, Bernasconi S, Bani MR, Garofalo A, Giavazzi R, Magnani J, Mantovani A, Menard S. Endothelial leukocyte adhesion molecule-1-dependent adhesion of colon carcinoma cells to vascular endothelium is inhibited by an antibody to Lewis fucosylated type I carbohydrate chain. Lab Invest. 1992;66(3):324–30. [PubMed] [Google Scholar]
- 12.Izumi Y, Taniuchi Y, Tsuji T, Smith CW, Nakamori S, Fidler IJ, Irimura T. Characterization of human colon carcinoma variant cells selected for sialyl Lex carbohydrate antigen: liver colonization and adhesion to vascular endothelial cells. Exp Cell Res. 1995;216(1):215–21. doi: 10.1006/excr.1995.1027. [DOI] [PubMed] [Google Scholar]
- 13.Brodt P, Fallavollita L, Bresalier RS, Meterissian S, Norton CR, Wolitzky BA. Liver endothelial E-selectin mediates carcinoma cell adhesion and promotes liver metastasis. Int J Cancer. 1997;71(4):612–619. doi: 10.1002/(sici)1097-0215(19970516)71:4<612::aid-ijc17>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 14.Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci U S A. 1998;95(16):9325–30. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YJ, Borsig L, Han HL, Varki NM, Varki A. Distinct selectin ligands on colon carcinoma mucins can mediate pathological interactions among platelets, leukocytes, and endothelium. Am J Pathol. 1999;155(2):461–72. doi: 10.1016/S0002-9440(10)65142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borsig L, Wong R, Hynes Richard O, Varki Nissi M, Varki A. Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proc Natl Acad Sci U S A. 2002;99(4):2193–8. doi: 10.1073/pnas.261704098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monzavi-Karbassi B, Whitehead TL, Jousheghany F, Artaud C, Hennings L, Shaaf S, Slaughter A, Korourian S, Kelly T, Blaszczyk-Thurin M, Kieber-Emmons T. Deficiency in surface expression of E-selectin ligand promotes lung colonization in a mouse model of breast cancer. Int J Cancer. 2005;117(3):398–408. doi: 10.1002/ijc.21192. [DOI] [PubMed] [Google Scholar]
- 18.Haier J, Nasralla M, Nicolson GL. Cell surface molecules and their prognostic values in assessing colorectal carcinomas. Ann Surg. 2000;231(1):11–24. doi: 10.1097/00000658-200001000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renkonen J, Paavonen T, Renkonen R. Endothelial and epithelial expression of sialyl Lewis(x) and sialyl Lewis(a) in lesions of breast carcinoma. Int J Cancer. 1997;74(3):296–300. doi: 10.1002/(sici)1097-0215(19970620)74:3<296::aid-ijc11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 20.Bresalier RS, Ho SB, Schoeppner HL, Kim YS, Sleisenger MH, Brodt P, Byrd JC. Enhanced sialylation of mucin-associated carbohydrate structures in human colon cancer metastasis. Gastroenterology. 1996;110(5):1354–1367. doi: 10.1053/gast.1996.v110.pm8613039. [DOI] [PubMed] [Google Scholar]
- 21.Mannori G, Crottet P, Cecconi O, Hanasaki K, Aruffo A, Nelson RM, Varki A, Bevilacqua MP. Differential colon cancer cell adhesion to E-, P-, and L-selectin: role of mucin-type glycoproteins. Cancer Res. 1995;55(19):4425–31. [PubMed] [Google Scholar]
- 22.Croce MV, Isla-Larrain M, Tur R, Rabassa ME, Segal-Eiras A. Antigenic differences between metastatic cells in bone marrow and primary tumours and the anti-MUC1 humoral immune response induced in breast cancer patients. Clin Exp Metastasis. 2004;21(2):139–147. doi: 10.1023/b:clin.0000024739.43297.ba. [DOI] [PubMed] [Google Scholar]
- 23.Wei J, Cui L, Liu F, Fan Y, Lang R, Gu F, Guo X, Tang P, Fu L. E-selectin and Sialyl Lewis X expression is associated with lymph node metastasis of invasive micropapillary carcinoma of the breast. Int J Surg Pathol. 2010;18(3):193–200. doi: 10.1177/1066896908320832. [DOI] [PubMed] [Google Scholar]
- 24.Walcheck B, Leppanen A, Cummings RD, Knibbs RN, Stoolman LM, Alexander SR, Mattila PE, McEver RP. The monoclonal antibody CHO-131 binds to a core 2 O-glycan terminated with sialyl-lewis x, which is a functional glycan ligand for P-selectin. Blood. 2002;99(11):4063–4069. doi: 10.1182/blood-2001-12-0265. [DOI] [PubMed] [Google Scholar]
- 25.Ihara S, Miyoshi E, Ko JH, Murata K, Nakahara S, Honke K, Dickson RB, Lin CY, Taniguchi N. Prometastatic effect of N-acetylglucosaminyltransferase V is due to modification and stabilization of active matriptase by adding β1–6 GlcNAc branching. J Biol Chem. 2002;277(19):16960–16967. doi: 10.1074/jbc.M200673200. [DOI] [PubMed] [Google Scholar]
- 26.Drake PM, Cho W, Li B, Prakobphol A, Johansen E, Anderson NL, Regnier FE, Gibson BW, Fisher SJ. Sweetening the pot: adding glycosylation to the biomarker discovery equation. Clin Chem (Washington, DC, U S) 2010;56(2):223–236. doi: 10.1373/clinchem.2009.136333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong L, Andrews D, Regnier F. Comparative proteomics of glycoproteins based on lectin selection and isotope coding. J Proteome Res. 2003;2(6):618–625. doi: 10.1021/pr0340274. [DOI] [PubMed] [Google Scholar]
- 28.Jung K, Cho W, Regnier FE. Glycoproteomics of Plasma Based on Narrow Selectivity Lectin Affinity Chromatography. Journal of Proteome Research. 2009;8(2):643–650. doi: 10.1021/pr8007495. [DOI] [PubMed] [Google Scholar]
- 29.Cho W, Jung K, Regnier FE. Use of Glycan Targeting Antibodies To Identify Cancer-Associated Glycoproteins in Plasma of Breast Cancer Patients. Analytical Chemistry (Washington, DC, United States) 2008;80(14):5286–5292. doi: 10.1021/ac8008675. [DOI] [PubMed] [Google Scholar]
- 30.Cho W, Jung K, Regnier FE. Screening antibody and immunosorbent selectivity by two-dimensional liquid chromatography-MS/MS (2-D LC-MS/MS) J Sep Sci. 2010;33(10):1438–1447. doi: 10.1002/jssc.200900860. [DOI] [PubMed] [Google Scholar]
- 31.Mechref Y, Madera M, Novotny MV. Methods Mol Biol (Totowa, NJ, U S) Vol. 424. 2008. Glycoprotein enrichment through lectin affinity techniques; pp. 373–396. (2D PAGE: Sample Preparation and Fractionation, Volume 1) [DOI] [PubMed] [Google Scholar]
- 32.Gendler SJ, Spicer AP, Lalani EN, Duhig T, Peat N, Burchell J, Pemberton L, Boshell M, Taylor-Papadimitriou J. Structure and biology of a carcinoma-associated mucin, MUC1. Am Rev Respir Dis. 1991;144(3 Pt 2):S42–S47. doi: 10.1164/ajrccm/144.3_pt_2.S42. [DOI] [PubMed] [Google Scholar]
- 33.Raz A, Lotan R. Endogenous galactoside-binding lectins: a new class of functional tumor cell surface molecules related to metastasis. Cancer Metastasis Rev. 1987;6(3):433–52. doi: 10.1007/BF00144274. [DOI] [PubMed] [Google Scholar]
- 34.St Hill CA, Farooqui M, Mitcheltree G, Gulbahce HE, Jessurun J, Cao Q, Walcheck B. The high affinity selectin glycan ligand C2-O-sLex and mRNA transcripts of the core 2 β-1,6-N-acetylglusaminyltransferase (C2GnT1) gene are highly expressed in human colorectal adenocarcinomas. BMC Cancer. 2009;9 doi: 10.1186/1471-2407-9-79. No pp given. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saldova R, Royle L, Radcliffe CM, Abd Hamid UM, Evans R, Arnold JN, Banks RE, Hutson R, Harvey DJ, Antrobus R, Petrescu SM, Dwek RA, Rudd PM. Ovarian Cancer is Associated with Changes in Glycosylation in Both Acute-Phase Proteins and IgG. Glycobiology. 2007;17(12):1344–1356. doi: 10.1093/glycob/cwm100. [DOI] [PubMed] [Google Scholar]
- 36.Wilson NL, Schulz BL, Karlsson NG, Packer NH. Sequential analysis of N- and O-linked glycosylation of 2D-PAGE separated glycoproteins. J Proteome Res. 2002;1(6):521–529. doi: 10.1021/pr025538d. [DOI] [PubMed] [Google Scholar]
- 37.He Z, Aristoteli LP, Kritharides L, Garner B. HPLC analysis of discrete haptoglobin isoform N-linked oligosaccharides following 2D-PAGE isolation. Biochem Biophys Res Commun. 2006;343(2):496–503. doi: 10.1016/j.bbrc.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Garbis SD, Tyritzis SI, Roumeliotis T, Zerefos P, Giannopoulou EG, Vlahou A, Kossida S, Diaz J, Vourekas S, Tamvakopoulos C, Pavlakis K, Sanoudou D, Constantinides CA. Search for Potential Markers for Prostate Cancer Diagnosis, Prognosis and Treatment in Clinical Tissue Specimens Using Amine-Specific Isobaric Tagging (iTRAQ) with Two-Dimensional Liquid Chromatography and Tandem Mass Spectrometry. J Proteome Res. 2008;7(8):3146–3158. doi: 10.1021/pr800060r. [DOI] [PubMed] [Google Scholar]
- 39.Yang Z, Hancock WS. Approach to the comprehensive analysis of glycoproteins isolated from human serum using a multi-lectin affinity column. J Chromatogr, A. 2004;1053(1–2):79–88. [PubMed] [Google Scholar]
- 40.Martin PM, Dussert C, Romain S, Ouafik LH. The plasmin/plasminogen system and cancer. Oncologie. 2010;12(5–6):322–340. [Google Scholar]
- 41.Janik ME, Litynska A, Vereecken P. Cell migration - The role of integrin glycosylation. Biochim Biophys Acta, Gen Subj. 2010;1800(6):545–555. doi: 10.1016/j.bbagen.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 42.Candinas D, Lesnikoski BA, Hancock WW, Otsu I, Koyamada N, Dalmasso AP, Robson SC, Bach FH. Inhibition of platelet integrin GPIIbIIIa prolongs survival of discordant cardiac xenografts. Transplantation. 1996;62(1):1–5. doi: 10.1097/00007890-199607150-00001. [DOI] [PubMed] [Google Scholar]
- 43.Klenotic PA, Huang P, Palomo J, Kaur B, Van Meir EG, Vogelbaum MA, Febbraio M, Gladson CL, Silverstein RL. Histidine-rich glycoprotein modulates the anti-angiogenic effects of vasculostatin. Am J Pathol. 2010;176(4):2039–2050. doi: 10.2353/ajpath.2010.090782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon JAR, Sodek J, Hunter GK, Goldberg HA. Bone sialoprotein stimulates focal adhesion-related signaling pathways: Role in migration and survival of breast and prostate cancer cells. J Cell Biochem. 2009;107(6):1118–1128. doi: 10.1002/jcb.22211. [DOI] [PubMed] [Google Scholar]
- 45.Aboghe DH, Bolduc C, Yoshioka M, St-Amand J. Effects of dihydrotestosterone on gene expression in mammary gland. J Steroid Biochem Mol Biol. 2008;111(3–5):225–231. doi: 10.1016/j.jsbmb.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Jonckheere N, Van Seuningen I. The membrane-bound mucins: From cell signalling to transcriptional regulation and expression in epithelial cancers. Biochimie. 2010;92(1):1–11. doi: 10.1016/j.biochi.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 47.Brinkman-van der Linden ECM, De Haan PF, Havenaar EC, Van Dijk W. Inflammation-induced expression of sialyl Lewisx is not restricted to α1-acid glycoprotein but also occurs to a lesser extent on α1-antichymotrypsin and haptoglobin. Glycoconjugate J. 1998;15(2):177–182. doi: 10.1023/a:1006972307166. [DOI] [PubMed] [Google Scholar]
- 48.Saldova R, Rudd PM, Kas J. Glycosylation changes of serum glycoproteins in ovarian cancer patients may contribute to disease pathogenesis. Chem Listy. 2009;103(5):386–393. [Google Scholar]
- 49.Saldova R, Wormald MR, Dwek RA, Rudd PM. Glycosylation changes on serum glycoproteins in ovarian cancer may contribute to disease pathogenesis. Dis Markers. 2008;25(4,5):219–232. doi: 10.1155/2008/601583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu X, Yang WC, Gao Q, Regnier F. Toward chromatographic analysis of interacting protein networks. J Chromatogr, A. 2008;1178(1–2):24–32. doi: 10.1016/j.chroma.2007.10.067. [DOI] [PubMed] [Google Scholar]
- 51.Gong Y, Li X, Yang B, Ying W, Li D, Zhang Y, Dai S, Cai Y, Wang J, He F, Qian X. Different Immunoaffinity Fractionation Strategies to Characterize the Human Plasma Proteome. J Proteome Res. 2006;5(6):1379–1387. doi: 10.1021/pr0600024. [DOI] [PubMed] [Google Scholar]
- 52.St Hill Catherine A, Farooqui M, Mitcheltree G, Gulbahce HE, Jessurun J, Cao Q, Walcheck B. The high affinity selectin glycan ligand C2-O-sLex and mRNA transcripts of the core 2 beta-1,6-N-acetylglucosaminyltransferase (C2GnT1) gene are highly expressed in human colorectal adenocarcinomas. BMC Cancer. 2009;9:79. doi: 10.1186/1471-2407-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reuning U, Magdolen V, Wilhelm O, Fischer K, Lutz V, Graeff H, Schmitt M. Multifunctional potential of the plasminogen activation system in tumor invasion and metastasis (review) Int J Oncol. 1998;13(5):893–906. doi: 10.3892/ijo.13.5.893. [DOI] [PubMed] [Google Scholar]
- 54.Slivova V, Zaloga G, DeMichele SJ, Mukerji P, Huang YS, Siddiqui R, Harvey K, Valachovicova T, Sliva D. Green tea polyphenols modulate secretion of urokinase plasminogen activator (uPA) and inhibit invasive behavior of breast cancer cells. Nutr Cancer. 2005;52(1):66–73. doi: 10.1207/s15327914nc5201_9. [DOI] [PubMed] [Google Scholar]
- 55.Simantov R, Febbraio M, Crombie R, Asch AS, Nachman RL, Silverstein RL. Histidine-rich glycoprotein inhibits the antiangiogenic effect of thrombospondin-1. J Clin Invest. 2001;107(1):45–52. doi: 10.1172/JCI9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shannan B, Seifert M, Leskov K, Willis J, Boothman D, Tilgen W, Reichrath J. Challenge and promise: roles for clusterin in pathogenesis, progression and therapy of cancer. Cell Death Differ. 2006;13(1):12–19. doi: 10.1038/sj.cdd.4401779. [DOI] [PubMed] [Google Scholar]
- 57.Cattaruzza S, Nicolosi PA, Perris R. Proteoglycans in the Control of Tumor Growth and Metastasis Formation. Connect Tissue Res. 2008;49(3–4):225–229. doi: 10.1080/03008200802143448. [DOI] [PubMed] [Google Scholar]
- 58.Mohamed E, Abdul-Rahman PS, Doustjalali SR, Chen Y, Lim BK, Omar SZ, Bustam AZ, Singh VA, Mohd-Taib NA, Yip CH, Hashim OH. Lectin-based electrophoretic analysis of the expression of the 35 kDa inter-α-trypsin inhibitor heavy chain H4 fragment in sera of patients with five different malignancies. Electrophoresis. 2008;29(12):2645–2650. doi: 10.1002/elps.200700828. [DOI] [PubMed] [Google Scholar]