Abstract
Orthotopic liver transplantation was performed in 60 recipient rats weighing 200 to 250 gm. Sixty rats of the same strain were used as liver donors, 30 weighing 100 to 140 gm (small for size) and the other 30 weighing 200 to 250 gm (same size). After 1, 2, 3, 4, 7 and 14 days (n = 5 each) DNA synthesis, nuclear thymidine labeling and mitoses were increased in both the small-for-size and same-size groups, but significantly more in the former. These changes were maximal after 48 to 72 hr, similar to but later than the well-known regeneration response after partial hepatectomy, which peaks at 24 hr in rats. Indirect indexes of regeneration of the transplanted livers also were measured: plasma or serum ornithine decarboxylase; insulin and glucagon serum levels; estradiol and testosterone serum levels (and their nuclear and cytosolic receptors); and transforming growth factor-β, c-Ha-ras and c-jun mRNA expressions. With the small-for-size transplantation, these followed the same delayed pattern as the direct regeneration parameters. The small livers gradually increased in size over the course of 1 to 2 wk and achieved a volume equal to that of the liver originally present in the recipient. In contrast, no significant liver weight gain occurred in the transplanted livers from same-size donors despite the evidence of regeneration by direct indexes, but not by most of the surrogate parameters, including ornithine decarboxylase.
It has previously been demonstrated, both in human beings (1) and in dogs (2), that a liver from a small donor transplanted into a larger recipient undergoes hypertrophic and hyperplastic changes that result in a progressive increase of liver volume, until the transplanted liver reaches the appropriate size for the recipient. In this study this process of liver growth was better characterized by examination of multiple parameters. These included the direct regeneration indexes of DNA synthesis, labeled nuclei and subsequent mitosis (3–5). Surrogate manifestations of regeneration also were monitored: systemic insulin and glucagon; circulating sex steroid hormones and their nuclear and cytosolic liver receptors (6–12); and the expression of transforming growth factor-β (TGF-β) mRNA (13, 14), c-Ha-ras mRNA (15) and c-jun mRNA (16, 17). These changes after transplantation of small-for-size livers were compared with those occurring after transplantation of size-matched livers.
MATERIALS AND METHODS
Animals
Male Fischer rats (F-344) weighing 180 to 220 gm or 90 to 120 gm were purchased from Zivic Miller Laboratories (Zelienople, PA). All the animals were maintained in a temperature- and light-controlled room (light from 6:30 AM to 6:30 PM) for at least 1 wk before being used, after their body weight had reached the appropriate ranges. They received food and water ad libitum. Our institution complies with all U.S. Department of Agriculture regulations and is fully accredited by the American Association for Accreditation of Laboratory Animal Care.
Surgical Procedures
Orthotopic liver transplantation (OLT) was performed by the procedure of Kamada and Calne (18). The suprahepatic vena cava was anastomosed end-to-end with 8-0 Novafil (American Cyanamid Co., D & G Monofil, Inc., Manati, Puerto Rico) suture. The intrahepatic vena cava and the portal vein were anastomosed by cuffs made with PE-240 and PE-200 polyethylene tubes, respectively.
All surgical procedures were performed between 8:30 AM and 11:30 AM. Because of the 1 to 2 hr required for each transplantation, equal-for-size and small-for-size procedures were always done in pairs at the same time. At the time of animal death, the patency of the portal vein and hepatic vein was routinely checked. At the same time as hormone measurements, blood hematocrit values were checked as a monitor for blood loss.
Study Design
Before transplantation, the body and liver weight of all donors and recipients were determined with a modified automatic Mettler PE-2000 balance (Mettler Instrument Corp., Hightstown, NJ). The recipient rats (body weight = 200 to 250 gm) were divided in two different groups. Thirty rats (control group) underwent transplantation with organs obtained from animals of the same size (200 to 250 gm body wt); the other 30 rats (experimental group) received livers from smaller size donors (100 to 140 gm body wt). At the time of transplantation, the donor livers weighed 5.38 ± 0.90 gm in the small-for-size cohort and 8.99 ± 1.02 gm in the same-size group.
At days 1, 2, 3, 4, 7 and 14 after OLT, five recipients of same-size livers and five animals with small-for-size livers were killed to obtain systemic blood and liver samples for biochemical determinations and liver weight. In addition, five normal rats were killed to collect systemic blood for serum basal hormone levels (insulin, glucagon, testosterone and estrogen), hepatic receptor level for androgen and estrogen and ornithine decarboxylase (ODC) plasma activity. Although some of the hormones are largely taken up on first pass through the liver (8, 19–21), systemic venous sampling was used because it was not feasible to repeatedly take portal venous collections.
Biochemical Determinations
Immunoreactive insulin plasma levels were determined by RIA, with a commercial kit obtained from Serono Diagnostics (Baintree, MA). The detection limit of the assay was 5 μU/ml. The coefficient of variation (CV) of replicate samples assayed in our laboratory with this test was 8.37%.
Immunoreactive glucagon plasma levels were determined with a commercial kit obtained from Serono Diagnostics. This kit was chosen specifically because of its high degree of accuracy, precision and specificity (22). Blood samples for the glucagon assay were collected in chilled tubes containing 500 units of a trypsin inhibitor and 1.2 mg sodium EDTA per milliliter of whole blood collected for assay. The detection limit for the assay was 15 pg/ml. The CV for this kit for replicate samples assayed in our laboratory was 9.6%. A gel filtration technique (23) was used to separately determine the amounts of immunoreactive glucagon having a molecular weight of 3,500 to 7,000 (biological) and 40,000 (glucagon aggregate) Da.
Serum estradiol and testosterone levels were determined with a 125I solid-phase assay. Direct RIA kits were obtained from Immunochem Corp. (Carson, CA). The sensitivity for estradiol and for testosterone was 1.0 pg/ml and 0.2 ng/ml, respectively. The CV for these kits in our laboratory, assaying replicate samples, was 6.0% and 10.9%, respectively. The interassay CV was less than 11% for all the above-mentioned determinations.
Plasma ODC activity, expressed in terms of disintegrations per minute per milliliter of plasma, was determined as previously described (24). To prevent enzyme degradation, blood (3 ml) was collected in chilled test tubes containing 60 μl of the following solution: pyridoxal phosphate 5 mmol/L, dithiotreitol 100 mmol/L, EDTA 0.5 mmol/L and Tween-80 0.2%.
Estrogen-receptor Assay
Cytosolic receptors were assayed by an immunoenzymatic method with a commercial kit (Abbott Laboratories, Chicago, IL) containing a specific monoclonal estrogen-receptor antibody (25). The receptor levels were expressed as picomoles per gram of fresh liver tissue. Nuclear receptors were evaluated as previously described (10, 11). Receptor levels were expressed as femtomoles per milligram of DNA.
Androgen-receptor Assay
Cytosolic and nuclear androgen receptors were evaluated following a standard method (11), and the values obtained were expressed as femtomoles per gram of fresh liver tissue and femtomoles per milligram DNA, respectively.
DNA Synthesis
At 8:30 AM on the day the rats were killed, they all received 50 μCi of [3H]thymidine (50 to 80 Ci/mmol; New England Nuclear, Boston, MA) by intraperitoneal injection. Two hours later the animals were killed, and DNA synthesis was measured by [3H] thymidine incorporation (11). The data were expressed as counts per minute per milligram DNA. The radioactivity content of samples was determined with a liquid scintillation counter (LKB 1219 RackBeta; LKB Instruments, Inc., Gaithersburg, MD) and an automatic gamma counter (LKB 1272; LKB Instruments, Inc.).
Northern-blot Analysis
Total cellular RNA was extracted with RNAzol (Biotech, Houston, TX). Twenty micrograms total RNA was electrophoresed in a 1% agarose gel in 10 mmol/L sodium phosphate buffer with constant recirculation. The fractionated RNA was transferred to a Zitabind nylon membrane (Waterman Lab, Hillsboro, OR) overnight in 20 × standard saline citrate. After transfer, the blot was fixed by UV light (short wave, 254 nm). cDNA probes were labeled with 32P with a random-primed labeling kit (Boehringer Mannheim Co., Indianapolis, IN). Prehybridization was done at 70° C from 30 min to 3 hr with Church buffer (1% BSA, 7% SDS, 0.5 mol/L sodium phosphate and 1 mmol/L EDTA) (14), and then the 32P-labeled probes were added to the Church buffer at 70° C for overnight hybridization. After hybridization, the membrane was washed in buffer A (1% BSA, 5% SDS, 40 mmol/L sodium phosphate and 1 mmol/L EDTA) at 70° C for 20 min twice and then in buffer B (1% SDS, 40 mmol/L sodium phosphate and 1 mmol/L EDTA) and 70° C for 20 min four times. The washed and air-dried membranes were autoradiographed at −70° C. Controls for equal loading of RNA to the various gel lanes were included by use of the internal probe for ribosomal 18S RNA.
cDNA Probes
c-Ha-ras was purchased from Oncogene Science Inc., Uniondale, NY; c-jun cDNA is a 2.0-kb EcoRI and BamHI fragment kindly provided by Dr. Frank Rauscher III, Wistar Institute, Philadelphia, PA; TGF-β cDNA is a 1.3-kb EcoRI fragment, a gift from Genentech Inc., San Francisco, CA; and c-myc cDNA is a 9.0-kb EcoI and HindIII fragment (American Type Culture Collection, Rockville, MD).
Histology and Autoradiography
After the rats were killed, specimens from each liver were taken and fixed in buffered formaldehyde. Paraffin sections were prepared for histological examination with hematoxylin and eosin, and the percentage of hepatocytes in mitosis was counted. Other sections were treated, as previously described, for autoradiographic studies (11).
Other Methods
Protein concentrations were determined by the method of Lowry et al. (26). DNA concentrations of homogenates and nuclear preparations were determined by the Burton method (27). Unweighted linear regression analyses of Scatchard plots were performed on an Olivetti MI-P81 computer. (Olivetti, S.p.A., Bari, Italy).
Statistical Analysis
Statistical evaluation of the data was performed with a two-way ANOVA on “Epistat Software” available on IBM personal computers (Pittsburgh, PA). Only a p value less than 0.05 was considered significant.
RESULTS
Hepatic Growth
When small-for-size livers were transplanted into larger recipients, an increase in liver mass was found, which became significant on day 4 and lasted until the organ reached the appropriate weight for the recipient (Fig. 1). Liver weight did not change significantly in the control (same-size) group over the same time period.
Fig. 1.
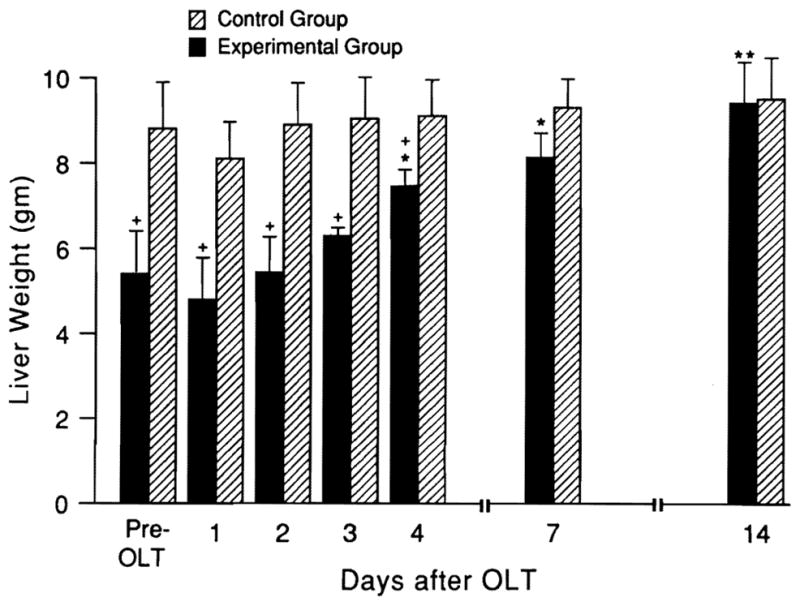
Weight changes (mean ± S.D.) of livers transplanted from same-size (hatched bars) vs. small donors (n = 5 rats at all time points). *p < 0.05 for increased weight of small-size livers at different times after transplantation vs. original weight. +p < 0.01 for weight gain of small-for-size vs. equal-size livers at same time points.
DNA Synthesis, Nuclear Labeling and Mitosis
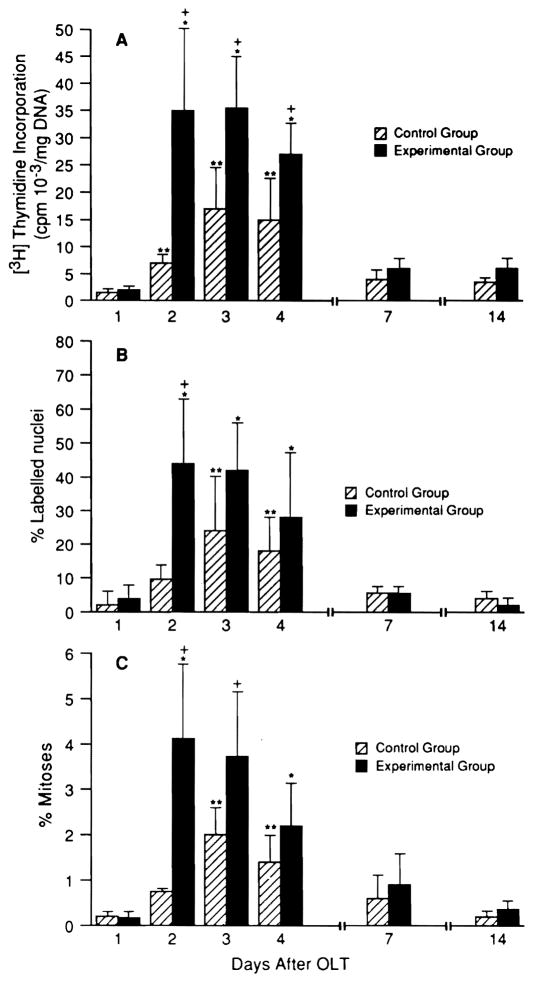
The weight changes in the small livers were preceded by significant rises in thymidine labeling, mitotic index and DNA synthesis that were obvious by day 2 (Fig. 2), with a beginning decline on day 4 when the actual weight increase was first significant. Similar but significantly smaller increases in the indexes of regeneration were also seen in the livers that were transplanted from same-size donors (Fig. 2). However, these were not reflected in increases in the liver weights (see Fig. 1).
Fig. 2.
[3H]thymidine incorporation, percentage of labeled nuclei and percentage of mitoses after OLT (mean ± S.D.). Hatched bars = equal-for-size donor; solid bars = small-for-size donor (n = 5 rats each time point). *p < 0.005 and **p < 0.01 for the increase in measures from postoperative day 2 onward vs. values on postoperative day 1. +p < 0.01 for differences in small vs. same-size livers at the same time points.
ODC
In contrast, ODC increased at 24 and 48 hr only when the small livers were used. The ODC changes were not correlated with contemporaneous plasma transaminase measures, which were increased to the same extent in both groups (data not shown). The evidence of regeneration activity in both the small and same-size livers had returned to baseline by 7 and 14 days (Fig. 3).
Fig. 3.
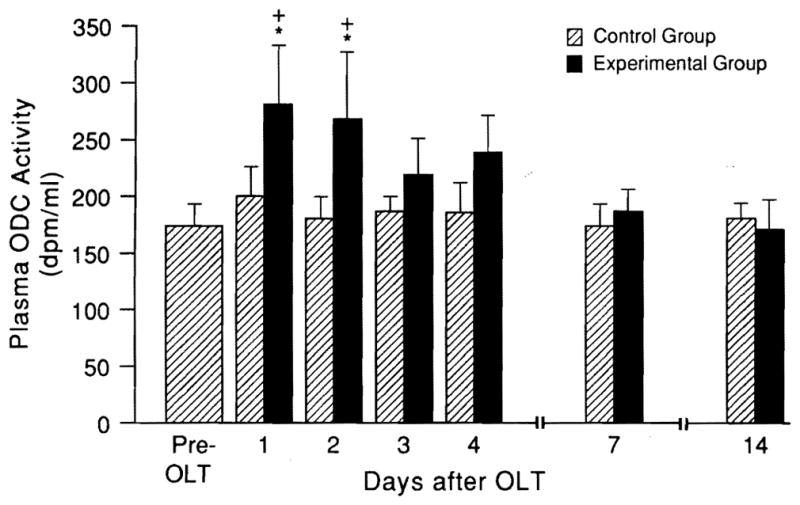
Systemic plasma ODC (mean ± S.D.) after OLT (n = 5 rats at each time point). Note that the ODC was unchanged when same-size livers were transplanted (hatched bars), but increased when small livers were used (*p < 0.01) and was significantly greater vs. the values at the same time in the same-size control group (+p < 0.05).
Hormone and Receptor Determinations
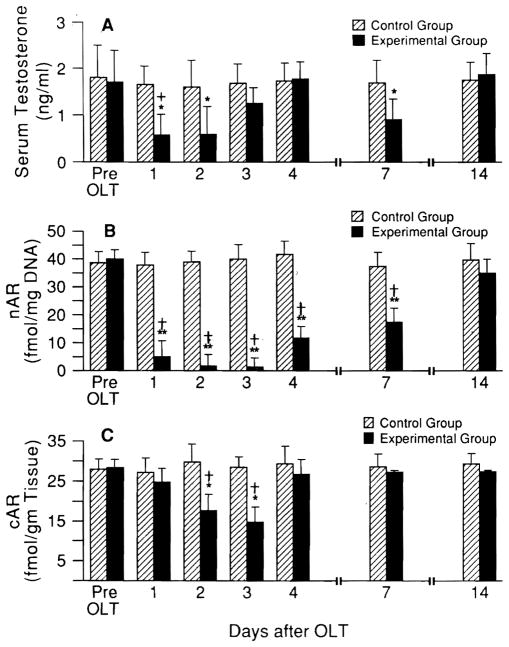
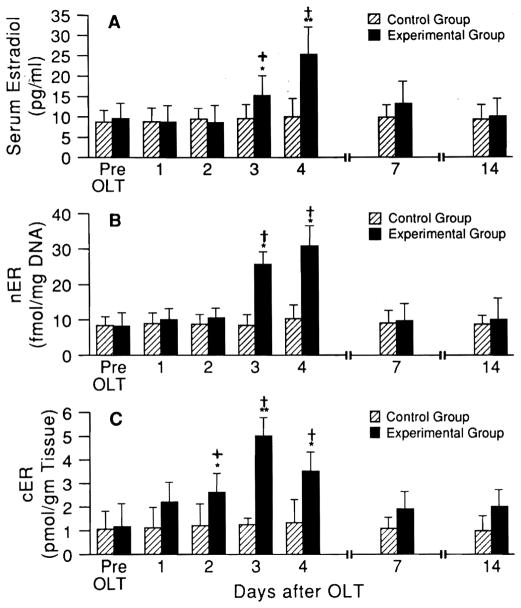
In serum prepared from systemic venous blood, testosterone levels decreased by more than 50% in the recipients of small livers but not in recipients of same-size livers, reaching a nadir at 24 to 48 hr and returning to near baseline levels by 4 days after OLT (Fig. 4A). The androgen-receptor levels of liver tissue in the small-for-size groups but not in the same-size livers also declined sharply with a similar time course (Fig. 4B and C). In contrast, estrogen serum levels (Fig. 5A) were increased, along with the hepatic tissue estrogen-receptor levels (nuclear and cytosolic estrogen receptors, Fig. 5B and C). Similar change of serum androgen and estrogen and their liver receptors has previously been described by us after hepatic regeneration after partial hepatectomy (PH) but with a more rapid onset and more prompt recovery (11).
Fig. 4.
(A) Serum testosterone levels and (B) liver nuclear (nAR) and (C) cytosolic (cAR) androgen-receptor levels after OLT (mean ± S.D.). Hatched bars = equal donor size; solid bars = small-for-size livers (n = 5 rats in each group). Pre-OLT vs. post-OLT values at different time points: *p < 0.01 and **p < 0.001. Small vs. same-size livers at same time points: +p < 0.01 and †p < 0.001.
Fig. 5.
(A) Serum testosterone levels and (B) nuclear (nER) and (C) cytosolic (cER) estrogen-receptor levels in liver after OLT (mean ± S.D.). Hatched bars = equal donor size; solid bars = small-for-size livers. *p < 0.01 and **p < 0.001 for pre-OLT vs. post-OLT value at different time points. +p < 0.01 and †p < 0.001 for small vs. same-size livers at the same time points.
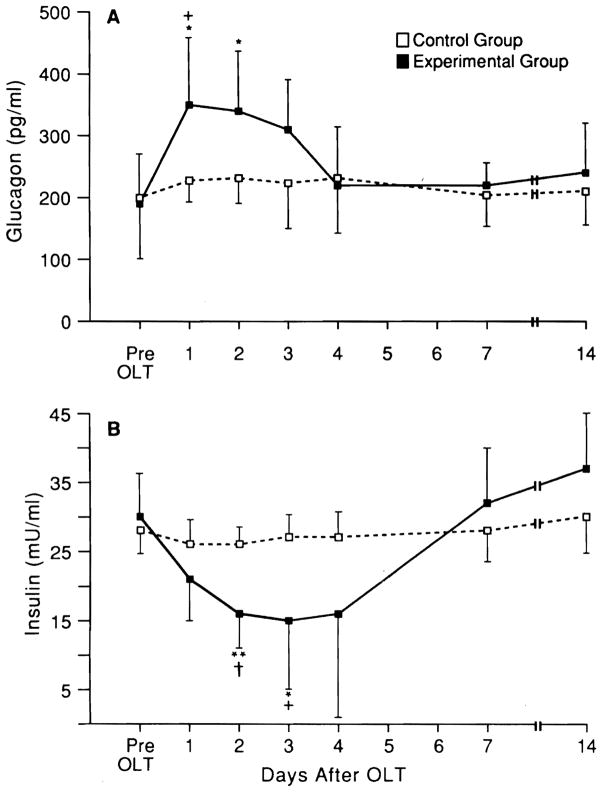
Significant changes were observed for glucagon and insulin levels in rat serum of the experimental group (Fig. 6A and B), but much less dramatically than those previously reported for hepatic regeneration after PH (9, 28). In the recipients of equal-size livers, no blood hormone or hepatic receptor changes were found.
Fig. 6.
Insulin and glycagon blood levels in liver after OLT (mean ± S.D.; n = 5 for each time point). *p < 0.01 and **p < 0.001 for pre-OLT vs. post-OLT values at different time points. +p < 0.01 and †p < 0.001 for small vs. same-size livers at the same time points.
Gene Expression Changes
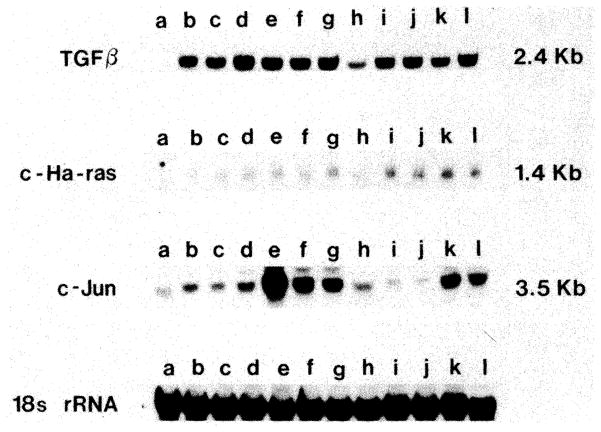
A prolonged increase was seen in TGF-β mRNA in the small transplanted livers, which became evident after 2 days. This was not distinguishably different from changes in the same-size livers. Similarly increased c-jun and c-Ha-ras expression was found in both the small and matched-sized livers, and both elevations of mRNA were later than that of the TGF-β (Fig. 7).
Fig. 7.
Northern blot of TGF-β mRNA, c-Ha-ras mRNA, c-jun mRNA and 18S rRNA (reference) after OLT. Lane a: normal liver; Lanes b through h: 1, 2, 3, 4, 7, 14 and 15 days after small-for-size OLT; lanes i through l: 2, 4, 15 and 30 days after equal-for-size OLT. For each of these experiments, the liver RNA was extracted from five rats and pooled for assay.
DISCUSSION
These experiments suggest that the events and presumably the mechanisms of hepatic mass adjustment are the same after the transplantation of disparate-size livers as those after PH. The principal differences between the PH and transplantation models appear to be quantitative, and a modified time frame. The identifiable hallmarks of classic posthepatectomy regeneration that also were seen after transplantation included an early increase in ODC, followed by increased thymidine incorporation, DNA synthesis and mitoses. The typical hormone changes that follow PH were present as well, including the falls in systemic blood insulin (8, 9) and androgen (11) and elevations of glucagon (8, 9, 29) and estrogen (13). The circulating sex hormone changes were congruent with simultaneous measurements of hepatic nuclear and cytosolic binding sites for androgens and estrogens (9–12, 29, 30). Finally, the molecular studies of the oncogenes c-jun and c-Ha-ras and the growth factor TGF-β showed similar evolving patterns as those described by others after PH (14–17, 31).
However, the pace of all these changes was slower in the transplantation model that has been observed after PH. The DNA synthesis in our transplant experiments did not peak until 2 to 3 days in contrast to the 24 hr that has been thoroughly documented after PH with or without arterialization (32). The same slow development of all other measured regeneration parameters and a more prolonged response were seen. Although the transplanted livers did not have reconstruction of their hepatic artery, this feature of the model did not explain the slow development of regeneration because previous studies have shown that liver regrowth is not affected by dearterialization (32).
Although these studies do not clarify the mechanisms of hepatic regeneration, they appear to identify factors that are not critical for hepatic growth control, including a major role of denervation (33–35), which by definition was complete in the transplantation model. They also weaken the possibility of an intrinsic autoregulatory role of the liver itself. Although the weanling rat liver has a heightened natural proliferation, the small livers in our experiments were from rats that weighed 100 to 140 gm, placing them beyond the weanling stage; their rate of DNA synthesis, thymidine incorporation and mitosis was not different from that of the same-size livers. The factors controlling the regeneration appeared to reside principally if not exclusively in the recipient environment. This conclusion has clinical relevance in view of the widespread clinical use of small liver fragments for pediatric transplantation, which have been reported to regenerate in the same way as whole livers (36–38). In either the experimental or clinical circumstances, both the initiation and cessation of the liver growth process appeared to be governed by the same rules as after PH.
It is tempting to relate these findings to those in the Eck fistula model, which has been used to identify growth-promoting (hepatotrophic) and inhibitory (anti-hepatotrophic) substances such as specific portal venous constituents in blood returning from splanchnic viscera and other growth or antigrowth factors that include some of the major immunosuppressive drugs (cyclosporine, FK 506 and rapamycin) used in clinical transplantation practice or in experimental models (39–41). With either PH or small-to-large liver transplantation, the growth impulse shuts off after the appropriate size is reached, presumably because the stimulus for regeneration is removed. In contrast, the heightened cell renewal that is characteristic after completing diversion of the portacaval shunt (21, 42) reaches a stable state in dogs over a 4-day period and then continues permanently thereafter. In this circumstance, the stimulus is apparently continuous, providing a background of heightened growth factor sensitivity against which biologically active growth (or antigrowth) substances can be more easily identified than in intact animals. Clarification of the link between the proliferation caused by PH, small-to-large liver transplantation and Eck fistula could lead to fruitful inquiries into the basic mechanisms of liver growth control.
After the transplantation of same-size livers, it was of interest that most of the direct and some of the surrogate regeneration parameters were seen, although less prominently than in the small-for-size livers. Notably absent was a rise in ODC. The evidence of regeneration, which presumably reflected ischemic or other injury during the organ transfer or afterward, did not culminate in a demonstrable gain in liver mass for unexplained reasons. This incongruity may have reflected the insensitivity of our method of liver mass measurement, or alternatively either or both a delayed signal and a “late” regeneration factor may have been missing under these conditions, without which the process of mass increase was not allowed to go forward.
Acknowledgments
This study has been supported by research grants from the Veterans Administration; project grants DK 29961 and CA 44602 from the National Institutes of Health, Bethesda, Maryland; and by Consiglio Nazionale delle Ricerche, Applicazioni Cliniche Ricerca Oncologica (ACRO) Program, grant number 92.02187, PF39.
References
- 1.Van Thiel DH, Gavaler JS, Kam I, Francavilla A, Polimeno L, Schade RR, Smith J, et al. Rapid growth of an intact human liver transplanted into a recipient larger than the donor. Gastroenterology. 1987;93:1414–1419. doi: 10.1016/0016-5085(87)90274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kam I, Lynch S, Svanas G, Todo S, Polimeno L, Francavilla A, Penkrot RJ, et al. Evidence that host size determines liver size: studies in dogs receiving orthotopic liver transplants. Hepatology. 1987;7:362–366. doi: 10.1002/hep.1840070225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinbren K. Regeneration of the liver. Gastroenterology. 1959;37:657–668. [PubMed] [Google Scholar]
- 4.Grisham JW. A morphologic study of deoxyribonucleic acid synthesis and cell proliferation in regenerating rat liver; autoradiography with thymidine-3H. Cancer Res. 1962;22:842–849. [PubMed] [Google Scholar]
- 5.Bucher NLR, Swaffield MN. The rate of incorporation of labeled thymidine into deoxyribonucleic acid of regenerating rat liver in relation to the amount of liver excised. Cancer Res. 1964;24:1611–1625. [PubMed] [Google Scholar]
- 6.Leffert HL. Growth control of differentiated fetal rat hepatocytes in primary monolayer culture. VII. Hormonal control of DNA synthesis and its possible significance to the problem of liver regeneration. J Cell Biol. 1974;62:792–801. doi: 10.1083/jcb.62.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bucher NLR, Weir GC. Insulin, glucagon, liver regeneration and DNA synthesis. Metabolism. 1976;25:1423–1425. doi: 10.1016/s0026-0495(76)80156-4. [DOI] [PubMed] [Google Scholar]
- 8.Francavilla A, Porter KA, Benichou J, Jones AF, Starzl TE. Liver regeneration in dogs: morphologic and chemical changes. J Surg Res. 1978;25:409–419. doi: 10.1016/s0022-4804(78)80005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pezzino V, Vigneri R, Cohen D, Goldifine ID. Regenerating rat liver, insulin and glucagon serum levels and receptor binding. Endocrinology. 1981;108:2163–2169. doi: 10.1210/endo-108-6-2163. [DOI] [PubMed] [Google Scholar]
- 10.Francavilla A, Di Leo A, Eagon PK, Wu SQ, Ove P, Van Thiel DH, Starzl TE. Regenerating rat liver: correlation between estrogen receptor localization and deoxyribonucleic acid synthesis. Gastroenterology. 1984;86:562–567. [PMC free article] [PubMed] [Google Scholar]
- 11.Francavilla A, Eagon PK, Di Leo A, Polimeno L. Sex hormone related functions in regenerating male rat liver. Gastroenterology. 1986;91:1262–1270. doi: 10.1016/s0016-5085(86)80026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francavilla A, Panella C, Polimeno L, Giangaspero A, Mazzaferro V, Pan CE, Van Thiel DH, et al. Hormonal and enzymatic parameters of hepatic regeneration in patients undergoing major liver resections. Hepatology. 1990;12:1134–1138. doi: 10.1002/hep.1840120510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun L, Mead JE, Panzica M, Mikumo R, Bell GI, Fausto N. Transforming growth factor β mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc Natl Acad Aci USA. 1988;85:1539–1543. doi: 10.1073/pnas.85.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson NL, Mead JE, Braun L, Goyette M, Shank PR, Fausto N. Sequential portooncogene expression during rat liver regeneration. Cancer Res. 1986;46:3111–3117. [PubMed] [Google Scholar]
- 15.Carr BI, Huang TH, Itakura K, Noel NO, Marceas N. TGF β gene transcription in normal and neoplastic liver growth. J Cell Biochem. 1989;39:477–487. doi: 10.1002/jcb.240390413. [DOI] [PubMed] [Google Scholar]
- 16.Alcorn JA, Feitelberg SP, Brenner DA. Transient induction of c-jun during hepatic regeneration. Hepatology. 1990;11:909–915. doi: 10.1002/hep.1840110602. [DOI] [PubMed] [Google Scholar]
- 17.Morello D, Fitzgerald MJ, Babinet C, Fausto N. C-myc, c-fos and c-jun regulation in the regenerating liver of normal and H-2K/c-myc transgene mice. Mol Cell Biol. 1990;10:3185–3193. doi: 10.1128/mcb.10.6.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamada N, Calne RY. A surgical experience with five hundred thirty liver transplants in the rat. Surgery. 1983;93:64–69. [PubMed] [Google Scholar]
- 19.Starzl TE, Francavilla A, Halgrimson CG, Francavilla FR, Porter KA, Brown TH, Putnam CW. The origin, hormonal nature, and action of hepatotrophic substances in portal venous blood. Surg Gynecol Obstet. 1973;137:179–199. [PMC free article] [PubMed] [Google Scholar]
- 20.Starzl TE, Watanabe K, Porter KA, Putnam CW. Effects of insulin, glucagon, and insulin/glucagon infusions on liver morphology and cell division after complete portacaval shunt in dogs. Lancet. 1976;1:821–825. doi: 10.1016/s0140-6736(76)90477-3. [DOI] [PubMed] [Google Scholar]
- 21.Francavilla A, Starzl TE, Porter K, Scotti-Foglieni C, Michalopoulos GK, Carrieri G, Trejo J, et al. Screening for candidate hepatic growth factors by selective portal infusion after canine Eck’s fistula. Hepatology. 1991;14:665–670. doi: 10.1016/0270-9139(91)90055-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenny AJ, Say RR. Glucagon-like activity extractable from the gastrointestinal tract of man and other animals. J Endocrinol. 1962;25:1–7. doi: 10.1677/joe.0.0250001. [DOI] [PubMed] [Google Scholar]
- 23.Chisholm DJ, Alford FP, Harewood MS, Findlay DM, Gray BN. Nature and biologic activity of “extrapancreatic glucagon” studies in pancreatectomized cats. Metabolism. 1978;27:261–273. doi: 10.1016/0026-0495(78)90106-3. [DOI] [PubMed] [Google Scholar]
- 24.Polimeno L, Azzarone A, Dell’Aquila P, Amoruso C, Barone M, Angelini A, Van Thiel DH, et al. Relationship between plasma and hepatic cytosolic levels of ornithine decarboxylase (ODC) and thymidine kinase (TK) in 70% hepatectomized rats. Dig Dis Sci. 1991;36:289–292. doi: 10.1007/BF01318198. [DOI] [PubMed] [Google Scholar]
- 25.Greene GL. Estrogen and progesterone receptor measurements with monoclonal antibodies. Int J Biol Markers. 1988;3:57–59. doi: 10.1177/172460088800300112. [DOI] [PubMed] [Google Scholar]
- 26.Lowry OH, Resebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;183:265–75. [PubMed] [Google Scholar]
- 27.Burton K. A study of the conditions and mechanisms of the diphenylamme reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956;62:315–320. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francavilla A, DiLeo A, Wu SK, Ove P, Van Thiel D, Sciascia C, Starzl TE. Discordance between glucokinase activity and insulin and glucagon receptor changes occurring during liver regeneration in the rat. Horm Metab Res. 1984;16:47–50. doi: 10.1055/s-2007-1014896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leffert H, Alexander NM, Faloona G, Rubalcava B, Unger R. Specific endocrine and hormonal receptor changes associated with liver regeneration in adult rats. Proc Natl Acad Sci USA. 1975;72:4033–4036. doi: 10.1073/pnas.72.10.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Francavilla A, Polimeno L, Eagon PK, DiLeo A, Panella C, Aquilino AM, Ingrosso M, et al. Androgen and estrogen receptors in regenerating male rat livers. In: Francavilla A, Panella C, DiLeo A, Van Thiel DH, editors. Liver and hormones. New York: Raven Press; 1987. pp. 277–286. [Google Scholar]
- 31.Fausto N, Mead JE. Regulation of liver growth: protooncogenes and transforming growth factors. Lab Invest. 1989;60:4–13. [PubMed] [Google Scholar]
- 32.Zhao D, Zimmermann A, Wheatley AM. Morphometry of the liver after liver transplantation in the rat: significance of an intact arterial supply. Hepatology. 1993;17:310–317. [PubMed] [Google Scholar]
- 33.Leffert HL, Koch KS, Moran T, Rubalcava B. Hormonal control of rat liver regeneration. Gastroenterology. 1979;76:1470–1482. [PubMed] [Google Scholar]
- 34.Cruise JL, Houck KA, Michalopoulos G. Induction of DNA synthesis in cultured rat hepatocytes through stimulation of alpha-1 adrenoreceptor by norepinephrine. Science. 1985;227:749–751. doi: 10.1126/science.2982212. [DOI] [PubMed] [Google Scholar]
- 35.Cruise JL, Knechtle SJ, Bollinger RR, Kuhn C, Michalopoulos GK. Alpha-1-adrenergic effects and liver regeneration. Hepatology. 1987;7:1189–1194. doi: 10.1002/hep.1840070604. [DOI] [PubMed] [Google Scholar]
- 36.Koch KS, Leffert HL. Do transplanted human livers regenerate? Hepatology. 1989;9:789–790. doi: 10.1002/hep.1840090520. [DOI] [PubMed] [Google Scholar]
- 37.Otte JB, De Ville de Goyet J, Alberti D, Balladur P, de Hemptinne B. The concept and technique of the split liver in clinical transplantation. Surgery. 1990;107:605–612. [PubMed] [Google Scholar]
- 38.Broelsch CE, Lloyd DM. Living related donors for liver transplants. Adv Surg. 1993;26:209–231. [PubMed] [Google Scholar]
- 39.Francavilla A, Starzl TE, Carr B, Azzarone A, Carrieri G, Zeng QH, Porter KA. The effects of FK 506, cyclosporine, and rapamycin on liver growth in vitro and in vivo. Transplant Proc. 1991;23:2817–2820. [PMC free article] [PubMed] [Google Scholar]
- 40.Mazzaferro V, Porter KA, Scotti-Foglieni CL, Venkataramanan R, Makowka L, Rossaro L, Francavilla A, et al. The hepatotrophic influence of cyclosporine. Surgery. 1990;107:533–539. [PMC free article] [PubMed] [Google Scholar]
- 41.Starzl TE, Schrieber SL, Albers MW, Porter KA, Foglieni CS, Francavilla A. Hepatotrophic properties in dogs of human FKBP, the binding protein for FK 506 and rapamycin. Transplantation. 1991;52:751–753. doi: 10.1097/00007890-199110000-00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Starzl TE, Watanabe K, Porter KA, Putnam CW. Effects of insulin, glucagon, and insulin/glucagon infusions on liver morphology and cell division after complete portacaval shunt in dogs. Lancet. 1976;1:821–825. doi: 10.1016/s0140-6736(76)90477-3. [DOI] [PubMed] [Google Scholar]