Abstract
The tetraspanin CD151 forms complexes in epithelial cell membranes with laminin-binding integrins α6 β4, α3 β1, and α6 β1, and modifies integrin-mediated cell migration in vitro. We demonstrate in this study that CD151 expression is upregulated in a distinct temporal and spatial pattern during wound healing, particularly in the migrating epidermal tongue at the wound edge, suggesting a role for CD151 in keratinocyte migration. We show that healing is significantly impaired in CD151-null mice, with wounds gaping wider at 7 days post-injury. The rate of re-epithelialization of the CD151-null wounds is adversely affected, with significantly less wound area being covered by migrating epidermal cells. Our studies reveal that although laminin levels are similar in wild-type and CD151-null wounds, the organization of the laminin in the basement membrane is impaired. Furthermore, upregulation of α6 and β4 integrin expression is adversely affected in CD151-null mice wounds. In contrast, we find no significant effect of CD151 gene knockout on α3 and β1 integrin expression in wound repair. We suggest that mice lacking the CD151 gene are defective in wound healing, primarily owing to impairment of the re-epithelialization process. This may be due to defective basement membrane formation and epithelial cell adhesion and migration.
INTRODUCTION
The tetraspanins are a family of membrane proteins characterized by four transmembrane domains, one small and one large extracellular loop, short intracellular N- and C-terminal domains, and highly conserved cysteine-containing motifs. Tetraspanins are expressed throughout phylogeny in multicellular organisms, indicating key roles in cell–cell communication and tissue organization. In mammals, there are approximately 30 different tetraspanins displaying 25–30% amino-acid identity mostly in the transmembrane domains. Most cell types express multiple tetraspanins, which associate with each other and with other membrane proteins giving rise to “tetraspanin-enriched microdomains” and modulating processes such as signal transduction and cell motility (Hemler, 2003; Wright et al., 2004a).
An important group of tetraspanin-interacting proteins are the integrins, which mediate cell–matrix and cell–cell adhesion, cell survival, and migration. Integrins are heterodimeric cell membrane proteins that are widely expressed throughout mammalian tissues. In mammals, 18 different α chains and eight β chains, which can combine to give at least 24 integrin heterodimers with different ligand binding specificities, have been described (Hynes, 2002). Integrins play key roles in epithelial function, as illustrated by the phenotypes of mice with targeted deletion of specific integrin genes. Four integrin chains, α3, α6, β1, and β4, have been shown to be particularly important for epidermal integrity. These chains combine to form heterodimers, α3β1, α6β1, and α6β4, which all bind to isoforms of laminin, a major component of the basement membrane. In particular, α6β4 is a component of hemidesmosomes, which are stable attachments linking the basement membrane to the keratin intermediate filaments (Jones et al., 1998a; Borradori and Sonnenberg, 1999). Consistent with this, mice lacking the α6 or β4 integrin chain display a phenotype similar to the human disorder epidermolysis bullosa, characterized by detachment of the skin from the basement membrane, and die neonatally (Dowling et al., 1996; Georges-Labouesse et al., 1996; van der Neut et al., 1996). Mice with deletion of the α3 integrin gene similarly die shortly after birth due to defects in kidney and lung epithelia and also display skin detachment due to rupture of the basement membrane (Kreidberg et al., 1996; DiPersio et al., 1997). The β1 integrin chain forms heterodimers with many different α chains and its deletion in mice results in early embryonic lethality (Fassler and Meyer, 1995). However, its function in skin has been studied by conditional knockout, and defects in epidermal proliferation, basement membrane formation, and hair follicle invagination have been observed (Brakebusch et al., 2000; Raghavan et al., 2000). Epidermal wound healing in vivo of β1 integrin conditional knockout mice is retarded owing to impaired cell migration (Grose et al., 2002).
The interactions of tetraspanins with β1 integrins and α6β4 integrin have been extensively characterized in vitro (Berditchevski, 2001). Based on sensitivity to detergents of different strengths, the tetraspanin CD151 is believed to be the primary mediator of tetraspanin interactions with α3β1 and α6β1 integrins (Yauch et al., 1998, 2000; Serru et al., 1999). CD151 binds to the stalk region of the α3 integrin chain via a region of the large extracellular loop that is proximal to the fourth transmembrane domain and includes a critical QRD motif (Yauch et al., 2000; Berditchevski et al., 2001; Kazarov et al., 2002). CD151 also associates strongly with the α6β4 complex (Sincock et al., 1999), localizes to the basolateral surface of basal keratinocytes in human skin (Sincock et al., 1997), and is a component of hemidesmosomes (Sterk et al., 2000; S. Fitter, R. Parton, and L. Ashman, unpublished data). In less stringent detergents such as CHAPS, CD151 also co-immunoprecipitates other basement membrane-binding integrins including α4β1 and α5β1 integrins, which bind fibronectin (Hasegawa et al., 1998; Fitter et al., 1999; Sincock et al., 1999). However, these associations have not been observed in some other studies (reviewed by Berditchevski, 2001), possibly due to the complex-selective nature of some CD151 antibodies employed (Geary et al., 2001) and/or cell type-specific differences.
In contrast to mice lacking its core binding integrin partners, CD151-null mice are viable and healthy (Wright et al., 2004b). They express α3 and α6 integrins normally, have morphologically normal hemidesmosomes, and do not display skin detachment. There is no evidence to date of abnormal kidney or lung function as observed in α3 integrin-null mice (Kreidberg et al., 1996). CD151-null mice do, however, display abnormal keratinocyte migration in vitro, a mild bleeding disorder resulting from defective signalling via another CD151 interaction partner, integrin αIIbβ3, as well as T-cell hyperproliferation of unknown mechanism (Lau et al., 2004; Wright et al., 2004b). Interestingly, rare cases of lack of functional CD151 in humans results in restricted (pretibial) epidermolysis bullosa, with biopsy specimens displaying detachment of the epidermis from the dermis (Karamatic Crew et al., 2004). These individuals also suffered renal failure, and electron microscopic examination of tissue from one patient revealed abnormalities of tubular and glomerular basement membranes.
Healing of epidermal wounds involves both proliferation and migration of keratinocytes as well as deposition of a provisional basement membrane rich in laminin 5, a ligand for α3β1 and α6β4 integrins (Goldfinger et al., 1999; Nguyen et al., 2000). The process involves the coordinated action of growth factors produced in response to wounding, integrins, and matrix metalloproteases (Santoro and Gaudino, 2005). We have now examined in vivo wound healing in CD151-null animals and found that it is significantly impaired compared with wild-type syngeneic mice. In wild-type mice, levels of α6 and β4 integrins, as well as CD151 itself, are markedly upregulated in wounds. This integrin upregulation is absent in CD151-null animals. Additionally, organization of laminin 5 appears to be impaired in CD151-null wounds, suggesting a function for CD151 in wound re-epithelialization and basement membrane formation.
RESULTS
CD151 is upregulated during normal wound healing
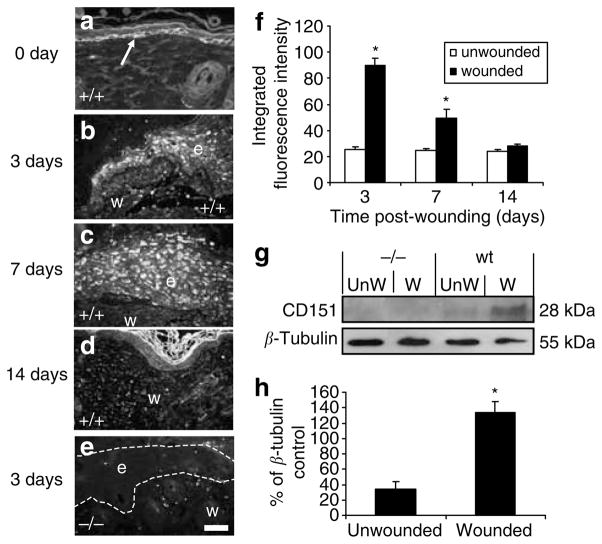
The temporal profile and cellular sources of CD151 expression during normal wound healing in wild-type C57BL/6 mice after full-thickness incisional wounding was assessed. Wounds were excised at 3, 7, and 14 days and revealed a distinct pattern of expression. In normal unwounded mouse skin, CD151 staining was observed predominantly in the basal keratinocytes (Figure 1a). Following wounding, CD151 staining was upregulated and observed throughout the migrating epidermis at the wound edge 3 days post-wounding (Figure 1b and f). CD151 staining persisted, although to a lesser extent, up to 7 days post-wounding (Figure 1c and f) until the wounds were full re-epithelialized but had returned to near basal levels by 14 days post-wounding (Figure 1d and f). Cellular staining of CD151 was also observed within newly forming granulation tissue within the wound in 3- and 7-day wounds (data not shown).
Figure 1. CD151 is upregulated in response to wounding.
CD151 localizes to (a) basal keratinocytes in normal unwounded skin and (b) the tip of the migrating epidermis at 3 days post-wounding. (c) CD151 expression remains elevated throughout the epidermis at 7 days post-wounding (d) but by 14 days has returned to basal expression (n=10 per group). CD151-null wounds were also stained with CD151-specific antibody. (e) A 3-day null wound with no positive CD151 staining observed. In all images, e denotes the position of epidermis and w indicates the position of wound. The arrow in (a) points to positive staining in basal keratinocytes. Bar=50 μm (given in (e), but refers to all images). (f) Epidermal immunofluorescence intensity was quantified at 3, 7, and 14 days post-wounding in normal and wounded wild-type and CD151- null skin. *P<0.05 versus unwounded skin. Protein was extracted from unwounded (day 0) and wounded (day 7) skin from wild-type mice and CD151-null mice. A 10 μg portion was loaded per lane and analyzed by Western blotting using an antibody specific to CD151. (g) Blots were stripped and reprobed with an antibody specific to β-tubulin. (h) Densitometric quantitation of gels showing normalized CD151 protein levels in wild-type 7-day wounds. n=3; *P<0.05 versus unwounded skin.
Consistent with the immunohistochemical pattern of increased wound staining intensity at days 3 and 7 declining at day 14 (Figure 1f), CD151 protein levels, as measured by Western blotting, were significantly upregulated in response to wounding at 7 days (Figure 1g and h). The marked temporal upregulation of CD151 during early wound healing suggests a role for this protein in the re-epithelialization process.
Wound healing is impaired in CD151-null wounds
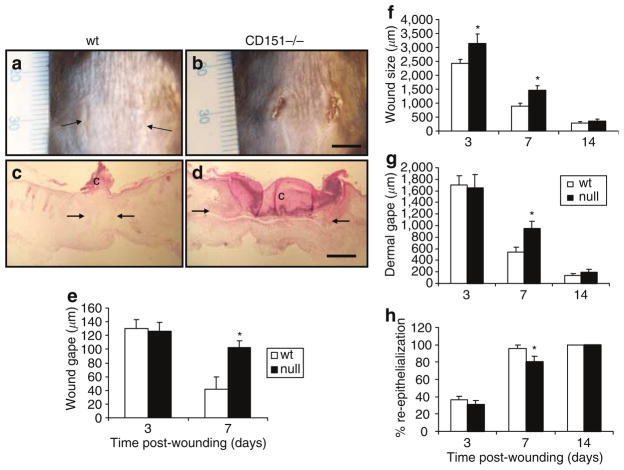
To determine directly the potential contribution of CD151 to wound healing, mice rendered null for the CD151 gene were used and their ability to heal full-thickness dorsal incisional wounds was compared with their wild-type counterparts. Healing was significantly impaired in CD151-null mice. Figure 2a and b shows representative wounds at day 7, with transverse sections shown in Figure 2c and d. The gape of the wounds was determined by measuring the width of the wounds at the midway point of the 1 cm incision at 0, 3, 7, and 14 days post-injury. Wound gape remained significantly elevated in the CD151-null mice 7 days post-wounding compared to the wild-type wounds (Figure 2e); however, by 14 days, all the wounds had closed and no differences between wild-type and CD151-null mice wounds were observed macroscopically.
Figure 2. Effect of CD151 gene deletion on wound healing in vivo.
Full-thickness, 1 cm incisions were made through the dorsal skin in wild-type (wt) and CD151-null mice. (a, b) Representative day-7 wounds. (e) Wound gape was measured at the midpoint of the wound at 3 and 7 days post-wounding. Wounds were completely closed in both groups after 14 days. Results represent means±SEM (n=10 for each group; *P<0.05 vs day-7 wild type). Hematoxylin- and eosin-stained sections of wild-type and CD151-null wounds were assessed. Representative day-7 (c) wild-type and (d) CD151 wounds are shown. Arrows demarcate wound edges and c indicates the position of clot. Bars (a,b)=5 mm; (c, d)=100 μm. (f) Wound sizes were significantly greater in CD151-null mice than in their wild-type counterparts at 3 and 7 days post-wounding. Results represent means±SEM (n=10 for each group; *P<0.05 vs wild type). Dermal wound gape was measured by measuring the distance between the dermal wound margins (arrows in a, b). (g) A significant increase in dermal gape was observed at 7 days in CD151-null mice. Results represent means±SEM (n=10 for each group; *P<0.05). (h) Wound re-epithelialization was evaluated by measuring the percentage of the wound that had epidermal covering at days 3, 7, and 14. A significant decrease in re-epithelialization was observed at day 7 in CD151-null mice. Results represent means±SEM (n=10 for each group; *P<0.05).
The distance between the wound margins was measured histologically at 3, 7, and 14 days post-wounding (Figure 2f). Wounds were significantly larger at both 3 and 7 days post-injury, but by 14 days all the wounds had healed and no difference in wound size was observed. The distance between the dermal wound margins was also measured at 3, 7, and 14 days post-wounding as an indicator of the dermal wound healing component, and significantly increased dermal gape was observed in CD151-null mice wounds 7 days post-wounding (Figure 2g).
Re-epithelialization is delayed in CD151-null wounds
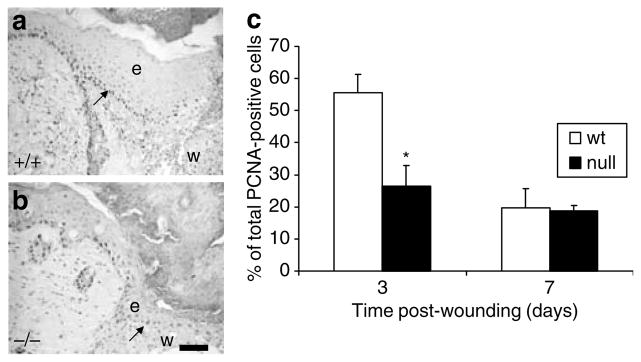
Re-epithelialization of the wound site is a crucial part of the wound healing process. The percentage of the wound covered by epidermis was measured at 3, 7, and 14 days post-wounding and the results are shown in Figure 2h. Re-epithelialization was impaired in CD151-null wounds at 3 and 7 days post-wounding with about 20% decrease in re-epithelialization occurring at 7 days (Figure 2h), indicating a potential function for CD151 in epidermal migration and adhesion. The number of proliferating keratinocytes in the epidermis at the edge of the wound and migrating across the wound surface was also determined using the proliferating cell nuclear antigen (PCNA) marker. Significantly fewer PCNA-positive cells were observed in the epidermis of 3-day CD151-null wounds compared to their wild-type counterparts (Figure 3a–c). However, by 7 days, no difference in keratinocyte proliferation between CD151-null or wild-type wounds was observed (Figure 3c).
Figure 3. CD151 affects keratinocyte proliferation in wound healing.
(a) Wild-type and (b) CD151-null wounds were stained with PCNA antibody at 3 and 7 days post-wounding. (c) Positive cells were counted in the migrating epithelial tongue at the wound edge and expressed as a percentage of the total number of keratinocytes in this area. A significant decrease in proliferation was observed at day 3 in CD151-null mice. Results represent means±SEM (n=6 for each group; *P<0.05). Bar=50 μm (given in (b), but refers to both the images). In both images, e denotes the position of epidermis and w indicates the position of wound. Arrows in (a) and (b) point to positive PCNA-stained cells in the migrating epithelial tongue.
Laminin organization is impaired in CD151-null wounds
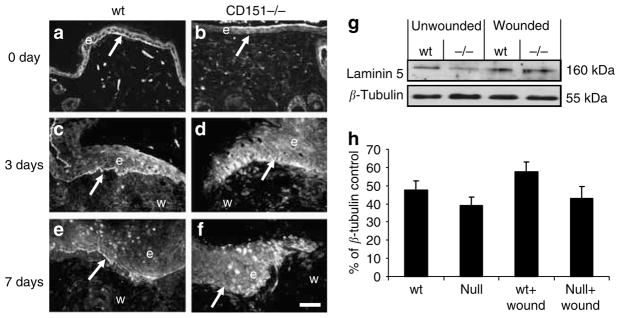
The formation of a new basement membrane is a key step in the re-epithelialization process. To characterize the effect of CD151 on deposition of the basement membrane, CD151-null and wild-type wounds were analyzed for laminin 5, a protein that localizes predominantly to the basement membrane in skin (Goldfinger et al., 1999; Nguyen et al., 2000). In normal, unwounded skin from wild-type mice, laminin 5 expression was observed predominantly at the basement membrane (Figure 4a). In contrast, normal skin from CD151-null mice showed little laminin 5 staining at the basement membrane (Figure 4b). Laminin 5 expression was increased, relative to adjacent normal tissue, at the wound edge 3 days post-wounding (Figure 4c), and this elevation persisted up to 7 days post-wounding (Figure 4e). The staining was observed as a sharp band underneath the basal keratinocytes in wounds of wild-type mice (Figure 4a and c). By contrast, in wounds of CD151-null mice, although laminin 5 levels were increased in response to wounding (Figure 4d and f), the localization of the protein was different. Laminin 5 was not restricted to the basal site of the keratinocytes, but looked dispersed and stained a more extended area within the migrating epidermis. Western blotting did not reveal a significant increase in laminin 5 subunit expression in 7-day wounds in wild-type mice compared to CD151-null mice (Figure 4g and h). This staining pattern was obtained with two independent laminin 5 antibodies. Therefore, it would appear that the tetraspanin CD151 is not required for laminin 5 production, but is necessary for its organization and deposition in the basement membrane.
Figure 4. Effect of CD151 deletion on laminin 5 expression in wound healing.
Representative immunofluorescence images of laminin 5 staining are shown in unwounded (a) wild-type (wt) and (b) CD151-null skin and at (c, d) 3 days and (e, f) 7 days post-wounding in (c, e) wild-type and (d, f) CD151-null wounds. Bar=50 μm (given in (f), but refers to all images). n=10 for each group. Arrows in (a, c, e) point to positive staining of basement membrane, which is not observed in (b, d, f). In all images, e denotes the position of epidermis and w indicates the position of wound. Protein was extracted from unwounded (day 0) and wounded (day 7) skin from wild-type mice and CD151-null mice. A 10 μg portion was loaded per lane and analyzed by Western blotting using an antibody specific to laminin 5. (g) Blots were stripped and reprobed with an antibody specific to β-tubulin. (h) Densitometric quantitation of gels showing normalized laminin 5 protein levels in wild-type 7-day wounds. n=3.
Altered expression of α6 and β4 integrins in CD151-null wounds
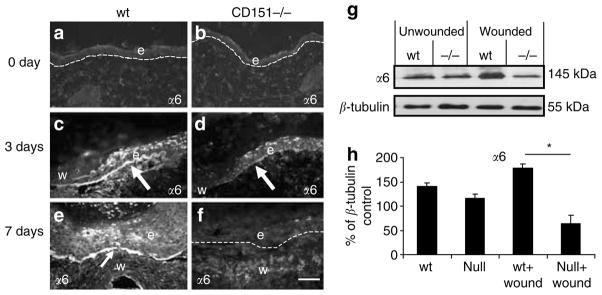
Immunostaining using an integrin α6-specific antibody revealed increased α6 immunostaining in response to wounding in wild-type mice wounds (Figure 5a, c, and e). This was particularly intense at the tip of the migrating epidermal tongue (Figure 5c). Integrin α6 was observed primarily staining basal keratinocytes attached to the basement membrane. Increased staining was observed in response to wounding, peaking at 3 days in wild-type wounds, remaining elevated until the wounds were completely re-epithelialized at 7 days (Figure 5e), and returning to basal levels by 14 days. In marked contrast, in CD151-null wounds, α6 integrin expression at 3 days post-wounding was very low and barely detectable at 7 days (Figure 5d and f). Western blotting also revealed a significant increase in α6 integrin protein expression 7 days post-wounding in wild-type mice; however, no increase in α6 expression was observed in CD151-null wounds (Figure 5g and h).
Figure 5. Effect of CD151 deletion on integrin α6 expression in wound healing.
Representative immunofluorescence images of integrin α6 staining in unwounded (a) wild-type (wt) and (b) CD151-null mouse skin. Increased α6 expression is observed in response to wounding at (c, d) 3 days and (e, f) 7 days post-wounding in (c, e) wild-type and (d, f) CD151-null wounds. Increased α6 staining is observed in wild-type wounds at the tip of the newly migrating epidermis compared to CD151-null wounds. Arrows point to positive α6 staining of the basement membrane. Protein was extracted from unwounded (day 0) and wounded (day 7) skin from wild-type and CD151-null mice. A 10 μg portion was loaded per lane and analyzed by Western blotting using an antibody specific to integrin α6. (g) Blots were stripped and reprobed with an antibody specific to β-tubulin. n=3 for each group. (h) Normalized densitometric quantification. Results represent means±SEM (*P<0.05). Bar=50 μm (given in (f), but refers to all images). In all images, e denotes the position of epidermis and w indicates the position of wound.
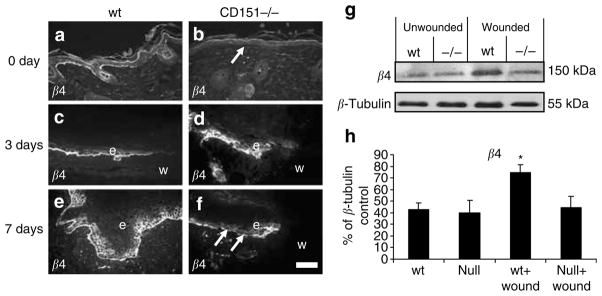
Integrin β4 is found on the cell surface as a heterodimeric complex with α6. This α6β4 integrin complex, which associates with CD151, is a receptor for laminin 5 and has a role in basement membrane adhesion. Therefore, the effect of CD151 gene deletion on β4 integrin was also investigated. Immunostaining using a β4 integrin-specific antibody showed specific staining along the basement membrane in normal unwounded skin of wild-type mice (Figure 6a). No staining along the basement membrane was observed in CD151-null unwounded skin (Figure 6b). Upregulation of β4 integrin expression was observed in response to wounding in normal wild-type wounds at 3 and 7 days post-injury (Figure 6c and e). Increased staining for β4 integrin was observed along the basement membrane, particularly at the wound edge where a marked increase in expression was observed 3 days post-wounding (Figure 6c). β4 integrin remained elevated in wild-type wounds at 7 days post-wounding (Figure 6e), with continuous positive staining of the basement membrane and basal keratinocytes. In CD151-null wounds, β4 integrin-positive staining was observed; however, the staining was not restricted to the basement membrane but haphazard within the basal keratinocytes (Figure 6d) By 7 days, staining of the basement membrane was observed but this was not continuous and gaps could be seen (arrows in Figure 6f). Consistent with the immunhistochemical staining, Western blotting also revealed a significant increase in β4 integrin protein expression 7 days post-wounding in wild-type mice but not in CD151-null wounds (Figure 6g and h).
Figure 6. Effect of CD151 deletion on integrin β4 expression in wound healing.
Representative immunofluorescence images of integrin β4 staining in unwounded (a) wild-type (wt) and (b) CD151-null mouse skin. Integrin β4 expression in response to wounding at (c, d) 3 days and (e, f) 7 days post-wounding in (c, e) wild-type and (d, f) CD151-null wounds. Integrin β4 staining is observed discretely staining basal keratinocytes and the basement membrane in wild-type wounds at the tip of the newly migrating epidermis. CD151-null wounds show impaired β4 staining. Arrows in (f) point to portions of basement membrane not staining positive for β4. Protein was extracted from unwounded (day 0) and wounded (day 7) skin from wild-type and CD151-null mice. A 10 μg portion was loaded per lane and analyzed by Western blotting using an antibody specific to integrin β4. (g) Blots were stripped and reprobed with an antibody specific to β-tubulin. n=3 for each group. (h) Densitometric quantification. Results represent means±SEM (*P<0.05). Bar=50 μm (given in (f), but refers to all images). In all images, e denotes the position of epidermis and w indicates the position of wound.
Integrin α3 expression in CD151-null wounds
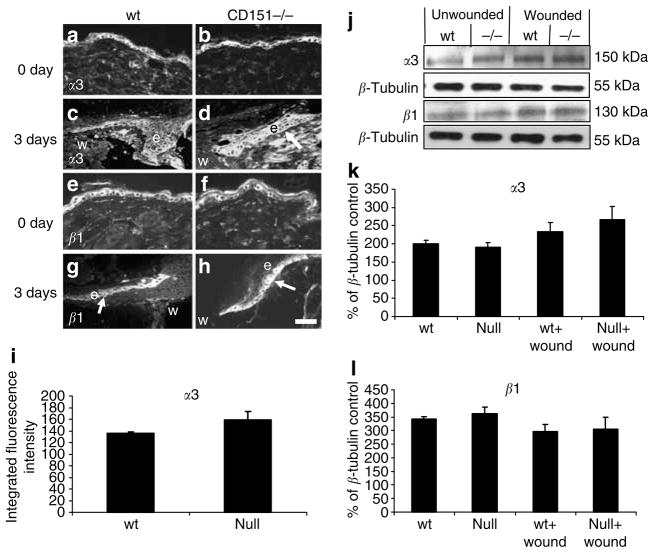
Integrin α3 expression increased in response to wounding in wild-type and CD151-null wounds (Figure 7a–d). This expression was throughout the migrating tip of the epidermis, with basal and suprabasal keratinocyte staining observed. This may suggest a mechanism by which the CD151-null wounds were able to re-epithelialize their wounds, albeit significantly slower than their wild-type counterparts. Immunofluorescent quantification of α3 expression in day-3 wounds showed increased α3 expression, although the results were not statistically significant. Western blotting of protein extracted from the wild-type and CD151-null wounds supported the immunostaining data, showing increased α3 expression in response to wounding (Figure 7g and h).
Figure 7. Effect of CD151 deletion on α3 and β1 expression in wound healing.
Representative immunofluorescence images of (a, b) integrin α3 and (e, f ) integrin β1 staining in unwounded skin. (c, d) Integrin α3 and (g, h) integrin β1 at 3 days post-wounding in (c, g) wild-type (wt) and (d, h) CD151-null wounds. (d) Increased suprabasal α3 staining is observed in CD151 wounds compared to wild type particularly at the tip of the newly migrating epidermis. (i) Epidermal immunofluorescence intensity was quantified in normal and wounded wild-type and CD151-null skin. No difference in β1 staining was observed between wild-type and CD151-null wounds. n=6 for each group. Bar=50 μm (given in (h), but refers to all images). In all images, e denotes the position of epidermis and w indicates the position of wound. Protein was extracted from unwounded (day 0) and wounded (day 7) skin from wild-type and CD151-null mice. A 10 μg portion was loaded per lane and analyzed by Western blotting using antibodies specific to integrins α3 and β1. (j) Blots were stripped and reprobed with an antibody to β-tubulin. n=3 for each group. (k, l) Normalized densitometric quantification.
Integrin β1 or α1 expression in CD151-null wounds
Integrin β1 is expressed on the cell surface as a heterodimer with integrin α1 through to α11 and αv. Expression of integrin β1 was induced in response to wounding in both wild-type and CD151-null wounds. Although the staining was predominantly in the basal keratinocytes, suprabasal cell staining was also observed along the newly forming epidermis from the wound margin to the migrating epidermal tip at 3 and 7 days post-wounding (Figure 7g and h). By 14 days, when the wounds were completely re-epithelialized, β1 staining had returned to basal levels. No differences were observed in either the localization or the intensity of staining between wild-type and CD151-null wounds. Western blotting showed no significant effect of CD151-null mutation on β1 protein levels (Figure 7e and h). No differences in α1 staining either in tissue localization or intensity were observed between wild-type and CD151-null wounds at 3, 7, or 14 days post-injury (data not shown).
DISCUSSION
Epidermal wound healing is a tightly regulated process involving proliferation and migration of keratinocytes at the wound margins and deposition of a provisional matrix on the wound bed. Laminin 5 and its integrin receptors, α3β1 and α6β4, have been reported to play a central role in this process (Goldfinger et al., 1999; Nguyen et al., 2000; Santoro and Gaudino, 2005). These laminin-binding integrins form strong complexes with the tetraspanin CD151, affecting integrin signalling (reviewed by Berditchevski, 2001; Hemler, 2003). Although the skin of mice with targeted deletion of CD151 is morphologically normal, we previously demonstrated defective outgrowth of keratinocytes from neonatal skin explants from these mice in vitro, a surrogate assay for epidermal wound healing (Wright et al., 2004b). We have now examined the role of CD151 in epidermal wound healing in vivo.
Here, we demonstrate that CD151 is expressed in a distinct spatial and temporal profile during murine wound healing. Upregulation of CD151 expression was observed in both the basal and suprabasal keratinocytes of the migrating epidermis and in wound tissue from wild-type mice (Figure 1), pointing to CD151 having an important role in the re-epithelialization process. The functional role of CD151 in wound healing was demonstrated by retarded re-epithelialization and wound closure in CD151-null mice. In these mice, healing was delayed, with wounds being significantly larger 7 days post-wounding (Figure 2), suggesting that CD151 may be an integral component of the healing response. Wounds in CD151-null mice also had a significantly slower rate of re-epithelialization and reduced keratinocyte proliferation, indicating impaired ability of the keratinocytes to proliferate and migrate across the wound bed. Our data are the first to show a functional effect of CD151 knockdown in epidermal wound healing in vivo.
During wound healing, two processes have to occur: the migration of cells to ensure that a wound site is covered and, at the same time, stabilization of epithelial–wound bed interactions. Previous studies with inhibitory antibodies, or overexpression in vitro, have established the importance of tetraspanins, including CD151, in cell migration (Boucheix and Rubinstein, 2001; Yanez-Mo et al., 2001). For example, Penas et al. (2000) showed that human keratinocyte migration in vitro can be inhibited using anti-CD151 monoclonal antibodies. Both migration and stabilization of keratinocyte–wound bed interactions involve binding of cell surface integrins to newly deposited extracellular matrix. Deposition of laminin-5 in wounded skin is essential for proper adhesion and migration of keratinocytes (Goldfinger et al., 1998). During migration, keratinocytes initially deposit large amounts of laminin 5 precursor in the matrix. A tightly regulated stepwise processing of laminin-5 modulates the affinity to different integrin receptors and thereby is essential to maintain the balance between adhesion and motility that is required for controlled migration (reviewed by Nguyen et al., 2000). Specifically, unprocessed laminin-5 preferentially binds α3β1, which is pro-migratory. In addition, ligation of α3β1 is required for processing and organization of laminin-5 in the extracellular matrix (deHart et al., 2003). Subsequently, processed laminin-5 preferentially binds to α6β4 integrin, stabilizing cell–substrate interaction. In epithelial cells, the laminin-5–α6β4 complex induces the formation of hemidesmosomes, which enforce the attachment of basal cells to the basement membrane in stratified epithelium (Jones et al., 1998b; Sterk et al., 2000). In the context of wound healing, the laminin-5–α6β4 complex may lead to the assembly of immature hemidesmosomes, which may be more dynamic than their mature counterparts allowing cell migration to occur while also providing stability for cell–wound bed interactions (Tsuruta et al., 2003).
Thus, an appropriately organized laminin-5 extracellular matrix is required for both keratinocyte migration into the wound and stabilization of cell–substrate interaction (Goldfinger et al., 1999). As CD151 regulates the functions of both α3 and α6 integrins, which are the major laminin-5-binding integrins, we examined the deposition of laminin-5 in epidermal wounds in both null and wild-type mice. In addition to delayed re-epithelialization, formation of the basement membrane was also affected in CD151-null wounds. Analysis of the 7-day wounds of CD151-null mice revealed significant abnormalities in the deposition of newly synthesized laminin-5. No discrete band of laminin-5 staining was observed in the migrating epithelium at the wound edge, as was observed in corresponding wild-type wounds. The staining pattern was more diffuse and disorganized, with laminin-5 staining observed throughout the epidermis at the wound edge (Figure 3). The data suggest that CD151 is required for correct organization of laminin-5 in wounds, contributing to delayed wound healing in CD151-null mice.
We went on to examine the levels and organization of α3, α6, β1, and β4 integrin chains in wound tissue. Several β1 integrins are potentially important in this system, notably the collagen receptor α2β1, the fibronectin receptors α5β1 and α9β1, and the laminin receptors α3β1 and α6β1 (reviewed by Nguyen et al., 2000; Singh et al., 2004). However, α3β1 and α6β1, together with α6β4, are the predominant binding partners of CD151 in epithelial cells. As previously reported for isolated keratinocytes (Wright et al., 2004b), expression of these integrins was normal in the unwounded skin of CD151-null mice (Figures 4 and 5). In epidermal wounds in wild-type mice, similar to previous reports (Hertle et al., 1992), β1 integrin was upregulated along the migrating edge of the epithelium, and although expression was observed primarily in the basal keratinocytes, suprabasal expression was observed in response to wounding. Similarly, in CD151-null wounds, β1 integrin expression was observed in the basal and suprabasal keratinocytes and was upregulated along the migrating epidermis, although total levels of β1 did not change (Figure 5). Suprabasal expression of α3 integrin was also observed in wounds, similar to previous wound healing studies (Clark 1990; Hertle et al., 1992). There was however no significant alteration in the distribution or total amount of α3 integrin in CD151-null mice relative to wild-type mice.
The function of β1 integrins in epidermal wound healing has been studied using conditional knockouts where β1 gene deletion was restricted to keratinocytes. Delayed wound healing due to impaired keratinocyte migration was also observed in those animals (Grose et al., 2002). Although our data suggest that the wound healing defect in CD151-null mice was not attributable to altered expression or localization of β1 integrins, these integrins likely contributed to the re-epithelialization of the wounds, which did occur in CD151-null mice, albeit more slowly than in normal mice. Furthermore, integrin α3-null mice display abnormal basement membrane architecture and function (DiPersio et al., 1997), and studies with keratinocytes from these mice have shown that α3β1 plays a key role in the organization of laminin-5 in the extracellular matrix in vitro (deHart et al., 2003). As CD151 modulates outside-in signalling via α3β1 and other integrins (Sawada et al., 2003; Lau et al., 2004), it remains possible that disorganization of the laminin-5 extracellular matrix in CD151-null mice may arise from altered signalling via α3β1, even though expression of this integrin was not significantly altered.
In contrast to the results with α3 and β1 integrin chains, abnormal expression of the integrins α6 and β4 was observed in wounds in CD151-null mice. In wild-type mice, expression of these integrins was significantly increased in the migrating epidermis at 3 days post-wounding and in the newly formed epidermis 7 days post-injury, similar to that previously reported (Larjava et al., 1993; Nguyen et al., 2000). However, in CD151-null wounds, no upregulation of either α6 or β4 expression was observed, either by immunofluorescence microscopy or Western blotting. Although α6 and β4 expression was observed in CD151-null wounds, the staining pattern was haphazard and disorganized (Figure 4). Consistent with studies by Russell et al. (2003), our data suggest that the failure to upregulate and correctly organize expression of α6β4 integrin plays a central role in defective wound healing in CD151-null mice.
In summary, our studies have revealed a functional role for CD151 in epidermal wound healing. In wild-type mice, CD151 protein expression levels were upregulated in wounds, suggesting a functional role in healing. Consistent with this, the gene knockout of CD151 resulted in impaired healing and delayed re-epithelialization. Organization of laminin-5 extracellular matrix was defective, and upregulation of integrins α6 and β4 in the wounds was prevented in the absence of CD151. The impaired deposition of laminin-5 and failure to upregulate expression of α6β4 may result in an altered balance between the different types of adhesion structures in CD151-null wounds, and may explain why wound healing is retarded in CD151-null mice.
MATERIALS AND METHODS
Antibodies
Rabbit anti-CD151 (sc-18753) was obtained from Santa Cruz (Santa Cruz, CA) and is an antibody raised against a peptide mapping near the amino terminus of human CD151. Integrin α1 (sc-6596) and α6 (sc-6596) were obtained from Santa Cruz (Santa Cruz, CA), and are antibodies raised against epitopes corresponding to an amino-acid sequence mapping at the carboxy terminus of the precursor form of integrin α1 and α6, respectively. Rabbit polyclonal anti-human integrin α3 was a kind gift from Fedor Berditchevski (Cancer Research UK, University of Birmingham, Birmingham, UK). The antibody was raised against the cytoplasmic tail of human α3 integrin, but crossreacts with mouse α3. Monoclonal rat anti-mouse integrin β4 was obtained from PharMingen (North Ryde, Australia (346-11A)). This antibody reacts with an epitope at the beginning of the cysteine-rich repeat region of the 200 kDa β4 integrin chain. The monoclonal antibody rat anti-mouse integrin β1 (9EG7) was from PharMingen and this antibody reacts with the 130 kDa integrin β1 chain (CD29). Laminin 5 (J18) polyclonal antiserum was raised in rabbit using rat laminin 5 purified from extracellular matrix preparations of 804G cells, as previously described (Langhofer et al., 1993). Mouse anti-PCNA was obtained from Sigma-Aldrich (Sydney, Australia).
Generation of CD151-null mice
The construction of CD151-null mice on a C57BL/6 background has been recently described (Wright et al., 2004b). These and normal syngeneic mice were bred at the Central Animal House, University of Newcastle, and were then housed in a specific-pathogen-free facility at the Women’s and Children’s Hospital Animal House Facility for the duration of the experiments.
Animal studies
All experiments were approved by the Adelaide Women’s and Children’s Hospital Animal Care and Ethics Committee following the Australian Code of Practice for the Care and the Use of Animals for Scientific Purposes.
Murine surgical techniques
Wild-type and CD151-null male mice (16–20 weeks old) were anesthetized with inhaled isofluorane, and the dorsum shaved and cleaned with 10% w/v povidine iodine solution. Two equidistant 1 cm full-thickness incisions were made through the skin and panniculus carnosus using fine scissors on the flanks of the animals extending 3.5–4.5 cm from the base of the skull, 1 cm on either side of the spinal column. The wounds were left to heal by secondary intention (i.e., the wound edges were not closed by sutures). Digital photographs were taken of the wounds at 0, 3, 7, and 14 days post-wounding. A ruler was aligned next to the wound to allow direct wound area and wound gape (midpoint of the 1 cm incision) measurements to be made. Wounds were harvested at 3, 7, and 14 days and were bisected. One half was fixed in 10% buffered formalin and processed so that the midpoint of the wound was sectioned and compared between groups. The other half was microdissected to remove any contaminating normal, unwounded skin and snap-frozen in liquid nitrogen for protein extraction and Western blot analysis.
Histology, immunohistochemistry, and image analysis
Histological sections were prepared from wound tissue fixed in 10% buffered formalin and embedded in paraffin. Four-micrometer sections were cut and floated onto Snowcoat™ slides (Surgipath, Richmond, IL), baked overnight at 55°C, and stored at room temperature. Sections were stained with hematoxylin and eosin or subjected to immunohistochemistry following antigen retrieval as follows.
The sections were deparaffinized in xylene for 10 minutes and rehydrated through a series of decreasing ethanol dilutions. Sections were placed in a target retrieval solution (DAKO Corporation, Botany, Australia), boiled for 2 minutes initially in a 900W microwave, and then heated on medium power for 2×5 minutes before being rapidly cooled to 50°C on ice. The sections were washed in phosphate-buffered saline (PBS) before incubation with 0.025% trypsin at 37°C for 3 minutes. After further PBS washes, the sections were blocked in 3% normal horse serum for 30 minutes. Primary antibodies α1, α6, β1, β4, and CD151 were applied at a 1:100 dilution and Laminin-5 at 1:200 and slides were incubated at 4°C overnight in a humidified chamber. Slides were rinsed three times in PBS and incubated for 1 hour at room temperature with species-specific biotinylated secondary antibodies (1:200). Slides were rinsed with 2–3 washes of PBS before final incubation of CY3-conjugated streptavidin (Sigma-Aldrich, Sydney, Australia) 1:200 dilution in PBS was applied for 40 minutes at room temperature. The stained sections were finally mounted in Dako fluorescent mounting medium (DAKO Corporation, Botany, Australia) and analysis of immunofluorescence was determined using AnalySIS software package (Soft Imaging System GmbH, Munster, Germany). For verification of staining, negative controls included replacement of the primary antibodies by either normal rabbit IgG (laminin), normal mouse (β1, β4), or normal goat IgG (α1, α6). On additional control sections, the primary and secondary antibodies were left out to determine nonspecific binding. All control sections had negligible immunofluorescence.
Kertinocyte proliferation was determined using the PCNA immunostaining technique (Geier et al., 2005). PCNA-positive cells were counted and expressed as a percentage of total keratinocytes in the migrating epithelial tongue at the wound edge.
Histological image analysis
Image analysis was performed using ImageProPlus program (MediaCybernetics Inc., Silver Spring, MD). Wound size was determined by manually drawing below the epidermis or clot between the wound margins (which can easily be seen where thick collagen bundles appear as representative of intact skin). The percentage of the wound that has re-epithelialized was determined by measuring the portion of the wound that was covered with epidermis as a percentage of the entire wound. Dermal gape was determined by drawing between the dermal wound margins. Blinded scoring of histological slides by two independent assessors was undertaken.
Western blotting
Protein was extracted from wild-type and CD151-null mouse wounds using standard protein extraction protocols. Briefly, wound tissue was microdissected to remove normal, unwounded skin, chopped and placed in lysis buffer (50mM Tris pH 7.5, 1mM EDTA, 50mM NaCl, 0.5% Triton X-100, containing protease inhibitor cocktail tablet (1 per 10 ml; Complete, Mini (Roche Products Pty Ltd, Dee Why, NSW, Australia)) and the samples were homogenized briefly. Samples were centrifuged (16,000×g, 10 minutes, at 4°C), with supernatants being transferred to fresh tubes and centrifuged for a further 10 minutes and the supernatants stored at −20°C. Sample proteins (10 μg) were run on 12.5% SDS-PAGE gels at 100V for 1 hour and then transferred to nitrocellulose by semidry transfer (Bio-Rad Laboratories, Regents Park, NSW, Australia) at 5 V for 1 hour. The membranes were stained with Poinceau Red to ensure even loading of the gels with protein. Membranes were blocked in 15% milk blocking buffer for 10 minutes and primary antibodies added in PBS containing 3% skimmed milk powder in PBS/0.3% Tween for 1 hour at room temperature. After two washes in PBS containing 3% skimmed milk powder/0.3% Tween, appropriate species-specific secondary horseradish peroxidase-conjugated antibodies were added for a further 1 hour at room temperature. Stringent washes for 1 hour were then performed before detection of horseradish peroxidase by enhanced chemiluminescence reagent (Amersham Biosciences UK Limited, Buckinghamshire, UK) and exposure to X-ray film. Membranes were stripped and reprobed for β-tubulin (Sigma-Aldrich, Sydney, Australia) for normalization of protein levels. Densitometric analysis of the resulting bands was performed using Image J software (NIH).
Statistical analysis
Statistical differences were determined using the t-test or an analysis of variance. For data not following a normal distribution, the Mann–Whitney U-test was used. A P-value of less than 0.05 was considered significant.
Acknowledgments
This work was supported in part by a grant from the National Health and Medical Research Council of Australia (NHMRC). A.J.C. is supported in part by the JB Reid Fellowship, University of Adelaide, Australia. L.K.A. is an NHMRC Principal Research Fellow and Brawn Professorial Fellow of the University of Newcastle, Australia. J.C.R.J. is supported by NIH grant RO1 DK60589. This work was performed in Adelaide, Australia.
Abbreviations
- PBS
phosphate-buffered saline
- PCNA
proliferating cell nuclear antigen
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Berditchevski F. Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci. 2001;114(Part 23):4143–51. doi: 10.1242/jcs.114.23.4143. [DOI] [PubMed] [Google Scholar]
- Berditchevski F, Gilbert E, Griffiths MR, Fitter S, Ashman L, Jenner SJ. Analysis of the CD151-alpha3beta1 integrin and CD151-tetraspanin interactions by mutagenesis. J Biol Chem. 2001;276:41165–74. doi: 10.1074/jbc.M104041200. [DOI] [PubMed] [Google Scholar]
- Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol. 1999;112:411–8. doi: 10.1046/j.1523-1747.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- Boucheix C, Rubinstein E. Tetraspanins. Cell Mol Life Sci. 2001;58:1189–205. doi: 10.1007/PL00000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Sorcclino JL, Pirro A, et al. Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. EMBO J. 2000;19:3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA. Fibronectin matrix deposition and fibronectin receptor expression in healing and normal skin. J Invest Dermatol. 1990;94(6 Suppl):128S–34S. doi: 10.1111/1523-1747.ep12876104. [DOI] [PubMed] [Google Scholar]
- deHart GW, Healy KE, Jones JC. The role of alpha3beta1 integrin in determining the supramolecular organization of laminin-5 in the extracellular matrix of keratinocytes. Exp Cell Res. 2003;283:67–79. doi: 10.1016/s0014-4827(02)00028-9. [DOI] [PubMed] [Google Scholar]
- DiPersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO. alpha3beta1 Integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137:729–42. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J, Yu Q-C, Fuchs E. Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol. 1996;137:729–42. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- Fitter S, Sincock PM, Jolliffe CN, Ashman LK. Transmembrane 4 superfamily protein CD151 (PETA-3) associates with beta 1 and alpha IIb beta 3 integrins in haemopoietic cell lines and modulates cell–cell adhesion. Biochem J. 1999;338(Part 1):61–70. [PMC free article] [PubMed] [Google Scholar]
- Geary SM, Cambareri AC, Sincock PM, Fitter S, Ashman LK. Differential tissue expression of epitopes of the tetraspanin CD151 recognised by monoclonal antibodies. Tissue Antigens. 2001;58:141–53. doi: 10.1034/j.1399-0039.2001.580301.x. [DOI] [PubMed] [Google Scholar]
- Geier MS, Tenikoff D, Yazbeck R, McCaughan GW, Abbott CA, Howarth GS. Development and resolution of experimental colitis in mice with targeted deletion of dipeptidyl peptidase IV. J Cell Physiol. 2005;204:687–92. doi: 10.1002/jcp.20333. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–3. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- Goldfinger LE, Hopkinson SB, deHart GW, Collawn S, Couchman JR, Jones C. The alpha3 laminin subunit, alpha6beta4 and alpha3beta1 integrin coordinately regulate wound healing in cultured epithelial cells and in the skin. J Cell Sci. 1999;112:2615–29. doi: 10.1242/jcs.112.16.2615. [DOI] [PubMed] [Google Scholar]
- Goldfinger LE, Stack MS, Jones JC. Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J Cell Biol. 1998;141:255–65. doi: 10.1083/jcb.141.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose R, Hutter C, Bloch W, Thorey I, Watt FM, Fassler R, et al. A crucial role of beta 1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development. 2002;129:2303–15. doi: 10.1242/dev.129.9.2303. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Nomura T, Kishimoto K, Yanagisawa K, Fujita S. SFA-1/PETA-3 (CD151), a member of the transmembrane 4 superfamily, associates preferentially with alpha 5 beta 1 integrin and regulates adhesion of human T cell leukemia virus type 1-infected T cells to fibronectin. J Immunol. 1998;161:3087–95. [PubMed] [Google Scholar]
- Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Ann Rev Cell Dev Biol. 2003;19:397–422. doi: 10.1146/annurev.cellbio.19.111301.153609. [DOI] [PubMed] [Google Scholar]
- Hertle MD, Kubler MD, Leigh IM, Watt FM. Aberrant integrin expression during epidermal wound healing and in psoriatic epidermis. J Clin Invest. 1992;89:1892–901. doi: 10.1172/JCI115794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Jones JC, Hopkinson SB, Goldfinger LE. Structure and assembly of hemidesmosomes. BioEssays. 1998a;20:488–94. doi: 10.1002/(SICI)1521-1878(199806)20:6<488::AID-BIES7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Jones JC, Skalli O, Goldman RD, Baker SE. What links laminin-5 to the keratin cytoskeleton in epithelial cells? Biol Bull. 1998b;194:371–2. doi: 10.2307/1543116. [DOI] [PubMed] [Google Scholar]
- Karamatic Crew V, Burton N, Kagan A, Green CA, Levene C, Flinter F, et al. CD151, the first member of the tetraspanin (TM4) superfamily detected on erythrocytes, is essential for the correct assembly of human basement membranes in kidney and skin. Blood. 2004;104:2217–23. doi: 10.1182/blood-2004-04-1512. [DOI] [PubMed] [Google Scholar]
- Kazarov AR, Yang X, Stipp CS, Sehgal B, Hemler ME. An extracellular site on tetraspanin CD151 determines alpha 3 and alpha 6 integrin-dependent cellular morphology. J Cell Biol. 2002;158:1299–309. doi: 10.1083/jcb.200204056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, et al. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–47. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- Langhofer M, Hopkinson SB, Jones JC. The matrix secreted by 804G cells contains laminin-related components that participate in hemidesmosome assembly in vitro. J Cell Sci. 1993;105:753–64. doi: 10.1242/jcs.105.3.753. [DOI] [PubMed] [Google Scholar]
- Larjava H, Salo T, Haapasalmi K, Kramer RH, Heino J. Expression of integrins and basement membrane components by wound keratinocytes. J Clin Invest. 1993;92:1425–35. doi: 10.1172/JCI116719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L-M, Wright MD, Moseley G, Hogarth PM, Ashman LK, Jackson DE. The tetraspanin superfamily member, CD151 regulates outside-in integrin αIIbβ3 signalling and platelet function. Blood. 2004;104:2368–75. doi: 10.1182/blood-2003-12-4430. [DOI] [PubMed] [Google Scholar]
- Nguyen BP, Ryan MC, Gil SG, Carter WG. Deposition of laminin 5 in epidermal wounds regulates integrin signaling and adhesion. Curr Opin Cell Biol. 2000;12:554–62. doi: 10.1016/s0955-0674(00)00131-9. [DOI] [PubMed] [Google Scholar]
- Penas PF, Garcia-Diez A, Sanchez-Madrid F, Yanez-Mo M. Tetra-spanins are localized at motility-related structures and involved in normal human keratinocyte wound healing migration. J Invest Dermatol. 2000;114:1126–35. doi: 10.1046/j.1523-1747.2000.00998.x. [DOI] [PubMed] [Google Scholar]
- Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol. 2000;150:1149–60. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AJ, Fincher EF, Millman L, Sinith R, Vela V, Waterman EA, et al. Alpha 6 beta 4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of alpha 3 beta 1 integrin. J Cell Sci. 2003;116(Part 17):3543–56. doi: 10.1242/jcs.00663. [DOI] [PubMed] [Google Scholar]
- Santoro MM, Gaudino G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res. 2005;304:274–286. doi: 10.1016/j.yexcr.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Sawada S, Yoshimoto M, Odintsova E, Hotchin NA, Berditchevski F. The tetraspanin CD151 functions as a negative regulator in the adhesion-dependent activation of Ras. J Biol Chem. 2003;278:26323–6. doi: 10.1074/jbc.C300210200. [DOI] [PubMed] [Google Scholar]
- Serru V, Le Naour F, Billard M, Azorsa DO, Lanza F, Boucheix C, et al. Selective tetraspan–integrin complexes (CD81/alpha4beta1, CD151/alpha3beta1, CD151/alpha6beta1) under conditions disrupting tetraspan interactions. Biochem J. 1999;340:103–11. [PMC free article] [PubMed] [Google Scholar]
- Sincock PM, Fitter S, Parton RG, Berndt MC, Gamble JR, Ashman LK. PETA-3/CD151, a member of the transmembrane 4 superfamily, is localised to the plasma membrane and endocytic system of endothelial cells, associates with multiple integrins and modulates cell function. J Cell Sci. 1999;112:833–44. doi: 10.1242/jcs.112.6.833. [DOI] [PubMed] [Google Scholar]
- Sincock PM, Mayrhofer G, Ashman LK. Localization of the transmembrane 4 superfamily (TM4SF) member PETA-3 (CD151) in normal human tissues: comparison with CD9, CD63, and alpha5beta1 integrin. J Histochem Cytochem. 1997;45:515–25. doi: 10.1177/002215549704500404. [DOI] [PubMed] [Google Scholar]
- Singh P, Reimer CL, Peters JH, Stepp MA, Hynes RO, Van De Water L. The spatial and temporal expression patterns of integrin alpha9beta1 and one of its ligands, the EIIIA segment of fibronectin, in cutaneous wound healing. J Invest Dermatol. 2004;123:1176–81. doi: 10.1111/j.0022-202X.2004.23485.x. [DOI] [PubMed] [Google Scholar]
- Sterk LM, Geuijen CA, Oomen LC, Calafat J, Janssen H, Sonnenberg A. The tetraspan molecule CD151, a novel constituent of hemidesmosomes, associates with the integrin alpha6beta4 and may regulate the spatial organization of hemidesmosomes. J Cell Biol. 2000;149:969–82. doi: 10.1083/jcb.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta D, Hopkinson SB, Jones JCR. Hemidesmosome protein dynamics in live epithelial cells. Cell Motil Cytoskel. 2003;54:122–34. doi: 10.1002/cm.10089. [DOI] [PubMed] [Google Scholar]
- van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nat Genet. 1996;13:366–9. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- Wright MD, Geary SM, Fitter S, Moseley GW, Lau LM, Sheng KC, et al. Characterization of mice lacking the tetraspanin superfamily member CD151. Mol Cell Biol. 2004b;24:5978–88. doi: 10.1128/MCB.24.13.5978-5988.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MD, Moseley GW, van Spriel AB. Tetraspanin microdomains in immune cell signalling and malignant disease. Tissue Antigens. 2004a;64:533–42. doi: 10.1111/j.1399-0039.2004.00321.x. [DOI] [PubMed] [Google Scholar]
- Yanez-Mo M, Mittelbrunn M, Sanchez-Madrid F. Tetraspanins and intercellular interactions. Microcirculation. 2001;8:153–68. doi: 10.1038/sj/mn/7800076. [DOI] [PubMed] [Google Scholar]
- Yauch RL, Berditchevski F, Harler MB, Reichner J, Hemler ME. Highly stoichiometric, stable, and specific association of integrin alpha3beta1 with CD151 provides a major link to phosphatidylinositol 4-kinase, and may regulate cell migration. Mol Biol Cell. 1998;9:2751–65. doi: 10.1091/mbc.9.10.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch RL, Kazarov AR, Desai B, Lee RT, Hemler ME. Direct extracellular contact between integrin alpha(3)beta(1) and TM4SF protein CD151. J Biol Chem. 2000;275:9230–8. doi: 10.1074/jbc.275.13.9230. [DOI] [PubMed] [Google Scholar]