Abstract
BACKGROUND
Nonalcoholic fatty liver disease is associated with hepatic insulin resistance and type 2 diabetes mellitus. Whether this association has a genetic basis is unknown.
METHODS
In 95 healthy Asian Indian men, a group known to have a high prevalence of non-alcoholic fatty liver disease, we genotyped two single-nucleotide polymorphisms (SNPs) in the gene encoding apolipoprotein C3 (APOC3) that are known to be associated with hypertriglyceridemia (rs2854116 [T-455C] and rs2854117 [C-482T]). Plasma apolipoprotein C3 concentrations, insulin sensitivity, and hepatic triglyceride content were measured. We also measured plasma triglyceride concentrations and retinyl fatty acid ester absorption as well as plasma triglyceride clearance after oral and intravenous fat-tolerance tests. Liver triglyceride content and APOC3 genotypes were also assessed in a group of 163 healthy non–Asian Indian men.
RESULTS
Carriers of the APOC3 variant alleles (C-482T, T-455C, or both) had a 30% increase in the fasting plasma apolipoprotein C3 concentration, as compared with the wild-type homozygotes. They also had a 60% increase in the fasting plasma triglyceride concentration, an increase by a factor of approximately two in the plasma triglyceride and retinyl fatty acid ester concentrations after an oral fat-tolerance test, and a 46% reduction in plasma triglyceride clearance. The prevalence of nonalcoholic fatty liver disease was 38% among variant-allele carriers and 0% among wild-type homozygotes (P<0.001). The subjects with nonalcoholic fatty liver disease had marked insulin resistance. A validation study involving non–Asian Indian men confirmed the association between APOC3 variant alleles and nonalcoholic fatty liver disease.
CONCLUSIONS
The polymorphisms C-482T and T-455C in APOC3 are associated with nonalcoholic fatty liver disease and insulin resistance.
Several studies have shown a strong relationship among nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes mellitus.1–3 Whether there is a genetic basis for this association remains unknown. Previous studies have suggested that two single-nucleotide polymorphisms (SNPs) in the gene encoding apolipoprotein C3 (APOC3) may be associated with hypertriglyceridemia.4–8 We therefore examined whether these SNPs might confer a predisposition to nonalcoholic fatty liver disease and insulin resistance.
We began by studying a cohort of healthy Asian Indian men, a group that has been shown to have a high prevalence of nonalcoholic fatty liver disease and insulin resistance.9 We screened this group for the two APOC3 SNPs and measured their hepatic triglyceride content and whole-body insulin sensitivity. We then examined the effects of the APOC3 SNPs on postprandial plasma triglyceride concentrations and retinyl fatty acid ester absorption after an oral fat-tolerance test and on plasma triglyceride clearance after an intravenous lipid infusion. In addition, we determined whether hepatic steatosis and whole-body insulin resistance in the risk-allele carriers could be reversed with weight loss. Finally, we performed a validation study in an independent group of healthy non–Asian Indian men.
METHODS
SUBJECTS
From 2004 through 2009, we prospectively recruited healthy, nonsmoking, sedentary Asian Indian men whose birth weights were known to be normal. These volunteers were drawn from the New Haven, Connecticut, community by means of local advertisement. Written informed consent was obtained from each subject, and the protocol was approved by the human subjects committee at Yale University. The participants answered a questionnaire about their usual daily intake of food and alcohol as well as any changes in body weight and eating habits over the previous 12 months. They were also asked to describe the food and snacks consumed on the day before the visit, and the composition of each participant’s diet was assessed. Physical activity (during work, leisure time, and exercise) was measured by means of a pedometer and a questionnaire.10
MEASUREMENT OF PLASMA GLUCOSE, INSULIN, AND LIPIDS
Fasting plasma glucose concentrations were measured with the use of the YSI STAT 2700 Select Biochemistry Analyzer (YSI Life Sciences). Fasting plasma concentrations of insulin were measured with the use of a double-antibody radioimmunoassay kit (Linco). Fasting plasma triglyceride, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and apolipoprotein C3 concentrations were measured enzymatically (Roche Cobas Mira Plus, Roche Diagnostics).
GENETIC SCREENING
Genomic DNA was extracted from whole blood, and participants were genotyped for the SNPs rs2854116 and rs2854117 in APOC3 (for details, see the Supplementary Appendix, available with the full text of this article at NEJM.org). These SNPs have previously been shown to be associated with hypertriglyceridemia.4–8 Since earlier studies have shown that the SNP rs651821 in the gene encoding apolipoprotein A5 (APOA5) is also associated with hyperlipidemia, we excluded subjects who were identified as having the high-risk variant for this polymorphism and who were homozygous for the low-risk variants in APOC3. In addition, we genotyped all study participants for the SNP rs738409 in the gene encoding patatin-like phospholipase domain–containing 3 (PNPLA3), which has also been shown to be associated with increased hepatic triglyceride content.11
GENETIC CLASSIFICATION
The two SNPs in APOC3 (rs2854116 and rs2854117) are located in the promoter region (upstream of the transcription start site) and are in strong linkage disequilibrium. The variant alleles at rs2854116 (T-455C) and at rs2854117 (C-482T) are each associated with higher apolipoprotein C3 expression in vitro than are the respective wild-type alleles.12 Because these associations are independent and not additive, we divided the study participants into two genetically defined groups for the purposes of analysis: those who were homozygous for the low-expression (wild-type) allele at both SNPs and those who were carriers of one or more high-expression (variant) alleles at either SNP.
ASSESSMENT OF INSULIN SENSITIVITY
Whole-body insulin sensitivity was assessed by means of a 3-hour oral glucose-tolerance test. A 75-g dextrose drink (Glucola, Curtin Matheson Scientific) was administered, and blood samples were collected at 10, 20, 30, 60, 90, 120, 150, and 180 minutes to determine plasma glucose and insulin concentrations. The insulin-sensitivity index was calculated as previously described.13
MEASUREMENT OF HEPATIC TRIGLYCERIDE CONTENT
Hepatic triglyceride content was measured with proton magnetic resonance spectroscopy on a BioSpec 4T system (Bruker Daltonics). Localized spectra of the liver were obtained with the use of a coil assembly composed of twin elliptical proton radiofrequency coils (13 cm by 9 cm) arranged in quadrature. Triglyceride content was measured by proton respiration-gated, stimulated echo acquisition mode spectroscopy in a voxel (15 mm3 by 15 mm3 by 15 mm3) placed in three different locations within the liver to account for the heterogeneity of hepatic tissue.14
FAT-TOLERANCE TESTING
Plasma triglyceride concentrations and retinyl fatty acid ester absorption were measured in a sub-group of the Asian Indian subjects after they had consumed a high-fat liquid meal. In another subgroup of the Asian Indian subjects, plasma triglyceride clearance was assessed during an intravenous fat-tolerance test. (Details of these methods are described in the Supplementary Appendix.)
ASSESSMENT OF HEPATIC STEATOSIS WITH WEIGHT REDUCTION
A subgroup of subjects with hepatic steatosis (hepatic lipid content >5.5%)15 were studied after programmed caloric restriction (approximate intake, 1200 kcal per day).3 After an overnight fast, the subjects’ body weight, plasma glucose and insulin concentrations, and liver triglyceride content were measured on a weekly basis. The weight-loss program continued until the hepatic triglyceride levels normalized. The hypocaloric period was followed by 4 weeks of weight stabilization on an isocaloric diet (35 kcal per kilogram of body weight; 60% carbohydrate, 20% protein, and 20% fat).
CONFIRMATION STUDY IN NON–ASIAN INDIAN MEN
To examine the relationship between the polymorphisms in APOC3 and hepatic steatosis, we performed a confirmation study involving a cohort of healthy, sedentary, nonsmoking, multiethnic non–Asian Indian men of normal birth weight. The subjects were recruited from the New Haven community by local advertisement, with the use of the same inclusion and exclusion criteria that were applied to the Asian Indian subjects.
STATISTICAL ANALYSIS
Plasma and liver triglyceride concentrations were log-transformed to fit a normal distribution; normality was tested with the use of a Kolmogorov–Smirnov test. The base-10 logarithm of liver triglyceride, the natural logarithm of plasma triglyceride, and levels of plasma apolipoprotein C3 were then adjusted for age and body-mass index by means of linear regression. To test for association under an additive model, two methods were used: a linear-regression trend test for correlation with the genotypes at rs2854116 and rs2854117 (with genotypes at each SNP coded as 0, 1, or 2) and a one-way analysis of variance. To test for association under a dominant model, we used a linear-regression trend test for correlation (with genotypes coded as 0 or 1) as well as an unequal-variance t-test. Nonparametric t-tests and Mann–Whitney U tests, as appropriate, were used to compare means between persons who were carrying one or more high-expression (variant) alleles for either or both of the APOC3 SNPs and persons who were homozygous for the low-expression (wild-type) allele at both SNPs. Paired t-tests were performed to compare means before and after weight loss. All analyses were done on SPSS software, version 16.0, or StatView (SAS Institute), and all tests were two-tailed, with an alpha level of 0.05. The difference in the prevalence of nonalcoholic fatty liver disease between groups was determined by means of a two-sided Fisher’s exact test, and linkage disequilibrium (D′) was calculated with the use of Haploview. All data are expressed as means ±SD (except where otherwise noted).
RESULTS
SUBJECTS AND GENOTYPES
We performed initial clinical evaluations of 102 Asian Indian men. Seven had high-risk variants at rs651821 in APOA5 and were therefore excluded. The clinical characteristics of the 95 subjects who met all the criteria for participation in the study are shown in Table 1. The mean age of the group was 32±13 years, and the mean body-mass index (the weight in kilograms divided by the square of the height in meters) was 24.7±3.6.
Table 1.
Clinical Characteristics of the Study Participants.*
| Characteristic | Asian Indian Cohort (N = 95) | Non–Asian Indian Cohort (N = 163) |
|---|---|---|
| Age (yr) | 32±13 | 28±13 |
| Height (m) | 1.73±0.09 | 1.77±0.07 |
| Weight (kg) | 74±11 | 75±10 |
| Body-mass index† | 24.7±3.6 | 24.1±2.9 |
| Fat intake (%)‡ | 27±12 | 31±9 |
| Carbohydrate intake (%)‡ | 57±13 | 49±11 |
| Protein intake (%)‡ | 15±6 | 18±5 |
| Alcohol intake (g/day) | 2±5 | 5±8 |
| Pedometer activity (km/day)§ | 5.38±3.06 | 6.42±2.95 |
| Exercise index | 2.5±1.0 | 2.5±0.9 |
| Fasting glucose (mg/dl) | 100±14 | 95±8 |
| Fasting insulin (μU/ml) | 13±1 | 9±5 |
| Fasting triglycerides (mg/dl) | 109±78 | 82±50 |
Plus–minus values are means ±SD.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Values given are a percentage of total daily caloric intake.
To convert kilometers to miles, multiply by 0.62.
Nineteen of the study subjects (20%) were homozygous for the common, low-expression (wild-type) alleles at both loci (CC at locus 482 and TT at locus 455); the other 76 carried at least one high-expression variant (C-482T, T-455C, or both). The number of subjects with each possible combination of haplotypes on the basis of these two loci are shown in Table 1 in the Supplementary Appendix. The 76 carriers of either high-expression variant allele were similar to the 19 wild-type homozygotes with respect to age, body weight, body-mass index, and physical activity level (see Table 2 in the Supplementary Appendix). The percentages of total daily caloric intake represented by fat, carbohydrate, and protein were also similar in the two groups.
PLASMA LIPIDS, HEPATIC LIPID CONTENT, AND INSULIN RESISTANCE
Plasma apolipoprotein C3 concentrations were approximately 30% higher in the variant-allele carriers than in the wild-type homozygotes (Table 2). In addition, fasting plasma triglyceride concentrations were approximately 60% higher in the carriers of variant alleles. There were no significant differences between the two groups in plasma concentrations of total cholesterol, HDL cholesterol, or LDL cholesterol.
Table 2.
Fasting Plasma Concentrations of Apolipoprotein C3, Triglycerides, and Cholesterol in APOC3 Wild-Type Homozygotes and Carriers of Variant Alleles.*
| Variable | Wild-Type Homozygotes (C-482 and T-455) (N = 19) | Variant-Allele Carriers (C-482T, T-455C, or both) (N = 75)† | P Value‡ | |
|---|---|---|---|---|
| Log-Transformed | Mann–Whitney Test | |||
| Apolipoprotein C3 (mg/dl) | 8.9±2.6 | 11.4±3.5 | 0.003 | 0.03 |
| Triglycerides (mg/dl) | 74±31 | 118±87 | 0.02 | 0.053 |
| Cholesterol (mg/dl) | ||||
| Total | 146±31 | 160±35 | 0.28 | 0.35 |
| HDL | 49±9 | 47±17 | 0.64 | 0.17 |
| LDL | 83±26 | 90±26 | 0.68 | 0.99 |
To convert the values for triglycerides to millimoles per liter, multiply by 0.01129. To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. HDL denotes high-density lipoprotein cholesterol, and LDL low-density lipoprotein cholesterol.
Data were not available for one carrier.
P values were adjusted for age and body-mass index.
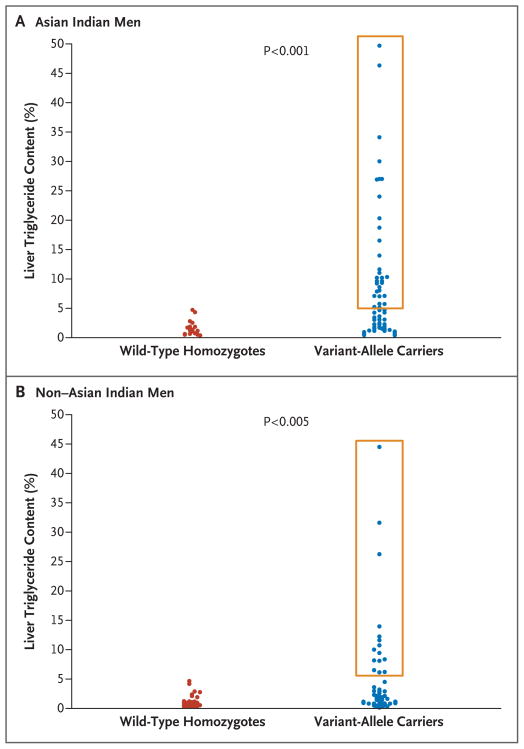
The average hepatic triglyceride content was markedly higher in the APOC3 variant-allele carriers than in the wild-type homozygotes (mean, 7.5±10.3% [median, 3.2%], vs. mean, 1.5±1.3% [median, 1.1%]; P<0.001) (Fig. 1A). These differences remained significant when the analysis was adjusted for age and body-mass index (P = 0.005 by Student’s t-test; P = 0.02 by the Mann–Whitney test). The prevalence of nonalcoholic fatty liver disease (hepatic lipid content >5.5%15) was 38% among the variant-allele carriers, whereas none of the wild-type homozygotes had nonalcoholic fatty liver disease (P<0.001) (Fig. 1A). Subjects with nonalcoholic fatty liver disease had marked insulin resistance as compared with those who did not have nonalcoholic fatty liver disease (insulin-sensitivity index, 2.0±2.0 [lower-third percentile for insulin sensitivity9], vs. 3.5±1.6; P<0.001).
Figure 1. Scattergrams of Liver Triglyceride Content in Asian Indian and Non–Asian Indian Men.
Carriers of APOC3 variant alleles (C-482T, T-455C, or both) were compared with APOC3 wild-type homozygotes (C-482 and T-455) in terms of liver triglyceride content. Results are shown for two groups of Asian Indian men (Panel A) and for two groups of non–Asian Indian men (Panel B). The boxes delineate subjects who had nonalcoholic fatty liver disease (hepatic lipid content >5.5%), all of whom were carriers of the variant allele.
PLASMA TRIGLYCERIDE AND RETINYL FATTY ACID ESTER CONCENTRATIONS
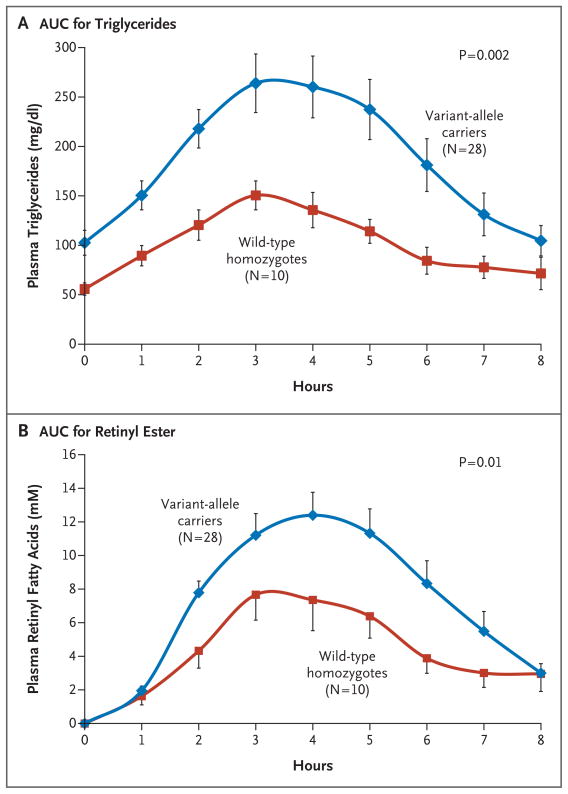
A total of 28 variant-allele carriers and 10 wild-type homozygotes underwent oral fat-tolerance testing. The average area under the curve (AUC) for the plasma triglyceride concentration was approximately doubled in the variant-allele carrier group as compared with the wild-type homozygote group (746±102 vs. 383±41 mg per deciliter per hour, P = 0.002) (Fig. 2A). Similarly, among the variant-allele carriers, the changes in the AUC for plasma concentrations of retinyl fatty acid ester (reflecting chylomicron remnants) was approximately twice those among the wild-type homozygotes (60±36 vs. 36±19 μmol per liter per hour, P = 0.01) (Fig. 2B).
Figure 2. Plasma Triglyceride and Retinyl Fatty Acid Ester Concentrations after an Oral Fat-Tolerance Test in Asian Indian Men.
Panel A shows the area under the curve (AUC) for plasma triglyceride concentrations after an oral fat-tolerance test in 28 carriers of APOC3 variant alleles (C-482T, T-455C, or both) as compared with 10 APOC3 wild-type homozygotes. Panel B shows the AUC for plasma retinyl ester concentrations in the two groups. Data are means ±SE.
PLASMA TRIGLYCERIDE CLEARANCE
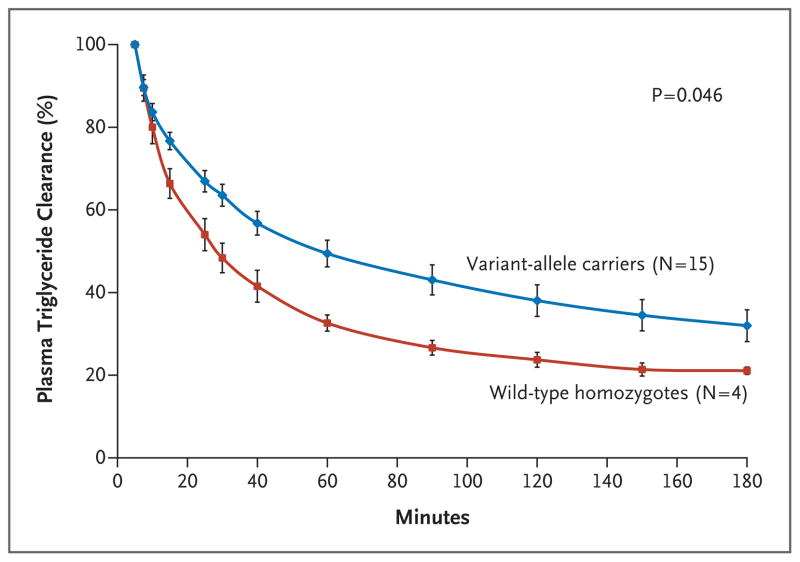
A total of 15 variant-allele carriers and 4 wild-type homozygotes underwent intravenous fat-tolerance testing. The variant-allele carriers had a 46% increase in the AUC for the relative plasma triglyceride concentration, reflecting decreased plasma triglyceride clearance, as compared with the wild-type homozygotes (171±21 vs. 249±71% per hour, P = 0.046) (Fig. 3).
Figure 3. Relative Plasma Triglyceride Concentrations after an Intravenous Fat-Tolerance Test in Asian Indian Men.
The relative plasma triglyceride concentrations are shown for 15 carriers of APOC3 variant alleles and 4 wild-type homozygotes. (Relative refers to the fact that starting plasma triglyceride levels were normalized to 100% for each subject.) The area under the curve was significantly higher for the variant-allele carriers (P = 0.046). Data are means ±SE.
EFFECT OF WEIGHT LOSS ON HEPATIC STEATOSIS AND INSULIN RESISTANCE
Seven of the subjects with hepatic steatosis and insulin resistance underwent a hypocaloric dietary intervention.3 Over a period of 3 to 6 months, their average weight decreased from 80.4±10.7 kg to 74.9±10.2 kg (P = 0.003). This weight loss was accompanied by a significant reduction in the liver triglyceride content (from 14.0 to 3.8%, P = 0.05). After weight loss, there was a marked improvement in insulin sensitivity on oral glucose-tolerance testing, with reductions in plasma glucose and plasma insulin concentrations (Fig. 1 in the Supplementary Appendix) and an increase in the insulin-sensitivity index (from 1.8±0.8 to 3.7±1.4, P<0.01).
EFFECT OF PNPLA3 GENOTYPE ON HEPATIC STEATOSIS
The liver triglyceride content was also increased in subjects of the CG and GG genotypes at rs738409 in PNPLA3, as compared with subjects of the CC genotype, with the use of both a dominant model (P = 0.002 by Student’s t-test; P = 0.003 by linear regression) and an additive model (P = 0.008 by analysis of variance; P = 0.04 by linear regression).11 Variants in APOC3 alone, PNPLA3 alone, and the two types combined accounted, respectively, for 11.0, 6.5, and 13.1% of the variance in the risk of nonalcoholic fatty liver disease.
CONFIRMATION STUDY IN NON–ASIAN INDIAN MEN
A confirmation study was performed in 163 healthy non–Asian Indian men (108 of whom were white, 26 Asian, 15 Hispanic, and 14 black) (Table 1, and Table 2 in the Supplementary Appendix). As was the case with the Asian Indian men, the prevalence of nonalcoholic fatty liver disease was higher among the APOC3 variant-allele carriers than among the wild-type homozygotes in this group (9% vs. 0%, P = 0.02) (Fig. 1B). Similarly, the non–Asian Indian men with nonalcoholic fatty liver disease had marked insulin resistance, as reflected by their lower insulin-sensitivity index as compared with that in the non–Asian men without nonalcoholic fatty liver disease (2.92±1.13 vs. 4.93±2.20, P<0.001).
DISCUSSION
We studied the relationship between genetic variants in APOC3 and the risk of nonalcoholic fatty liver disease and insulin resistance. The APOC3 variants we studied (C-482T and T-455C) were associated with approximately a 30% increase in the fasting plasma apolipoprotein C3 concentration, approximately a 60% increase in the fasting plasma triglyceride concentration, and an increase by a factor of approximately two in postprandial plasma triglyceride and retinyl fatty acid ester concentrations after an oral fat-tolerance test. Thirty-eight percent of the Asian Indian men with APOC3 variants had nonalcoholic fatty liver disease and marked insulin resistance, whereas no APOC3 wild-type homozygotes had nonalcoholic fatty liver disease. This association was confirmed in an independent group of non–Asian Indian men.
We also measured plasma triglyceride concentrations after an intravenous lipid infusion and found a 46% reduction in plasma triglyceride clearance in carriers of APOC3 variant alleles as compared with wild-type homozygotes. These findings support the hypothesis that the APOC3 variants we studied promote both fasting and postprandial hypertriglyceridemia by reducing triglyceride clearance. These data are consistent with previous studies showing that transgenic mice with overexpression of human apolipoprotein C3 had hypertriglyceridemia due to decreased lipoprotein lipase activity,16 as well as a recent study by Pollin et al. showing that a different mutation in the apolipoprotein C3 gene resulted in lower plasma triglyceride levels and had cardioprotective effects in Amish subjects.17
Taken together, these results suggest that the APOC3 variants C-482T and T-455C lead to increased plasma concentrations of apolipoprotein C3, which in turn inhibit lipoprotein lipase and triglyceride clearance, thus conferring a predisposition to both fasting and postprandial hypertriglyceridemia due to an increase in chylomicron-remnant particles. Increased concentrations of circulating chylomicron-remnant particles are then preferentially taken up by the liver by means of a receptor-mediated process,18–22 resulting in non-alcoholic fatty liver disease and hepatic insulin resistance.
There is now strong evidence that nonalcoholic fatty liver disease has an important role in causing hepatic insulin resistance.1–3,23–27 Recent studies have shown that increased hepatocellular concentrations of diacylglycerol in nonalcoholic fatty liver disease lead to activation of the protein kinase C epsilon isoform, resulting in reduced insulin signaling and hepatic insulin resistance.23–27 Furthermore, the reversal of nonalcoholic fatty liver disease by modest weight reduction corrects hepatic insulin resistance and fasting hyperglycemia in patients with type 2 diabetes mellitus.3 Consistent with these studies was our finding that modest weight reduction (approximately 6 kg) in Asian Indian men eliminated hepatic steatosis and markedly improved whole-body insulin sensitivity, as reflected by marked reductions in their plasma glucose and insulin concentrations and a doubling of their insulin-sensitivity index.
We found that the prevalence of variant APOC3 alleles was similar among Asian Indian men and non–Asian Indian men, suggesting that other factors are responsible for the increased prevalence of nonalcoholic fatty liver disease among the Asian Indian men.9 In this regard, we also genotyped the Asian Indian men for the SNP rs738409 in PNPLA3, which has recently been shown to be associated with nonalcoholic fatty liver disease by means of an unknown mechanism.11 We found that the CG and GG genotypes of this SNP were also associated with increased liver triglyceride content, as compared with the CC genotype in this population.
We also examined the fraction of variance in the risk of nonalcoholic fatty liver disease attributable to APOC3, PNPLA3, or both and found that they accounted for 11.0, 6.5, and 13.1%, respectively, of the variance, suggesting that these gene variants have an interactive effect. However, in contrast to the APOC3 variants described here, the PNPLA3 variants have not been found to be associated with insulin resistance.11
In summary, we found that genetic variants in APOC3 were associated with nonalcoholic fatty liver disease and insulin resistance in a cohort of Asian Indian men. We confirmed this observation in a second cohort of non–Asian Indian men.
Supplementary Material
Acknowledgments
Supported by grants from the U.S. Public Health Service (R01 AG-23686, R01 DK-49230, P01 DK-068229, P30 DK-45735, and UL1 RR-024139) and by Distinguished Clinical Scientist Awards from the American Diabetes Association (to Dr. Petersen and Dr. Shulman).
We thank Donna Caseria, B.S., R.D., Carolyn Canonica, B.S., Christopher Cunningham, B.S., Yanna Kosover, Irina Smolgovsky, Mikhail Smolgovsky, Donna D’Eugenio, R.N., Gina Solomon, R.N., Caitlyn Schalich, B.S., and the staff of the Yale Center for Clinical Investigation Hospital Research Unit for their technical support, as well as all the volunteers for their participation in these studies.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Petersen KF, Oral EA, Dufour S, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–50. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seppälä-Lindroos A, Vehkavaara S, Häkkinen AM, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–8. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 3.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–8. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Dijk KW, Rensen PC, Voshol PJ, Havekes LM. The role and mode of action of apolipoproteins CIII and AV: synergistic actors in triglyceride metabolism? Curr Opin Lipidol. 2004;15:239–46. doi: 10.1097/00041433-200406000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Olivieri O, Bassi A, Stranieri C, et al. Apolipoprotein C-III, metabolic syndrome, and risk of coronary artery disease. J Lipid Res. 2003;44:2374–81. doi: 10.1194/jlr.M300253-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Olivieri O, Stranieri C, Bassi A, et al. ApoC-III gene polymorphisms and risk of coronary artery disease. J Lipid Res. 2002;43:1450–7. doi: 10.1194/jlr.m200145-jlr200. [DOI] [PubMed] [Google Scholar]
- 7.Guettier JM, Georgopoulos A, Tsai MY, et al. Polymorphisms in the fatty acid-binding protein 2 and apolipoprotein C-III genes are associated with the metabolic syndrome and dyslipidemia in a South Indian population. J Clin Endocrinol Metab. 2005;90:1705–11. doi: 10.1210/jc.2004-1338. [DOI] [PubMed] [Google Scholar]
- 8.Miller M, Rhyne J, Chen H, et al. APOC3 promoter polymorphisms C-482T and T-455C are associated with the metabolic syndrome. Arch Med Res. 2007;38:444–51. doi: 10.1016/j.arcmed.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen KF, Dufour S, Feng J, et al. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci U S A. 2006;103:18273–7. doi: 10.1073/pnas.0608537103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 11.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–5. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li WW, Dammerman MM, Smith JD, Metzger S, Breslow JL, Leff T. Common genetic variation in the promoter of the human apo CIII gene abolishes regulation by insulin and may contribute to hypertriglyceridemia. J Clin Invest. 1995;96:2601–5. doi: 10.1172/JCI118324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 14.Petersen KF, Dufour S, Savage DB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:12587–94. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Browning JD. Statins and hepatic steatosis: perspectives from the Dallas Heart Study. Hepatology. 2006;44:466–71. doi: 10.1002/hep.21248. [DOI] [PubMed] [Google Scholar]
- 16.Ito Y, Azrolan N, O’Connell A, Walsh A, Breslow JL. Hypertriglyceridemia as a result of human apo CIII gene expression in transgenic mice. Science. 1990;249:790–3. doi: 10.1126/science.2167514. [DOI] [PubMed] [Google Scholar]
- 17.Pollin TI, Damcott CM, Shen H, et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–5. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper AD. Role of the liver in the degradation of lipoproteins. Gastroenterology. 1985;88:192–205. doi: 10.1016/s0016-5085(85)80155-4. [DOI] [PubMed] [Google Scholar]
- 19.Windler EE, Greeve J, Daerr WH, Greten H. Binding of rat chylomicrons and their remnants to the hepatic low-density-lipoprotein receptor and its role in remnant removal. Biochem J. 1988;252:553–61. doi: 10.1042/bj2520553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherrill BC, Dietschy JM. Characterization of the sinusoidal transport process responsible for uptake of chylomicrons by the liver. J Biol Chem. 1978;253:1859–67. [PubMed] [Google Scholar]
- 21.Nagata Y, Chen J, Cooper AD. Role of low density lipoprotein receptor-dependent and -independent sites in binding and uptake of chylomicron remnants in rat liver. J Biol Chem. 1988;263:15151–8. [PubMed] [Google Scholar]
- 22.Weintraub MS, Eisenberg S, Breslow JL. Different patterns of postprandial lipoprotein metabolism in normal, type IIa, type III, and type IV hyperlipoproteinemic individuals: effects of treatment with cholestyramine and gemfibrozil. J Clin Invest. 1987;79:1110–9. doi: 10.1172/JCI112926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuel VT, Liu ZX, Qu X, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–53. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 24.Samuel VT, Liu ZX, Wang A, et al. Inhibition of protein kinase Cε prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117:739–45. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi CS, Savage DB, Kulkarni A, et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem. 2007;282:22678–88. doi: 10.1074/jbc.M704213200. [DOI] [PubMed] [Google Scholar]
- 26.Savage DB, Choi CS, Samuel VT, et al. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by anti-sense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest. 2006;116:817–24. doi: 10.1172/JCI27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang D, Liu ZX, Choi CS, et al. Mitochondrial dysfunction due to long-chain Acyl-CoA dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance. Proc Natl Acad Sci U S A. 2007;104:17075–80. doi: 10.1073/pnas.0707060104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.