Until about 2 years ago, a transplanted organ was seen as an island in a hostile recipient sea. Then, a different perspective emerged about the reason for organ graft acceptance.1 This was based on the startling observation that donor leukocytes from these transplanted organs had migrated and survived throughout the body of the recipient for as long as three decades.2–6 The events following transplantation then were seen as a two-way cell interaction—one direction being a graft-vs-host reaction and the other the conventional host-vs-graft (rejection) reaction.
Because the so-called passenger leukocytes from the graft were multilineage and derived originally from the bone marrow, the organ transplantation was in effect a mini bone marrow transplantation. At the vast interface between the coexisting donor and recipient cell populations, we suggested that changes occurred in the way each cell population viewed the other, and that these changes defined the mysterious suppressor and veto cells about which hundreds of articles have been written.1,6
We believe that the liver is the most tolerogenic transplanted organ because of its much larger total load as well as its lineage profile of the migratory leukocytes. With the liver and other leukocyte-rich organs, the duality of the immunologic reactions— graft-vs-host and host-vs-graft—were thought to be especially important.5 However, with all successful transplantations, no matter what the organ, the graft as well as the recipient became genetic composites following the migration, composed of cells of both parties.
This transition within the graft is particularly dramatic in the successfully transplanted intestine in which the donor epithelium sits on a bed of leukocytes that can be identified as recipient with specific monoclonal antibodies. 7,8 The presence of a double cell population also is dramatically evident in the liver9,10 and less so in kidney11 and heart.
No matter what organ is transplanted, there are, of course. David and Goliath proportions of the donor and recipient cell populations at the outset, but in each, the interaction of the two cell populations can be envisioned as a teeter-totter in which each side can cancel the immunologic effect of the other in what we have called mutual natural immunosuppression. We believe that this reciprocal interaction blindfolds the MHC effect and explains very well why tissue matching, which is crucial for successful clinical bone marrow transplantation, does not accurately predict outcome after transplantation of whole organs.12
In our original description of the spontaneous chimerism, we emphasized that graft acceptance by this mechanism may “become stable without further treatment, or (in other cases) only when continued immunosuppression is provided. ” 1 Failure was defined as instability of the chimerism which could be manifested by rejection (most commonly), but also by graft-vs-host disease (GVHD). Although organ acceptance was different than the acquired tolerance of Billingham et al,13,14 the relation of the two was easy to understand.
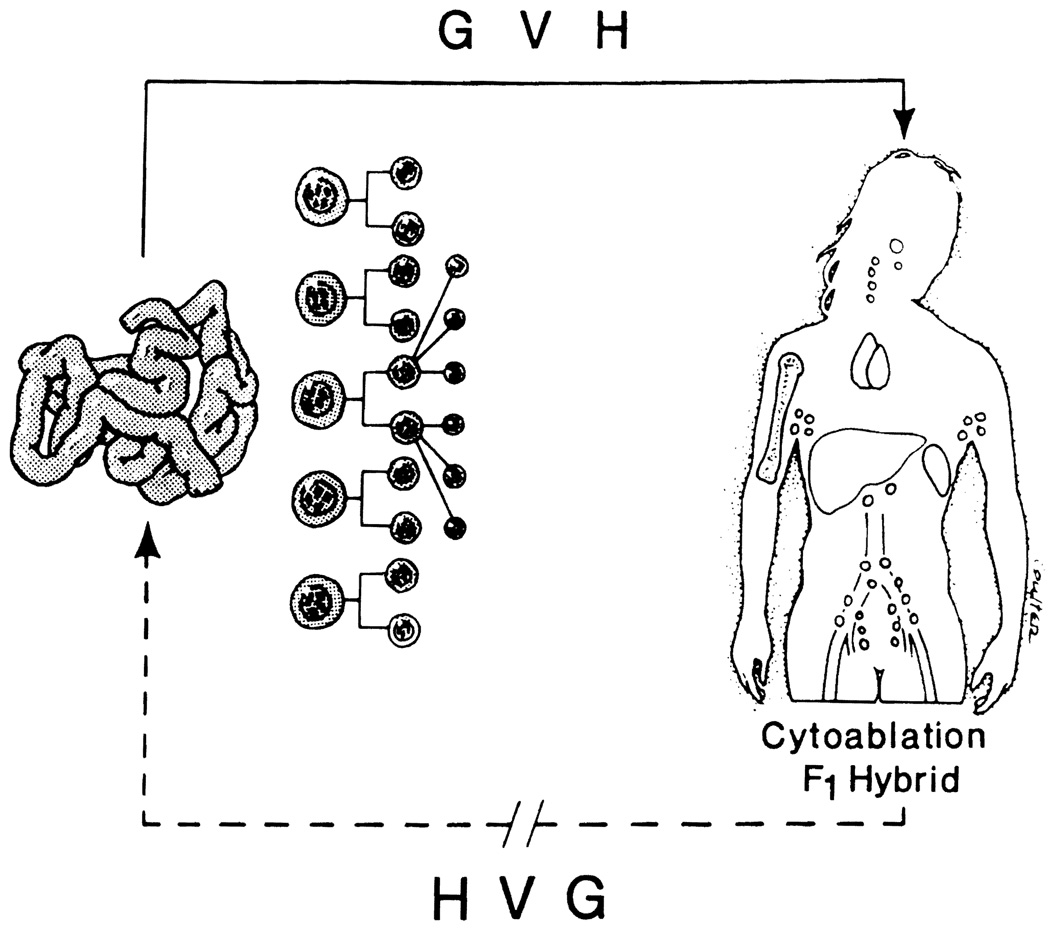
The experimental model of Billingham et al was unbalanced by reason of recipient immunologic immaturity. Imbalance can also be achieved genetically in the F1 hybrid parent-to-offspring animal model, and iatrogenically by recipient cytoablation with irradiation or cytotoxic drugs. Then, if the immunocytes in the transplanted organ are sufficiently numerous (as in the intestine), one of the censoring limbs is absent and GVHD follows in the same way as with bone marrow transplantation (Fig 1). However, if neither the recipient nor the graft is leukocyte depleted, it is possible to routinely perform intestinal or even multivisceral transplantation without an exorbitant risk of graft-vs-host disease. 15
Fig 1.
Transplantation of a leukocyte-rich organ (eg, small bowel) into a recipient who has been cytoablated with irradiation or cytotoxic drugs, or else into a F1 hybrid parent-to-off-spring animal will lead to graft-vs-host-disease (GVH) but will not initiate host-vs-graft reactions (HVG).
The natural chimerism concept defines the difference between bone marrow and whole organ transplantation entirely in terms of the therapeutic strategies that are used. Bone marrow transplantation is conceptually derived directly from the original Billingham et al model in that one of the immunologic reaction limbs is iatrogenically disabled. This leaves the recipient GVHD prone, and requires MHC matching for survival. Success is called tolerance. In contrast, the treatment strategy evolved empirically for whole organ transplantation leaves both cell populations intact, thereby removing MHC matching as a requisite for success, and largely eliminates the threat of GVHD. Engraftment of the transplanted organ, which depends on survival of the donor leukocyte population, commonly is called graft acceptance.
ALL NATURE IS BUT ART UNKNOWN TO THEE*
Two important clues that cell migration or something like it must be occurring were observed in the first patients to be treated with the combination of azathioprine and prednisone. Rejection could be easily reversed in these kidney recipients, and more importantly, there was a subsequent ability to lighten immunosuppressive doses, often to surprisingly low levels. 16 This was referred to in the title of the article as tolerance. Although the term was bitterly criticized at the time, it has proved to be conceptually correct (see later). The implication was not that drugs could be stopped but that the evolution of donor-specific nonreactivity had begun.
The use of living volunteer kidney donors for these pioneer patients made their prototype course easy to characterize. After a few days or weeks of good function of the transplanted kidney, the initially high creatinine clearance and satisfactorily low BUN obtained in most cases deteriorated with the onset of a rejection. These adverse events were easily reversed with what were then thought to be astronomical doses of prednisone. More importantly, the steroids could be weaned or in some cases stopped, and usually the eventual azathioprine dose was lowered to less than what had failed at the outset to prevent rejection of variable severity. A number of these patients then had stable graft function for more than 30 years. 17
These observations crystallized the central therapeutic dogma upon which whole organ transplantation is based. It calls for baseline therapy with one or two drugs, secondary adjustments as needed with steroids, and then individualized drug weaning to whatever maintenance levels are required to have stable graft function. The baseline agents have improved through the years (Table 1), but the dogma has remained the same.
Table 1.
Central Therapeutic Dogma of Immunosuppression
| Strategy | Baseline Agents | Mode of Action |
|---|---|---|
| Baseline therapy with one or two drugs | Azathioprine | ↓ DNA synthesis |
| Cyclophosphamide | ↓ DNA synthesis | |
| CyA | ↓ IL-2 production | |
| FK 506 | ↓ IL-2 production | |
| Secondary adjustments with steroids or antilymphoid agents | ||
| Case by case trial (and potential error) of weaning |
In the ensuing three decades, many details of rejection were clarified: its dependence on antigen-presenting cells, the necessity for a costimulatory molecule, the role of accessory molecules, and the way that cytokines controlled clonal expansion of T helper and the cytotoxic T cells that are the agents of allograft destruction. However, the curious thing, which has been most completely documented in experimental animals, was the diversity of agents with which long-term or permanent graft survival could be induced with a short course of therapy no matter what the level of intervention in the immune reaction. 18 Deoxyspergualin was said to alter the antigen-presenting cell. The antimetabolites prevented clonal expansion by inhibition of DNA synthesis. Cyclosporine (CyA) and FK 506 disrupted T-cell receptor signals to the nucleus. Monoclonal antibodies interrupted the immune reaction at various specific targets, including accessory molecules, and rapamycin interdicted the action of normally formed cytokines. These nonspecific drugs appeared to be permissive of a natural event that became specific only by virtue of the presence of donor antigens.
What was happening was revealed in retrospect by studies nearly 30 years posttransplantation of a group of patients treated at the University of Colorado in 1962 and 1963 with the then new azathioprine-prednisone protocol. Both the donors and recipients underwent preoperative delayed hypersensitivity skin tests with tuberculin, histoplasmin, and other antigens. Recipients who were negative to these antigens preoperatively but whose donors were positive acquired the positive skin tests if the kidney transplantation succeeded, but not if it failed. The explanation offered was adoptive transfer of immunity, which explicitly meant the migration of donor leukocytes.19 However, this was not considered plausible because the kidney at that time was thought to be a leukocyte-poor organ.
However, the memory of the earlier observations lingered on, and in the spring and summer of 1992, five of these original patients whose grafts still functioned normally were studied for chimerism in their blood, skin, and lymph nodes. Their donors, who still were alive, cooperated. All five recipients were found to be chimeras.4 The identity of the donor and recipient cells was established with either cytostaining, allowing the donor cells to be seen in the tissues, or by polymerase chain reaction (PCR). which identified donor DNA. The markers were donor-specific HLA alleles of chromosome 6 and the Y chromosome when there had been a male donor to female recipient.
Chimerism also was found in all 22 liver recipients who were studied from 10-1/2 to 21 years after liver replacement. Its generalized nature was evident from a more complete tissue sampling than in the kidney recipients.5 In addition to possessing donor HLA alleles in the chimeric cells. nine of these hepatic recipients were women who had been given male livers. The chimerism was confirmed in all nine cases by the additional presence of Y chromosomes. 2 The identifiable chimeric cells usually were sparse, estimated to be 1/1000 or fewer. However, confirmatory PCR studies in all of the kidney and liver recipients left no doubt about the validity of the cytochemical findings.
Before long. chimerism also was proved in recipients of thoracic organs.5 An important study was reported last week in Venice by the Pittsburgh group who stratified 15 lung transplant recipients followed 1 to 5 years into a favorable group of 8 with no bronchiolitis obliterans and 7 who had the ominous finding of chronic rejection. The patients without bronchiolitis obliterans had dense chimerism—positive in seven of seven lymph nodes, seven of eight skin biopsies, and six of eight blood samples. Chimerism was demonstrable in the less favored group, but less regularly and with a generally lower quantitative grade. Using the cryopreserved donor spleen cells as stimulators, donor-specific nonreactivity was demonstrated in all but one of the densely chimeric recipients, but in only one of the less favored group (Table 2).
Table 2.
Donor Cell Chimerism and In Vitro Immune Reactivity; Correlation With Bronchiolitis Obliterans in Lung Allograft Recipients 1–5 Years After Transplantation
| Donor Cell Chimerism | |||||
|---|---|---|---|---|---|
| Bronchiolitis Obliterans |
MLR Donor-Specific Hyporeactivity |
||||
| n | Blood | Skin | Lymph Node |
||
| No | 8 | 6/8 | 7/8 | 7/7 | 6/7* |
| Yes | 7 | 3/7 | 2/7 | 3/7 | 1/4† |
From Keenan et al (see text above).
One patient had persistently low pre- and posttransplant MLR responses.
In vitro immune testing was only possible in five of seven recipients: of the five tested, one had persistently low pre- and posttransplant MLR responses (perfectly matched MHC class II).
CAN DRUGS BE STOPPED?
Liver Recipients
Once it is conceded that organ graft acceptance is mechanistically associated with the surviving donor multilineage passenger leukocytes, it is possible to envision the engraftment of any whole organ in the same context as a small bone marrow transplantation. Then, the natural question is if some of these patients could have their immunosuppression stopped all together. At the time (in April–June 1992) of the chimerism investigations, there were 43 patients who had survived for 12 to 23 years after liver replacement. Six of them had long since discontinued therapy, to whom four more have been added. Presently, 10 of the remaining 43 long-term survivors have been drug free for 1 to 15 years.
Because complications of immunosuppression have been the principal causes of late death in our chronically surviving liver recipient population, a prospective weaning trial was begun. Some patients were excluded for a variety of reasons, but a total of 59 were entered. Twenty-one already have had complete weaning from 3 months to 13 years. Twenty-four more have had reductions to homeopathic doses over 4 to 14 months. Immunosuppression has been resumed in only 24% of cases because of mild rejection in nine patients, moderate rejection in three, and histopathologically severe rejection in two. No patients became jaundiced, no grafts were lost, and there was no permanent loss of graft function. The patients who flunked the trial were restored to preexisting immunosuppression. Our conclusion is that cautious weaning can be safely undertaken under careful surveillance 5 or 10 years after liver transplantation.
Kidney Recipients
Drug discontinuance is far more dangerous in kidney recipients, but it is well known to be feasible in isolated cases. Among 10 of our patients from 1962 to 1963, including a man bearing the longest continuously functioning kidney allograft in the world and about 2/3 of such survivors left from the world’s experience preceding 1964, 17 4 were MHC matched but the other 6 had one or two haplotype mismatches. When tested in 1992 along with their donors. all of these patients had donor-specific nonreactivity by mixed lymphocyte reaction (MLR) and cell-mediated lympholysis (CML) testing, which was absolute in 8 of the 10 cases and pronounced in the other 2.
Five of these 10 patients are off immunosuppression and have been for 1, 1-2/3, 14, 28, and 30 years. Three of the five drug-free patients did not have HLA identity and had been shown by detection of donor HLA alleles to have chimerism in their tissues. Thus, chimerism may be stable without further treatment, instead of requiring immunosuppression for lifetime as we have always assumed in the past. Although trying to predict which patients can come off drugs is an unpredictable and dangerous exercise, the fact that kidney recipients can achieve this state is the point to be made.
IATROGENIC AUGMENTATION OF CHIMERISM
If chimerism is a seminal event in graft acceptance, it would be advantageous to add to the minimal dose of the so-called passenger leukocytes, which are of bone marrow origin (see earlier), by giving unaltered donor bone marrow at the time the natural chimerism occurs, namely perioperatively. Such a trial is well underway in Pittsburgh and now includes 30 patients. 20 The donor bone marrow cells are obtained from the thoracico-lumbar vertebrae of the cadaveric donor. This is a rich source of leukocytes that contains fewer mature T leukocytes than in other locations.
The first 18 patients given 3 × 108 cells/kg included 7 liver recipients (1 also given pancreatic islets), 10 kidney recipients (2 with pancreatic islets), and 1 recipient of a heart. The patients were not preconditioned, and postoperative immunosuppression was with standard FK 506 and prednisone. All 18 patients have had a very good clinical result.
Seventeen of the 18 patients have demonstrable macro-chimerism, the only exception being a kidney recipient who had no markers to be studied because of a perfect MHC match with a donor of the same sex. The use of different technologies allowed cross-confirmation of results. The highest yield was with PCR, showing chimerism in 16 of the 18 cases. Quantitation of the chimerism also was done with a technique of PCR coamplification developed by Dr Massimo Trucco and his associates.20 After male-to-female transplantation in four cross-sex combinations, all four recipients had Y chromosomes detectable, and in these cases there was an excellent correlation with the results obtained using donor HLA allele detection.
The yield with flow cytometry was 14/18. showing .9% to 6.4% circulating donor leukocytes 3 months to 1 year after transplantation. After the marrow-organ transplantation, the initial wave of circulating donor cells usually recedes to a nadir after 2 or 3 months and then increases progressively to a stable level thereafter. The density of chimerism was estimated to be generally 1000× or greater than that occurring spontaneously. The follow-up on these patients is now 4 to 16 months.
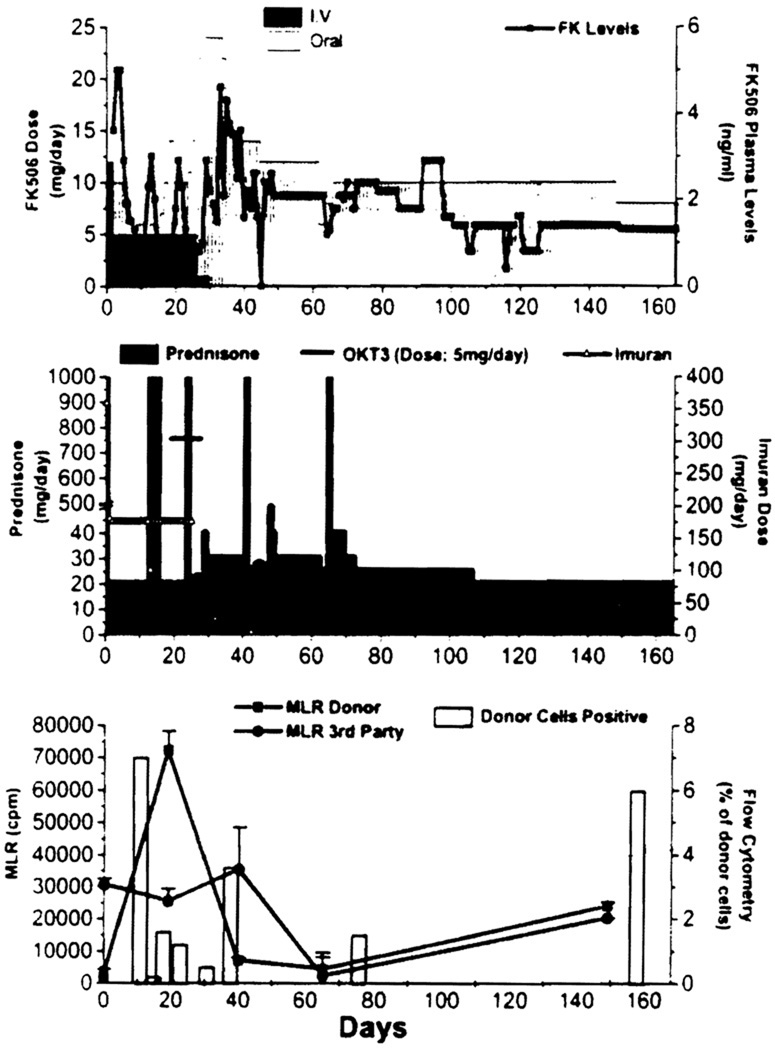
Twelve of the 17 patients who could be studied have lower donor-specific reactivity by MLR to third party cells, and in 9 there was multiple other in vitro evidence of donor-specific nonreactivity, as early as 50 days following transplantation. The exceptional patient in the series who maintained vigorous donor-specific reactivity was the heart recipient who had two rejections, the first at 1 month and the second at 60 days. These were treated with OKT3 and/or the addition of azathioprine. However, throughout the course, this patient always maintained blood chimerism. Circulating donor leukocytes were initially in the 10% range, and stabilized at 6% at the end of 160 days (Fig 2). Despite this high level of chimerism and a good clinical result, donor and third party-specific reactivity have remained parallel.
Fig 2.
Immunosuppression profile, serial in vitro donor (■), and third party (●) specific MLR responses and the level of donor cell chimerism (as determined by flow cytometry) in a simultaneous heart and bone marrow transplant recipient.
There have been no serious complications in this trial, now totalling 30 patients. As was expected, rejection has been diagnosed in 50% of cases, but this was controlled with no more difficulty than usual. In two patients, a mild skin rash, which previously would have been passed off as a drug rash in a nonmarrow patient, was proved by biopsy to be minimal GVHD. These rashes, both in liver recipients, resolved spontaneously in one patient and in the other involuted after a minimal increase in her routine immunosuppression.
With these results in hand, it is worth briefly reviewing earlier efforts to use donor leukocytes to induce organ graft acceptance. The most extensive have been with the strategy developed by Monaco et al 21 in which donor bone marrow was stored and given about 3 weeks later by Barber et al of Alabama22 to a large group of kidney recipients and by Rolles et al in England23 to liver recipients. The results were disappointing, particularly in the English liver trials, reported in The Lancet last January 15.23 Inexplicably, chimerism could not be found in the British patients, either in the control or bone marrow augmented recipients. With what we now know, the delayed timing of the bone marrow must he questioned.
Donor-specific transfusion, such as advocated by Salvatierra et al,24 is another example of the leukocyte augmentation principle, but in these trials (especially those elsewhere than in San Francisco), the timing was highly variable—frequently well before organ transplantation—and often with no attention to preserving the white cells or even with their deliberate destruction or removal.
SEARCH FOR THE TOLEROGENIC CELL
The clinical portion of this discussion has been presented first because it provided the pathway of discovery. However, much additional information has been added. In immunosuppressed rats. Demetris et al25 have shown how the migratory cells begin to home to the central lymphoid organs within minutes, where their identification can be facilitated by injecting gamma interferon (to increase antigen expression) a few hours before the animals are killed.26 After a pause of 2 or 3 weeks, the leukocytes break out and become generalized.
These studies and those in mice27 showed that the migration is multilineage, following the same routes as syngeneic cells—B cells to B-cell areas of the lymph nodes, spleen, and thymus, T cells to T-cell-rich regions, and the dendritic cells and macrophages to their normal destinations. A bonus in the mouse experiments was the finding that permanent survival of liver allografts occurred without the need for treatment with all MHC disparities. These mouse liver recipients could accept donor strain skin and heart, but not grafts from third parry strains. The degree of natural chimerism was similar to that in rats.
Having obtained so much suggestive evidence that chimerism is the fundamental explanation of graft acceptance, the obvious question was how the migratory leukocytes induced tolerance. To address the question, the mouse liver was chosen as the source of nonparenchymal leukocytes.28 After separating the hepatocytes and duct cells from the leukocytes, about 1.0 × 107 nonparenchymal cells (NPCs) could be obtained from one mouse liver. Using the technique described by Inaba et al,29 these NPCs were cultured in granulocyte macrophage colony stimulating factor (GMCSF)-enriched medium, which gives a selective growth advantage to leukocytes of myeloid lineage. After 4 or 5 days of culture, there was a subpopulation of approximately 2 million cells which expressed cell surface markers characteristic of dendritic cells (NLDC-145+, 33D1 +, and N418+).
From the cluster of these cells that formed on the bottom of the liquid culture wells, cells that floated free were picked out for further culture and studied. These had the light and electron microscopic appearance of a precursor of dendritic cells. However, it was difficult at first to prove that they actually were dendritic cells because it was impossible to drive them to maturation, even after pulsing the culture with gamma interferon and tumor necrosis factor. They were not allostimulatory, could not be made to express high levels of class II antigen, and were avidly phagocytic.
The impasse was broken after it was pointed out (A.J.D.) that the majority of dendritic cells in normal livers are located in the areas that are rich in type I collagen. When this microenvironment was simulated by coating the culture wells with type I collagen, the precursor cells promptly assumed the properties of dendritic cells, now strongly expressing class II antigen. However, it was still not known if these cells would mature and express class II antigen in vivo. This question was answered by injecting purified precursor cells (class II-depleted) from fully allogeneic B10.BR livers into the footpad of B10 mouse recipients. They migrated promptly to the T-cell areas of the central lymphoid organs where they were easily identified as donor with donor-specific monoclonal antibody staining and shown to express class II antigen. 28 Thus, their character depended on the microenvironment in which they were placed.
The crucial next step explains (we believe) how chimeric dendritic cells, which have been thought to have a life expectancy of only a few days or weeks, could be perpetuated in the tissues of our patients for up to 30 years. In the first phase of these mouse experiments, liver transplantation was carried out in the fully allogeneic but nonrejecting mouse strain (combination B10→C3H). As expected, the recipient animals became chimeric. Samples containing the mixed donor and recipient cells were collected from the spleen, thymus, and lymph nodes. In these tissues, donor as well as recipient dendritic cells at variable stages of maturation could be demonstrated with the same culture techniques as had been used for study of the liver cell population (Lu, personal communication. March 22, 1994). Our assumption is that these cells were derived from precursor dendritic cells or even pluripotent stem cells in these widely distributed recipient foci. In the liver recipients, the profile of both donor and recipient cells was much the same 4, 14, or 150 days after the transplantation.
Thus, the liver grafts had exported leukocytes that generated multiple active niduses that included donor as well as similar recipient precursor cells. The result was the creation of widespread and persistent cellular oasis. Even though heart grafts were rejected in similar but separate experiments, they also initiated the same process, but apparently not vigorously or extensively enough for it to be self-sustaining. These remarkable findings suggest a mechanism for perpetuation of the migratory dendritic cells, and along with an independent line of inquiry by Hara et al,30 they have suggested a means by which the chimeric cells can be tolerogenic.
Hara et al have shown that the anterior chamber of the eye, which is an immunologically privileged site, is lined by immature dendritic cells. In the anterior chamber, which Hara et al described as being rich in transforming growth factor beta (TGFβ), antigenic peptides are engulfed by these cells and travel to the spleen where they evoke a tolerogeneic instead of antigenic response. We suspect that our subpopulation of hepatic NPCs, smaller numbers of similar cells in other organs, and Hara et al’s tolerogenic anterior chamber cells are fundamentally the same. Verbanac et al31 have obtained evidence that the veto cell is an immature dendritic cell whose function is TGFβ linked.
COMMENT
It is hard to understand how at least the crude outlines of a phenomenon as obvious and fundamental as the spontaneous chimerism of this discussion could have escaped our notice for all these years. When clinical organ transplantation was first performed on a large scale beginning in 1962, it was predicted to fail by most immunologists. Yet it succeeded, understandably to the special bewilderment of those who ostensibly knew the most. The reasons why it succeeded now seem to us self-evident. The clues were always there, in every clinic around the world, but they were ignored because they did not fit preconceived patterns. Having now seen both sides of the bidirectional cell reaction that is implicit with cell migration and chimerism after whole organ transplantation, we have crawled intellectually inside of the system. We believe that this will allow us to improve the ways that we manage our patients.
Acknowledgments
Supported by research grants from the Veterans Administration and Project grant DK 29961 from the National Institutes of Health, Bethesda, Maryland.
Footnotes
All Nature IS but Art unknown to thee;
All Chance direction which thou cans’t not see;
All Discord Harmony not understood;
All partial Evil universal Good.
And spite of Pride, in erring Reason’s spite,
One truth is clear, “Whatever is, is RIGHT. ”
Alexander Pope (Essay on Man): 1730 A.D.
REFERENCES
- 1.Starzl TE, Demetris AJ, Murase N, et al. Lancet. 1992;339:1579. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Demetris AJ, Trucco M, et al. Lancet. 1992;340:876. doi: 10.1016/0140-6736(92)93286-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Demetris AJ, Trucco M, et al. N Engl J Med. 1993;328:745. doi: 10.1056/NEJM199303183281101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Demetris AJ, Trucco M, et al. Transplantation. 1993;55:1272. doi: 10.1097/00007890-199306000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE, Demetris AJ, Trucco M, et al. Hepatology. 1993;17:1127. [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Demetris AJ, Murase N, et al. Immunol Today. 1993;14:326. doi: 10.1016/0167-5699(93)90054-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murase N, Demetris AJ, Matsuzaki T, et al. Surgery. 1991;110:87. [PMC free article] [PubMed] [Google Scholar]
- 8.Iwaki Y, Starzl TE, Yagihashi A, et al. Lancet. 1991;337:818. doi: 10.1016/0140-6736(91)92517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter KA. In: Experience in Hepatic Transplantation. Starzl TE, editor. Philadelphia, PA: WB Saunders Co.; 1969. p. 464. [Google Scholar]
- 10.Kashiwagi N, Porter KA, Penn I, et al. Surg Forum. 1969;20:374. [PMC free article] [PubMed] [Google Scholar]
- 11.Randhawa PS, Starzl T, Ramos HC, et al. Am J Kidney Dis. doi: 10.1016/s0272-6386(12)80162-4. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starzl TE, Demetris A, Trucco M. Clin Transplant. 1993;7:353. [PMC free article] [PubMed] [Google Scholar]
- 13.Billingham RE, Brent L, Medawar PB. Nature. 1953;172:603. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 14.Billingham R, Brent L, Medawar P. Philos Trans R Soc Lond (Biol) 1956;239:357. [Google Scholar]
- 15.Todo S, Tzakis AG, Abu-Elmagd K, et al. Ann Surg. 1992;216:223. doi: 10.1097/00000658-199209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starzl TE, Marchioro TL, Waddell WR. Surg Gynecol Obstet. 1963;117:385. [PMC free article] [PubMed] [Google Scholar]
- 17.Starzl TE, Schroter GPJ, Hartmann NJ, et al. Transplant Proc. 1990;22:2361. [PMC free article] [PubMed] [Google Scholar]
- 18.Thomson AW, Starzl TE, editors. Immunosuppressive Drugs: Developments in Anti-Rejection Therapy. Edward Arnold; 1994. p. 1. [Google Scholar]
- 19.Wilson WEC, Kirkpatrick CH. In: Experience in Renal Transplantation. Starzl TE, editor. Philadelphia, PA: WB Saunders Co; 1964. p. 239. [Google Scholar]
- 20.Fontes P, Rao A, Demetris AJ, et al. Lancet. 1994;344:151. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monaco AP, Clark AW, Wood ML, et al. Surgery. 1976;79:384. [PubMed] [Google Scholar]
- 22.Barber WH, Mankin JA, Laskow DA, et al. Transplantation. 1991;51:70. doi: 10.1097/00007890-199101000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Rolles K, Burrough AK, Davidson BR, et al. Lancet. 1994;343:263. doi: 10.1016/s0140-6736(94)91113-4. [DOI] [PubMed] [Google Scholar]
- 24.Salvatierra O, Jr, Melzer J, Potter D, et al. Transplantation. 1985;40:654. doi: 10.1097/00007890-198512000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Demetris AJ, Murase N, Fujisaki S, et al. Transplant Proc. 1993;25:3337. [PMC free article] [PubMed] [Google Scholar]
- 26.Rao AS, Demetris AJ, Qian S, et al. Cell Transplant. doi: 10.1177/096368979400300412. (in press) [DOI] [PubMed] [Google Scholar]
- 27.Qian S, Demetris AJ, Murase N, et al. Hepatology. 1994;19:916. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu L, Woo J, Rao AS, et al. J Exp Med. (in press) [Google Scholar]
- 29.Inaba K, Steinman RM, Pack MW, et al. J Exp Med. 1992;175:1157. doi: 10.1084/jem.175.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hara Y, Okamoto S, Rouse B, et al. J Immunol. 1993;151:5162. [PubMed] [Google Scholar]
- 31.Verbanac KM, Carver FM, Haisch CE, et al. Transplantation. doi: 10.1097/00007890-199403270-00022. (in press) [DOI] [PubMed] [Google Scholar]