IN recent years more and more attention has been paid to the so-called vanishing bile duct syndrome, a progressive loss of intrahepatic bile ducts observed in various liver diseases, including chronic rejection of allografts.1–5 As yet, the factors responsible for causing this condition still remain mostly unknown. In a series of analysis on transplanted livers,6–7 we found that in failed grafts, vascular lesions particularly of hepatic arteries occur and underlie the bile duct damages, suggesting ischemia to be an important causative factor. However, little is unknown about the normal vascular supply of small bile ducts; the anatomical observations made so far only dealt with periductal vessels of rodents,8–11 not of man. Not disclosed have been the nature and the predisposed site of such vascular injuries and their anatomical correlation with the ductular diseases.
In the present report, we describe our recent studies on the 3-D microstructure of normal peribiliary small vessels and how and to what extent they could undergo obstructive changes in chronic rejection, on which basis to shed light on the blood-vessel-dependent aspect of bile duct injuries in transplanted livers. Bile duct loss will be shown closely related with diseases of supplying vessels in the peribiliary plexus, reflecting the significance of ischemia as a co-factor underlying chronic rejection. Analysis was further extended to hepatic lesions in patients with primary biliary cirrhosis (PBC); this is to understand what this condition has in common with chronic rejection, since there appear to be similarities between the two diseases, as long as the microscopic appearance of the liver is concerned.
MATERIALS AND METHODS
Studied were four normal autopsy livers and five surgical specimens of liver, the latter having been obtained from two patients with failed allografts and three with PBC.
Clinical data of these patients are summarized in Table 1. Two of the four normal livers were fixed by perfusing with 2.5% glutaraldehyde via the hepatic artery and simultaneously via the portal vein; the other specimens, including the remaining two autopsy livers, were simply fixed by immersion in 10% formaldehyde. All the specimens were embedded in celloidin-paraffin. Three hundred to six hundred serial sections were prepared from each block at 3 μm thickness and stained with Elastica-Goldner stain.
Table 1.
Cases Examined
| •Material Case | Age | Sex | |
|---|---|---|---|
| 1 | 19 | F | Chronic Rejection Transplanted for Cirrhosis |
| 2 | 27 | M | Chronic Rejection Transplanted for CAH |
| 3 | 58 | F | PBC (Stage I + II) |
| 4 | 49 | F | PBC (Stage I + II) |
| 5 | 49 | F | PBC (Stage II) |
| 6 | 19 | M | Normal liver (autopsy) |
| 7 | 40 | F | Normal liver (autopsy) |
| 8 | 59 | M | Normal liver (autopsy) |
| 9 | 61 | M | Normal liver (autopsy) |
PBC, primary biliary cirrhosis; CAH, chronic active heoatitis.
Three-D reconstruction of small bile ducts and supplying vessels was performed with the aid of a computer system (PAS-310) specially designed for the study of 3-D microstructure at the Department of Pathology, the Institute for Tuberculosis and Cancer, Tohoku University.12 Using a profile projector (Reichert, model Visopan), a portal tract in the first section from a set of serial sections was projected onto a sheet of tracing paper at ×380, and the contours of bile ducts and blood vessels were delineated as faithfully as possible. The picture was placed on a digitizer, and the contours of bile ducts and blood vessels were input into the computer by tracing with a cursor. The process was repeated one after another until the last section was input; then the computer was ready to integrate in its display a 3-D picture rotated at any assigned angle around the x, y, and z axes. Different structures such as bile ducts, hepatic arteries, portal veins, and periductal small vessels were expressed in different colors. In the 3-D graphics, the loss of intrahepatic ducts was anatomically corrected with that of periductal small vessels. This correlation was further substantiazed by morphometry. The serial sections from the failed liver allografts were entirely scanned to show the difference in the density of periductal blood vessels between injured and uninjured ducts, by counting their number in each step of serial sections. These results were classified into three groups (D < 40 μm, 40 μm < D < 80 μm, and 80 μm < D) based on the sizes of the bile ducts subjected to observation.
RESULTS
Normal Microvasculature Supplying the Intrahepatic Bile Ducts
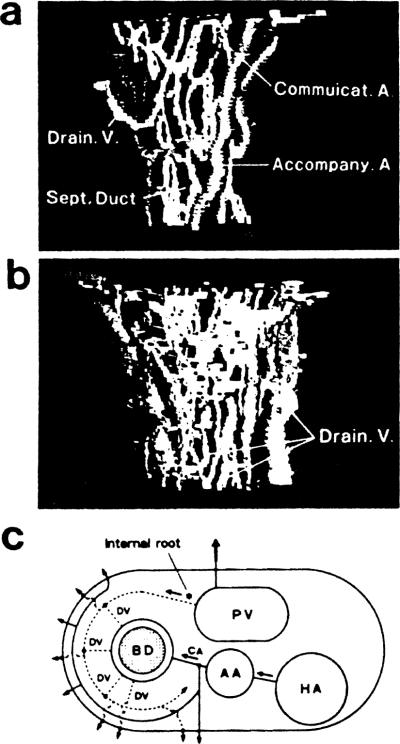
Fig 1a and 1b are computer-assisted 3-D pictures from a normal liver, showing a septal bile duct, about 80 μm in diameter, and peribiliary blood vessels. We confirmed that the basic pattern of vasculature is the same in more peripheral ducts, including the interlobular ducts. In Fig 1a, portal veins were omitted from the graphic presentation for the sake of better perspective of arterioles and capillaries. A dense capillary plexus, the peribiliary capillary network, is demonstrated surrounding a duct. Basically, this network is supplied by arterioles branching from the arteries that run parallel with the ducts. We call the latter the accompanying arteries (AA), and we call the former the communicating arterioles (CA). Usually, other arterioles are found dividing from AA and directly leading to sinusoids, without having any contact with the peribiliary network. In Fig 1b, the peribiliary network is shown drained into sub-branches of portal veins that surround the duct at a distance; these correspond to what has been called the “internal roots” of the portal system. The network also has a small number of venules that directly lead to sinusoids independently of the “internal roots.” In Fig 1c, the above pattern of vascular supply for a septal (or interlobular) duct is schematically presented. Probably this may be the first demonstration of the peribiliary vasculature of man, of which previous knowledge has been more or less partial. Of the result, special weight should be given to the finding that it is the communicating arterioles (CA) that finally supply the peribiliary network.
Fig 1.
Computer-aided 3-D pictures of normal peribiliary microvasculature showing (a) the afferent system; (b) the draining system; (c) a schema showing the basic pattern of periductal vasculature. BD, bile duct; AA, accompanying artery; CA, communicating arteriole; HA, larger hepatic artery; PV, portal vein; DV, draining venule.
Changes of Microvasculature and Their Relation to Ductal Injuries
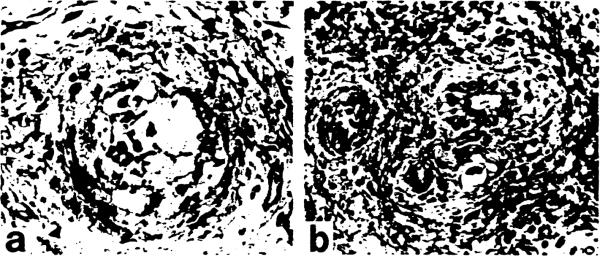
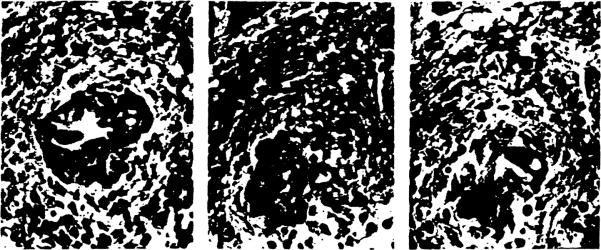
In the failed allografts (Casel, 2), severe obstructive arteriopathy proved to involve various levels of small arteries, including not only the accompanying arteries (AA) but the communicating arterioles (CA) around and in the peribiliary network (Fig 2a,b). In these, besides intimal thickening due to proliferating foam cells, the media were shown destroyed with infiltrating macrophages. Fig 3 is a 3-D picture from the failed graft (Casel) visualizing the changes of small ducts and peribiliary microvasculature. The upper one third of the interlobular duct has undergone marked atrophy, where communicating arterioles (CA) are no longer found leading to the peribiliary plexus. Correspondingly, the plexus in this region has been severely destroyed with significantly reduced density of capillaries. The concrete histologic appearances of its phenomenon are shown in Fig 4a–c. Therefore, it appears likely that the severer the arteriolar injuries and loss of peribiliary capillaries, the more marked the ductal damage.
Fig 2.
Severe obstructive arteriopathies in (a) an accompanying artery and (b) a proximal part of a communicating arteriole. Elastica-Goldner stain, ×260.
Fig 3.

A 3-D picture of peribiliary vessels from a failed graft (Case 1). The interlobular duct has undergone marked atrophy in the upper ⅓, where CAs are destroyed and periductal capillaries are mostly missing.
Fig 4.
(a) An interlobular duct with signs of marked atrophy (b) and (c) A communicating arteriole in b dwindles and disappears (c) after several steps of serial sections.
Also in the livers of PBC, there was a similar relationship between vascular and ductal injuries in the portal tracts where loss of CAs and capillaries was remarkable around the inflamed ducts. The vascular injuries appeared more remarkable when there was granulomatous reaction of epithelioid cells. However, there was an unmistakable difference from the allografts. In PBC, the AAs remained uninjured, even in the areas with fulminant ductal lesions. It appears that in this disease, the vessels are involved secondarily in the inflammatory process taking place in the surroundings, whereas in chronic rejection, arteriopathies are attributable not only to periportal inflammation but to other factors, including immune reaction against the small vessels themselves due to proximal arterial stenosis.
Morphometry
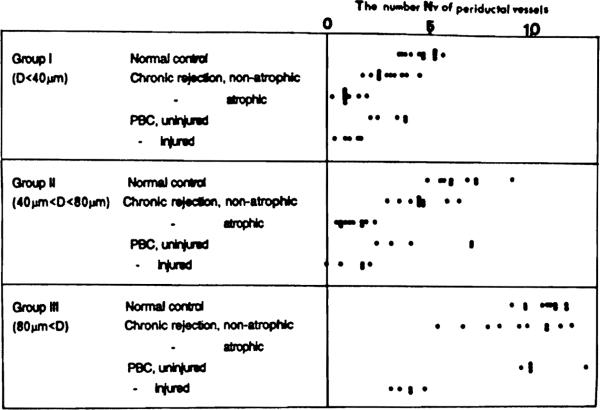
The number (Nv) of vessels around a duct, including capillaries and CAs, was counted at 60 ductular segments. These were sampled so as to contain each 30 segments with and without atrophy. In addition, another 30 ducts were sampled from PBC and control cases, respectively. In making statistical treatments of the data, the ducts were classified into three classes according to the external diameter D. The result is shown in Fig 5. In the failed allografts. Nvs were markedly low around segmental atrophic ducts for the ranges of less than 40 μm and between 40 μm and 80 μm. On the other hand, non-atrophic ducts showed moderately reduced Nvs for the same ranges. A similar result was obtained from the PBC. The differences proved to be significant by Student's t test, implying a correlation between the ductal and vascular damages.
Fig 5.
The number (Nv) of small vessels around a duct. For explanation, see text.
DISCUSSION
It has been known since the previous century11 that the intrahepatic bile ducts are equipped with a peribiliary vascular network that is supplied by hepatic arterial twigs and drained into sinusoids by the “internal roots,” an alternative pathway of the terminal portal vein. This was followed by many studies, most of which, however, were focused only on whether and to what degree the network functions as an arterio-portal shunt. Moreover, these studies were done on rodent livers, not on human livers, except for the observations by Mitra8 and Murakami9; the former was performed on materials intravascularly injected with india ink, and the latter on corrosion cast of hepatic vessels using the SEM. However, these techniques only allow one to visualize the vascular contours, while the surrounding structures, particularly the bile ducts, are left unvisualized in the void spaces. In this respect, a computer-assisted 3-D reconstruction appears to be a technique indispensable for the studies of microvasculature.
The technique allowed us to establish an overall view of the vasculature supplying and draining the peribiliary network in an integrated schema.
Worthy of special attention may be the presence of the communicating arterioles that have not been described in previous studies. These, periodically dividing off from the mother artery (AA) at intervals of 100 μm to 200 μm, appear especially susceptible to injuries because of their fragile structure, thus bringing ischemia to ducts and ductules. Also, the draining system can be affected by chronically persisting periportal inflammation. The blockade of venules by insults from within or without will further contribute to the impediment of peribiliary blood flow.
The basic anatomy of the normal peribiliary vasculature, once established, greatly helps understand the mechanism of “vanishing bile duct syndrome.” Oguma et al6,7 reported that in chronically rejected livers, there is a correlation of duct loss and the damage of hepatic arterial branches; the present report is an extension of that study where the observation was rather confined to AAs, not covering the whole terminal vasculature. In the present 3-D reconstructions, obstructive vascular diseases were shown involving more peripheral arterioles as well, including the CAs. Moreover, the loss of ducts and peribiliary capillaries proved to be exactly in the areas supplied by the diseased CAs, as shown in both 3-D pictures and morphometry, which strongly suggests that, at least partially, ischemia is responsible for causing these injuries.
On the other hand, no attention has been paid to the bile duct loss in terms of ischemia in PBC. The association of duct loss with the loss of CAs and peribiliary capillaries again raised the possibility of ischemia as a significant factor. However, in PBC, peribiliary vascular diseases appear to be secondary to immune responses against ducts.
Much has yet to be studied about the significance of vascular factors involved in the rejection of transplanted organs. We think, however, that the present study has provided a possible breakthrough into the pathology of rejection of transplanted organs. We are looking forward to gaining more insight into this aspect of organ transplantation.
REFERENCES
- 1.Sherlock S. Lancet. 1987;i:493. doi: 10.1016/s0140-6736(87)91802-2. [DOI] [PubMed] [Google Scholar]
- 2.Ludwig J. Semin Liver Dis. 1987;7:293. doi: 10.1055/s-2008-1040584. [DOI] [PubMed] [Google Scholar]
- 3.Snover DC, Freese DK, Sharp HL, Bloomer JR, Najarian JS, Ascher NL. Am J Pathol. 1987;11:1. doi: 10.1097/00000478-198701000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Vierling JM, Fennel RH, Batts KP, Perkins JD, Krom RAF. Hepatology. 1985;5:1076. [Google Scholar]
- 5.Ludwig J, Wiesner RH, Batts KP, Perkins JD, Krom RAF. Hepatology. 1987;7:476. doi: 10.1002/hep.1840070311. [DOI] [PubMed] [Google Scholar]
- 6.Oguma S, Zerbe T, Banner B, Belle S, Starzl TE, Demetris AJ. Transplant Proc. 1989;21:2203. [PMC free article] [PubMed] [Google Scholar]
- 7.Oguma S, Belle S, Starzl TE, Demetris AJ. Hepatology. 1989;9:204. doi: 10.1002/hep.1840090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitra SK. J Anat. 1966;100:651. [PMC free article] [PubMed] [Google Scholar]
- 9.Murakami T, Itoshima T, Shimada Y. Arch Histol Cytol. 1974;37:245. doi: 10.1679/aohc1950.37.245. [DOI] [PubMed] [Google Scholar]
- 10.Yaegashi H, Takahashi T, Kawasaki M. J Microsc. 1987;146:55. doi: 10.1111/j.1365-2818.1987.tb01326.x. [DOI] [PubMed] [Google Scholar]
- 11.Kiernan F. Philos Trans R Soc Lond. 1833:711. [Google Scholar]