Abstract
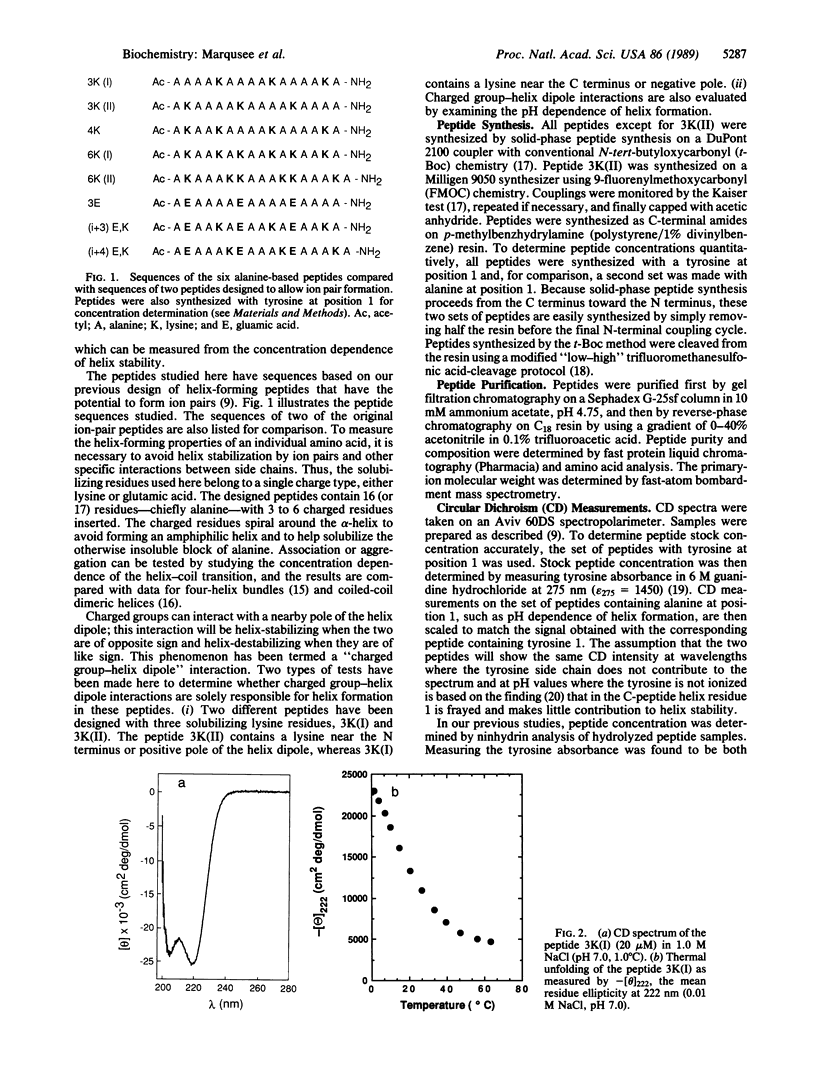
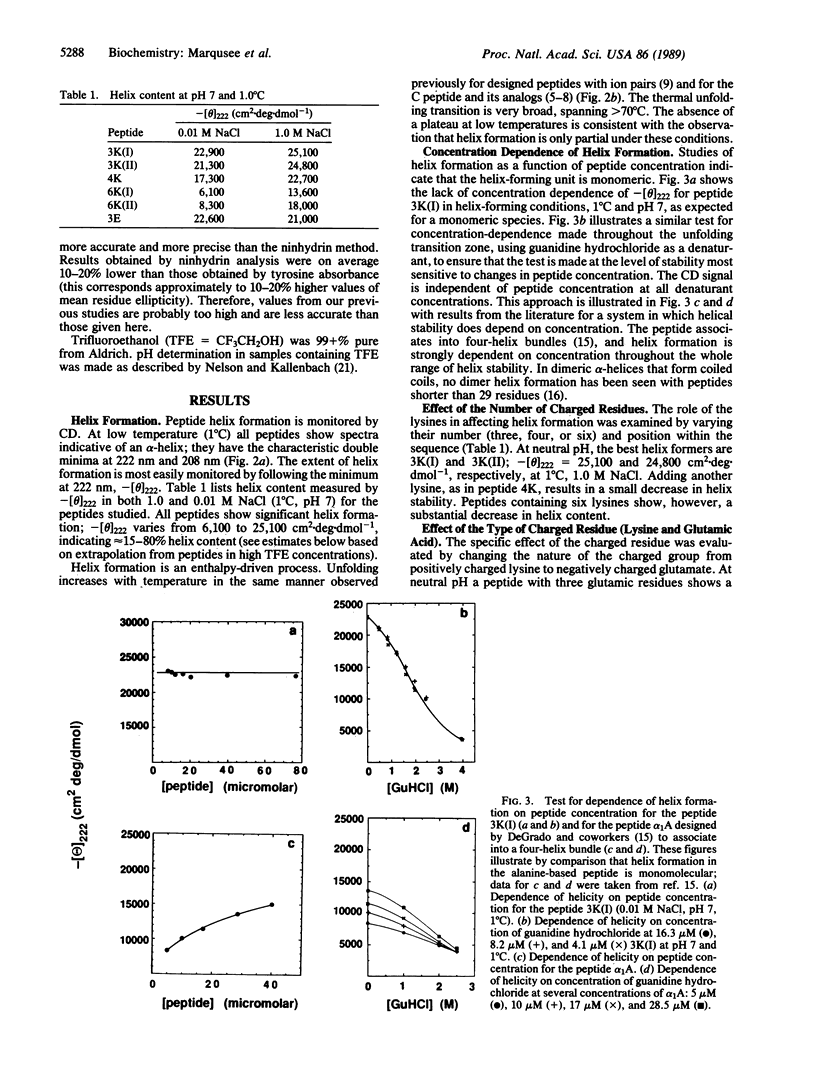
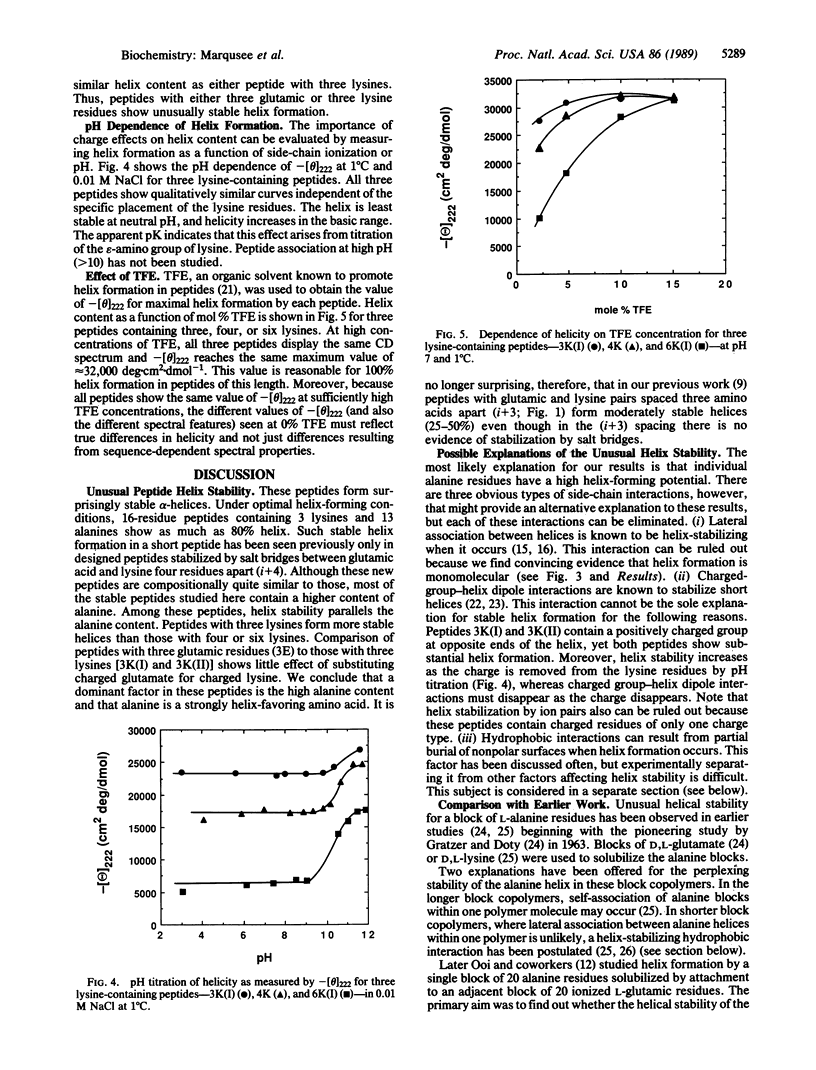
Short, 16-residue, alanine-based peptides show stable alpha-helix formation in H2O. This result is surprising when contrasted with the classical view that regards the alpha-helix as a marginally stable structure in H2O and considers short helices unstable. The alanine-based peptides are solubilized by insertion of three or more residues of a single charge type, lysine (+) or glutamic acid (-). The results cannot be explained by helix stabilization resulting from concentration-dependent association or by the interaction of charged residues with the helix dipole. Our results are not predicted by the parameters for alanine and lysine that have been determined by the "host-guest" method: these parameters predict that a 16-residue peptide should not show measurable alpha-helix formation. Analysis of the role of the hydrophobic interaction in alpha-helix formation [Richards, F.M. & Richmond, T. (1978) in Molecular Interactions and Activity in Proteins, Ciba Foundation Symposium 60, ed. Wolstenholme, G.E. (Excepta Medica Amsterdam), pp. 23-25] does not show an unusually strong hydrophobic interaction in a helical block of alanine residues. The likely explanation for our results is, therefore, that individual alanine residues have a high helical potential. It is not yet known whether any other amino acids show this property, and the origin of this property is also unknown.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bierzynski A., Kim P. S., Baldwin R. L. A salt bridge stabilizes the helix formed by isolated C-peptide of RNase A. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2470–2474. doi: 10.1073/pnas.79.8.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack A., Orgel L. E. Beta structures of alternating polypeptides and their possible prebiotic significance. Nature. 1975 Jul 31;256(5516):383–387. doi: 10.1038/256383a0. [DOI] [PubMed] [Google Scholar]
- Brandts J. F., Kaplan L. J. Derivative sspectroscopy applied to tyrosyl chromophores. Studies on ribonuclease, lima bean inhibitors, insulin, and pancreatic trypsin inhibitor. Biochemistry. 1973 May 8;12(10):2011–2024. doi: 10.1021/bi00734a027. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Klee W. A. Helix-coil transition of the isolated amino terminus of ribonuclease. Biochemistry. 1971 Feb 2;10(3):470–476. doi: 10.1021/bi00779a019. [DOI] [PubMed] [Google Scholar]
- Chothia C. Structural invariants in protein folding. Nature. 1975 Mar 27;254(5498):304–308. doi: 10.1038/254304a0. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Wells M., Fasman G. D. Conformational studies on copolymers of hydroxypropyl-L-glutamine and L-leucine. Circular dichroism studies. Biochemistry. 1972 Aug 1;11(16):3028–3043. doi: 10.1021/bi00766a015. [DOI] [PubMed] [Google Scholar]
- Fairman R., Shoemaker K. R., York E. J., Stewart J. M., Baldwin R. L. Further studies of the helix dipole model: effects of a free alpha-NH3+ or alpha-COO- group on helix stability. Proteins. 1989;5(1):1–7. doi: 10.1002/prot.340050102. [DOI] [PubMed] [Google Scholar]
- Ingwall R. T., Scheraga H. A., Lotan N., Berger A., Katchalski E. Conformational studies of poly-L-alanine in water. Biopolymers. 1968;6(3):331–368. doi: 10.1002/bip.1968.360060308. [DOI] [PubMed] [Google Scholar]
- Kim P. S., Baldwin R. L. A helix stop signal in the isolated S-peptide of ribonuclease A. 1984 Jan 26-Feb 1Nature. 307(5949):329–334. doi: 10.1038/307329a0. [DOI] [PubMed] [Google Scholar]
- Lau S. Y., Taneja A. K., Hodges R. S. Synthesis of a model protein of defined secondary and quaternary structure. Effect of chain length on the stabilization and formation of two-stranded alpha-helical coiled-coils. J Biol Chem. 1984 Nov 10;259(21):13253–13261. [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Marqusee S., Baldwin R. L. Helix stabilization by Glu-...Lys+ salt bridges in short peptides of de novo design. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8898–8902. doi: 10.1073/pnas.84.24.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. W., Kallenbach N. R. Stabilization of the ribonuclease S-peptide alpha-helix by trifluoroethanol. Proteins. 1986 Nov;1(3):211–217. doi: 10.1002/prot.340010303. [DOI] [PubMed] [Google Scholar]
- Osterman D. G., Kaiser E. T. Design and characterization of peptides with amphiphilic beta-strand structures. J Cell Biochem. 1985;29(2):57–72. doi: 10.1002/jcb.240290202. [DOI] [PubMed] [Google Scholar]
- Ptitsyn O. B. Thermodynamic parameters of helix-coil transitions in polypeptide chains. Pure Appl Chem. 1972;31(1):227–244. doi: 10.1351/pac197231010227. [DOI] [PubMed] [Google Scholar]
- Rico M., Nieto J. L., Santoro J., Bermejo F. J., Herranz J., Gallego E. Low-temperature 1H-NMR evidence of the folding of isolated ribonuclease S-peptide. FEBS Lett. 1983 Oct 17;162(2):314–319. doi: 10.1016/0014-5793(83)80779-0. [DOI] [PubMed] [Google Scholar]
- Shoemaker K. R., Kim P. S., Brems D. N., Marqusee S., York E. J., Chaiken I. M., Stewart J. M., Baldwin R. L. Nature of the charged-group effect on the stability of the C-peptide helix. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2349–2353. doi: 10.1073/pnas.82.8.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker K. R., Kim P. S., York E. J., Stewart J. M., Baldwin R. L. Tests of the helix dipole model for stabilization of alpha-helices. Nature. 1987 Apr 9;326(6113):563–567. doi: 10.1038/326563a0. [DOI] [PubMed] [Google Scholar]
- Strehlow K. G., Baldwin R. L. Effect of the substitution Ala----Gly at each of five residue positions in the C-peptide helix. Biochemistry. 1989 Mar 7;28(5):2130–2133. doi: 10.1021/bi00431a025. [DOI] [PubMed] [Google Scholar]


