Abstract
Background and aims
Crohn's disease (CD) ileal lesions are colonised by pathogenic adherent-invasive Escherichia coli (AIEC) producing outer membrane vesicles (OMVs) that contribute to the bacterial invasion process. In addition, increased expression of endoplasmic reticulum (ER)-localised stress response proteins, due to ER stress, is observed in patients with CD. The expression of the ER-localised stress response protein Gp96 in patients with CD and its biological role with regards to the ability of AIEC to invade intestinal epithelial cells were analysed.
Methods and results
Immunohistochemistry on tissue arrays showed that, together with CEACAM6 (carcinoembryonic antigen-related cell adhesion molecule 6) or the ER stress protein Grp78, Gp96 is also strongly expressed at the apical plasma membrane of the ileal epithelial cells of 50% of patients with CD. Invasion experiments in the presence of antibodies raised against Gp96, or after transfection of Intestine-407 cells with gp96 small interfering RNA (siRNA), indicated that Gp96 is essential to promote AIEC LF82 invasion, allowing, via the recognition of the outer membrane protein OmpA, OMVs to fuse with intestinal epithelial cells.
Conclusions
Gp96 is overexpressed on the apical surface of ileal epithelial cells in patients with CD and acts as a host cell receptor for OMVs, promoting AIEC invasion. From the results shown here, it is speculated that AIEC could take advantage of the abnormal expression of Gp96 in patients with CD to invade the ileal mucosa.
Keywords: Gp96, Crohn's disease, adherent-invasive E coli, outer membrane vesicles, invasion, bacterial interactions, intestinal epithelium
Significance of this study.
What is already known about this subject?
Colonisation of Crohn's disease (CD) ileal lesions by pathogenic adherent-invasive Escherichia coli (AIEC).
Contribution of outer membrane vesicles (OMVs) to the invasion process of the reference AIEC strain LF82 with intestinal epithelial cells.
Presence on the OMVs surface membrane of OmpA: a multifaceted protein with many diverse roles in adhesion, invasion and persistence of intracellular bacteria.
Expression of the endoplasmic reticulum (ER)-localised stress response chaperone Gp96 by intestinal epithelial cells.
Key role of ER stress in patients with CD.
What are the new findings?
Overexpression of Gp96 on the apical surface of ileal epithelial cells in patients with CD.
Role of OmpA in the AIEC invasion process.
Role of Gp96 to promote invasion of the AIEC reference strain LF82 allowing, via the recognition of the outer membrane protein OmpA, OMVs to fuse with intestinal epithelial cells.
How might it impact on clinical practice in the foreseeable future?
From the results shown here, we speculate that AIEC could take advantage of the abnormal expression of Gp96 in patients with CD to invade the ileal mucosa. Strategies to inhibit the OmpA–Gp96 interaction would be helpful to develop new treatment options for preventing inflammation resulting from gut mucosa invasion by AIEC but also by virulent bacteria using Gp96 as a host cell receptor.
Introduction
Inflammatory bowel disease (IBD) mainly comprises two disorders, ulcerative colitis (UC) and Crohn's disease (CD), with a combined prevalence of ∼150–200 cases per 100 000 in Western countries. The abnormal inflammatory response observed in IBD requires interplay between host genetic factors and the intestinal microbiota.1 2 Several lines of evidence support the notion that IBD results from an excessive immune response to gut commensal organisms.1 3 However, the disease could result from a problem in the composition of the microflora leading to generalised or localised dysbiosis. Increased numbers of mucosa-associated Escherichia coli are observed in patients with CD.4–9 These mucosa-associated E coli, called AIEC for adherent-invasive E coli, are able to adhere to and invade intestinal epithelial cells (IECs),6 10 and to colonise the ileal mucosa of patients with CD.11 AIEC are able to promote their own colonisation in genetically predisposed patients who develop ileal CD by inducing increased expression of CEACAM6 (carcinoembryonic antigen-related cell adhesion molecule 6), which acts as a receptor for these bacteria.12
Among the virulence factors harboured by AIEC strains, outer membrane vesicles (OMVs), by delivering bacterial effectors to host cells, play a role in the invasive ability of AIEC reference strain LF82.13 Through their interaction with eukaryotic cells, OMVs, which are 50–200 nm proteoliposomes, can deliver vesicle components and virulence factors to or into host cells.14 15 One of the major proteins on the surface membrane of OMVs is the OmpA protein, a multifaceted protein with many diverse roles in adhesion, invasion and persistence of intracellular bacteria.16–18 Among its biological roles, it is implicated in the ability of meningitis-associated E coli to invade brain microvascular endothelial cells (BMECs) via interaction with the endothelial cell glycoprotein Ecgp96.19 20 A homologue of Ecgp96 glycoprotein is expressed by IECs: the endoplasmic reticulum (ER)-localised stress response chaperone Gp96.21 Interestingly, ER stress was recently reported to have a key role in both patients with UC and those with CD after the discovery of single nucleotide polymorphisms within the XBP1 gene encoding the transcription factor XBP1, a key component of the ER stress response.22 23 Therefore, in the present study, we analysed Gp96 expression in patients with CD and its biological role with regards to AIEC colonisation and invasion of CD ileal mucosa.
Materials and methods
Patients and biopsy specimens
All patients included in this study were hospitalised in the Department of Gastroenterology (Archet II Hospital, University of Nice Sophia Antipolis, France) and provided a signed agreement for this study, and the protocol was approved by the local ethics committee of the University of Nice Sophia Antipolis. Intestinal biopsies were obtained from macroscopically inflamed mucosa of the terminal ileum and of the colon in 65 patients with CD (active CD) and from macroscopically non-inflamed mucosa of the terminal ileum and of the colon in 55 patients with CD (quiescent phase of the disease). There were 67 men and 53 women, with a mean age of 40 years (range 19–60) and mean disease duration of 10 years (range 2–23). Patients were all French Caucasians. In addition, biopsies were taken from the ileum and colon of 40 control patients consisting of individuals who had no significant pathological findings following endoscopic examination for changes in stool habits, abdominal pain, upper gastrointestinal bleeding or cancer surveillance.
Tissue microarray (TMA) construction and immunohistochemistry
Representative intestinal biopsies obtained for each individual in building TMAs were selected from H&E-stained sections. Briefly, one tissue core (600 μm in diameter) was obtained from each specimen from the upper part of the mucosa; pits and glands were always cut longitudinally. The tissue cores were arrayed into a new paraffin block using a fine steel needle. The two final TMAs consisted of one 480–600 μm diameter tissue core and one 480, 600 μm diameter tissue core, for ileum mucosa or colonic mucosa, respectively, from patients and controls. Immunohistochemical methods were performed on serial 4 μm deparaffinised TMA sections processed as described.24 Monoclonal rat anti-Gp96 (SPA-850, Stressgen Biotechnologies, San Diego, California, USA), monoclonal anti-CEACAM6 clone 9A6 (Genovac, Freiburg, Germany) and polyclonal goat anti-Grp78 (N-20, Santa-Cruz Biotechnology, Santa Cruz, California, USA) were used. For measurement of histological disease activity, the scoring system for histological abnormalities in CD mucosal biopsy specimens was used.25 After immunostaining, slides were analysed with an image analysis workstation (Spot Browser version 7; Alphelys, Plaisie, France), as described previously.24
Bacterial strains, plasmids and culture conditions
Bacterial strains, plasmids and oligonucleotides used in this study are listed in tables 1 and 2. For bacterial growth, isogenic mutant constructions26 27 and transcomplementation experiments, see supplemental methods online.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
| Strains | ||
| LF82 | E coli isolated from an ileal biopsy of a patient with CD | 6 |
| LF82-ΔompA | LF82 isogenic mutant with the ompA gene deleted | This study |
| K-12 MG1655 | Non-pathogenic E coli strain | Laboratory stock |
| Plasmids | ||
| pKOBEG | pBAD cloning vector harbouring the λ phage redγβα operon, chloramphenicolr | 27 |
| pBAD33 | E coli cloning vector, chloramphenicolr | 47 |
| pPBI13 | pBAD33 harbouring the 1.1 kb HindIII–SalI fragment with the entire ompA gene of strain LF82 | This study |
| pPBI14 | pBAD33 harbouring the 1.1 kb HindIII–SalI fragment with the entire ompA gene of strain MG1655 | This study |
| pSUPER.neo | Vector system for expression of small interfering RNA | Oligoengine |
| gp96 siRNA | pSUPER.neo harbouring the oligonucleotide specific to the gp96 mRNA | This study |
| Control siRNA | pSUPER.neo harbouring the oligonucleotide control | This study |
Table 2.
Oligonucleotides used for PCR experiments
| Primer | Oligonucleotide sequence (5′–3′) | PCR product size (bp) | Use |
| A2GBL-3 | AAAGCCACGTTGTGTCTCAA | 957 | Kanamycin resistance cassette amplification |
| B2GBLnp5 | TTAGAAAAACTCATCGAGCA | ||
| MIompA(R) | AAAGGCAAAAAAAACCCCGCAGCGGGGTTTTTCTACCAGACGAGAACTTAGAAAAACTCATCGAGCA | 1097 | ΔompA isogenic mutant construction |
| MIompA(F) | CTCGTTGGAGATATTCATGGCGTATTTTGGATGATAACGAGGCGCAAAAAAAAGCCACGTTGTGTCTCAA | ||
| OmpA1 | GGAGCCGGAGCAACTACTGG | 205 | Isogenic mutant verification |
| OmpA2 | ACGACACCGGCGTTTCTCCG | ||
| OmpA3 | GCAGGCATTGCTGGGTAAGG | 1232 | Isogenic mutant verification and sequencing |
| OmpA4 | AATATTGAGCAGATCCCCCGG | ||
| OmpASalI | ACGCGTCGACCGTTGGAGATATTCATGGCG | 1098 | Cloning of ompA gene |
| OmpAHindIII | CCCAAGCTTGGGAGACGAGAACTTAAGCCTGC | ||
| gp96-upstream | GGGTGTGGTGGACTCAGATG | 669 | gp96 quantification by qRT–PCR48 |
| gp96-downstream | GTTGCCAGACCATCCGTACT | ||
OMV preparation
OMVs were isolated as described previously.13 The culture supernatants were filtered and OMVs were collected by ultracentrifugation at 150 000 g for 3 h at 4°C. OMV pellets were resuspended in 10 mM Tris–HCl pH 8.0, 150 mM NaCl.
Cell culture, transfection and invasion assays
Intestine-407 and Caco-2 cells were obtained from the American Type Culture Collection (ATCC). The 19-mer oligonucleotides (5′-UCAGUUGGAUGGAUUAAAU-3′ specific to the gp96 mRNA and 5′-UUCUCCGAACGUGUCACGU-3′ as a control) were selected for synthesis of small interfering RNA (siRNA), cloned into pSUPER vector (Oligoengine, Seattle, Washington, USA) and transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, California, USA) according to the manufacturer's instructions. Bacterial invasion was quantified as described previously.10 Monolayers were infected for 3 h at a multiplicity of infection (MOI) of 10 bacteria per cell and the number of intracellular bacteria was determined as described.28 Inhibition invasion assays were performed after a 30 min pretreatment of cells at 37°C using anti-Gp96 (H-212, Santa Cruz Biotech) or rabbit immunoglobulin G (IgG; isotype control, C-20, Santa Cruz Biotech). Pretreatment of IECs with OMVs was performed as previously described.13 For pretreatment of IEC with antibodies and OMVs, monolayers were first incubated for 30 min with anti-Gp96 (diluted 1:500), and next for 1 h with OMVs, then washed and infected.
mRNA quantification, protein preparation and analysis, ligand overlay assay and pull-down assay
See supplemental methods.
Yeast cell aggregation assays
Yeast cell aggregation assays were performed as described.13
Confocal microscopy
After 10 min incubation in the presence of OMVs, cells were washed, fixed and immunostained with rat anti-Gp96 monoclonal antibody (SPA-850) and rabbit antiserum against E coli lipopolysaccharide O83 (generously provided by Lothar Beutin). Secondary antibodies used were fluorescein isothiocyanate (FITC)-conjugated donkey antirabbit IgG (Sigma-Aldrich, St Louis, Missouri, USA) and Cy3-conjugated AffiniPure goat antirat IgG (Jackson Immuno Research Laboratories, Westgrove, Pennsylvania, USA). Cells were observed with a Zeiss LSM 510 Meta confocal microscope.
Statistical analysis
For analysis of the significance of difference in Gp96 immunostaining, assays were compared using the Student t test. Values are expressed as the mean±SEM of ‘n’ number of experiments. The association of Gp96 expression with categorical pathological features was made using χ2 analysis. Calculations and analyses were performed with SPSS 11.5 for Windows, and, where appropriate, were two-tailed. Student t test was used for analysis of the statistical significance between invasion levels. p Values ≥0.05 were considered statistically significant.
Results
Gp96 protein expression is strongly increased in the ileal intestinal epithelium of patients with CD
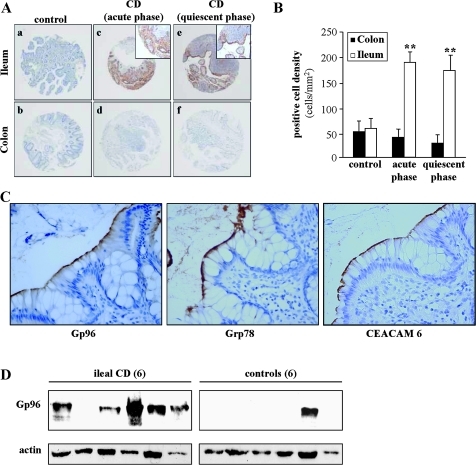
Intestinal biopsies were processed for Gp96 immunostaining on ileal and colonic mucosa of 65 patients with CD with macroscopically inflamed mucosa, of 55 patients with CD with non-inflamed mucosa and of 40 controls using anti-Gp96 monoclonal antibody (figure 1A). Immunohistochemistry showed a very strong staining of Gp96 in the ileal epithelium of patients in the acute phase of CD, in contrast to control ileal biopsies that showed very weak or no Gp96 expression. The Gp96 expression level in the ileum from patients in the quiescent phase of CD was still increased compared with that in the ileum from controls. In patients with CD, Gp96 was mainly observed at the apical plasma membrane of the epithelium. Conversely, a weak staining or an absence of staining was noted in colon biopsies taken both in controls and in patients with CD. In patients with CD in the active phase, Gp96 immunostaining was observed in 33/65 (50%) individuals, whereas in patients in the quiescent phase Gp96 immunostaining was observed in 19/55 (34%) individuals. Concerning positive cell densities in ileal biopsies, quantification of Gp96 immunostaining indicated that the numbers of Gp96-positive cells in patients in the acute or quiescent phase of CD were similar and significantly higher than those observed in controls (figure 1B). Conversely, very few positive cells were observed in colon biopsies from controls and subjects with CD (figure 1B). As CEACAM6 is abnormally expressed in ileal mucosa of patients with CD12 and as the expression of Grp78, another ER-localised stress response chaperone, is also increased in inflamed ileal CD mucosa and in inflamed colonic UC and CD mucosa,23 29 we analysed the expression of Gp96, CEACAM6 and Grp78 in CD ileal and colonic specimens. As shown in figure 1C, very strong staining of Gp96 was observed together with that of Grp78 and of CEACAM6 at the apical plasma membrane of the ileal epithelium of patients with CD. Western blot analysis of total protein extracts from CD ileal specimens taken in involved areas and of controls showed a strong expression of Gp96 in five out of six CD ileal mucosa specimens and only one out of six ileal mucosa specimens of controls (figure 1D).
Figure 1.
Gp96 expression in the intestinal biopsies of patients with Crohn's disease (CD) and controls. (A) Gp96 immunohistochemical staining of tissues microarrays (TMAs) from ileum and colon biopsies of controls (a, b), patients in the acute inflamed phase (c, d) or patients in the quiescent phase (e, f) of CD. Each spot shows representative tissue immunostaining for Gp96 (a–f, immunoperoxidase ×40; c and e, inset, immunoperoxidase ×200). (B) Quantification of Gp96 immunostaining using the Spot Browser software, in TMAs from colon and ileum biopsies of controls, and patients in the acute or quiescent phase of CD, **p<0.01. (C) Carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6), Grp78 and Gp96 immunostaining of TMAs from ileum biopsies of patients with CD, magnification ×400. (D) Western blot analysis of whole protein extracts from ileal biopsies taken in involved areas of six patients with CD and from six ileal biopsies from controls using anti-Gp96 and anti-β-actin antibodies.
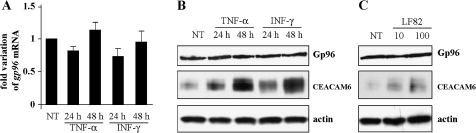
Stimulation by interferon γ (IFNγ) and tumour necrosis factor α (TNFα) or bacterial infection do not modify Gp96 expression in IECs
To investigate whether abnormal Gp96 expression in patients with CD resulted from stimulation by proinflammatory cytokines or E coli infection of IECs, we analysed Gp96 expression in cultured IECs after stimulation with IFNγ or TNFα or after infection with AIEC. No modification in gp96 mRNA levels was observed in Caco-2 cells after TNFα or IFNγ stimulation for 24 or 48 h compared with non-treated cells (figure 2A). Western blot analysis indicated that, unlike CEACAM6 increased expression under proinflammatory cytokine stimulation, similar Gp96 expression was observed in proinflammatory cytokine-stimulated or unstimulated Caco-2 cells (figure 2B). In addition, the Gp96 protein level was also not modified in Caco-2 cells after 3 h of infection by AIEC strain LF82 at an MOI of 10 or 100 (figure 2C). Similar results were observed with Intestine-407 cells (data not shown).
Figure 2.
Gp96 expression in Caco-2 cells. (A) Fold variation of gp96 mRNA levels in Caco-2 cells after 24 or 48 h of stimulation with tumour necrosis factor α (TNFα) or interferon γ (IFNγ) relative to that in non-treated cells (NT) using RT–PCR. gapdh (glyceraldehyde phosphate dehydrogenase) mRNA levels were measured as controls. Data are the mean±SEM of three separate experiments. (B and C). Western blot analysis showing expression levels of Gp96 and carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6) by Caco-2 cells after 24 or 48 h of stimulation with 50 ng/ml TNFα or IFNγ (B) or after a 3 h infection period with adherent-invasive Escherichia coli (AIEC) strain LF82 (C) at a multiplicity of infection of 10 or 100. As loading control, labelling was performed using anti-β-actin polyclonal antibodies.
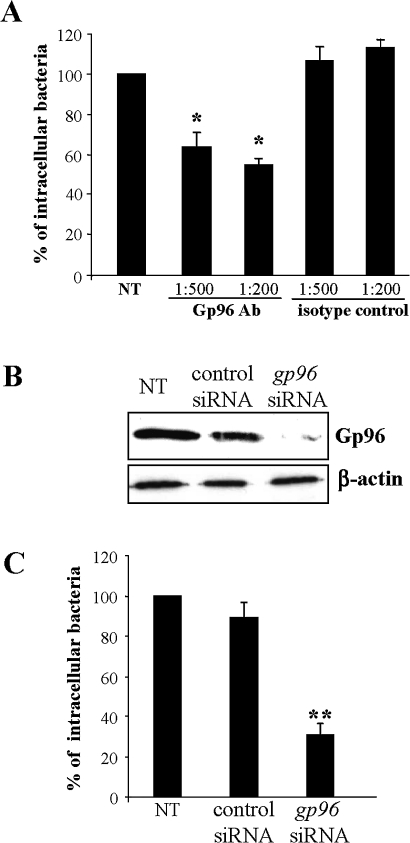
Gp96 expression supports LF82 invasion
The role of Gp96 in AIEC invasion of IECs was investigated using invasion inhibition assays in the presence of anti-Gp96 polyclonal antibodies and by invasion assays using IECs with decreased levels of gp96 by RNA silencing. When the IECs were pretreated with anti-Gp96 antibodies (dilution 1:200), the invasion level of LF82 bacteria was 54.5±3.3% of that of strain LF82 on untreated cells (figure 3A). In contrast, pretreatment with anti-IgG isotype control had no effect on LF82 invasion. Interestingly, reduced Gp96 expression by transfecting IECs with gp96 siRNA (figure 3B) induced decreased LF82 invasion levels (figure 3C). Indeed, the invasion levels of LF82 bacteria on Intestine-407 cells transfected with gp96 siRNA were 30.9±5.0% of that of strain LF82 on untreated cells. In contrast, transfection of cells with control siRNA that did not reduce the Gp96 protein level did not affect the ability of AIEC strain LF82 to invade IECs. Taken together, these results strongly suggest that Gp96 plays a major role in AIEC invasion.
Figure 3.
Gp96 expression supports LF82 invasion. (A) Effect of pretreatment of Intestine-407 epithelial cells with anti-Gp96 antibodies on the invasive level of LF82. Intestine-407 cells were pretreated with rabbit polyclonal antibodies raised against Gp96 (Gp96 Ab) or with rabbit polyclonal antibodies (isotype control) diluted 1:200 or 1:500 for 30 min and then infected by LF82 bacteria. Invasion was determined after a 3 h infection period and after gentamicin treatment for an additional hour. Results are expressed as intracellular bacteria relative to those obtained for strain LF82 on non-treated cells (NT), taken as 100%. Each value is the mean±SEM of at least four separate experiments. *p<0.05 compared with the wild-type strain on untreated cells. (B) Western blot analysis of whole protein extracts from Intestine-407 cells using anti-Gp96 and anti-β-actin antibodies. Intestine-407 cells were non-transfected (NT), or transfected with 10 ng of small interfering RNA blocking Gp96 (gp96 siRNA), or non-specific siRNA as control. (C) Effect of gp96 siRNA on the invasive level of the wild-type strain LF82. Invasive bacteria were quantified as described in A. **p<0.01 compared with the wild-type strain on untreated cells.
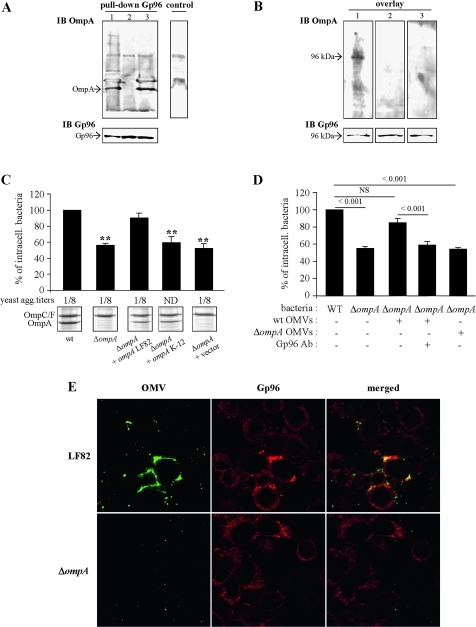
OmpA binds Gp96 and is involved in AIEC strain LF82 invasion ability
To investigate whether OmpA expressed by LF82 bacteria binds Gp96 receptor expressed by Caco-2 IECs, we used pull-down and a ligand overlay approach. As shown in figure 4A, OmpA from AIEC strain LF82 was detected after pull-down using rat anti-Gp96 monoclonal antibody. A similar result was observed with OmpA from E coli K-12 strain MG1655. The control reaction without anti-Gp96 antibody captured non-specific proteins but not OmpA. However, using the ligand overlay approach, we observed one band of ∼100 kDa, the predicted size of Gp96, after incubation with OMP extracted from wild-type strain LF82, but not from the LF82-ΔompA mutant or from E coli K-12 (figure 4B). Analysis of OmpA sequences revealed that the LF82 strain OmpA differed from that of the non-pathogenic E coli K-12 strain MG1655 by five amino acids: two amino acids located in one of the periplasmic domains, one in the transmembrane domain and two in the third extracellular loop of the OmpA protein (Supplemental figure S1).
Figure 4.
Gp96-dependent invasion involves adherent-invasive Escherichia coli (AIEC) outer membrane protein A (OmpA). Analysis of the interaction between OmpA and Gp96 by pull-down assay and ligand overlay. (A) For pull-down, total cell extracts from Caco-2 cells were incubated with Omp extracted from AIEC LF82 (1), LF82-ΔompA (2) and E. coli K-12 MG1655 (3), and immunoprecipitated with anti-Gp96 and Sepharose beads. In the control assay, the incubation step with anti-Gp96 was omitted. Captured proteins were separated on a 12% polyacrylamide gel, transferred onto a nitrocellulose membrane and probed with serum raised against OmpA. (B) For overlay, total extracts of Caco-2 cells were separated on a 12% polyacrylamide gel, transferred onto a nitrocellulose membrane and probed successively with Omp extracted from AIEC LF82 (1), LF82-ΔompA (2) and E. coli K-12 MG1655 (3), with anti-OmpA and detected with peroxidase-conjugated secondary antibody. (C) Invasion abilities with Intestine-407 epithelial cells of the LF82-ΔompA mutant, the LF82-ΔompA mutant transformed with the cloned LF82 ompA gene or the cloned K-12 MG1655 ompA gene, or the pBAD33 vector alone. Invasive bacteria were quantified as described in figure 3A. **p<0.01 compared with the wild-type strain. Expression of type 1 pili was determined visually by yeast aggregation and the titre was recorded as the last dilution giving a positive aggregation results. Whole-cell lysates of LF82, LF82-ΔompA bacteria transformed with the cloned LF82 ompA gene, with the cloned K-12 ompA gene, or with the pBAD33 vector alone and grown in medium with l-arabinose, were separated by sodium dodecylsulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and stained with Coomassie blue. The positions of OmpC/F and OmpA are marked. (D) Effect of pretreatment of Intestine-407 epithelial cells with outer membrane vesicles (OMVs) and anti-Gp96 antibodies (Gp96 Ab) on the invasive level of LF82-ΔompA. Intestine-407 cells were pretreated with rabbit polyclonal antibodies raised against Gp96 diluted 1:500 for 30 min, then pretreated with OMVs from wild-type (WT) or LF82-ΔompA bacteria for 1 h and, after washing, cells were infected with bacteria. Invasive bacteria were quantified as described in figure 3A. (E) Analysis of the interaction of LF82 or ΔompA OMVs with the cytoplasmic membrane of Intestine-407 epithelial cells. The fusion of OMVs with the membrane Intestine-407 cells was analysed by confocal microscopy. Cells were incubated with OMVs at 37°C for 10 min. After washing and fixation, cells were labelled with rat antibodies raised against Gp96 and Cy3-conjugated goat anti-rat IgG secondary antibodies. OMVs fused with the cytoplasmic membrane of Intestine-407 cells were labelled with rabbit antibodies raised against E coli lipopolysaccharide O83 and fluorescein isothiocyanate (FITC)-conjugated donkey anti-rabbit immunoglobulin G (IgG). Each confocal microscopy image is representative of three independent experiments.
Since OmpA plays a major role in the ability of meningitis-associated E coli K1 to invade human BMECs via its binding to the Gp96 receptor,19 20 we investigated the role of OmpA in the ability of AIEC strain LF82 to invade IECs. The LF82 isogenic mutant with the ompA gene deleted presented no defect in type 1 pili expression. Electronic microscopic examination of negatively stained bacteria indicated that the bacteria expressed type 1 pili (data not shown), and similar titres (1/8) of yeast cell aggregation were obtained compared with the wild-type strain LF82 (figure 4C), indicating that the LF82-ΔompA isogenic mutant synthesised levels of functional type 1 pili similar to the wild-type strain LF82 (figure 4C). A quantitative invasion assay showed that the LF82-ΔompA mutant has a reduced ability to invade Intestine-407 epithelial cells, with a 56.3±2.6% residual invasion level compared with the wild-type strain LF82, taken as 100% (figure 4C). Transcomplementation with the cloned ompA gene of AIEC strain LF82 fully restored the invasion of the mutant. Transformation of the LF82-ΔompA mutant with the cloned ompA gene of E coli K-12 strain MG1655 did not restore invasion to a level similar to that of the wild-type LF82 (figure 4C). This was not due to defects in OmpA expression since the amounts of OmpA produced were similar in LF82-ΔompA bacteria transcomplemented with cloned ompA from AIEC strain LF82 or cloned ompA from E coli K-12 (figure 4C). Thus amino acid substitution in LF82 OmpA favours the interaction of OmpA with Gp96 in order to promote IEC invasion.
The ability of LF82 OMVs to promote invasion involves OmpA and Gp96
The ability of LF82 OMVs to restore the invasion of the LF82-ΔompA isogenic mutant was analysed. The invasion level of the LF82-ΔompA mutant was increased when the IECs were pretreated with LF82 OMVs, reaching 85.2±5.3%, and was not significantly different from that of strain LF82 taken as 100% (figure 4D). On the host side, this involved Gp96 as addition of anti-Gp96 polyclonal antibodies blocked the restoration of the invasion level of the LF82-ΔompA mutant with LF82 OMV-treated cells. On the bacterial side, this involved OmpA, as no increase in the invasion level of the LF82-ΔompA mutant was observed with IECs pretreated with LF82-ΔompA OMVs (figure 4D). This was confirmed by confocal analysis of the interaction of LF82 OMVs with Intestine-407 cells using anti-O83 to label OMVs. As shown in figure 4E, a co-localisation of OMVs with plasma membrane of some Gp96-expressing Intestine-407 cells was observed, as the result of the fusion of LF82 OMVs with the host cell membrane. In addition, confocal images indicated a recruitment or accumulation of Gp96 at the cell surface at the sites where the OMVs were observed. No fusion event of OMVs with the host cell membrane was observed when experiments were performed with Intestine-407 cells transfected with gp96 siRNA (data not shown) or with LF82-ΔompA OMVs (figure 4E), indicating a role for OmpA–Gp96 interaction in the fusion of LF82 OMVs with the host cell membrane.
Discussion
We show in the present study that the ER-localised stress response chaperone Gp96 is overexpressed at the apical plasma membrane of IECs of patients with CD, and plays a role in the internalisation of AIEC mainly associated with the ileal mucosa of patients with CD. Such a result illustrates the hypothesis that the pathogenesis of CD involves a complex interplay between host genetic profile and microbes. The heat shock glycoprotein Gp96 was reported to be highly expressed in rheumatoid arthritis tissues30 and in advanced tumour stage in oesophageal adenocarcinomas.31 This is the first report showing a connection between modified expression of Gp96 in IECs and IBD. In good accordance with Grp78, another ER-localised stress response chaperone whose expression is increased in inflamed ileal CD mucosa and in inflamed colonic UC and CD mucosa,23 29 the increased expression of Gp96 in ileal epithelium of patients with CD could indicate an ER stress. The idea that ER stress is involved in IBD is quite novel; however, it is now well recognised that unresolved ER stress, as the consequence of genetic abnormalities of the unfolded protein response or of a variety of secondary (inflammation and environmental) factors, leads to IEC, goblet cell and Paneth cell dysfunction.22 23 Several single nucleotide polymorphisms within the XBP1 gene encoding the transcription factor XBP1, a key component of the ER stress response as shown in a knock-out mouse model,23 were observed both in patients with CD and in those with UC.23 32–35
The increased expression of the ER stress response chaperone Gp96 in CD could be either a primary cause or a consequence of intestinal inflammation, since it was reported that Gp96 is transcriptionally upregulated in IFNγ-treated human Burkitt's lymphoma Daudi cells and HeLa cells.36 Here using cultured intestinal epithelial Caco-2 or Intestine-407 cells stimulated with TNFα or IFNγ, we did not notice any modification of Gp96 expression. This latter observation is in good agreement with the fact that Gp96 was found to be highly expressed in patients in both acute and quiescent phases of CD. We also investigated whether infection with AIEC pathogenic bacteria can interfere with Gp96 expression, but we did not observe any increase in response to AIEC infection. The biological function of Gp96 in the interaction of AIEC with IECs was determined by invasion experiments in the presence of antibodies raised against Gp96 or after transfection of Intestine-407 cells with gp96 siRNA. Both experiments showed a decreased invasion of AIEC strain LF82, indicating that Gp96 promotes AIEC LF82 invasion. Gp96 has already been reported to be a key mediator of the innate immune response due to its ability to bind pathogenic bacteria or their products.19–21 37 38 Indeed, Gp96 is a plasma membrane receptor for Vip, a Listeria monocytogenes virulence factor that is required for cell invasion and downstream signalling events.21 In addition, a Gp96 homologue, Ecgp96, the expression of which is increased during meningitis-associated E coli K1 infection of human BMECs, promotes invasion of these pathogenic bacteria.19 20 37
AIEC are able to adhere to and to invade IECs; a process which involves several virulence factors. Among them, the type 1 pili are essential, through the recognition of CEACAM6,12 to promote the adhesion of bacteria; however, they are not sufficient to promote invasion, since their expression in a non-pathogenic E coli K-12 does not confer invasive ability.28 We have previously reported that OMVs contribute to the invasion process of E coli LF82 with IECs.13 OMVs are a compartment where some virulence factors undergo full maturation, such as the E coli pore-forming cytotoxin ClyA,39 and a potential vehicle for intercellular transport of toxins, as shown for the Helicobacter pylori VacA toxin,40 the E coli heat-labile toxin41 or the Actinobacillus actinomycetemcomitans leukotoxin.42 We have previously reported that pretreatment of IECs with E coli LF82 OMVs partially restores the invasion level of a non-invasive mutant and that the effect of LF82 OMVs was specific, since no increase in the invasion level was observed when cells were pretreated with OMVs from non-pathogenic E coli K-12.13 The ability of native bacterial OMVs to fuse with host cells and deliver their contents directly into the cytosol has been theorised based on their ability to fuse with bacterial membranes. This has been illustrated for neisserial OMVs binding to the CEACAM1 receptor expressed on the surface of T lymphocytes,43 and for OMVs from Pseudomonas aeruginosa fusing with respiratory epithelial cells.44 In the present study, we report that AIEC strain LF82 OMVs can fuse with IECs. In addition, we identified Gp96 as the host membrane OMV receptor and observed that it was recruited at the OMV fusion sites.
The outer membrane protein, OmpA, is a major protein of OMVs and we observed that in AIEC strain LF82, deletion of the ompA gene induced (1) a significant decrease in the ability of the bacteria to invade Intestine-407 epithelial cells compared with a wild-type strain; (2) a loss of fusion of OMVs with the host cell membrane; and (3) a loss of the ability of OMVs to restore invasion of a non-invasive mutant. Alignment of amino acid sequences of OmpA from AIEC LF82 and non-pathogenic E coli K-12 MG1655 revealed five modifications in OmpA, and two amino acid substitutions, specific to AIEC LF82, are located in the third extracellular loop. The invasion levels of the transformed LF82-ΔompA mutant expressing LF82 OmpA after transcomplementation with a cloned copy of the LF82 ompA gene were restored. In contrast, when this mutant was transformed to express K-12 OmpA, it was still impaired in its invasive ability. Furthermore, the invasion level of the LF82-ΔompA mutant was restored to that of the wild-type strain when the IECs were pretreated with LF82 OMVs, but not with LF82-ΔompA OMVs. In addition, in the presence of anti-Gp96 antibodies, LF82 OMVs were unable to restore the invasive defect of the LF82-ΔompA mutant, suggesting the need for an interaction between the eukaryotic Gp96 receptor and the bacterial OmpA protein from AIEC OMVs for fusion of vesicles with IECs and delivery into host cells of virulence factors that contribute to the invasion process.
Co-localisation of Gp96 and CEACAM6 was observed in patients with CD. Gp96 participates in the folding and assembly of many secretory and membrane proteins and has been shown to be essential for the cell surface expression of Toll-like receptors (TLRs), including TLR2 and TLR4.45 Therefore, we can consider whether it participates in the cell membrane targeting of CEACAM6. Such a co-localisation of Gp96 and CEACAM6 in patients with CD at the apical side of ileal epithelial cells is of great interest, since we previously reported that CEACAM6 acts as a receptor for E coli type 1 pili and therefore allows AIEC to colonise the ileal mucosa12 and promote gut inflammation as shown in transgenic CEABAC10 mice expressing the human CEACAM6 molecule.46 Increased expression of Gp96 at the same site as CEACAM6 should increase AIEC virulence, since the bacteria would be able to colonise the mucosa by binding to CEACAM6 and can better invade the ileal epithelium through AIEC OMV–Gp96 interaction. In addition, we can speculate that increased expression of Gp96 in patients with CD could also contribute to an abnormal colonisation of the mucosa by other bacteria using Gp96 as a plasma membrane receptor allowing the bacteria to bind to or to enter inside host cells, as already reported for L monocytogenes.21
In conclusion, our findings highlight increased expression of the ER-localised stress response protein Gp96 in ileal epithelial cells of patients with CD. From this result and as demonstrated in the present study, the AIEC mostly associated with the ileal form of CD are able to take advantage of Gp96 overexpression, since we show here that Gp96 acts as a host cell receptor for AIEC invasion via OMVs rich in OmpA protein. We hypothesise that patients at high risk for developing severe ileal CD are those who, in addition to expressing CEACAM6,12 overexpress Gp96 in the ileal mucosa. Therefore, in light of our findings, strategies to inhibit the OmpA–Gp96 interaction would be helpful to develop new treatment options for preventing inflammation resulting from gut mucosa invasion by AIEC but also by virulent bacteria using Gp96 as a host cell receptor.
Acknowledgments
We thank Lothar Beutin (department of Biological safety, Robert Koch Institut, Berlin, Germany) for rabbit antiserum against E coli lipopolysaccharride O83, Roland Lloubes (CNRS UPR 9027, Institut de Biologie Structurale et Microbiologie, Marseille, France) for OmpA antibodies, and Benoit Chassaing and Jessica Thompson (CMMI2, Imperial College of London, UK) for helpful reading of the manuscript. The confocal microscope was the property of the Institut Fédératif de Recherche (IFR) Santé Université d'Auvergne, Clermont-Ferrand, France.
Footnotes
Funding: This study was supported by the Ministère de la Recherche et de la Technologie (JE2526), by the INRA (USC 2018) and by grants from the Association F. Aupetit (AFA), Institut de Recherche des Maladies de l'Appareil Digestif (IRMAD, Laboratoire Astra France) and Project INCa INFLACOL 2008.
Competing interests: None
Ethics approval: The protocol was approved by the local ethics committee of the University of Nice Sophia Antipolis.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest 2007;117:514–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007;448:427–34 [DOI] [PubMed] [Google Scholar]
- 3.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008;134:577–94 [DOI] [PubMed] [Google Scholar]
- 4.Baumgart M, Dogan B, Rishniw M, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn's disease involving the ileum. ISME J 2007;1:403–18 [DOI] [PubMed] [Google Scholar]
- 5.Conte MP, Schippa S, Zamboni I, et al. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut 2006;55:1760–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darfeuille-Michaud A, Neut C, Barnich N, et al. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology 1998;115:1405–13 [DOI] [PubMed] [Google Scholar]
- 7.Kotlowski R, Bernstein CN, Sepehri S, et al. High prevalence of Escherichia coli belonging to the B2+D phylogenetic group in inflammatory bowel disease. Gut 2007;56:669–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin HM, Campbell BJ, Hart CA, et al. Enhanced Escherichia coli adherence and invasion in Crohn's disease and colon cancer. Gastroenterology 2004;127:80–93 [DOI] [PubMed] [Google Scholar]
- 9.Sasaki M, Sitaraman SV, Babbin BA, et al. Invasive Escherichia coli are a feature of Crohn's disease. Lab Invest 2007;87:1042–54 [DOI] [PubMed] [Google Scholar]
- 10.Boudeau J, Glasser AL, Masseret E, et al. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect Immun 1999;67:4499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darfeuille-Michaud A, Boudeau J, Bulois P, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology 2004;127:412–21 [DOI] [PubMed] [Google Scholar]
- 12.Barnich N, Carvalho FA, Glasser AL, et al. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest 2007;117:1566–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolhion N, Barnich N, Claret L, et al. Strong decrease in invasive ability and outer membrane vesicle release in Crohn's disease-associated adherent-invasive Escherichia coli strain LF82 with the yfgL gene deleted. J Bacteriol 2005;187:2286–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host–pathogen interaction. Genes Dev 2005;19:2645–55 [DOI] [PubMed] [Google Scholar]
- 15.Mashburn-Warren LM, Whiteley M. Special delivery: vesicle trafficking in prokaryotes. Mol Microbiol 2006;61:839–46 [DOI] [PubMed] [Google Scholar]
- 16.Nicholson TF, Watts KM, Hunstad DA. OmpA of uropathogenic Escherichia coli promotes post-invasion pathogenesis of cystitis. Infect Immun 2009;77:5245–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres AG, Kaper JB. Multiple elements controlling adherence of enterohemorrhagic Escherichia coli O157:H7 to HeLa cells. Infect Immun 2003;71:4985–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiser JN, Gotschlich EC. Outer membrane protein A (OmpA) contributes to serum resistance and pathogenicity of Escherichia coli K-1. Infect Immun 1991;59:2252–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prasadarao NV. Identification of Escherichia coli outer membrane protein A receptor on human brain microvascular endothelial cells. Infect Immun 2002;70:4556–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prasadarao NV, Srivastava PK, Rudrabhatla RS, et al. Cloning and expression of the Escherichia coli K1 outer membrane protein A receptor, a gp96 homologue. Infect Immun 2003;71:1680–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cabanes D, Sousa S, Cebria A, et al. Gp96 is a receptor for a novel Listeria monocytogenes virulence factor, Vip, a surface protein. EMBO J 2005;24:2827–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heazlewood CK, Cook MC, Eri R, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med 2008;5:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaser A, Lee AH, Franke A, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 2008;134:743–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofman P, Butori C, Havet K, et al. Prognostic significance of cortactin levels in head and neck squamous cell carcinoma: comparison with epidermal growth factor receptor status. Br J Cancer 2008;98:956–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology 2002;122:512–30 [DOI] [PubMed] [Google Scholar]
- 26.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 2000;97:6640–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaveroche MK, Ghigo JM, d'Enfert C. A rapid method for efficient gene replacement in the filamentous fungus Aspergillus nidulans. Nucleic Acids Res 2000;28:E97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boudeau J, Barnich N, Darfeuille-Michaud A. Type 1 pili-mediated adherence of Escherichia coli strain LF82 isolated from Crohn's disease is involved in bacterial invasion of intestinal epithelial cells. Mol Microbiol 2001;39:1272–84 [DOI] [PubMed] [Google Scholar]
- 29.Shkoda A, Ruiz PA, Daniel H, et al. Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology 2007;132:190–207 [DOI] [PubMed] [Google Scholar]
- 30.Huang QQ, Sobkoviak R, Jockheck-Clark AR, et al. Heat shock protein 96 is elevated in rheumatoid arthritis and activates macrophages primarily via TLR2 signaling. J Immunol 2009;182:4965–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langer R, Feith M, Siewert JR, et al. Expression and clinical significance of glucose regulated proteins GRP78 (BiP) and GRP94 (GP96) in human adenocarcinomas of the esophagus. BMC Cancer 2008;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barmada MM, Brant SR, Nicolae DL, et al. A genome scan in 260 inflammatory bowel disease-affected relative pairs. Inflamm Bowel Dis 2004;10:513–20 [DOI] [PubMed] [Google Scholar]
- 33.Hampe J, Schreiber S, Shaw SH, et al. A genomewide analysis provides evidence for novel linkages in inflammatory bowel disease in a large European cohort. Am J Hum Genet 1999;64:808–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hampe J, Shaw SH, Saiz R, et al. Linkage of inflammatory bowel disease to human chromosome 6p. Am J Hum Genet 1999;65:1647–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vermeire S, Rutgeerts P, Van Steen K, et al. Genome wide scan in a Flemish inflammatory bowel disease population: support for the IBD4 locus, population heterogeneity, and epistasis. Gut 2004;53:980–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson SL, Shen T, Lou J, et al. The endoplasmic reticular heat shock protein gp96 is transcriptionally upregulated in interferon-treated cells. J Exp Med 1994;180:1565–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mittal R, Prasadarao NV. Nitric oxide/cGMP signalling induces Escherichia coli K1 receptor expression and modulates the permeability in human brain endothelial cell monolayers during invasion. Cell Microbiol 2010;12:67–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Na X, Kim H, Moyer MP, et al. gp96 is a human colonocyte plasma membrane binding protein for Clostridium difficile toxin A. Infect Immun 2008;76:2862–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wai SN, Lindmark B, Soderblom T, et al. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 2003;115:25–35 [DOI] [PubMed] [Google Scholar]
- 40.Fiocca R, Necchi V, Sommi P, et al. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J Pathol 1999;188:220–6 [DOI] [PubMed] [Google Scholar]
- 41.Horstman AL, Kuehn MJ. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J Biol Chem 2000;275:12489–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demuth DR, James D, Kowashi Y, et al. Interaction of Actinobacillus actinomycetemcomitans outer membrane vesicles with HL60 cells does not require leukotoxin. Cell Microbiol 2003;5:111–21 [DOI] [PubMed] [Google Scholar]
- 43.Lee HS, Boulton IC, Reddin K, et al. Neisserial outer membrane vesicles bind the coinhibitory receptor carcinoembryonic antigen-related cellular adhesion molecule 1 and suppress CD4+ T lymphocyte function. Infect Immun 2007;75:4449–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bomberger JM, Maceachran DP, Coutermarsh BA, et al. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog 2009;5:e1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Randow F, Seed B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat Cell Biol 2001;3:891–6 [DOI] [PubMed] [Google Scholar]
- 46.Carvalho FA, Barnich N, Sivignon A, et al. Crohn's disease adherent-invasive Escherichia coli colonize and induce strong gut inflammation in transgenic mice expressing human CEACAM. J Exp Med 2009;206:2179–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guzman LM, Belin D, Carson MJ, et al. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol 1995;177:4121–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schreiter K, Hausmann M, Spoettl T, et al. Glycoprotein (gp) 96 expression: induced during differentiation of intestinal macrophages but impaired in Crohn's disease. Gut 2005;54:935–43 [DOI] [PMC free article] [PubMed] [Google Scholar]