Abstract
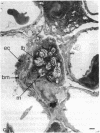
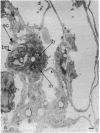
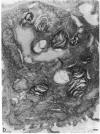
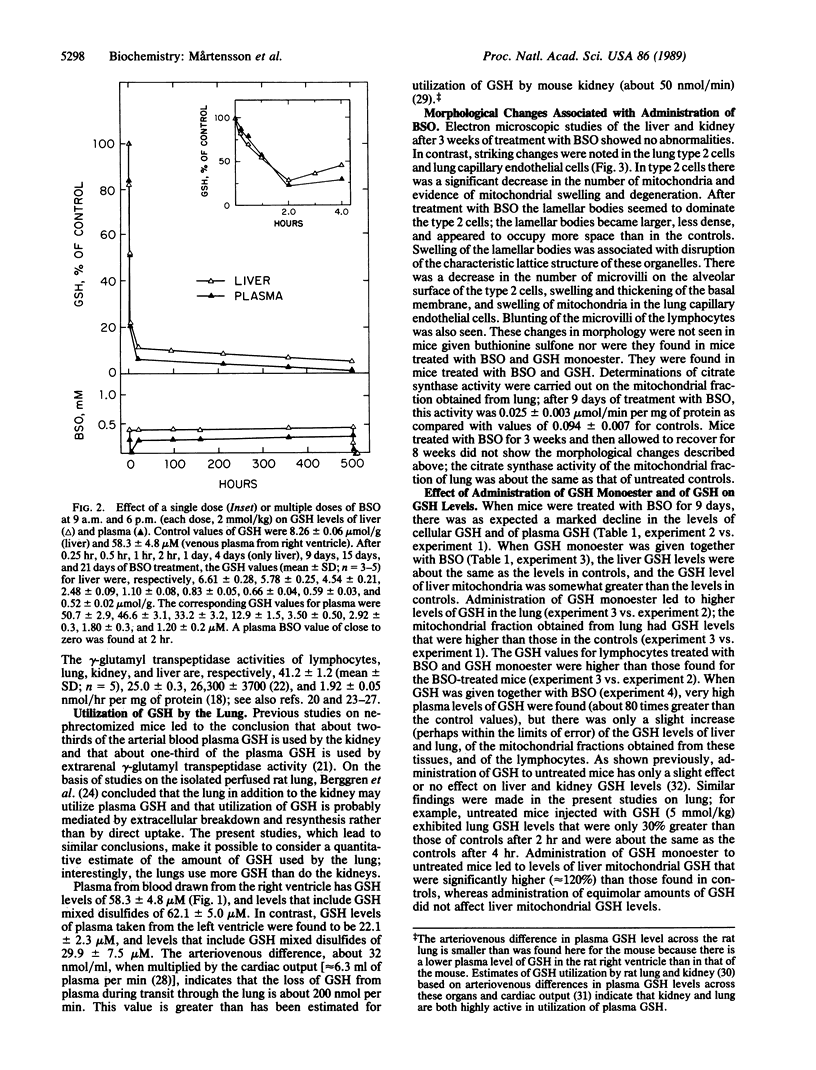
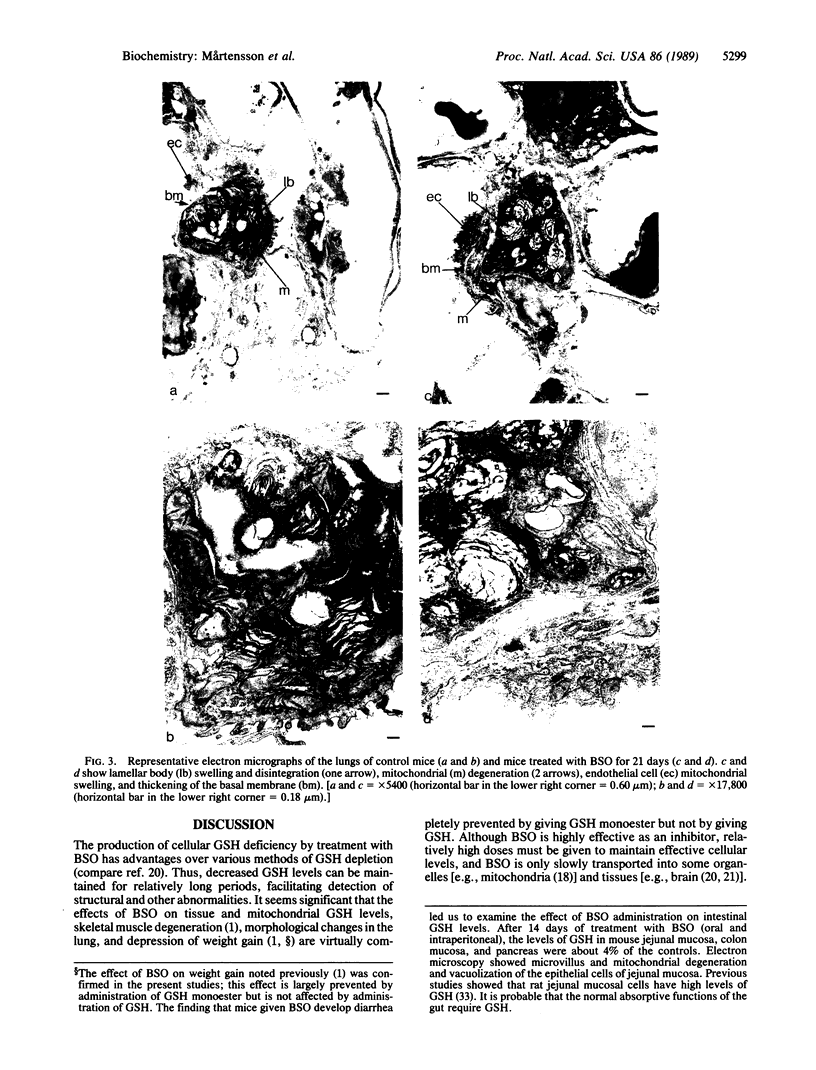
Mice treated with buthionine sulfoximine, an inhibitor of glutathione synthesis, showed striking alterations of morphology of lung type 2 cell lamellar bodies (swelling and disintegration) and mitochondria (degeneration) and of lung capillary endothelial cells (mitochondrial swelling). These effects probably may be ascribed to glutathione deficiency; administration of glutathione monoester protects against them. Measurements of arteriovenous plasma glutathione levels across the lung indicate that the net uptake of glutathione by this organ is substantial. Thus, glutathione exported from the liver to the blood plasma is utilized by the lung which, like the liver, kidney, and lymphocytes (and unlike skeletal muscle), exhibits a high overall rate of glutathione turnover. Intraperitoneal injection of glutathione into buthionine sulfoximine-treated mice leads to very high levels of plasma glutathione without significant increase in the glutathione levels of liver, lung, and lymphocytes; on the other hand, administration of glutathione monoester leads to markedly increased tissue and mitochondrial levels of glutathione. Administration of glutathione monoester (in contrast to glutathione) to control mice also increases mitochondrial glutathione levels. The findings indicate that glutathione is required for mitochondrial integrity and that it probably also functions in the processing and storage of surfactant in lamellar bodies. The morphological changes observed after treatment with buthionine sulfoximine and their prevention by glutathione monoester as well as findings on glutathione metabolism indicate that this tripeptide plays an important role in the lung. The previously observed failure of buthionine sulfoximine-treated mice to gain weight is mainly due to glutathione deficiency in the intestinal mucosa.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Aguayo L. G., Warnick J. E., Weinstein H., Glick S. D., Maayani S., Ickowicz R. K., Blaustein M. P. The behavioral effects of phencyclidines may be due to their blockade of potassium channels. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7792–7796. doi: 10.1073/pnas.78.12.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. E., Bridges R. J., Meister A. Direct evidence for inter-organ transport of glutathione and that the non-filtration renal mechanism for glutathione utilization involves gamma-glutamyl transpeptidase. Biochem Biophys Res Commun. 1980 Sep 30;96(2):848–853. doi: 10.1016/0006-291x(80)91433-3. [DOI] [PubMed] [Google Scholar]
- Anderson M. E., Meister A. Dynamic state of glutathione in blood plasma. J Biol Chem. 1980 Oct 25;255(20):9530–9533. [PubMed] [Google Scholar]
- Anderson M. E., Powrie F., Puri R. N., Meister A. Glutathione monoethyl ester: preparation, uptake by tissues, and conversion to glutathione. Arch Biochem Biophys. 1985 Jun;239(2):538–548. doi: 10.1016/0003-9861(85)90723-4. [DOI] [PubMed] [Google Scholar]
- Berggren M., Dawson J., Moldéus P. Glutathione biosynthesis in the isolated perfused rat lung: utilization of extracellular glutathione. FEBS Lett. 1984 Oct 15;176(1):189–192. doi: 10.1016/0014-5793(84)80938-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cantin A. M., North S. L., Hubbard R. C., Crystal R. G. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol (1985) 1987 Jul;63(1):152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- Cornell J. S., Meister A. Glutathione and gamma-glutamyl cycle enzymes in crypt and villus tip cells of rat jejunal mucosa. Proc Natl Acad Sci U S A. 1976 Feb;73(2):420–422. doi: 10.1073/pnas.73.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross C. E., Watanabe T. T., Hasegawa G. K., Goralnik G. N., Roertgen K. E., Kaizu T., Reiser K. M., Gorin A. B., Last J. A. Biochemical assays in lung homogenates: artifacts caused by trapped blood after perfusion. Toxicol Appl Pharmacol. 1979 Mar 30;48(1 Pt 1):99–109. doi: 10.1016/s0041-008x(79)80012-5. [DOI] [PubMed] [Google Scholar]
- Dawson J. R., Vähäkangas K., Jernström B., Moldéus P. Glutathione conjugation by isolated lung cells and the isolated, perfused lung. Effect of extracellular glutathione. Eur J Biochem. 1984 Feb 1;138(3):439–443. doi: 10.1111/j.1432-1033.1984.tb07935.x. [DOI] [PubMed] [Google Scholar]
- English D., Andersen B. R. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J Immunol Methods. 1974 Aug;5(3):249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Anderson M. E., Meister A. Inhibition of glutathione biosynthesis by prothionine sulfoximine (S-n-propyl homocysteine sulfoximine), a selective inhibitor of gamma-glutamylcysteine synthetase. J Biol Chem. 1979 Feb 25;254(4):1205–1210. [PubMed] [Google Scholar]
- Griffith O. W. Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem. 1982 Nov 25;257(22):13704–13712. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5606–5610. doi: 10.1073/pnas.76.11.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Origin and turnover of mitochondrial glutathione. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. A simple specific method for the determination of the hemoglobin content of tissue homogenates. Clin Chim Acta. 1979 Mar 1;92(2):229–234. doi: 10.1016/0009-8981(79)90117-7. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Mårtensson J., Meister A. Mitochondrial damage in muscle occurs after marked depletion of glutathione and is prevented by giving glutathione monoester. Proc Natl Acad Sci U S A. 1989 Jan;86(2):471–475. doi: 10.1073/pnas.86.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J. Method for determination of free and total glutathione and gamma-glutamylcysteine concentrations in human leukocytes and plasma. J Chromatogr. 1987 Sep 4;420(1):152–157. doi: 10.1016/0378-4347(87)80166-4. [DOI] [PubMed] [Google Scholar]
- Mårtensson J. The effect of fasting on leukocyte and plasma glutathione and sulfur amino acid concentrations. Metabolism. 1986 Feb;35(2):118–121. doi: 10.1016/0026-0495(86)90110-1. [DOI] [PubMed] [Google Scholar]
- Nedergaard J., Cannon B. Overview--preparation and properties of mitochondria from different sources. Methods Enzymol. 1979;55:3–28. doi: 10.1016/0076-6879(79)55003-4. [DOI] [PubMed] [Google Scholar]
- Novogrodsky A., Tate S. S., Meister A. gamma-Glutamyl transpeptidase, a lymphoid cell-surface marker: relationship to blastogenesis, differentiation, and neoplasia. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2414–2418. doi: 10.1073/pnas.73.7.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M., Wilk S. Intermediates of the gamma-glutamyl cycle in mouse tissues. Influence of administration of amino acids on pyrrolidone carboxylate and gamma-glutamyl amino acids. Eur J Biochem. 1975 May 6;53(2):581–590. doi: 10.1111/j.1432-1033.1975.tb04101.x. [DOI] [PubMed] [Google Scholar]
- Puri R. N., Meister A. Transport of glutathione, as gamma-glutamylcysteinylglycyl ester, into liver and kidney. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5258–5260. doi: 10.1073/pnas.80.17.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. B., Jr, Srere P. A. Organization of Krebs tricarboxylic acid cycle enzymes in mitochondria. J Biol Chem. 1985 Sep 5;260(19):10800–10805. [PubMed] [Google Scholar]
- Smith J. E. Relationship of in vivo erythrocyte glutathione flux to the oxidized glutathione transport system. J Lab Clin Med. 1974 Mar;83(3):444–450. [PubMed] [Google Scholar]
- Tate S. S., Grau E. M., Meister A. Conversion of glutathione to glutathione disulfide by cell membrane-bound oxidase activity. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2715–2719. doi: 10.1073/pnas.76.6.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate S. S., Meister A. gamma-Glutamyl transpeptidase from kidney. Methods Enzymol. 1985;113:400–419. doi: 10.1016/s0076-6879(85)13053-3. [DOI] [PubMed] [Google Scholar]