Abstract
Preterm infants are at an increased risk of invasive pneumococcal disease infection and, additionally, have a diminished response to Haemophilus influenzae type b (Hib) conjugate vaccines. There are little data examining the response of preterm infants to a seven-valent pneumococcal conjugate vaccine (PCV7). We examined the responses of preterm infants immunized at 2, 3, and 4 months of age to PCV7. A total of 133 preterm and 54 term infants were immunized with PCV7 and the Neisseria meningitidis group C (MCC), diphtheria, tetanus, pertussis, polio, and Hib vaccines. Pneumococcal serotype-specific IgG was measured by enzyme-linked immunosorbent assay (ELISA) pre- and postimmunization and at 12 months or following a booster of PCV7. Term and preterm responses were compared using linear and logistic regression analyses. Term infants had higher preimmunization geometric mean concentrations (GMCs) for all serotypes. Preterm infants had lower postimmunization GMCs for serotype 23F. Gestational age affected postimmunization GMCs for serotypes 4, 6B, and 23F. Preterm infants were as likely to have levels of ≥0.35 μg/ml as term infants for all serotypes except 23F. The proportions of infants with titers of ≥0.35 μg/ml for all 7 serotypes were comparable between groups. A total of 28 of 29 term infants who received a booster had levels of ≥0.35 μg/ml for all serotypes. One infant had undetectable levels for serotype 6B. Of the 32 preterm infants boosted, 9 had levels of <0.35 μg/ml for 1 serotype, and 1 had levels of <0.35 μg/ml for 2 serotypes. In nonboosted infants, GMCs for all serotypes except 6B had fallen by 12 months of age. These results support the need for a booster dose in the second year of life.
The primary immunization schedule of the United Kingdom (UK) is continually evolving. While a vaccine may be demonstrated to be immunogenic in one population when administered according to one schedule, apparently, minor changes to that schedule can have an adverse effect on vaccine response. Preterm infants are at an increased risk of many of the infections we immunize against, for example, pertussis (9). Almost half the children who develop pertussis are under 4 months of age (9). Preterm infants are currently recommended to be vaccinated at the same chronological age as term infants rather than at the same age postconception.
The UK primary immunization schedule in place between September 2004 and September 2006 consisted of a combined vaccine against diphtheria, tetanus, pertussis, polio, and Haemophilus influenzae type b (diphtheria-tetanus-acellular pertussis [DTaP]/inactivated polio vaccine [IPV]/Haemophilus influenzae type b [Hib]) (Pediacel; Aventis Pasteur MSD) and a conjugate vaccine against Neisseria meningitidis group C (MCC) given at 2, 3, and 4 months of age, with no booster in the second year of life (5). In 2002, the chief medical officer advised that children under 2 years of age at risk of invasive pneumococcal disease (IPD) should receive three doses of the seven-valent pneumococcal conjugate vaccine (PCV7; pneumococcal capsular polysaccharide conjugated to the carrier protein CRM197), with their primary immunizations followed by a booster in the second year of life (4). Infants were considered to be at increased risk of IPD if they had a chronic respiratory, cardiac, renal, or liver disease or an immunodeficiency. Many preterm infants are included in these categories.
A postal questionnaire survey of 73 UK neonatal intensive care units highlighted the fact that many preterm infants who are at an increased risk of IPD were not being adequately immunized because of the lack of evidence that these infants are protected by the conjugate pneumococcal vaccine (11). This survey indicated that many infants who were immunized were not receiving the recommended booster dose in the second year of life. In the UK immunization schedule at this time, none of the other vaccines in the primary schedule were boosted.
The immunogenicity of PCV7 when administered to preterm infants according to the then-current UK immunization schedule was examined and compared to the response of a cohort of term infants that was previously described. As many preterm infants were not routinely receiving their 12 -month booster, we also measured antibody levels at 12 months of age.
MATERIALS AND METHODS
This study was approved by the Newcastle and North Tyneside Local Research Ethics Committees and the Medicines and Healthcare Research Authority.
Power calculation.
The power calculation was carried out a priori. Previous studies have demonstrated 97% efficacy of the PCV7 vaccine in term infants immunized at 2, 4, and 6 months of age, according to the U.S. schedule (5). Therefore, assuming 90% of control subjects and 78% of preterm infants would achieve putatively protective levels, a sample size of 200 preterm subjects and 50 term subjects would have 91% power using a 5% two-sided test.
Subjects.
All infants were recruited from the Royal Victoria Infirmary (RVI), Newcastle upon Tyne, UK. Recruitment of preterm infants took place between June 2004 and April 2006, and recruitment of term infants took place between April and June 2005. All infants at <36 weeks of gestation who had required respiratory support during the neonatal period were eligible if the majority of their neonatal care occurred at the RVI. Healthy infants at ≥37 weeks of gestation born at the RVI during the recruitment period were eligible as controls. Written informed consent was obtained from the parents of all participants.
Exclusion criteria were significant congenital abnormalities. In addition, any term infants who had required any medical intervention except for phototherapy prior to recruitment were excluded.
Immunization of preterm infants.
The study design did not intervene in the timing of vaccine administration or brand of vaccine used for the preterm infants. This observational design allowed immunization of all study infants as per normal local practice and according to the standard UK schedule. Infants who did not complete their primary series by the end of their first year of life or who had not received one dose of PCV7 by 6 months of age were excluded from the study; infants were not otherwise excluded on the basis of timing of their immunizations.
The immunization schedule at the time of the study included vaccines against diphtheria, tetanus, polio, pertussis, Hib, and meningococcal serogroup C (MenC) at 2, 3, and 4 months of age for all infants and PCV7 at the same times for infants considered to be at an increased risk of IPD. In addition, some infants also received immunization against influenza and monoclonal immunoglobulin against respiratory syncytial virus (palivizumab).
Prior to September 2004, DTaP/Hib combination vaccines were supplied packaged as a single vaccine (ActHib DTaP). Neonatal inpatients received the inactivated polio vaccine (IPV) simultaneously with their other immunizations, and outpatients received the live oral polio vaccine. Following this time, all infants received the combined “5 in 1” 5-component acellular pertussis vaccine DTaP/IPV/Hib (Pediacel; Aventis Pasteur MSD).
Information regarding the timing, brands, and batches of immunizations that preterm infants received were collected from hospital records, general practitioner (GP) feedback forms, and parent-held patient records. The majority of immunizations were carried out by the GPs.
Immunization of term infants.
Term infants were immunized at 2, 3, and 4 months of age by the research team in the patients' homes. They received immunizations from the same batch of DTaP/IPV/Hib (Pediacel; Aventis Pasteur), as well as a MCC-CRM197 conjugate vaccine, Meningitec (Wyeth Vaccines), and Prevenar (Wyeth Vaccines), with no additional immunizations.
Infants who received a booster dose of PCV7.
The study was not initially designed to examine the responses of infants to a booster dose of the vaccine. However, when the preliminary analysis of the term infants was performed, their response was lower than expected. Therefore, infants who had not achieved putatively protective levels to all serotypes following the primary immunization course were recommended to receive a booster dose of the vaccine as soon as these results were available, regardless of their age. Some preterm infants had already had their 12-month blood samples taken before the results post-primary immunization were known. These infants were included in the analysis of the group of infants who had not received a booster but were recommended to receive a booster dose of PCV7 at the end of the study. The research team administered the vaccine to term infants who fell into this category, and GPs were asked to administer the vaccine to the preterm infants.
Serology.
Venous blood samples were obtained prior to the first immunization, 4 weeks following the third immunization, and either at 12 months of age or 4 weeks after a booster dose of PCV7. Blood samples were allowed to clot at room temperature, and the sera were separated and stored at −80°C until analysis. Pneumococcal serotype-specific IgG antibody concentrations for all PCV7 serotypes and 2 control serotypes (serotypes 1 and 5) were analyzed by enzyme-linked immunosorbent assay (ELISA) at Newcastle University using a standardized protocol which included capsular polysaccharide and serotype 22F capsular polysaccharide adsorption testing (www.vaccine.uab.edu).
Statistical analysis.
Differences in timings of immunizations and sampling were compared using the Mann-Whitney U test.
For statistical analysis, all samples below the lower limit of detection of an assay were assigned values of half the lower limit of detection. Antibody concentrations were log transformed, and geometric mean concentrations (GMCs) were calculated with 95% confidence intervals (CI). Putatively protective levels for all pneumococcal serotypes were defined as pneumococcal serotype-specific titers of ≥0.35 μg/ml, in line with WHO recommendations (15).
The probability of achieving pneumococcal serotype-specific titers of ≥0.35 μg/ml after 3 immunizations was modeled using logistic regression and presented as an odds ratio (OR). The effect of prematurity on GMCs was analyzed using censored linear regression with normally distributed residuals and presented as GMC ratios. Normality was assessed using residual plots. The regression analysis was modeled with entry in blocks. The factors known a priori to influence antibody response are, in this case, the time between the first and third PCV7 administration, time between completion of immunizations and postimmunization blood sampling for the postimmunization results, age at 12-month sampling, and interval between booster and postbooster sampling for 12-month and postbooster results, which were entered in the first block, with the major study variable entered in the second block (term or preterm and gestation in weeks). Analyses were performed with SPSS (version 10).
RESULTS
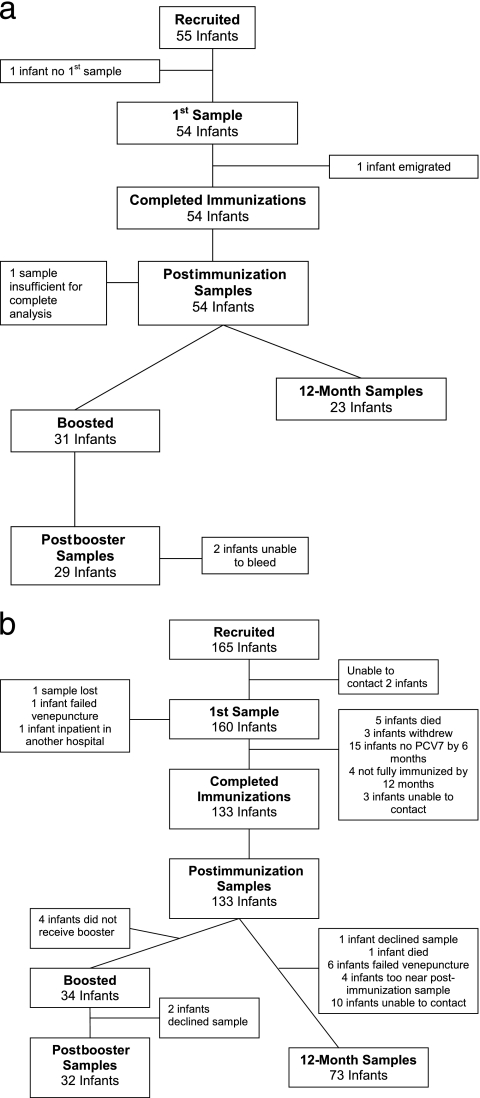
Parents of 189 preterm and 197 term infants were approached to take part in the study; 165 preterm and 55 term infants were recruited. Recruitment of preterm infants was consistent throughout the study period. The progress of all infants throughout the study is given in Fig. 1. There were 132 preterm and 52 term infants who had both pre- and postimmunization samples collected, 32 preterm and 29 term infants who had sera collected following a fourth dose of PCV7, and 73 preterm and 23 term infants who had sera collected at 12 months without having received a fourth dose of PCV7.
FIG. 1.
Progress of infants throughout the study. (a) Term infants; (b) preterm infants.
Demographic data for the study population and for infants who received a fourth dose of PCV7 are given in Tables 1 and 2, respectively. A total of 64 of the 132 preterm infants were born at 28 weeks of gestation or earlier. Despite recommendations during the study period that all infants should receive their primary immunizations at a postnatal age of 2, 3, and 4 months, there was considerable variability, especially among preterm infants (Table 2). Due to the time between postimmunization samples being collected and analyzed as well as problems contacting parents of older infants, there was also significant variation in the timing of booster immunizations for those who required it and the postbooster blood sampling (Table 2).
TABLE 1.
Population demographics of infants
| Characteristic | Value for infants: |
|||
|---|---|---|---|---|
| Completing study |
Receiving fourth dose of PCV7 |
|||
| Term (n = 54) | Preterm (n = 133) | Term (n = 29) | Preterm (n = 32) | |
| Gestation (wk)a | 39.7 (36.9-42) | 28.6 (23.9-35.9) | 39.9 (37.3-51.6) | 27.9 (24.0-32.4) |
| Birth wt (g)a | 3,300 (2,060-4,400) | 1,060 (430-3,390) | 3,315 (2,060-4,380) | 925 (555-2,030) |
| No. of males (%) | 25 (46) | 74 (56) | 15 (52) | 16 (50) |
| No. of multiple births (%) | 4 (7) | 39 (29) | 1 (3) | 9 (28) |
Median (range).
TABLE 2.
Timing of immunizations and blood sampling
| Characteristic | Value for infantsa |
|
|---|---|---|
| Term | Preterm | |
| Age, first samplingb | 61 (56-71) | 54 (28-78) |
| Age, first PCV7 | 61 (56-71) | 61 (50-109) |
| Age, second PCV7b | 89.5 (81-104) | 96 (81-246) |
| Age, third PCV7b | 118 (112-132) | 138 (109-308) |
| Age, second samplingb | 147 (139-172) | 182 (145-337) |
| Interval between first and third PCV7 | 56 (55-70) | 73 (54-243) |
| Interval between third PCV7 and second samplingb | 28 (21-51) | 35 (14-189) |
| Age at boosterb | 342 (325-361) | 416.5 (193-617) |
| Age, postbooster samplingb | 375 (354-402) | 463.5 (246-665) |
| Interval between booster and sampling | 32 (27-56) | 38.5 (14-141) |
| Age, 12-mo nonboosted sampling | 373 (361-420) | 402 (350-526) |
Values shown are the median numbers (ranges) of days.
P < 0.05.
Pre- and postimmunization GMCs and the number of infants achieving putatively protective levels against each serotype are given in Table 3. Although some infants did have putatively protective antibody levels against some serotypes prior to immunization, none had these levels against all 7 vaccine serotypes. Although preimmunization antibody titers were lower in preterm infants for 8 of 9 serotypes tested, the significantly lower postnatal age at which preterm infants had this sample collected may identify a more marked difference. When age in days was included into the regression model, preimmunization titers of all serotypes, except those of serotype 1, remained significantly lower for preterm infants than term infants (P < 0.01). Preimmunization GMCs for all 9 serotypes increased significantly with each week of gestation (P < 0.02).
TABLE 3.
Pre- and postimmunization GMCs and proportions of infants achieving putatively protective levels for all serotypes
| Serotype | IgG GMC (95% CI)a |
No. of infants achieving putatively protective levels (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| Preimmunization |
Postimmunization |
Preimmunization |
Postimmunization |
|||||
| Term (n = 53) | Preterm (n = 132) | Term | Preterm | Term (n = 53) | Preterm (n = 133) | Term | Preterm | |
| 4 | 0.04 (0.03-0.05) | 0.02 (0.02-0.02) | 1.61 (1.26-2.07) | 1.02 (0.87-1.19) | 0 | 0 | 50 (96) | 117 (88) |
| 6B | 0.08 (0.06-0.10) | 0.05 (0.05-0.06) | 0.29 (0.20-0.42) | 0.20 (0.15-0.27) | 6 (12) | 9 (7) | 20 (38) | 52 (39) |
| 9V | 0.07 (0.05-0.11) | 0.04 (0.03-0.05) | 1.07 (0.82-1.39) | 0.94 (0.79-1.10) | 9 (17) | 1 (1) | 47 (90) | 114 (86) |
| 14 | 0.33 (0.22-0.49) | 0.19 (0.16-0.23) | 3.80 (2.79-5.19) | 3.80 (3.12-4.64) | 23 (44) | 34 (26) | 51 (98) | 127 (96) |
| 18C | 0.26 (0.20-3.04) | 0.09 (0.07-0.11) | 1.39 (1.11-1.75) | 1.05 (0.91-1.21) | 23 (44) | 11 (8) | 50 (96) | 121 (91) |
| 19F | 0.45 (0.36-0.56) | 0.21 (0.18-0.25) | 2.64 (2.06-3.39) | 1.67 (1.44-1.94) | 33 (63) | 41 (31) | 52 (100) | 128 (96) |
| 23F | 0.12 (0.08-0.16) | 0.06 (0.05-0.07) | 1.46 (1.07-2.00) | 0.58 (0.44-1.94) | 11 (21) | 10 (8) | 50 (96) | 94 (71) |
| 1 | 0.03 (0.02-0.03) | 0.02 (0.02-0.03) | 0.02 (0.01-0.02) | 0.01 (0.01-0.02) | 1 (2) | 0 | 0 | 0 |
| 5 | 0.11 (0.08-0.16) | 0.06 (0.06-0.07) | 0.07 (0.06-0.09) | 0.03 (0.03-0.04) | 1 (2) | 0 | 1 (2) | 1 (1) |
Values shown are in μg/ml.
For term and preterm infants, GMCs increased from pre- to postimmunization for all 7 vaccine serotypes (4, 6B, 9V, 14, 18C, 19F, and 23F) but not for the 2 control serotypes (1 and 5). However, only 19 (36%) term infants and 45 (34%) preterm infants achieved putatively protective levels against all 7 vaccine serotypes, with only 38 and 39% of infants, respectively, achieving these levels against serotype 6B. Preterm delivery was associated with a lower GMC to serotype 23F and reduced odds of achieving putatively protected levels to that serotype (P < 0.01) but did not affect the response to the other vaccine serotypes. The effect of gestation in weeks on GMCs and the proportion of infants achieving putatively protective levels postimmunization for each serotype are given in Table 4. There was no significant difference in the odds of being protected against all 7 vaccine serotypes between term and preterm infants or with increasing gestational age.
TABLE 4.
Effect of gestation in weeks on GMCs and ORs of infants achieving titers of ≥0.35 μg/ml for each serotype postimmunization
| Serotype | Factor increase for each wk of gestationb |
|
|---|---|---|
| GMC (95% CI) | OR (95% CI) | |
| 1 | 1.03 (1.01-1.05)a | |
| 4 | 1.03 (1.00-1.05)a | 1.05 (0.95-1.16) |
| 5 | 1.08 (1.05-1.10)a | |
| 6B | 1.07 (1.03-1.12)a | 1.06 (1.00-1.12) |
| 9V | 1.00 (0.98-1.03) | 1.01 (0.93-1.10) |
| 14 | 1.03 (1.00-1.06) | 1.13 (0.99-1.30) |
| 18C | 1.01 (0.99-1.03) | 1.00 (0.90-1.12) |
| 19F | 1.02 (1.00-1.05) | 0.90 (0.75-1.08) |
| 23F | 1.09 (1.05-1.13)a | 1.16 (1.07-1.23)a |
P < 0.04.
Values shown are in μg/ml. Boldface, statistically significant results for vaccine serotypes.
The GMCs at 12 months of age were significantly lower than postimmunization GMCs for all serotypes (P < 0.03), except serotypes 1, 5 (nonvaccine control serotypes), and 6B (P < 0.06) (Table 5). There was no significant difference in rate of decline in titers for term and preterm infants (data not shown). Interestingly, some individuals demonstrated an increase in titers from postimmunization to 12 months of age for some serotypes, and the GMC for the control serotype 5 in both cohorts increased significantly (P < 0.01) over this time period. The proportion of infants achieving putatively protective levels to serotypes 4, 9V, 18C, and 23F decreased significantly (P < 0.03) from postimmunization to 12 months for all infants, as did the proportions of term infants and preterm infants achieving putatively protective levels to serotypes 6B and 19F, respectively. The proportion of infants achieving these levels against serotype 14 remained the same. At 12 months of age, only 5 (22%) term infants and 8 (11%) preterm infants had putatively protective levels against all vaccine serotypes. The effect of preterm delivery resulted in significantly lower GMCs to serotypes 4, 9V, 18C, 19F, and 23F (P < 0.05) and reduced the odds of achieving putatively protective levels to serotypes 18C, 19F, and 23F at 12 months of age. There was a positive relationship between gestational age in weeks and GMC for serotypes 4, 6B, 9V, 18C, 19F, and 23F and between gestational age in weeks and the odds of achieving putatively protective levels to serotypes 18C, 19F, and 23F (P < 0.03) at 12 months of age. There was no significant difference in the odds of being protected against all 7 vaccine serotypes between term and preterm infants or with increasing gestational age.
TABLE 5.
Summary data for 12-month antibody statuses of infants who did not receive a fourth dose of PCV7
| Serotype | IgG GMC (95% CI)a |
No. of infants achieving putatively protective levels (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| Postimmunization |
At 12 months |
Postimmunization |
At 12 months |
|||||
| Term (n = 22) | Preterm (n = 73) | Term (n = 23) | Preterm (n = 73) | Term | Preterm | Term | Preterm | |
| 4 | 2.31 (1.72-3.11) | 1.21 (1.00-1.48) | 0.38 (0.25-0.59) | 0.23 (0.19-0.28) | 22 (100) | 69 (95) | 13 (57) | 24 (33) |
| 6B | 0.90 (0.54-1.50) | 0.34 (0.24-0.48) | 0.50 (0.29-0.86) | 0.26 (0.19-0.36) | 17 (77) | 38 (52) | 7 (30) | 30 (41) |
| 9V | 1.71 (1.26-2.33) | 1.15 (0.97-1.37) | 0.46 (0.29-0.75) | 0.29 (0.24-0.35) | 22 (100) | 69 (95) | 11 (48) | 30 (41) |
| 14 | 6.40 (4.27-9.59) | 4.39 (3.43-5.63) | 2.09 (1.47-2.96) | 1.58 (1.25-1.99) | 22 (100) | 71 (97) | 23 (100) | 69 (95) |
| 18C | 2.05 (1.43-2.93) | 1.28 (1.07-1.52) | 0.60 (0.38-0.94) | 0.31 (0.25-0.39) | 21 (96) | 69 (95) | 16 (70) | 30 (41) |
| 19F | 3.82 (2.88-5.07) | 2.22 (1.89-2.61) | 1.59 (0.80-3.18) | 0.50 (0.38-0.66) | 22 (100) | 73 (100) | 20 (87) | 43 (59) |
| 23F | 2.33 (1.55-3.50) | 0.75 (0.54-1.04) | 0.36 (0.26-0.51) | 0.21 (0.17-0.27) | 22 (100) | 57 (78) | 11 (48) | 22 (30) |
| 1 | 0.02 (0.01-0.03) | 0.01 (0.01-0.02) | 0.03 (0.02-0.06) | 0.03 (0.02-0.03) | 0 | 0 | 1 (4) | 0 |
| 5 | 0.09 (0.06-0.15) | 0.03 (0.03-0.04) | 0.19 (0.12-0.30) | 0.13 (0.10-0.17) | 1 (5) | 0 | 7 (30) | 12 (16) |
Values shown are in μg/ml.
There was a significant increase in GMCs for both term and preterm infants for all serotypes, including the 2 control serotypes from post-primary immunizations to post-fourth dose (P ≤ 0.01) (Table 6). Twenty-eight term infants achieved putatively protective levels against all 7 vaccine serotypes, and the remaining infant had undetectable levels against serotype 6B. However, 9 preterm infants failed to achieve putatively protective levels against one serotype, and 1 infant did not have protective levels to two serotypes following a fourth dose of the vaccine. Preterm birth adversely affected the GMCs to serotypes 4, 6B, 14, 19F, and 23F (P < 0.01). There was a significant difference between the proportions of preterm and term infants who achieved putatively protective levels to serotype 23F (P < 0.05) but not to other serotypes postbooster.
TABLE 6.
GMCs and proportions of infants achieving putatively protective levels following a fourth dose of PCV7
| Serotype | IgG GMC (95% CI)a |
No. of infants achieving putatively protective levels (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| Postimmunization |
Postbooster |
Postimmunization |
Postbooster |
|||||
| Term (n = 29) | Preterm (n = 32) | Term | Preterm | Term | Preterm | Term | Preterm | |
| 4 | 1.27 (0.88-1.84) | 1.27 (0.88-1.84) | 3.84 (2.72-5.42) | 2.08 (1.47-2.93) | 26 (90) | 24 (75) | 29 (100) | 32 (100) |
| 6B | 0.13 (0.10.0.17) | 0.07 (0.04-0.10) | 5.95 (3.28-10.78) | 2.13 (1.31-3.46) | 3 (10) | 2 (6) | 28 (97) | 31 (97) |
| 9V | 0.81 (0.57-1.16) | 0.52 (0.38-0.72) | 3.60 (2.73-4.75) | 2.29 (1.64-3.21) | 24 (83) | 21 (66) | 29 (100) | 31 (97) |
| 14 | 2.62 (1.70-4.04) | 2.39 (1.57-3.64) | 10.54 (7.88-14.10) | 6.46 (4.32-9.84) | 27 (93) | 29 (91) | 29 (100) | 32 (100) |
| 18C | 1.08 (0.84-1.37) | 0.69 (0.53-0.89) | 1.99 (1.57-2.51) | 1.19 (0.84-1.68) | 28 (97) | 27 (84) | 29 (100) | 29 (91) |
| 19F | 2.09 (1.45-2.99) | 1.05 (0.78-1.42) | 4.51 (3.13-6.51) | 1.83 (1.25-2.70) | 28 (97) | 30 (94) | 29 (100) | 30 (94) |
| 23F | 1.01 (0.65-1.57) | 0.27 (0.16-0.43) | 4.79 (3.11-7.37) | 1.60 (0.95-2.70) | 27 (93) | 17 (53) | 29 (100) | 28 (88) |
| 1 | 0.02 (0.01-0.02) | 0.01 (0.01-0.02) | 0.05 (0.03-0.11) | 0.02 (0.02-0.03) | 0 | 0 | 8 (28) | 0 |
| 5 | 0.07 (0.05-0.10) | 0.03 (0.02-0.04) | 0.29 (0.20-0.43) | 0.08 (0.05-0.13) | 0 | 0 | 15 (52) | 5 (16) |
Values shown are in μg/ml.
DISCUSSION
Preterm infants have been shown to have a diminished response to Hib conjugate vaccines (1), and this coupled with their increased respiratory morbidity (2) highlights the need to ensure that they respond adequately to immunization against pneumococcus. The findings from this study contribute important data.
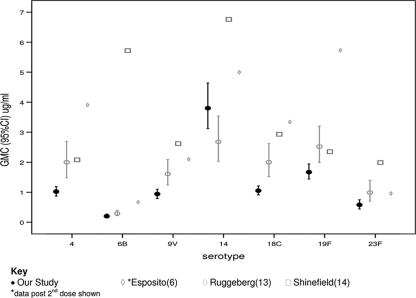
Three previously published studies examining the immunogenicity of PCV7 in preterm infants vary in their methodology, including the timing of vaccine administration, and their findings (6, 13, 14). Additionally, the inclusion in our study of infants who have required some form of respiratory support resulted in the subjects being more preterm and more sick than those in two of the other studies. A comparison of the postimmunization GMCs from our study and those from the studies by Ruggeberg et al. (13), Shinefield et al. (14), and Esposito et al. (6) is given in Fig. 2.
FIG. 2.
Comparison of postimmunization GMCs in preterm infants between our study and those of Esposito et al., Ruggeberg et al., and Shinefield et al.
When comparing the proportion of preterm infants achieving putatively protective levels to each serotype from our study and those from the studies by Ruggeberg et al. (13) and Esposito et al. (6), we used the cutoff of ≥0.35 μg/ml. In the study by Esposito et al., a higher proportion of infants achieved putatively protective levels to serotypes 4 and 6B, while in the study by Ruggeberg et al., a higher proportion of infants had these levels to serotype 23F. Both our study and that of Ruggeberg et al. found that >90% of preterm infants had putatively protective levels against 5 or more serotypes. Shinefield et al. and Esposito et al. used a cutoff of ≥0.15 μg/ml as a correlate of protection, and both studies found that ≥95% of infants achieved these levels against each serotype. The results from our study were comparable for all serotypes (data not shown), except serotypes 6B and 23F, for which only 60% and 83% of infants, respectively, achieved these levels.
All of these studies also compare the results of the preterm infants to those of a contemporaneous term cohort. Neither Shinefield et al. (14) nor Esposito et al. (6) found a difference between the responses of preterm and term infants to the PCV7 following the primary course. In contrast, Ruggeberg et al. (13) found that preterm infants had significantly lower GMCs to all serotypes except 18C and that statistically fewer preterm infants achieved levels of ≥0.35 μg/ml for all serotypes except 14 and 18C. In our study, preterm infants had lower GMCs and were less likely to achieve putatively protective levels to serotype 23F only.
The only other study that examines the responses of preterm infants to a preterm booster dose of PCV7 is that of Esposito et al. (6), which found higher GMCs postbooster for all vaccine serotypes. Furthermore, all of the preterm infants included in that study except one achieved putatively protective levels against all 7 vaccine serotypes following a booster dose of PCV7, whereas 10 of the 32 infants who received a booster in our study did not achieve these levels to 1 or more vaccine serotypes.
Preterm infants have previously been demonstrated to have a diminished response to Hib conjugate vaccines and, in some cases, to MCC vaccines (1, 3). It is therefore unsurprising that preterm infants have a reduced response to PCV7 compared to that of term infants. There was, however, marked variation between different serotypes, demonstrating that like polysaccharides, some polysaccharide-protein conjugates are more immunogenic in infants than others. It is possible that we did not see a difference between the preterm and term cohorts for serotypes 4 and 6B because of the relatively small difference in gestational age between the most mature preterm infants and the least mature term infants.
Although the exact mechanisms of the immune response to conjugate vaccines remain unclear, it is apparent that it is more complicated than the immune response to a simple protein antigen. The differences in lymphocyte phenotypes between term and preterm infants could therefore be more important in the response to conjugate vaccines. The fact that there was a relationship between gestational age at birth as well as corrected gestational age and GMC for three of the vaccine serotypes suggests that at least for these serotypes, the ability to mount an immune response to the polysaccharide protein conjugate is a developmental process rather than an on/off process.
The methodology of our study allowed the preterm infants to be immunized as they would normally have been in the community. This resulted in considerable variation in the timing of immunizations, brand of other vaccines administered (especially the MCC vaccine), and whether or not vaccines were administered simultaneously. It is possible that the many variations that arose from the study design have masked some differences between term and preterm infants, even though efforts were made to control them during regression analysis. As discussed previously, the responses of term infants in this study were less than expected (12). This could therefore mask any differences between the term and preterm cohorts. We also failed to recruit as many preterm infants to the study as we had hoped, which again could have resulted in type 2 error. The postimmunization results of some preterm infants were not known prior to their 12-month blood samples being collected; therefore, some infants who we recommended to have boosters did not have these boosters until the end of the study. This may have resulted in the results of the preterm cohort that had blood samples taken at 12 months being lower than those of the term cohort.
In September 2006, a new primary immunization schedule was introduced in the UK. This new schedule recommends that all infants receive DTaP/IPV/Hib at 2, 3, and 4 months of age, PCV7 at 2 and 4 months of age, and the MCC vaccine at 3 and 4 months of age. Infants are boosted with a combined Hib/MCC vaccine at 12 months of age and a third dose of PCV7 at 13 months of age, with the measles, mumps, and rubella vaccine. Although the schedule examined in our study differed from this new immunization program, the results are still relevant. Preterm infants have significantly less maternally derived antibody than term infants. Early effective immunization is therefore important to decrease the window of disease vulnerability. This is supported by the increased incidence of IPD among preterm and low-birth-weight infants in the study by Shinefield et al. (14). Our study found that in addition to a poor response to serotype 6B, preterm infants had a diminished response to serotype 23F, and several infants remained unprotected to at least one serotype following a booster dose of the vaccine. The implications for preterm infants immunized according to the new UK schedule remain unclear. Although studies have demonstrated equivalent immunogenicity of conjugate vaccines with a reduced number of doses but larger interval (7), it is unlikely that reducing the number of doses an infant receives will result in improved immunogenicity of the vaccine. However, if the majority of healthy term infants are well protected, it is possible that with good herd immunity and low levels of IPD, the response of a specific vulnerable individual, such as a preterm infant, to the vaccine will become less important.
Until the efficacy of the new immunization schedule is established, it will be important to consider the protection of preterm infants against IPD. Given that the majority of infants had an adequate response to a fourth dose, it is likely that the reduced response of our preterm cohort is due at least in part to immunological immaturity. A practical solution would be to offer to examine the response to PCV7 in preterm infants post-primary immunization and, if they are unprotected, to offer them an early booster dose. However, this would have significant cost implications and would require rigorous systems to ensure that at-risk infants were identified. An alternative approach would be to ensure that household contacts were immunized against pneumococcus. There is evidence that immunization with PCV7 decreases nasal carriage (8), and therefore, if close contacts of vulnerable individuals are immunized with PCV7, those individuals are likely to be exposed to pneumococcus. In addition, there has been a decrease in the incidence of IPD in older adults following the introduction of PCV7 in the United States (10), and it has been hypothesized that this is a result of reduced transmission of pneumococcus from children to their grandparents.
Acknowledgments
This study was kindly funded by Wyeth Vaccines, the Children's Research Fund, and the Bubble Foundation.
Footnotes
Published ahead of print on 22 September 2010.
REFERENCES
- 1.Berrington, J. E., A. J. Cant, J. N. Matthews, M. O'Keeffe, G. P. Spickett, and A. C. Fenton. 2006. Haemophilus influenzae type b immunization in infants in the United Kingdom: effects of diphtheria/tetanus/acellular pertussis/Hib combination vaccine, significant prematurity, and a fourth dose. Pediatrics 117:e717-e724. [DOI] [PubMed] [Google Scholar]
- 2.Broughton, S., R. Bhat, A. Roberts, M. Zuckerman, G. Rafferty, and A. Greenough. 2006. Diminished lung function, RSV infection, and respiratory morbidity in prematurely born infants. Arch. Dis. Child. 91:26-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cant, A., J. E. Berrington, A. C. Fenton, Q. Zang, and A. Finn. 2005. Reduced IgG antibody response to Neisseria meningitidis type C (MenC) immunisation in significantly preterm UK infants, ES2. Abstr. 23rd Annu. Meet. Eur. Soc. Paediatr. Infect. Dis. (ESPID), 18 to 20 May 2005, Valencia, Spain.
- 4.CMO. 2002, posting date. Pneumococcal vaccine for at-risk under 2 year olds. http://www.doh.gov.uk/cmo/cmo0201.htm.
- 5.Department of Health Immunisation against Infectious Diseases. 1996. The green book. Department of Health Immunisation against Infectious Diseases, London, United Kingdom.
- 6.Esposito, S., L. Pugni, S. Bosis, A. Proto, L. Cesati, C. Bianchi, C. Cimino, F. Mosca, and N. Principi. 2005. Immunogenicity, safety and tolerability of heptavalent pneumococcal conjugate vaccine administered at 3, 5 and 11 months post-natally to pre- and full-term infants. Vaccine 23:1703-1708. [DOI] [PubMed] [Google Scholar]
- 7.Goldblatt, D., J. Southern, L. Ashton, P. Richmond, P. Burbidge, J. Tasevska, A. Crowley-Luke, N. Andrews, R. Morris, R. Borrow, K. Cartwright, and E. Miller. 2006. Immunogenicity and boosting after a reduced number of doses of a pneumococcal conjugate vaccine in infants and toddlers. Pediatr. Infect. Dis. J. 25:312-319. [DOI] [PubMed] [Google Scholar]
- 8.Klugman, K. P. 2001. Efficacy of pneumococcal conjugate vaccines and their effect on carriage and antimicrobial resistance. Lancet Infect. Dis. 1:85-91. [DOI] [PubMed] [Google Scholar]
- 9.Langkamp, D. L., and J. P. Davis. 1996. Increased risk of reported pertussis and hospitalization associated with pertussis in low birth weight children. J. Pediatr. 128:654-659. [DOI] [PubMed] [Google Scholar]
- 10.Lexau, C. A., R. Lynfield, R. Danila, T. Pilishvili, R. Facklam, M. M. Farley, L. H. Harrison, W. Schaffner, A. Reingold, N. M. Bennett, J. Hadler, P. R. Cieslak, C. G. Whitney, and the Active Bacterial Core Surveillance Team. 2005. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 294:2043-2051. [DOI] [PubMed] [Google Scholar]
- 11.Moss, S. J., A. C. Fenton, and A. R. Gennery. 2005. Use of conjugate pneumococcal vaccine by United Kingdom neonatal intensive care units. Arch. Dis. Child. Fetal Neonatal Ed. 90:F187-F188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moss, S. J., A. C. Fenton, J. Toomey, A. Grainger, R. Borrow, P. Balmer, J. Smith, and A. R. Gennery. 2010. Immunogenicity of a heptavalent conjugate pneumococcal vaccine administered concurrently with a combination diphtheria, tetanus, five-component acellular pertussis, inactivated polio, and Haemophilus influenzae type b vaccine and a meningococcal group C conjugate vaccine at 2, 3, and 4 months of age. Clin. Vaccine Immunol. 17:311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruggeberg, J. U., C. Collins, P. Clarke, N. Johnson, R. Sinha, N. Everest, J. Chang, E. Stanford, P. Balmer, R. Borrow, S. Martin, M. J. Robinson, E. R. Moxon, A. J. Pollard, and P. T. Heath. 2007. Immunogenicity and induction of immunological memory of the heptavalent pneumococcal conjugate vaccine in preterm UK infants. Vaccine 25:264-271. [DOI] [PubMed] [Google Scholar]
- 14.Shinefield, H., S. Black, P. Ray, B. Fireman, J. Schwalbe, and E. Lewis. 2002. Efficacy, immunogenicity and safety of heptavalent pneumococcal conjugate vaccine in low birth weight and preterm infants. Pediatr. Infect. Dis. J. 21:182-186. [DOI] [PubMed] [Google Scholar]
- 15.WHO. 2005. Recommendations for the production and control of pneumococcal conjugate vaccines. WHO technical report series no. 927. World Health Organization, Geneva, Switzerland.