Abstract
The most advanced malaria vaccine, RTS,S, is comprised of an adjuvant portion of the Plasmodium falciparum circumsporozoite (CS) protein fused to and admixed with the hepatitis B virus surface antigen. This vaccine confers short-term protection against malaria infection, with an efficacy of about 50%, and induces particularly B-cell and CD4+ T-cell responses. In the present study, we tested by the hypothesis that the Th1 immune response to CS protein, in particular the CD8+ T-cell response, which is needed for strong and lasting malaria immunity, is boosted to sustainable levels vectors adenovirus and 26 with an homologous insert 35 (Ad35.CS/Ad26.CS). In this study, we evaluated immune responses induced with vaccination regimens based on an adjuvant-containing, yeast-produced complete CS protein followed by two recombinant low-seroprevalence adenoviruses expressing P. falciparum CS antigen, Ad35.CS (subgroup B) and Ad26.CS (subgroup D). Our results show that (i) the yeast (Hansenula polymorpha)produced, adjuvanted full-length CS protein is highly potent in inducing high CS-specific humoral responses in mice but produces poor T-cell responses, (ii) the Ad35.CS vector boosts the gamma interferon-positive (IFN-γ+) CD8+ T-cell response induced by the CS protein immunization and shifts the immune response toward the Th1 type, and (iii) a three-component heterologous vaccination comprised of a CS protein prime followed by boosts with Ad35.CS and Ad26.CS elicits an even more robust and sustainable IFN-γ+ CD8+ T-cell response than one- or two-component regimens. The Ad35.CS/Ad26.CS combination boosted particularly the IFN-γ+ and tumor necrosis factor alpha-positive (TNF-α+) T cells, confirming the shift of the immune response from the Th2 type to the Th1 type. These results support the notion of first immunizations of infants with an adjuvanted CS protein vaccine, followed by a booster Ad35.CS/Ad26.CS vaccine at a later age, to induce lasting protection against malaria for which the Th1 response and immune memory is required.
Almost 40 years after the feasibility of vaccination against malaria was first demonstrated by means of irradiated sporozoites (9), a vaccine modality that efficiently induces long-lived protective immunity remains elusive. The most advanced circumsporozoite (CS)-based malaria vaccine candidate to date is RTS,S, a vaccine based on a fragment of Plasmodium falciparum circumsporozoite (CS) protein fused to and admixed with hepatitis B virus surface protein. In adults, RTS,S with the adjuvant AS02 has consistently conferred 40% protection against malaria infection upon sporozoite challenge (54). Even though RTS,S/AS02 induces high-level CS-specific antibody responses, the induced T-cell responses are weak (21). As the Th1 response, particularly gamma interferon (IFN-γ) and CD8+ T cells, is associated with protection, novel adjuvant systems were developed with the aim of improving the induced T-cell response while maintaining potent levels of CS-specific antibody responses. One of these novel adjuvant systems, AS01, demonstrated its suitability in mice, as it improved CS-specific CD4+ T-cell responses and led to induction of CD8+ T cells (32). Nonhuman primate studies also demonstrated that RTS,S with AS01 adjuvant induces strong CS-specific antibody responses as well as mean higher frequencies of IFN-γ- and tumor necrosis factor alpha (TNF-α)-producing CD4+ T cells than those generated by RTS,S with AS02 adjuvant. However, the induction of CD8+ T cells was not confirmed in this nonhuman primate study (32). In humans, RTS,S/AS01 has been shown to induce high titers of CS-specific antibodies and higher numbers of Th1 CD4+ T cells than those generated by RTS,S/AS02 but no CS-specific CD8+ T cells (22). However, RTS,S/AS01 was able to afford 50% protection against malaria infection in adults upon sporozoite challenge (22) and 53% efficacy against disease in children between the ages of 5 and 17 months (5). These results, albeit far from being optimal, supported the progress of RTS,S/AS01 to phase III clinical trial testing in early 2009, and these trials enrolled children at the age of 6 weeks to 17 months at multiple sites in sub-Saharan Africa. It is anticipated that RTS,S/AS01 will be the first licensed malaria vaccine, provided its efficacy is confirmed in the phase III trial.
Although our understanding about the correlate(s) of protection for malaria is limited, there is ample evidence that CS protein-specific antibodies, CD8+ T cells, and Th1 cytokines, particularly IFN-γ, play a central role in controlling the preerythrocytic and early liver stages of malaria (19, 20, 35, 47, 57). Adenovirus (Ad) vectors are particularly suited for induction of IFN-γ-producing CD8+ T cells required to combat malaria infection (33, 43), due to intracellular expression of a transgene inserted in the vector genome and efficient routing of expressed protein toward the class I presentation pathway. Recently, we demonstrated the advantage of utilizing two recombinant adenoviral vectors derived from distinct serotypes, Ad type 35 CS (Ad35.CS) and Ad5.CS, in a heterologous prime-boost regimen in mice and nonhuman primates (46). This heterologous prime-boost regimen elicited a high-level CS-specific IFN-γ+ T-cell response as well CS-specific Th1-type antibodies able to bind malaria parasites. Though the Ad5-based vectors are very potent vaccines, the high prevalence of preexisting immunity toward Ad5 in the human population hampers their immunogenicity and clinical utility (8, 38). The low seroprevalence of Ad5-neutralizing antibodies in infants of 6 months to 1.5 years of age offers an opportunity to administer Ad5-based vaccines to this population without antibodies interfering and neutralizing the vaccine efficacy (42); however, acceptance of this approach by regulatory agencies may remain difficult to obtain. Novel vaccine vectors based on rare low-seroprevalence Ad serotypes have an advantage of not being hampered by anti-Ad5 immunity while inducing a strong immune response (1, 4, 28, 33, 41).
Within this study, we evaluated whether vaccination with Ad35.CS and Ad26.CS can enhance the CS-specific immune response induced by a yeast-produced full-length CS protein vaccine and, in particular, whether the combined vaccination sustainably potentiates the Th1 responses necessary for protection against malaria. The Ad35.CS vaccine candidate is currently being evaluated in a phase 1 clinical study, in partnership with the National Institute of Allergy and Infectious Diseases, and so far, it has been shown to be safe. Candidate Ad35-based vaccines against other infectious diseases, i.e., tuberculosis and HIV infection, have also been clinically evaluated and demonstrated to be safe and immunogenic. Recently, an Ad26 vector vaccine against HIV was also clinically assessed in a phase I study, which showed that a 3-dose regimen of this HIV candidate vaccine is safe and immunogenic. Based upon encouraging results, the clinical testing of the combination of Ad35- and Ad26-based vaccines against malaria and HIV is in preparation.
MATERIALS AND METHODS
Vector and protein construction, production, and purification.
E1/E3-deleted, replication-incompetent Ad26 and Ad35 vectors expressing the same P. falciparum CS gene were generated in E1-complementing PER.C6 cells and purified using CsCl gradients as previously described (1, 16). Viral particles (VP) were quantified by high-performance liquid chromatography (HPLC). The P. falciparum CS gene is a synthetic, mammalian-codon-optimized insert encoding a CS protein based on the EMBL protein sequence CAH04007, and it is truncated for the last 14 amino acids at the C terminus. The N terminus of the CS protein is a consensus assembled by alignment of various sequences present in GenBank, while the repeat region and the C terminus are based on the sequence of the 3D7 P. falciparum clone. The CS repeat region consisted of 27 NANP repeats, a cluster of 3 NVDP sequences, and one separate NVDP sequence. CS protein of the same sequence as in the adenovirus vectors has been produced in Hansenula polymorpha RB11 clone by ARTES Biotechnology GmbH (Germany). A C-terminal His tag sequence was introduced into the construct to facilitate Ni-column purification of the CS protein from the culture supernatant.
Characterization of the yeast-produced CS protein.
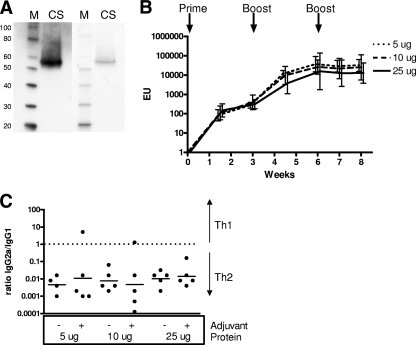
The yeast-produced CS protein was analyzed by CS-specific Western blotting and InstantBlue staining, which demonstrated the identity and purity of the CS protein (more than 80% pure) (Fig. 1A). For the Western blot, rabbit polyclonal antibody against P. falciparum CS (MRA-24; the Malaria Research and Reference Reagent Resource Center, American Type Culture Collection) was used in combination with goat anti-rabbit immunoglobulin G (IgG) conjugated to horseradish peroxide (HRP; Bio-Rad), and enhanced chemiluminescence (ECL+; GE Health Care) was used to detect CS expression. The InstantBlue staining was performed according to protocol provided by the manufacturer (Expedeon).
FIG. 1.
Characterization of the yeast-produced CS protein. (A) The yeast-produced CS protein was analyzed by CS-specific Western blotting (left two lanes) and InstantBlue staining (right two lanes). M, magic marker; CS, CS protein. (B) BALB/c mice (5 per group) were immunized subcutaneously (s.c.) three times with 5, 10, or 25 μg CS protein with a Montanide ISA720 adjuvant at 3-week intervals. CS-specific humoral responses were assessed by an ELISA every 1.5 weeks up to 8 weeks after the initial immunization. Mean titers with 95% confidence intervals are depicted. EU, ELISA units. (C) IgG2a/IgG1 ratios upon measurement of CS-specific IgG2a and IgG1 responses 8 weeks after the initial immunization. Bars represent geometric mean IgG2a/IgG1 ratios.
A dose of the yeast-produced CS protein for prime-boost immunogenicity studies was selected using immunization of BALB/c mice (5 per group) with increasing dosages of CS protein (5 μg, 10 μg, and 25 μg), formulated with the Montanide ISA 720 (Seppic, France) at a 30:70 volume-based ratio, at 3-week intervals. The CS-specific humoral response were assessed using an enzyme-linked immunosorbent assay (ELISA), which demonstrated that the yeast-produced CS protein induced maximal CS-specific antibody responses at the lowest tested dose (5 μg) and after two immunizations (Fig. 1B). The induced IgG response consisted predominantly of IgG1 antibodies, indicating the Th2-type response (Fig. 1C). Analysis of the CS-specific cellular immunity using an enzyme-linked immunospot (ELISPOT) assay revealed poor induction of IFN-γ+ T cells for all doses (data not shown).
Animals and vaccination regimens.
Our study sought to evaluate whether vaccination with Ad35.CS and Ad26.CS can enhance the CS-specific immune response induced by a protein-based vaccine (eventually RTS,S) as a potential vaccination strategy for malaria. Since the RTS,S vaccine was not available, for these studies, we used a yeast-produced full-length CS protein vaccine.
All animal experiments were approved in advance by an independent ethical review board. Six- to 8-week-old female BALB/c mice were purchased from Harlan (Zeist, Netherlands) and kept at an institutional animal facility under specific-pathogen-free conditions during the experiment.
To evaluate the immunogenicity of the heterologous CS protein/Ad prime-boost regimens, BALB/c mice (8 per group) were primed at week 0 with 5 μg CS protein with adjuvant and boosted at week 4 with 109 VP Ad35.CS. The optimal immunization doses of Ad.CS for immunization were selected from earlier dose-response experiments (data not shown). Another group of mice (n = 8) received a homologous prime-boost regimen of 5 μg CS protein with adjuvant. For a negative-control group, BALB/c mice (n = 6) were injected at week 0 with adjuvant Montanide ISA720 and at week 4 with 109 VP Ad35.Empty (adenovirus vector without insert, indicated as the sham immunization group).
To evaluate the 3-component heterologous prime-boost, BALB/c mice (n = 8) were immunized at week 0 with 5 μg CS protein with adjuvant and boosted at week 4 with 109 VP Ad35.CS and at week 8 with 1010 VP Ad26.CS. Comparator groups of BALB/c mice (8 per group) started immunization at week 4 with 5 μg CS protein with adjuvant and were boosted after 4 weeks (at week 8) with either 109 VP Ad35.CS or 5 μg CS protein with adjuvant. For a negative-control group, mice (n = 3) received the adjuvant Montanide ISA720 at week 0, 109 VP Ad35.Empty (Ad35.Empty) at week 4 and 1010 VP Ad26.Empty at week 8.
CS-specific T-cell assays.
CS-specific cellular immune responses in vaccinated mice were assessed using an IFN-γ ELISPOT assay, intracellular cytokine staining in combination with surface staining of CD4 and CD8 markers (ICS) as described previously elsewhere (4, 45), and a cytometric bead array (CBA) assay.
For the stimulation of splenocytes in the ELISPOT assay and ICS, a peptide pool consisting of 11-amino-acid-overlapping 15-mer peptides spanning the whole sequence of the P. falciparum CS protein was used. The pool contained a highly immunodominant CD8+ T-cell epitope (NYDNAGTNL; H-2Kd), which is responsible for the main part of measured responses in the ELISPOT assay and the CD8+ responses in the ICS. This was confirmed with an experiment wherein the splenocytes were stimulated with the 9-mer peptides, which generated responses virtually identical to those generated with the peptide pool (data not shown). For the ELISPOT assay, 96-well multiscreen plates (Millipore, Bedford, MA) were coated overnight with 100 μl/well of 10 μg/ml anti-mouse IFN-γ (BD Pharmingen, San Diego, CA) in endotoxin-free Dulbecco's phosphate-buffered saline (D-PBS). The plates were then washed three times with D-PBS containing 0.05% Tween 20 (D-PBS-Tween), blocked for 2 h with D-PBS containing 5% fetal bovine serum (FBS) at 37°C, and rinsed with RPMI 1640 containing 10% FBS. Splenocytes from individual mice were stimulated with the CS peptide pool for 18 h at 37°C. Following incubation, the plates were washed six times with D-PBS-Tween and once with distilled water. The plates were then incubated with 2 μg/ml biotinylated anti-mouse IFN-γ (BD Pharmingen, San Diego, CA) for 2 h at room temperature, washed six times with D-PBS-Tween, and incubated for 2 h with a 1:500 dilution of streptavidin-alkaline phosphatase (Southern Biotechnology Associates, Birmingham, AL). Following six washes with D-PBS-Tween and one with PBS, the plates were developed with nitroblue tetrazolium-5-bromo-4-chloro-3-indolyl-phosphate chromogen (Pierce, Rockford, IL), the reaction was stopped with tap water, air dried, and read using an ELISPOT reader (Aelvis GmbH). The numbers of spot-forming units (SFU) per 106 cells were calculated. In the case of the ICS, splenocytes from individual animals were stimulated with the CS peptide pool or cultured with medium alone. All cultures contained monensin (GolgiStop; BD Biosciences) as well as 1 μg/ml anti-CD49d (BD Biosciences). The cultured cells were stained with monoclonal antibodies specific for cell surface molecules (CD4 and CD8). After fixing with Cytofix/Cytoperm solution (BD Biosciences), cells were permeabilized and stained with antibodies specific for mouse IFN-γ. Approximately 200,000 to 1,000,000 events were collected per sample. The background levels of cytokine staining were typically lower than 0.01% for CD4+ T cells and lower than 0.05% for CD8+ T cells.
The T-helper response induced by the different vaccination regimens was evaluated using a cytometric bead array (CBA) assay. Splenocytes from individual mice were stimulated with 5 μg/ml yeast-produced CS protein. After 48 h of incubation at 37°C, supernatants were harvested and analyzed for the presence of the Th1 (IFN-γ, TNF-α, and interleukin-2 [IL-2]), Th2 (IL-4, IL-6, and IL-10), and Th17 (IL-17) cytokines by using a mouse Th1/Th2/Th17 cytokine kit according to the protocol provided by the manufacturer (BD Biosciences).
CS-specific antibody assays.
CS-specific antibody responses were assessed by an ELISA as previously described (34). Ninety-six-well microtiter plates (Maxisorp; Nunc) were coated overnight at 4°C with 2 μg/ml of CS-specific (NANP)6C peptide in 0.05 M carbonate buffer (pH 9.6). Plates were washed three times and blocked with PBS containing 1% bovine serum albumin (BSA) and 0.05% Tween 20 for 1 h at 37°C. After the plates were washed three times, 1:100-diluted individual serum samples were added to the wells and diluted 2-fold serially in PBS containing 0.2% BSA and 0.05% Tween 20. Plates were incubated for 2 h at 37°C. Plates were washed three times and incubated with biotin-labeled anti-mouse or anti-rabbit IgG (Dako, Denmark), followed by horseradish peroxidase-conjugated streptavidin (Pharmingen, San Diego, CA) for 30 min each at 37°C. For detection of the IgG subclasses, samples were incubated with horseradish peroxidase-labeled anti-mouse IgG1 or IgG2a antibodies (Southern Biotech, Birmingham, AL). Finally, the plates were washed and 100 μl of o-phenylenediamine dihydrochloride (OPD) substrate (Pierce, Rockford, IL) was added to each well. After 10 min, the reaction was stopped by the addition of 100 μl/well of 1 M H2SO4. The optical density was measured at 492 nm by using a Bio-Tek reader (Bio-Tek Instruments, Winooski, VT). The ELISA units were calculated relative to the optical density (OD) curve of the serially diluted standard serum, with one ELISA unit corresponding to the serum dilution at 50% of the maximum of the standard curve. The IgG2a/IgG1 ratio was determined using titer values of IgG1 and IgG2a antibodies, which are expressed as the inverse of serum dilution.
Statistical analyses.
Comparisons of geometric mean immune responses were performed by a Student t test after logarithmic transformation to account for two test groups. Comparisons of geometric mean immune responses were performed by analyses of variance (ANOVA) with Tukey adjustments after logarithmic transformation to account for multiple comparisons. In all cases, P values lower than 0.05 were considered significant.
RESULTS
Immunogenicity of CS protein prime followed by Ad35.CS boost.
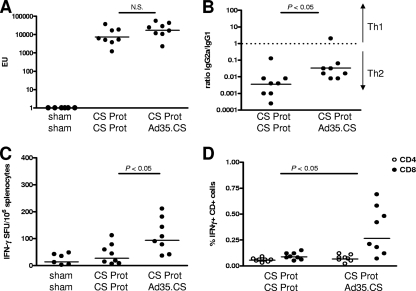
CS-specific humoral response induced in BALB/c mice with a CS protein prime and Ad35.CS boost at 2 weeks postimmunization was assessed by ELISA (Fig. 2A and B), while the cellular immune responses were measured using an IFN-γ ELISPOT assay (Fig. 2C) and ICS (Fig. 2D). The homologous prime-boost regimen with the CS protein elicited a very potent CS-specific IgG response. The levels of the antibody response elicited by the heterologous CS protein/Ad35.CS regimen were comparable to those seen for the homologous CS protein prime-boost regimen (P value of >0.05 for comparison of CS-specific IgG levels by ANOVA). Besides determining the total CS-specific IgG levels, we determined the IgG2a/IgG1 ratio to obtain indications of the type of T-helper responses induced by the different prime-boost regimens (Fig. 2B). The homologous CS protein prime-boost regimen elicited primarily IgG1 antibody responses, indicating a more Th2-type immune response, while replacing the protein boost with an Ad35.CS boost resulted in a more pronounced induction of IgG2a antibodies, indicating a shift toward a Th1-type response (P value of <0.05 for comparison of IgG2a/IgG1 ratios by ANOVA).
FIG. 2.
Immunogenicity of heterologous prime-boost regimen comprised of the yeast-produced CS protein (Prot) and Ad35.CS. BALB/c mice (8 per group) were immunized as indicated in the graphs. A negative-control group received the adjuvant and Ad35.Empty vector (sham). Two weeks after the boost immunization, CS-specific humoral immune responses were assessed by CS-specific IgG responses by using an ELISA (A) and IgG2a/IgG1 ratios upon measurement of CS-specific IgG2a and IgG1 responses (B). CS-specific CD8+ T-cell immune responses were assessed by an IFN-γ ELISPOT assay (C) and IFN-γ ICS (D). Bars represent geometric means of ELISA units (EU; A), IgG2a/IgG1 ratios (B), spot-forming units (SFU; C), or percentages of IFN-γ+ CD4+- or IFN-γ+ CD8+-positive cells (D). The background level of cytokine staining was typically lower than 0.01% for the CD4+ T cells and lower than 0.05% for the CD8+ T cells. N.S., not significant.
Evaluation of the CS-specific T-cell responses by using ELISPOT (Fig. 2C) and ICS (Fig. 2D) assays showed that the homologous CS protein regimen evoked a poor but measurable CS-specific T-cell response. The inclusion of Ad35.CS as a boost to the CS protein prime resulted in significantly increased levels of CS-specific IFN-γ-producing CD8+ T cells (P value of <0.05 for comparison of CS-specific CD8+ T-cell levels by ANOVA). This correlated to the more Th1-type response for the CS protein/Ad35.CS regimens as determined by CS-specific IgG2a/IgG1 ratio. It should be noted that the IFN-γ+ CD4+ response might have been underestimated using the stimulation with the 15-mer peptides, as we observed this in another study (40). Stimulation of splenocytes with the CS protein in the current study did show higher CD4+ responses; however, the background in the assay was unacceptably high (data not shown).
Immunogenicity of a three-component heterologous prime-boost regimen.
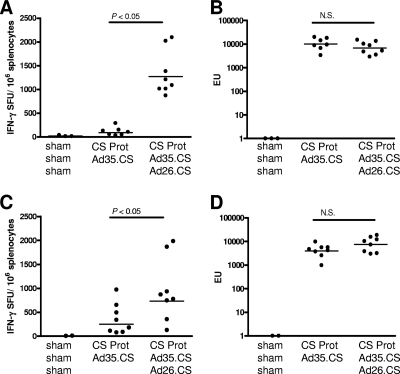
The combination of the yeast-produced CS protein with the Ad35.CS in a heterologous prime-boost regimen resulted in the induction of a high level of IFN-γ+ CD8+ T cells and maintained a high-level CS-specific IgG response, and the antibody response was shifted toward the Th1 type. We next investigated whether a prime-boost regimen comprised of the three components, CS protein, Ad35.CS, and Ad26.CS, might result in an even more robust and sustained Th1 immune response. Our earlier experiments demonstrated that the Ad35.CS/Ad26.CS combination induced significantly higher immune responses than the Ad35.CS/Ad35.CS combination (data not shown), and therefore, the homologous Ad35.CS/Ad35.CS adenovirus vector combination was not included as a booster in the current study. A group of mice received a prime with a CS protein with adjuvant and a boost with Ad35.CS followed by a second boost with Ad26.CS (three-component heterologous prime-boost). A comparator group of mice received a prime with a CS protein with adjuvant followed by an Ad35.CS boost. At 2 weeks after the final boost immunization, mice receiving the three-component heterologous prime-boost regimen showed significantly higher levels of CS-specific IFN-γ-producing CD8+ T cells than the mice receiving the CS protein prime and Ad35.CS boost regimen (P value of <0.05 for comparison of CS-specific IFN-γ-producing CD8+ T-cell levels by ANOVA; Fig. 3A). At 8 weeks after the final boost immunization, the IFN-γ+ CD8+ T-cell response induced by the three-component prime-boost regimen was still significantly higher than that induced by the CS protein/Ad35.CS regimen (P value of <0.05 for comparison of CS-specific IFN-γ-producing CD8+ T-cell levels by ANOVA; Fig. 3C). Importantly, at both time points, the levels of CS-specific IgG responses induced by the three-component prime-boost regimen were comparable to those seen for the CS protein/Ad35.CS regimen (P value of >0.05 for comparison of CS-specific IgG levels by ANOVA; Fig. 3B and D). The IgG2a/IgG1 ratio of CS-specific antibodies induced with the CS protein/Ad35.CS/Ad26.CS vaccine regimen was comparable to the ratio induced with the CS protein/Ad35.CS immunization (data not shown).
FIG. 3.
Immunogenicity of a three-component heterologous prime-boost regimen. BALB/c mice (8 per group) were immunized as indicated in the graphs. A negative-control group received the adjuvant and Ad.Empty vectors (sham). Two weeks (A) and eight weeks (C) after the final boost immunization, CS-specific IFN-γ+ CD8+ T-cell responses were assessed using an ELISPOT assay. Two weeks (B) and eight weeks (D) after the final boost immunization, CS-specific humoral immune responses were assessed by use of an IgG ELISA. Bars represent geometric mean numbers of spot-forming units (SFU; A and C) or ELISA units (EU; B and D).
Cytokine profiles induced by the different vaccination regimens.
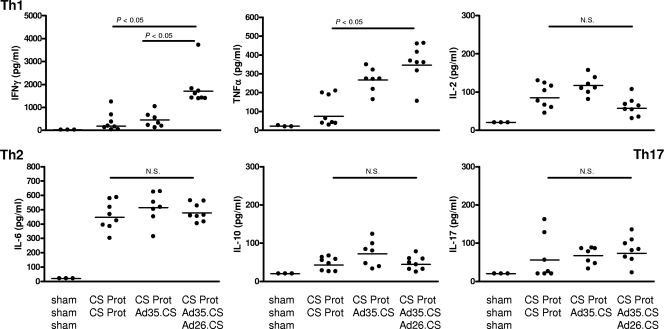
The total number of CS-specific CD4+ T cells expressing two or more immune markers, namely, Th1 cytokines IFN-γ, TNF-α, IL-2, and activation marker CD40L, induced upon immunization with RTS,S, has been associated with protection to malaria infection in the human challenge model (22). We investigated cytokine profile breadth induced in CS-specific T cells with a three-component malaria vaccine, CS protein/Ad35.CS/Ad26.CS, and compared it to the cytokine profiles induced with a CS protein/CS protein or CS protein/Ad35.CS regimen. Two weeks after the final boost immunization, expression levels of the Th1 (IFN-γ, TNF-α, and IL-2), Th2 (IL-4, IL-6, and IL-10), and Th17 (IL-17) cytokines were determined using the cytometric bead array (CBA) assay upon 48-h in vitro stimulation of splenocytes with the CS protein. The CBA assay with protein stimulation provides a blueprint of the types of T-helper cells that have been induced with the vaccination regimen. All vaccination regimens, except for the sham, induced the tested cytokines, with the exception of IL-4, which was not detected (Fig. 4 and data not shown). The CS protein/Ad35.CS/Ad26.CS regimen induced significantly higher levels of IFN-γ and TNF-α than either the CS protein or the CS protein/Ad35.CS regimen (P value of <0.05 for comparison of cytokine levels by ANOVA; Fig. 4). The levels of other cytokines (IL-2, IL-6, IL-10, and IL-17) were comparable for all immunization regimens (P value of >0.05 for comparison of cytokine levels by ANOVA; Fig. 4).
FIG. 4.
Cytokine profiles induced by the different vaccination regimens. BALB/c mice (8 per group) were immunized as indicated in the graphs. A negative-control group received the adjuvant and Ad.Empty vectors (sham). Two weeks after the final boost immunization, cytokine expression was assessed by a CBA assay upon 48-h in vitro stimulation of splenocytes with the CS protein. Bars represent geometric means (pg/ml) of IFN-γ, TNF-α, IL-2, IL-6, IL-10, or IL-17 cytokine levels. Measurable levels of IL-4 were not detected in any of the immunized mice.
In summary, these data confirm that a prime-boost regimen comprising of the three components, CS protein, Ad35.CS, and Ad26.CS, results in a robust and broad Th1-type immune response.
DISCUSSION
Immunizations with a CS protein vaccine elicit potent antibody responses but poor cellular responses. In this study, we demonstrated that vaccination with the CS protein followed by an Ad35.CS vector in a heterologous prime-boost regimen results in enhancement of IFN-γ+ CD8+ T-cell responses. The boost with Ad35.CS did not hamper the level of CS-specific humoral response induced with the protein vaccination but shifted the Ig isotypes toward a Th1-type response. In addition, we established that a heterologous prime-boost regimen comprising a CS protein prime followed by boosts with Ad35.CS and Ad26.CS elicits strong CS-specific Th1-type responses, with a durable enhancement of the IFN-γ+ CD8+ T cells and potent antibody responses.
The ongoing phase III trial of RTS,S, a CS-protein based vaccine with an AS01 adjuvant, represents a breakthrough for malaria vaccine development. The vaccine induces primarily antibody responses and has been shown to partially protect young children and infants in areas of malaria endemicity, reducing the risk of clinical episodes of malaria by 53% over an 8-month follow-up period (5). While RTS,S gives a better chance of surviving to the most vulnerable part of the population, it is clear that more must be done in order to develop vaccines that will provide greater and more sustainable levels of protection to fully eradicate malaria.
IFN-γ+ CD8+ T cells have been associated with protection against liver-stage malaria parasites, as they inactivate and eliminate intracellular parasites through IFN-γ-induced production of nitric oxide and through cell-mediated cytotoxicity (6, 12, 44, 48). Therefore, it is widely accepted that persistent protective immunity against malaria likely requires high levels of Th1-type immune responses targeting the preerythrocytic stage of the malaria parasites. Such a complex immunity is not easily achieved by single-vaccine modalities, as demonstrated by the low number of malaria vaccine regimens in advanced clinical trials (39).
Adenoviral vectors are known to induce high levels of antigen-specific IFN-γ+ CD8+ T cells (53). Combining adenoviral vectors with other vaccine types has proven highly efficient in eliciting strong and sustainable T-cell immunity as well as humoral responses (7, 14, 26, 49-51). Indeed, within this study, we showed that priming with a yeast-produced CS protein with adjuvant followed by the Ad35.CS boost resulted in the induction of high-level CS-specific IFN-γ+ CD8+ T-cell responses compared to those induced by an exclusively protein-based vaccine regimen. Importantly, while the overall CS-specific IgG levels were not affected relative to the responses induced with an entirely CS protein-based vaccination regimen, the CS protein/Ad35.CS regimen elicited a more Th1-type response. These results corroborated our earlier findings in which prime-boost regimens comprised of Ad35 vaccine vectors expressing CS or LSA-1 and RTS,S or a LSA-1 protein vaccine resulted in potent Th1-type T-cell responses and high-level humoral responses (45, 52).
Previously, we reported on the heterologous prime-boost regimen utilizing the Ad35.CS and Ad5.CS vaccine vectors that elicited high levels of CS-specific IFN-γ-producing T cells in both mice and nonhuman primates (46). These results demonstrated the potential of adenoviral-vector-based heterologous prime-boost regimens to induce the type of immunity required to combat malaria. Because of the high Ad5 seroprevalence in the human population, considerable effort has been directed toward the development of novel low-seroprevalence adenoviral vaccine vectors that are able to circumvent anti-Ad5 immunity and are highly immunogenic (1, 4, 13, 17, 25, 36, 56). The Ad26-based vaccine vector has been shown to be a particularly interesting vector, considering its ability to induce immune responses in mice (1), nonhuman primates (27, 28), and humans (3), and it is particularly suited as a boost to other adenoviral-vector-based vaccines that utilize different adenovirus serotypes. Given the wide diversity of adenoviruses in nature, many different serotypes are potentially available. In our study, the inclusion of the Ad26.CS boost to the CS protein/Ad35.CS prime-boost regimen elicited an overall higher and more sustainable CS-specific IFN-γ+ CD8+ immune response than the homologous or the 2-component heterologous prime-boost regimens.
The recent association of Th1 cytokine-expressing CD4+ T cells, induced with the RTS,S vaccine, with protection against malaria infection in the human challenge model has reinforced the view that induction of a broad immune response of the Th1 type is required for development of efficient malaria vaccines (22). Induction of balanced proinflammatory and regulatory immune responses is also a key factor determining the outcome of malaria infection. Failure to develop an effective proinflammatory response might result in unrestricted parasite replication, whereas failure to control this response can lead to the development of severe immunopathology (10). Boosting of the CS protein vaccine with Ad35.CS and, in particular, with the Ad35.CS/Ad26.CS combination strongly enhanced the levels of Th1 cytokines IFN-γ and TNF-α, while the levels of Th1 cytokine IL-2, Th2 cytokines IL-6 and IL-10, and Th17 cytokine IL-17 were comparable to the levels induced with the CS protein vaccine alone. This result indicated the capacity of the three-component regimen to stimulate an overall balanced cytokine response, with a strong shift toward the Th1 responses compared to the homologous CS protein regimen, which induced a primarily Th2-biased response. While the role for the Th1-type response in protection against malaria has been well documented (6, 12, 19, 20, 44, 48), to our knowledge, there are no reports concerning the role of Th17 cells in malaria infection. However, there is mounting evidence that IL-17 might be relevant for protection against parasitic infections, as has been indicated by the induction of this cytokine in response to infection with a number of parasites, such as Eimeria maxima (18), Nippostrongylus brasiliensis (29), and Leishmania donovani (37). For instance, production of IL-17 and IL-22 in humans was shown to have a strong and independent association with protection from kala-azar disease, caused by L. donovani (37). Other studies have also indicated a role for Th17 cells in protection against other pathogens, such as Mycobacterium tuberculosis (2, 23, 24), Streptococcus pneumoniae (30, 31, 59), Helicobacter pylori (11, 55), and influenza virus (15, 58). In the current study, although no significant difference was observed between the mean levels of the IL-17 cytokines of different groups, the adenoviral-vector-containing regimens induced more-uniform IL-17 responses than protein immunization.
The limited and short-lived protection induced with the CS protein vaccine points in two directions of improvement using Ad.CS-based vaccines. One direction is administration of an Ad35.CS vaccine as a priming vaccine for the CS protein vaccine, to strongly increase Th1 cellular responses, as described by Stewart et al. (52). The second direction, as demonstrated in the current study, is administration of the Ad35.CS/Ad26.CS combination as a booster vaccine (in the second year of life or even at school age) following an early-in-life protein CS vaccine to induce long-lasting protection for which the Th1-type response and immune memory are required.
Acknowledgments
We acknowledge financial support from the European Commission (FP6 grant for the project PRIBOMAL).
Footnotes
Published ahead of print on 8 September 2010.
REFERENCES
- 1.Abbink, P., A. A. Lemckert, B. A. Ewald, D. M. Lynch, M. Denholtz, S. Smits, L. Holterman, I. Damen, R. Vogels, A. R. Thorner, K. L. O'Brien, A. Carville, K. G. Mansfield, J. Goudsmit, M. J. Havenga, and D. H. Barouch. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81:4654-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aujla, S. J., Y. R. Chan, M. Zheng, M. Fei, D. J. Askew, D. A. Pociask, T. A. Reinhart, F. McAllister, J. Edeal, K. Gaus, S. Husain, J. L. Kreindler, P. J. Dubin, J. M. Pilewski, M. M. Myerburg, C. A. Mason, Y. Iwakura, and J. K. Kolls. 2008. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 14:275-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barouch, D. 2009. First-in-human phase 1 safety and immunogenicity of an adenovirus serotype 26 HIV-1 vaccine vector Oral abstract session 5, new vaccinal approaches. In AIDS Vaccine Conf., 19 to 22 October 2009, Paris, France.
- 4.Barouch, D. H., M. G. Pau, J. H. Custers, W. Koudstaal, S. Kostense, M. J. Havenga, D. M. Truitt, S. M. Sumida, M. G. Kishko, J. C. Arthur, B. Korioth-Schmitz, M. H. Newberg, D. A. Gorgone, M. A. Lifton, D. L. Panicali, G. J. Nabel, N. L. Letvin, and J. Goudsmit. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 172:6290-6297. [DOI] [PubMed] [Google Scholar]
- 5.Bejon, P., J. Lusingu, A. Olotu, A. Leach, M. Lievens, J. Vekemans, S. Mshamu, T. Lang, J. Gould, M. C. Dubois, M. A. Demoitie, J. F. Stallaert, P. Vansadia, T. Carter, P. Njuguna, K. O. Awuondo, A. Malabeja, O. Abdul, S. Gesase, N. Mturi, C. J. Drakeley, B. Savarese, T. Villafana, W. R. Ballou, J. Cohen, E. M. Riley, M. M. Lemnge, K. Marsh, and L. von Seidlein. 2008. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N. Engl. J. Med. 359:2521-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carvalho, L. J., C. T. Daniel-Ribeiro, and H. Goto. 2002. Malaria vaccine: candidate antigens, mechanisms, constraints and prospects. Scand. J. Immunol. 56:327-343. [DOI] [PubMed] [Google Scholar]
- 7.Casimiro, D. R., A. J. Bett, T. M. Fu, M. E. Davies, A. Tang, K. A. Wilson, M. Chen, R. Long, T. McKelvey, M. Chastain, S. Gurunathan, J. Tartaglia, E. A. Emini, and J. Shiver. 2004. Heterologous human immunodeficiency virus type 1 priming-boosting immunization strategies involving replication-defective adenovirus and poxvirus vaccine vectors. J. Virol. 78:11434-11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catanzaro, A. T., R. A. Koup, M. Roederer, R. T. Bailer, M. E. Enama, Z. Moodie, L. Gu, J. E. Martin, L. Novik, B. K. Chakrabarti, B. T. Butman, J. G. Gall, C. R. King, C. A. Andrews, R. Sheets, P. L. Gomez, J. R. Mascola, G. J. Nabel, and B. S. Graham. 2006. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J. Infect. Dis. 194:1638-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clyde, D. F., H. Most, V. C. McCarthy, and J. P. Vanderberg. 1973. Immunization of man against sporozite-induced falciparum malaria. Am. J. Med. Sci. 266:169-177. [DOI] [PubMed] [Google Scholar]
- 10.Couper, K. N., D. G. Blount, M. S. Wilson, J. C. Hafalla, Y. Belkaid, M. Kamanaka, R. A. Flavell, J. B. de Souza, and E. M. Riley. 2008. IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog. 4:e1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLyria, E. S., R. W. Redline, and T. G. Blanchard. 2009. Vaccination of mice against H pylori induces a strong Th-17 response and immunity that is neutrophil dependent. Gastroenterology 136:247-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doolan, D. L., and N. Martinez-Alier. 2006. Immune response to pre-erythrocytic stages of malaria parasites. Curr. Mol. Med. 6:169-185. [DOI] [PubMed] [Google Scholar]
- 13.Fitzgerald, J. C., G. P. Gao, A. Reyes-Sandoval, G. N. Pavlakis, Z. Q. Xiang, A. P. Wlazlo, W. Giles-Davis, J. M. Wilson, and H. C. Ertl. 2003. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J. Immunol. 170:1416-1422. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert, S. C., J. Schneider, C. M. Hannan, J. T. Hu, M. Plebanski, R. Sinden, and A. V. Hill. 2002. Enhanced CD8 T cell immunogenicity and protective efficacy in a mouse malaria model using a recombinant adenoviral vaccine in heterologous prime-boost immunisation regimes. Vaccine 20:1039-1045. [DOI] [PubMed] [Google Scholar]
- 15.Hamada, H., L. Garcia-Hernandez Mde, J. B. Reome, S. K. Misra, T. M. Strutt, K. K. McKinstry, A. M. Cooper, S. L. Swain, and R. W. Dutton. 2009. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J. Immunol. 182:3469-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Havenga, M., R. Vogels, D. Zuijdgeest, K. Radosevic, S. Mueller, M. Sieuwerts, F. Weichold, I. Damen, J. Kaspers, A. Lemckert, M. van Meerendonk, R. van der Vlugt, L. Holterman, D. Hone, Y. Skeiky, R. Mintardjo, G. Gillissen, D. Barouch, J. Sadoff, and J. Goudsmit. 2006. Novel replication-incompetent adenoviral B-group vectors: high vector stability and yield in PER.C6 cells. J. Gen. Virol. 87:2135-2143. [DOI] [PubMed] [Google Scholar]
- 17.Holterman, L., R. Vogels, R. van der Vlugt, M. Sieuwerts, J. Grimbergen, J. Kaspers, E. Geelen, E. van der Helm, A. Lemckert, G. Gillissen, S. Verhaagh, J. Custers, D. Zuijdgeest, B. Berkhout, M. Bakker, P. Quax, J. Goudsmit, and M. Havenga. 2004. Novel replication-incompetent vector derived from adenovirus type 11 (Ad11) for vaccination and gene therapy: low seroprevalence and non-cross-reactivity with Ad5. J. Virol. 78:13207-13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong, Y. H., H. S. Lillehoj, E. P. Lillehoj, and S. H. Lee. 2006. Changes in immune-related gene expression and intestinal lymphocyte subpopulations following Eimeria maxima infection of chickens. Vet. Immunol. Immunopathol. 114:259-272. [DOI] [PubMed] [Google Scholar]
- 19.John, C. C., A. M. Moormann, D. C. Pregibon, P. O. Sumba, M. M. McHugh, D. L. Narum, D. E. Lanar, M. D. Schluchter, and J. W. Kazura. 2005. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am. J. Trop. Med. Hyg. 73:222-228. [PubMed] [Google Scholar]
- 20.John, C. C., A. J. Tande, A. M. Moormann, P. O. Sumba, D. E. Lanar, X. M. Min, and J. W. Kazura. 2008. Antibodies to pre-erythrocytic Plasmodium falciparum antigens and risk of clinical malaria in Kenyan children. J. Infect. Dis. 197:519-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kester, K. E., J. F. Cummings, C. F. Ockenhouse, R. Nielsen, B. T. Hall, D. M. Gordon, R. J. Schwenk, U. Krzych, C. A. Holland, G. Richmond, M. G. Dowler, J. Williams, R. A. Wirtz, N. Tornieporth, L. Vigneron, M. Delchambre, M. A. Demoitie, W. R. Ballou, J. Cohen, and D. G. Heppner, Jr. 2008. Phase 2a trial of 0, 1, and 3 month and 0, 7, and 28 day immunization schedules of malaria vaccine RTS,S/AS02 in malaria-naive adults at the Walter Reed Army Institute of Research. Vaccine 26:2191-2202. [DOI] [PubMed] [Google Scholar]
- 22.Kester, K. E., J. F. Cummings, O. Ofori-Anyinam, C. F. Ockenhouse, U. Krzych, P. Moris, R. Schwenk, R. A. Nielsen, Z. Debebe, E. Pinelis, L. Juompan, J. Williams, M. Dowler, V. A. Stewart, R. A. Wirtz, M. C. Dubois, M. Lievens, J. Cohen, W. R. Ballou, and D. G. Heppner, Jr. 2009. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J. Infect. Dis. 200:337-346. [DOI] [PubMed] [Google Scholar]
- 23.Khader, S. A., G. K. Bell, J. E. Pearl, J. J. Fountain, J. Rangel-Moreno, G. E. Cilley, F. Shen, S. M. Eaton, S. L. Gaffen, S. L. Swain, R. M. Locksley, L. Haynes, T. D. Randall, and A. M. Cooper. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8:369-377. [DOI] [PubMed] [Google Scholar]
- 24.Khader, S. A., J. E. Pearl, K. Sakamoto, L. Gilmartin, G. K. Bell, D. M. Jelley-Gibbs, N. Ghilardi, F. deSauvage, and A. M. Cooper. 2005. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J. Immunol. 175:788-795. [DOI] [PubMed] [Google Scholar]
- 25.Lemckert, A. A., J. Grimbergen, S. Smits, E. Hartkoorn, L. Holterman, B. Berkhout, D. H. Barouch, R. Vogels, P. Quax, J. Goudsmit, and M. J. Havenga. 2006. Generation of a novel replication-incompetent adenoviral vector derived from human adenovirus type 49: manufacture on PER.C6 cells, tropism and immunogenicity. J. Gen. Virol. 87:2891-2899. [DOI] [PubMed] [Google Scholar]
- 26.Letvin, N. L., Y. Huang, B. K. Chakrabarti, L. Xu, M. S. Seaman, K. Beaudry, B. Korioth-Schmitz, F. Yu, D. Rohne, K. L. Martin, A. Miura, W. P. Kong, Z. Y. Yang, R. S. Gelman, O. G. Golubeva, D. C. Montefiori, J. R. Mascola, and G. J. Nabel. 2004. Heterologous envelope immunogens contribute to AIDS vaccine protection in rhesus monkeys. J. Virol. 78:7490-7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, J., B. A. Ewald, D. M. Lynch, M. Denholtz, P. Abbink, A. A. Lemckert, A. Carville, K. G. Mansfield, M. J. Havenga, J. Goudsmit, and D. H. Barouch. 2008. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J. Virol. 82:4844-4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, J., K. L. O'Brien, D. M. Lynch, N. L. Simmons, A. La Porte, A. M. Riggs, P. Abbink, R. T. Coffey, L. E. Grandpre, M. S. Seaman, G. Landucci, D. N. Forthal, D. C. Montefiori, A. Carville, K. G. Mansfield, M. J. Havenga, M. G. Pau, J. Goudsmit, and D. H. Barouch. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457:87-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, Z., H. Lin, Q. Liu, G. Mousavi, and W. C. Gause. 2008. The parasite Nippostrongylus brasiliensis induces multiple regulatory pathways that control increases of IL-17 expression and associated pathology in the lung. FASEB J. 22:848.36. [Google Scholar]
- 30.Lu, Y. J., J. Gross, D. Bogaert, A. Finn, L. Bagrade, Q. Zhang, J. K. Kolls, A. Srivastava, A. Lundgren, S. Forte, C. M. Thompson, K. F. Harney, P. W. Anderson, M. Lipsitch, and R. Malley. 2008. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 4:e1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malley, R., A. Srivastava, M. Lipsitch, C. M. Thompson, C. Watkins, A. Tzianabos, and P. W. Anderson. 2006. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect. Immun. 74:2187-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mettens, P., P. M. Dubois, M. A. Demoitie, B. Bayat, M. N. Donner, P. Bourguignon, V. A. Stewart, D. G. Heppner, Jr., N. Garcon, and J. Cohen. 2008. Improved T cell responses to Plasmodium falciparum circumsporozoite protein in mice and monkeys induced by a novel formulation of RTS,S vaccine antigen. Vaccine 26:1072-1082. [DOI] [PubMed] [Google Scholar]
- 33.Ophorst, O. J., K. Radosevic, M. J. Havenga, M. G. Pau, L. Holterman, B. Berkhout, J. Goudsmit, and M. Tsuji. 2006. Immunogenicity and protection of a recombinant human adenovirus serotype 35-based malaria vaccine against Plasmodium yoelii in mice. Infect. Immun. 74:313-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ophorst, O. J., K. Radosevic, K. Ouwehand, W. van Beem, R. Mintardjo, J. Sijtsma, J. Kaspers, A. Companjen, L. Holterman, J. Goudsmit, and M. J. Havenga. 2007. Expression and immunogenicity of the Plasmodium falciparum circumsporozoite protein: the role of GPI signal sequence. Vaccine 25:1426-1436. [DOI] [PubMed] [Google Scholar]
- 35.Overstreet, M. G., I. A. Cockburn, Y. C. Chen, and F. Zavala. 2008. Protective CD8 T cells against Plasmodium liver stages: immunobiology of an ‘unnatural’ immune response. Immunol. Rev. 225:272-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinto, A. R., J. C. Fitzgerald, W. Giles-Davis, G. P. Gao, J. M. Wilson, and H. C. Ertl. 2003. Induction of CD8+ T cells to an HIV-1 antigen through a prime boost regimen with heterologous E1-deleted adenoviral vaccine carriers. J. Immunol. 171:6774-6779. [DOI] [PubMed] [Google Scholar]
- 37.Pitta, M. G., A. Romano, S. Cabantous, S. Henri, A. Hammad, B. Kouriba, L. Argiro, M. el Kheir, B. Bucheton, C. Mary, S. H. El-Safi, and A. Dessein. 2009. IL-17 and IL-22 are associated with protection against human kala azar caused by Leishmania donovani. J. Clin. Invest. 119:2379-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Priddy, F. H., D. Brown, J. Kublin, K. Monahan, D. P. Wright, J. Lalezari, S. Santiago, M. Marmor, M. Lally, R. M. Novak, S. J. Brown, P. Kulkarni, S. A. Dubey, L. S. Kierstead, D. R. Casimiro, R. Mogg, M. J. DiNubile, J. W. Shiver, R. Y. Leavitt, M. N. Robertson, D. V. Mehrotra, and E. Quirk. 2008. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin. Infect. Dis. 46:1769-1781. [DOI] [PubMed] [Google Scholar]
- 39.Radošević, K., A. Rodriguez, A. Lemckert, and J. Goudsmit. 2009. Heterologous prime-boost vaccinations for poverty-related diseases: advantages and future prospects. Expert Rev. Vaccines 8:577-592. [DOI] [PubMed] [Google Scholar]
- 40.Radošević, K., A. Rodriguez, R. Mintardjo, D. Tax, K. L. Bengtsson, C. Thompson, M. Zambon, G. J. Weverling, F. Uytdehaag, and J. Goudsmit. 2008. Antibody and T-cell responses to a virosomal adjuvanted H9N2 avian influenza vaccine: impact of distinct additional adjuvants. Vaccine 26:3640-3646. [DOI] [PubMed] [Google Scholar]
- 41.Radošević, K., C. W. Wieland, A. Rodriguez, G. J. Weverling, R. Mintardjo, G. Gillissen, R. Vogels, Y. A. Skeiky, D. M. Hone, J. Sadoff, T. van der Poll, M. Havenga, and J. Goudsmit. 2007. Protective immune responses to a recombinant adenovirus type 35 tuberculosis vaccine in two mouse strains: CD4 and CD8 T-cell epitope mapping and role of gamma interferon. Infect. Immun. 75:4105-4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts, D. M., A. Nanda, M. J. Havenga, P. Abbink, D. M. Lynch, B. A. Ewald, J. Liu, A. R. Thorner, P. E. Swanson, D. A. Gorgone, M. A. Lifton, A. A. Lemckert, L. Holterman, B. Chen, A. Dilraj, A. Carville, K. G. Mansfield, J. Goudsmit, and D. H. Barouch. 2006. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 441:239-243. [DOI] [PubMed] [Google Scholar]
- 43.Rodrigues, E. G., F. Zavala, D. Eichinger, J. M. Wilson, and M. Tsuji. 1997. Single immunizing dose of recombinant adenovirus efficiently induces CD8+ T cell-mediated protective immunity against malaria. J. Immunol. 158:1268-1274. [PubMed] [Google Scholar]
- 44.Rodrigues, M. M., A. S. Cordey, G. Arreaza, G. Corradin, P. Romero, J. L. Maryanski, R. S. Nussenzweig, and F. Zavala. 1991. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int. Immunol. 3:579-585. [DOI] [PubMed] [Google Scholar]
- 45.Rodríguez, A., J. Goudsmit, A. Companjen, R. Mintardjo, G. Gillissen, D. Tax, J. Sijtsma, G. J. Weverling, L. Holterman, D. E. Lanar, M. J. Havenga, and K. Radosevic. 2008. Impact of recombinant adenovirus serotype 35 priming versus boosting of a Plasmodium falciparum protein: characterization of T- and B-cell responses to liver-stage antigen 1. Infect. Immun. 76:1709-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodríguez, A., R. Mintardjo, D. Tax, G. Gillissen, J. Custers, M. G. Pau, J. Klap, S. Santra, H. Balachandran, N. L. Letvin, J. Goudsmit, and K. Radosevic. 2009. Evaluation of a prime-boost vaccine schedule with distinct adenovirus vectors against malaria in rhesus monkeys. Vaccine 27:6226-6233. [DOI] [PubMed] [Google Scholar]
- 47.Schofield, L., J. Villaquiran, A. Ferreira, H. Schellekens, R. Nussenzweig, and V. Nussenzweig. 1987. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 330:664-666. [DOI] [PubMed] [Google Scholar]
- 48.Seguin, M. C., F. W. Klotz, I. Schneider, J. P. Weir, M. Goodbary, M. Slayter, J. J. Raney, J. U. Aniagolu, and S. J. Green. 1994. Induction of nitric oxide synthase protects against malaria in mice exposed to irradiated Plasmodium berghei infected mosquitoes: involvement of interferon gamma and CD8+ T cells. J. Exp. Med. 180:353-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiver, J. W., and E. A. Emini. 2004. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu. Rev. Med. 55:355-372. [DOI] [PubMed] [Google Scholar]
- 50.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 51.Skeiky, Y. A., and J. C. Sadoff. 2006. Advances in tuberculosis vaccine strategies. Nat. Rev. Microbiol. 4:469-476. [DOI] [PubMed] [Google Scholar]
- 52.Stewart, V. A., S. M. McGrath, P. M. Dubois, M. G. Pau, P. Mettens, J. Shott, M. Cobb, J. R. Burge, D. Larson, L. A. Ware, M. A. Demoitie, G. J. Weverling, B. Bayat, J. H. Custers, M. C. Dubois, J. Cohen, J. Goudsmit, and D. G. Heppner, Jr. 2007. Priming with an adenovirus 35-circumsporozoite protein (CS) vaccine followed by RTS,S/AS01B boosting significantly improves immunogenicity to Plasmodium falciparum CS compared to that with either malaria vaccine alone. Infect. Immun. 75:2283-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tatsis, N., and H. C. Ertl. 2004. Adenoviruses as vaccine vectors. Mol. Ther. 10:616-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vekemans, J., A. Leach, and J. Cohen. 2009. Development of the RTS,S/AS malaria candidate vaccine. Vaccine 27(Suppl. 6):G67-G71. [DOI] [PubMed] [Google Scholar]
- 55.Velin, D., L. Favre, E. Bernasconi, D. Bachmann, C. Pythoud, E. Saiji, H. Bouzourene, and P. Michetti. 2009. Interleukin-17 is a critical mediator of vaccine-induced reduction of Helicobacter infection in the mouse model. Gastroenterology 136:2237-2246. [DOI] [PubMed] [Google Scholar]
- 56.Vogels, R., D. Zuijdgeest, R. van Rijnsoever, E. Hartkoorn, I. Damen, M. P. de Bethune, S. Kostense, G. Penders, N. Helmus, W. Koudstaal, M. Cecchini, A. Wetterwald, M. Sprangers, A. Lemckert, O. Ophorst, B. Koel, M. van Meerendonk, P. Quax, L. Panitti, J. Grimbergen, A. Bout, J. Goudsmit, and M. Havenga. 2003. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 77:8263-8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weiss, W. R., M. Sedegah, R. L. Beaudoin, L. H. Miller, and M. F. Good. 1988. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc. Natl. Acad. Sci. U. S. A. 85:573-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williman, J., E. Lockhart, L. Slobbe, G. Buchan, and M. Baird. 2006. The use of Th1 cytokines, IL-12 and IL-23, to modulate the immune response raised to a DNA vaccine delivered by gene gun. Vaccine 24:4471-4474. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, Z., T. B. Clarke, and J. N. Weiser. 2009. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J. Clin. Invest. 119:1899-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]